Abstract
The aim of this study was to determine if progesterone modulates object and spatial memory consolidation in young ovariectomized C57BL/6 mice. Object memory was tested in an object recognition task using 24- and 48-hr delays. Spatial memory was tested in a 2-day version of the Morris water maze in which retention was tested 24 or 48 hrs after training. Immediately after training in each task, mice received a single intraperitoneal injection of vehicle or 5, 10, or 20 mg/kg water-soluble progesterone. Mice were then tested 24 or 48 hrs later in the absence of circulating progesterone. Post-training injections of 10 and 20 mg/kg progesterone enhanced object recognition, but not memory in the spatial water maze. These findings suggest that object memory consolidation in young female mice is more sensitive to the modulatory effects of progesterone than spatial memory consolidation, at least using the tasks, doses, and delays tested. As such, these findings may have important implications for the design of progesterone therapies intended to reduce age-related memory decline.
Keywords: Morris water maze, Reference memory, Object recognition, Non-spatial memory, Progestin, Mouse
1. Introduction
Investigations into the effects of estrogen and progestin therapy on memory have yielded conflicting results. Although some studies have reported that treatment with estrogen and progestin improves spatial and verbal memory [10, 25], other studies, including the Women’s Health Initiative (WHI) Memory Study and WHI Study of Cognitive Aging, report little or no benefit of estrogen plus progestin therapy on measures of both global cognitive function and specific memory abilities [23, 33, 34, 40]. These inconsistencies highlight the need to more clearly understand how estrogens and progestins (e.g., progesterone) influence cognitive function.
In rodents, the preponderance of evidence suggests that the potent estrogen 17β-estradiol promotes neural plasticity and improves certain types of memory (for reviews, see [8, 42]). However, fewer studies have investigated the effects of progesterone on memory and, therefore, far less is known about how this important ovarian hormone modulates cognitive function. Some studies suggest that, like 17β-estradiol, progesterone can enhance memory. For example, a single injection of progesterone given immediately after training (i.e., post-training) enhances object recognition, working memory, and inhibitory avoidance in young ovariectomized rats [17, 39]. However, chronic treatment with progesterone prior to training (i.e., pre-training) impairs spatial working memory and footshock avoidance learning in young ovariectomized rats and mice [2, 13]. Further, a single injection of progesterone 4 hrs before training has no beneficial effect on spatial memory in young ovariectomized rats [5, 35].
Together, these studies suggest that the mnemonic effects of progesterone may depend on the timing of administration. Studies reporting beneficial effects of progesterone on memory administered the hormone post-training, whereas those reporting no effect or an impairing effect administered progesterone pre-training. The difference in timing of treatment relative to testing may be important because progesterone can have anxiolytic and analgesic effects [3, 16] which could adversely impact both mnemonic and non-mnemonic (e.g., motor activity, motivation) aspects of task performance. As such, the results of pre-training progesterone administration on performance in memory tasks may not accurately reflect the impact of this hormone on memory, per se. In contrast, treatment immediately post-training, with training and testing conducted in the absence of circulating progesterone, allows for direct observation of the memory consolidating effects of progesterone in the absence of non-mnemonic confounds during training.
To date, single post-training injections of progesterone have been shown to enhance novel object recognition in young female rats [17, 39]. However, previous post-training studies used progesterone dissolved in oil, thus making it unlikely that hormone would be fully metabolized by testing 4 [39] or 24 [17] hrs later. As such, it is unclear whether these effects should be attributed to effects of progesterone on memory consolidation immediately after training or to circulating progesterone during retention testing. In addition, prior studies used a version of the object recognition task in which the total time to explore the objects was limited to 3 min [12]. The primary disadvantage of this procedure is that intrinsic differences among mice in motivation and neophobia may lead to differential exposure to the objects and, thus, influence the time spent with the objects during retention testing. Further, circulating progesterone at the time of retention testing in previous studies [17, 39] may have also contributed to group differences in total exploration time during testing. In general, the differential object exploration confound can be eliminated using a protocol in which total exploration time, rather than total trial time, is fixed. Such a protocol has been shown in previous studies to involve the hippocampus [1,7]. Understanding the effects of progesterone on hippocampal memory is particularly important, given that this type of memory is vulnerable to the detrimental effects of aging [9]. Further, progesterone significantly alters hippocampal physiology by modulating CA1 dendritic spine density [41], synaptic proteins [6], and intracellular signaling [27, 28]. Interestingly, no previous study has examined effects of post-training progesterone on consolidation of spatial memory, which is clearly dependent on the hippocampus [26]. Because acute intraperitoneal injections of 17β-estradiol given immediately after spatial Morris water maze training can significantly enhance spatial memory consolidation in young ovariectomized rats [30] and mice [20], it is possible that post-training progesterone might also facilitate hippocampal-dependent spatial memory. Examining the effects of progesterone on tasks that have been demonstrated to involved the hippocampus would help to clarify the role of this hormone in modulating memory and could provide relevant information for future hormone therapies in aging women.
To eliminate the potential impact of circulating progesterone and differential object exposure on the effects of post-training progesterone, the present study examined effects of acute post-training progesterone injection on object memory consolidation using a rapidly metabolized water-soluble form of progesterone and an object recognition protocol in which total exploration time is fixed. To provide comparison with another type of hippocampal memory [26], spatial memory consolidation was also tested using the Morris water maze. Immediately after training in each task, young ovariectomized mice progesterone were injected intraperitoneally (i.p.) with vehicle or a progesterone-2-hydroxypropyl-β-cyclodextrin (HBC) inclusion complex. HBC-bound hormones are metabolized within 24 hrs [32, 37], allowing retention to be assessed in the absence of circulating progesterone. Spatial memory was tested 24 or 48 hrs after training, and object recognition memory was tested both 24 and 48 hrs after training. Although this post-training study design does not mimic either the natural secretion of progesterone or the effects of chronic hormone treatment, it permits a determination of the specific effects of progesterone on memory in the absence of non-mnemonic confounds during training or testing inherent to circulating progesterone. Distinguishing between the effects of progesterone on memory and other performance factors will be critically important for the design and use of future hormone therapies in women.
2. Materials and Methods
2.1. Subjects
Subjects were C57BL/6 mice (n = 78) ovariectomized by Taconic (Germantown, NY) at 8 weeks of age and shipped to Yale at 9 weeks of age. Mice were housed in a room with a 12:12 light/dark cycle (lights on at 07:00). All behavioral testing occurred during the light phase. Mice were housed up to 5 per shoebox cage and had ad libitum access to food (Harlan 2018 18% Protein Rodent Diet) and water. Prior to behavioral testing, mice were handled 5 times for 5 min per handling session to familiarize them to being picked up by the experimenter. All procedures were approved by the Institutional Animal Care and Use Committee of Yale University, and conformed to the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2. Hormone treatment
Upon arrival at the laboratory, mice were randomly assigned to one of the following four treatment groups: vehicle control, 5 mg/kg progesterone, 10 mg/kg progesterone, or 20 mg/kg progesterone. We have used the 10 and 20 mg/kg doses previously in aged ovariectomized mice and these doses were estimated in physiological range [24]. The 5 mg/kg dose was used in case young females were more sensitive to lower doses. Vehicle-treated mice received acute intraperitoneal (i.p.) injections of 2-hydroxypropyl-β-cyclodextrin (HBC) dissolved in physiological saline (Sigma, St. Louis, MO). Progesterone-treated mice received i.p. injections of a progesterone-HBC inclusion complex dissolved in physiological saline. HBC enhances the solubility for hydrophobic steroid hormones [31] and is metabolized within 24 hrs [32, 37].
An initial set of 42 mice was tested in both object recognition delays and the Morris water maze task with a 24-hour delay. Each bout of testing was separated by two weeks. Samples sizes for this set of mice were as follows: vehicle control (n = 10), 5 mg/kg progesterone (n = 12), 10 mg/kg progesterone (n = 10), or 20 mg/kg progesterone (n = 10). A separate set of mice was tested in the Morris water maze task with a 48-hour delay, with sample sizes as follows: vehicle control (n = 10), 5 mg/kg progesterone (n = 9), 10 mg/kg progesterone (n = 7), or 20 mg/kg progesterone (n = 10).
2.3. Object recognition task
Non-spatial object memory was tested using a novel object recognition task previously shown to involve the hippocampus [1, 7]. The testing apparatus and procedures were conducted as previously described [14, 20]. The testing apparatus was a white open field box (58 cm long × 58 cm wide × 46 cm high) in the center of a dimly lit room. Activity in the open field box was recorded using a video monitor and computer (running custom-written software) located outside of the room. Testing was conducted in three phases: habituation, sample, and choice.
During habituation, each mouse explored the empty open field for 5 min to habituate it to the apparatus. No data were collected during habituation. During the sample phase, each mouse was allowed to freely explore two identical objects in the open field until it had accumulated 30 s of time exploring the objects. Once the mouse spent 30 s exploring the objects, it was removed from the box, injected immediately with vehicle or progesterone, and returned to its home cage.
The choice phase took place 24 or 48 hrs after completion of the sample phase. Vehicle-treated mice typically remember the original object after 24 hrs [19, 22], but not 48 hrs [20, 21]. Therefore, the 24-hr delay permitted observation of impairing effects of progesterone on object recognition, whereas the 48-hr delay permitted observation of progesterone-induced improvements in memory consolidation. At both delays, each mouse was placed in the field with an object they explored during the sample phase (familiar object) and a new (novel) object. The mice were allowed to accumulate a total of 30 s exploring the objects. Time (s) spent with each object was recorded. Because mice inherently explore novel stimuli, more time than chance (15 s) spent with the novel object indicated memory for the familiar object. One mouse in the 20 mg/kg progesterone group did not accumulate 30 s of exploration time within 20 min of the sample phase of 48-hr delay training and was excluded from the data analyses for the 48-hr delay only. Duration (time) of object exploration and elapsed time to accumulate 30 sec of exploration (elapsed time) were recorded during the choice phase to assess treatment effects on exploration.
2.4. Morris water maze
Spatial and non-spatial reference memory was assessed using a 2-day Morris water maze task as previously described [20, 24]. The apparatus was a white circular tank (97 cm in diameter) filled with water (24°C ± 2°C) and made opaque with white nontoxic paint. The maze was surrounded by various extramaze cues. Data were collected using an HVS 2020 (Hampton, England) automated tracking system.
One day prior to testing, mice learned to find a platform using a shaping procedure. Shaping was conducted over four consecutive trials in which a smaller ring (55 cm) was placed inside of the larger (97 cm) ring to decrease the total swimming area. Mice were first placed on a 10 × 10 cm platform covered in red tape and raised just above the surface of the water. They were allowed to sit on the platform for 10 s and then were removed. They were then placed at three distances progressively further from the platform and allowed to swim to the platform. If the mouse did not find the platform within 60 s, then it was led to the platform by the experimenter. No data were collected during shaping.
2.4.1. Spatial trials
During the spatial trials, mice had to learn the location of a transparent lucite platform (10 × 10 cm) submerged just underneath the surface of the water that remained in the same location for all trials. Eight consecutive spatial trials were conducted on Day 1 of testing. Mice were placed in the pool at one of four different start positions, which varied for each mouse on each trial, and given 60 s to find the platform. Once the platform was located, the mouse was allowed to sit on the platform for 10 s. If the mouse did not find the platform within 60 s, then the experimenter led it to the platform by its tail and let it sit on the platform for 10 s. Upon removal from the platform, mice were placed in a holding cage on a heating pad for an intertrial interval of 45 s. Mice received i.p. injections of vehicle or progesterone immediately after trial 8 and then were returned to their home cages.
Memory for the platform location was assessed during four consecutive spatial trials on Day 2, which took place 24 or 48 hrs after injection. Spatial memory consolidation is most accurately measured in the first trial of Day 2 because re-exposure to the platform in this trial serves as a powerful reminder of the platform location [24, 30]. The remaining three trials were used to determine if mice could re-learn the platform location.
For all spatial trials, swim distance (m) and swim speed (m/s) were recorded. Lower swim distances indicated better memory. Swim speed was recorded as a control for differences in swimming ability.
2.4.2. Non-spatial (cued) trials
Four cued water maze trials began immediately after the last spatial trial on Day 2. The cued task is a non-spatial water maze task conducted to control for non-mnemonic aspects of task performance [20, 24]. For these four trials, the platform was made visible with yellow tape and a white circular cue attached to the side of the platform (7 ½ cm in diameter). The platform was moved to a different location in the tank for each trial. All other aspects of the procedure were the same as the spatial task. Swim distance (m) and swim speed (m/s) were recorded.
2.5. Data Analysis
For object recognition, separate one-sample t-tests (SPSS, SPSS Inc., Chicago, IL) were performed to determine if the time spent with the novel object differed from chance (15 s) during the choice phase of the object recognition task, as previously described [14]. Elapsed time was analyzed using one-way repeated measures analysis of variance (ANOVA) with Treatment as the independent variable and Elapsed Time as the dependent variable (SuperANOVA, Abacus Concepts; Berkeley, CA, USA).
Water maze data were analyzed using one-way ANOVAs with Treatment as the independent variable and Trial as the repeated measure, as previously described [24]. Three separate sets of repeated-measures analyses were performed for the eight trials of Day 1, the four spatial trials of Day 2, and the four cued trials. In addition, to verify that the groups did not differ immediately prior to hormone injection, a one-way ANOVA without repeated measures was conducted on trial 8 of Day 1. Finally, additional repeated measures ANOVAs were performed to compare performance in trial 8 of Day 1 (last trial before injection) to trial 1 of Day 2 (first trial after injection) to examine progesterone effects on memory consolidation.
Following each ANOVA, Fisher’s Protected Least Significant Difference (PLSD) post-hocs were performed on all significant main effects of Treatment. An alpha level of 0.05 was used to reject the null hypothesis.
3. Results
3.1. Object recognition task
3.1.1. Twenty-four hour delay
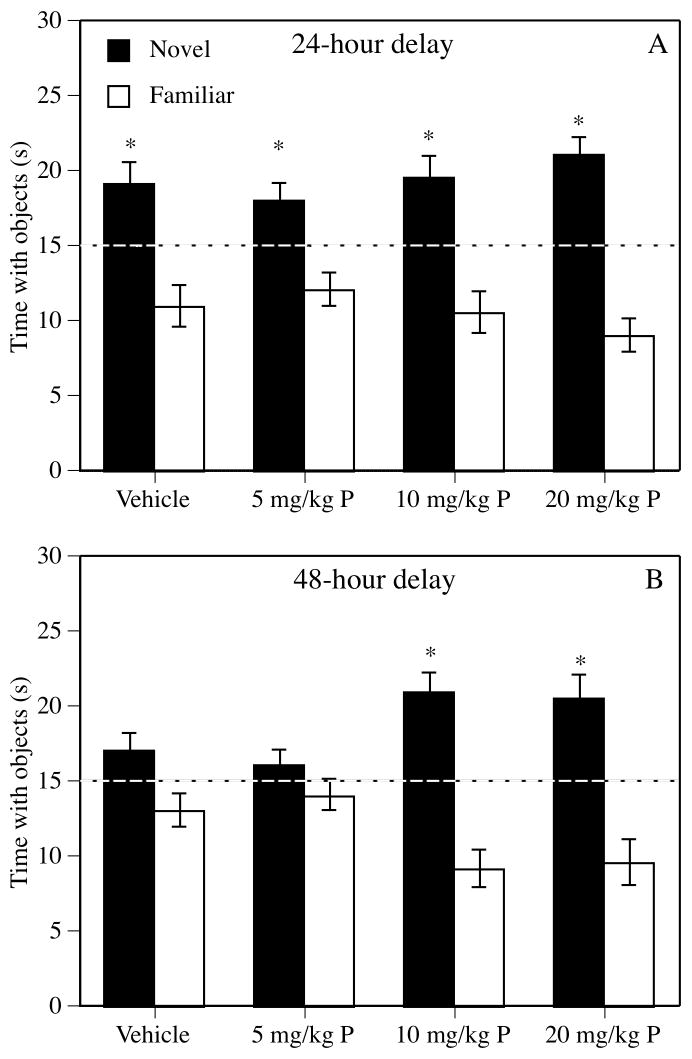
All groups demonstrated intact memory for the familiar object 24 hrs after the sample phase, as indicated by the fact that all groups spent significantly more time than chance with the novel object during the choice phase (vehicle, t(9) = 2.95, P = 0.02; 5 mg/kg progesterone, t(11) = 2.60, P = 0.03; 10 mg/kg progesterone, t(9) = 3.33, P = 0.009; 20 mg/kg progesterone, t(9) = 5.20, P = 0.001; Figure 1A). The groups also displayed similar levels of exploration during the choice phase, as the main effect of Treatment on elapsed time was not significant (F(3,38) = 2.30, P = 0.09). Mean (± standard error of the mean; S.E.M.) elapsed times (s) for each group were as follows: vehicle = 269.0 ± 21.4, 5 mg/kg progesterone = 256.1 ± 34.5, 10 mg/kg progesterone = 210.6 ± 25.9, and 20 mg/kg progesterone = 173.9 ± 26.8.
Figure 1.
(A) During the 24-hr choice phase of the object recognition task, all groups spent significantly more time than chance (dotted line at 15 s) with the novel object (*P < 0.05), demonstrating intact memory for the familiar object. (B) During 48-hr delay testing, the 10 and 20 mg/kg progesterone groups spent significantly more time with the novel object (* P < 0.05), whereas the vehicle and 5 mg/kg progesterone groups spent similar amounts of time with both objects. Each bar represents the mean (± the standard error of the mean (S.E.M.)) time spent with the novel object during each choice phase. Progesterone is abbreviated “P” on the X-axis.
3.1.2. Forty-eight hour delay
Forty-eight hrs after the sample phase, the 10 and 20 mg/kg progesterone groups spent significantly more time with the novel object, suggesting that these doses significantly enhanced object recognition (10 mg/kg progesterone, t(9) = 4.56, P = 0.001; 20 mg/kg progesterone, t(8) = 3.62, P = 0.007, Figure 1B). The vehicle and 5 mg/kg progesterone groups spent similar amounts of time with the novel and familiar objects (vehicle, t(9) = 1.85, P = 0.10; 5 mg/kg progesterone, t(11) = 0.96, P = 0.36), suggesting no effect of the 5 mg/kg dose on object recognition. Elapsed time to accumulate 30 s of exploration during the choice phase did not differ among the groups (F(3,37) = 2.38, P = 0.09). Mean (± S.E.M.) elapsed times (s) were as follows: vehicle = 364.2 ± 35.7, 5 mg/kg progesterone = 392.6 ± 51.8; 10 mg/kg progesterone = 260.7 ± 50.3; 20 mg/kg progesterone = 252.9 ± 41.6.
3.2. Morris water maze
3.2.1. Twenty-four hour delay
Spatial trials
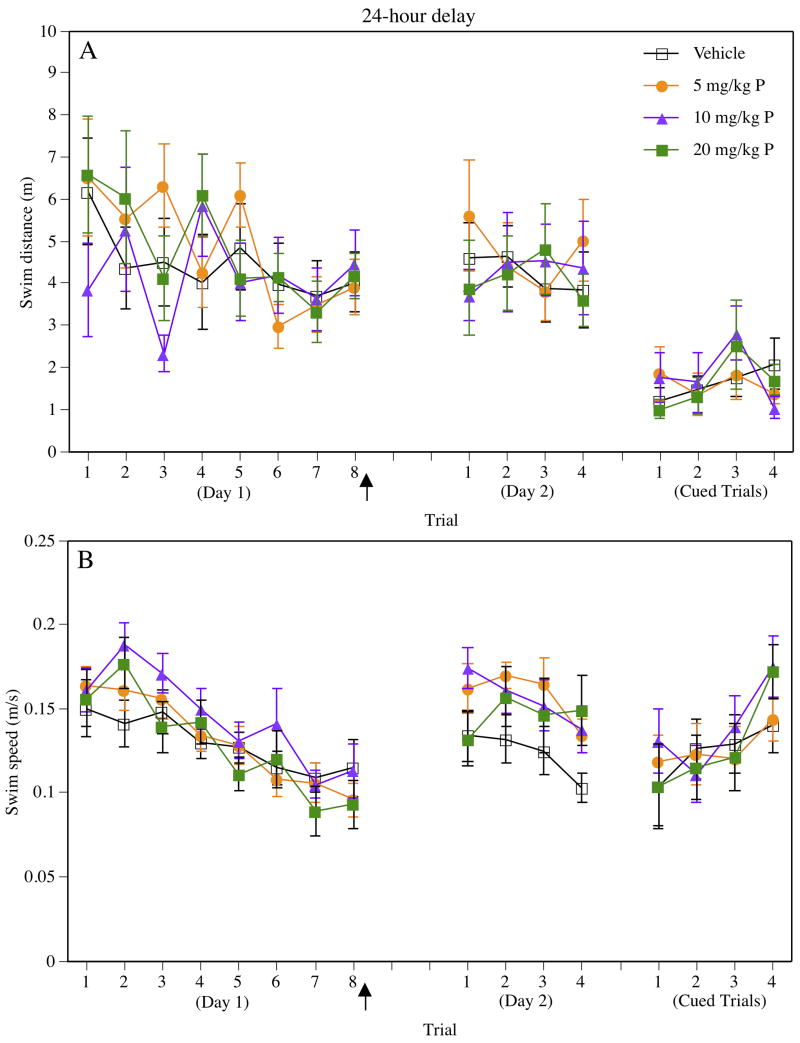
Morris water maze data from training and 24-hour testing are presented in Figure 2. During training on Day 1, there were no group differences in performance, as illustrated by non-significant main effects of Treatment for swim distance (Figure 2A) and swim speed (Figure 2B). However, as expected, the main effects of Trial on Day 1 were significant for both swim distance (F(7,266) = 2.45, P = 0.02) and swim speed (F(7,266) = 22.38, P = 0.0001), indicating that distances improved and speeds decreased across trials. The Treatment × Trial interaction was not significant for either measure, suggesting similar rates of acquisition among the groups. All groups performed similarly on the final trial of Day 1, as demonstrated by non-significant main effects of Treatment for swim distance (F(3,38) = 0.13, P > 0.05) and swim speed (F(3,38) = 0.66, P > 0.05) in this trial. Therefore, vehicle and progesterone groups did not differ in performance of the spatial task before progesterone treatment.
Figure 2.
Performance in the Morris water maze using the 24-hour delay, as assessed by swim distance (A) and swim speed (B) during eight spatial water maze training trials on Day 1, four retention trials on Day 2, and four cued trials on Day 2. Swim distance and swim speed decreased in all groups across trials on Day 1. On the first trial of Day 2, vehicle- and progesterone-treated groups performed similarly, indicating that post-training progesterone injections did not affect spatial memory consolidation. Swim distance and swim speed did not differ among the groups during the cued trials. Each point represents the mean (± S.E.M.) for one trial for each group. The filled arrow indicates that injections were given immediately after testing on Day 1. Progesterone is abbreviated “P” in the figure legend.
On Day 2, mice were re-tested in the spatial task to determine if post-training progesterone injections enhanced memory for the platform location. One-way ANOVAs conducted on the first trial of Day 2 indicated no significant differences among the groups (swim distance, F(3,38) = 0.72, P > 0.05; swim speed, F(3,38) = 2.01, P > 0.05). A repeated-measures ANOVA including the last trial of Day 1 and the first trial of Day 2 also indicated no differential change in swim distances among the groups (Figure 2A), as suggested by non-significant main effects and Treatment × Trial interaction. The same repeated-measures ANOVA for swim speed indicated a significant main effect of Trial (F(1,38) = 34.88, P = 0.0001), but non-significant Treatment and Treatment × Trial effects. The significant Trial effect was driven by the fact that mice swam slower on the last trial of Day 1 than the first trial of Day 2. Swim distances on Day 2 remained similar across trials and between groups, as suggested by non-significant main effects and Treatment × Trial interaction for the four retention trials on Day 2. The main effect of Trial was significant for swim speed on Day 2 (F(3,114) = 3.62, P = 0.02), but the main effect of Treatment and Treatment × Trial interaction were not.
Cued trials
The main effects of Treatment were not significant for swim distance (F(3,38) = 0.11, P > 0.05) or swim speed (F(3,38) = 0.29, P > 0.05), indicating that all groups performed similarly during the cued trials (Figure 2). The main effect of Trial (F(3,114) = 2.05, P > 0.05) and the Treatment × Trial interaction (F(9,114) = 0.75, P > 0.05) were also not significant for swim distance. For swim speed, the main effect of Trial was significant (F(3,114) = 5.80, P = 0.001), but the Treatment × Trial interaction was not (F(9,114) = 0.59, P > 0.05), suggesting that all groups swam faster as testing progressed.
3.2.2. Forty-eight hour delay
Spatial trials
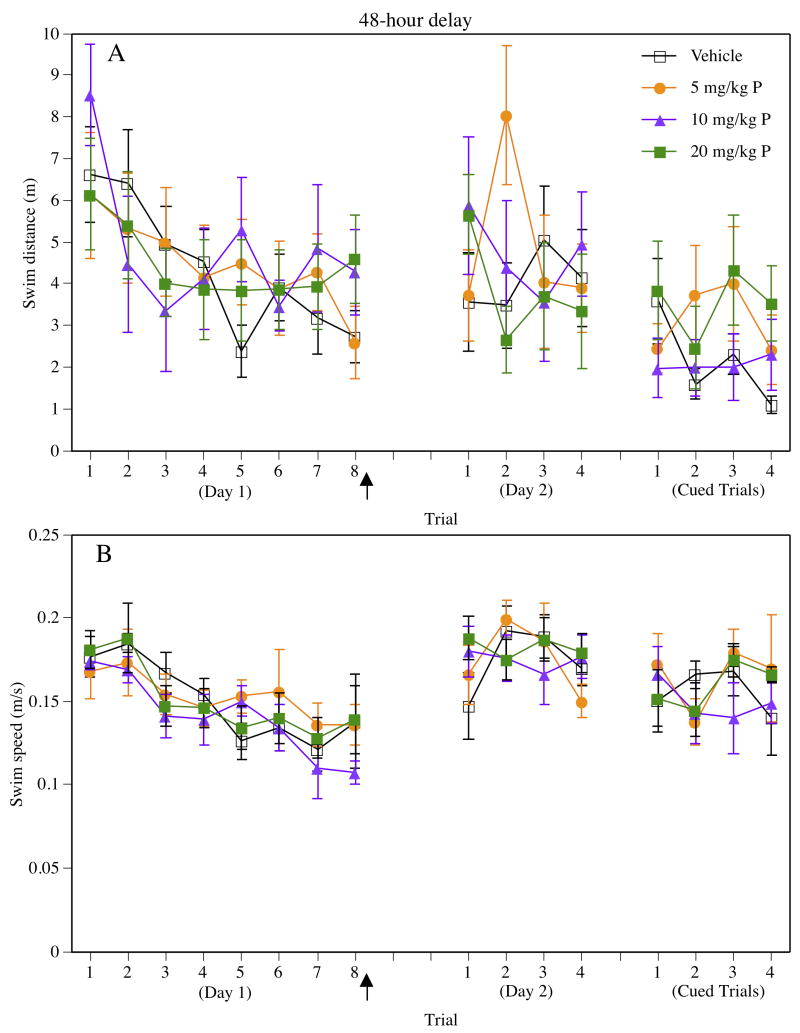
Data from training and 48-hour testing are presented in Figure 3. Similar to training with the 24-hour delay, there were no group differences in performance during training on Day 1, as indicated by non-significant main effects of Treatment for swim distance (Figure 3A) and swim speed (Figure 3B). Although the main effects of Trial on Day 1 were significant for both swim distance (F(7,224) = 4.12, P = 0.0003) and swim speed (F(7,224) = 9.88, P = 0.0001), the Treatment × Trial interactions were not significant for either measure, suggesting similar rates of acquisition among the groups. Non-significant main effects of Treatment for swim distance (F(3,32) = 0.63, P > 0.05) and swim speed (F(3,32) = 0.49, P > 0.05) in the final trial of Day 1 suggested that the groups performed similarly this trial. Together, these data indicate that the performance of the vehicle and progesterone groups did not differ prior to progesterone treatment.
Figure 3.
Performance in the Morris water maze using the 48-hour delay, as assessed by swim distance (A) and swim speed (B) during eight spatial water maze training trials on Day 1, four retention trials on Day 2, and four cued trials on Day 2. Vehicle- and progesterone-treated groups did not differ in any phase of testing. Each point represents the mean (± S.E.M.) for one trial each group. The filled arrow indicates that injections were given immediately after testing on Day 1. Progesterone is abbreviated “P” in the figure legend.
During the first trial of Day 2, no significant differences were observed among the groups in swim distance (F(3,32) = 1.05, P > 0.05) or swim speed (F(3,32) = 1.18, P > 0.05). The repeated-measures ANOVA comparing performance in the last trial of Day 1 with the first trial of Day 2 also indicated no differential change in swim distances among the groups (Figure 3A), as suggested by non-significant main effects and Treatment × Trial interaction. Although the swim distances of the vehicle and 5 mg/kg progesterone groups appeared to be higher than those of the 10 mg/kg and 20 mg/kg progesterone groups in both trials, the Treatment effect was not significant (F(3,32) = 2.47, P = 0.08) as described above. The repeated-measures ANOVA for swim speed revealed a significant main effect of Trial (F(1,32) = 9.07, P = 0.005), which was driven by the fact that all groups swam slower on the last trial of Day 1 than the first trial of Day 2 (Figure 3B). However, non-significant Treatment and Treatment × Trial effects suggested no effect of progesterone on performance during the first trial of Day 2. Despite the anomalous increase in swim distance by the 5 mg/kg progesterone group on trial 2 of Day 2, swim distances on Day 2 remained similar across trials and between groups, as suggested by non-significant main effects and Treatment × Trial interaction for the four Day 2 retention trials. Likewise, the non-significant main effects and Treatment × Trial interaction observed for swim speed indicated that swim speeds remained similar among the groups throughout Day 2 testing.
Cued trials
The main effect of Treatment and the Treatment × Trial interaction were not significant for swim distance (Figure 3A) or swim speed (Figure 3B), indicating that all groups performed similarly during the cued trials. Further, the non-significant main effect of Trial for both measures indicated no overall change in swim distance or swim speed throughout testing.
4. Discussion
The present study demonstrates that a single i.p. injection of progesterone can enhance object recognition memory, but not spatial memory in the Morris water maze, among young ovariectomized mice. Specifically, 10 and 20 mg/kg progesterone facilitated memory consolidation at a 48-hr delay in the object recognition task, whereas no dose affected memory in the spatial water maze task at either delay. The 48-hr object recognition data are consistent with a previous report indicating that a single post-training progesterone injection can enhance object recognition tested 4 [39] or 24 [17] hrs after administration in young ovariectomized rats. Although the 24-hr object recognition data would seem inconsistent with these previous studies, it is important to note that vehicle-treated mice were not impaired at this delay, so it was not possible to observe an improvement by progesterone at this time point. Importantly, the fact that these present study utilized a rapidly metabolized water-soluble form of progesterone, and an object recognition protocol in which total exploration time is fixed, eliminated the potential confounding influence of circulating progesterone and differential object exposure on the results. As such, the present data point towards a specific beneficial effect of progesterone on object memory consolidation, rather than on non-mnemonic aspects of recognition task performance. In addition to object recognition, other work has shown that post-training progesterone treatment can enhance memory consolidation in the Y-maze and inhibitory avoidance tasks in young ovariectomized rats [17]. Together, the data from these tasks and from object recognition suggest that single injections of progesterone administered after training can enhance non-spatial memory consolidation in multiple tasks and in both rats and mice.
In contrast, the present study reported no effect of post-training progesterone on spatial memory in the Morris water maze tested after 24 or 48 hours. Although this finding is consistent with previous reports demonstrating that pre-training progesterone treatment does not affect spatial memory in the Morris water maze in young ovariectomized rats [5, 11], it differs from the beneficial effects of 17β-estradiol on spatial memory consolidation in female rats [30] and mice [20] tested using this same protocol. However, a conclusion that the two hormones differentially affect spatial memory in the water maze cannot yet be supported because performance issues may have contributed to the apparent differences among studies. For example, vehicle-treated mice in the present study did not forget the platform location overnight after either delay like vehicle-treated mice in previous reports using the 2-day spatial Morris water maze task with a 24-hour delay [20, 24]; swim distances for vehicle-treated mice in previous studies were about 6 m, whereas those in this study ranged from 3.56–4.62 m. This relative lack of forgetting may have left less room for progesterone to improve memory in this study relative to vehicle controls. One benefit of the strong performance of vehicle-treated mice is that it allowed for detrimental effects of progesterone on spatial memory to be observed at both delays. Interestingly, no such effects were found, indicating that progesterone does not impair spatial memory in young female mice. Nevertheless, considerably longer delays beyond 48 hours may be necessary to observe beneficial effects of progesterone in this task. In addition, higher doses of progesterone than those used in this study may have improved spatial memory in the Morris water maze, as a recent study from our laboratory found that higher doses of progesterone were necessary in aged ovariectomized mice to enhance spatial memory relative to object memory [29]. Therefore, it is possible that progesterone administered at a dose greater than 20 mg/kg may enhance spatial memory performance in young mice. Together, the present results suggest that the effects of post-training progesterone on spatial memory may be more sensitive to methodological factors than object memory.
When comparing between pre-training and post-training studies, it would appear that the timing of progesterone administration is critical to the mnemonic effects of this hormone. This study and others [17, 39] have reported memory-enhancing effects of post-training progesterone injections, whereas previous studies that have administered progesterone pre-training have reported no effect or an impairing effect of the hormone on memory [2, 13, 35]. The differential effects of pre-training and post-training progesterone treatment may be due to the fact that pre-training progesterone can interfere with cognitive processes during training. For instance, pre-training progesterone treatment in female human subjects impairs memory for faces and reduces neural activity in the amygdala, fusiform gyrus, and prefrontal cortex during memory processing [38]. Progesterone and progesterone metabolites may reduce neural activity by binding to the GABAA receptor [4], thereby producing anxiolytic and analgesic effects that may hinder cognitive performance [3, 16]. As such, the effects of pre-training progesterone on memory must be interpreted with these findings in mind. For studies in which the specific effects of progesterone on memory are of interest, post-training administration should be used to enable the observation of the mnemonic effects of progesterone without confounds produced by GABAA binding on training.
The object memory enhancements observed in the present study were likely due to the effects of progesterone on the hippocampus given that the object recognition task used in this study has been shown to depend on an intact hippocampus [36]. Indeed, progesterone is known to produce numerous beneficial effects on hippocampal function, including enhanced extracellular signal-regulated kinase (ERK) activation [27, 28] and neuroprotection [18, 27]. Further, preliminary data from our laboratory suggest that bilateral infusions of progesterone into the dorsal hippocampus immediately post-training can enhance object memory consolidation in young ovariectomized mice using the same object recognition protocol as in this study (P. Orr, personal communication). Nevertheless, progesterone did not enhance spatial memory in the Morris water maze, which is also a hippocampal task [26], and this finding may suggest that the mnemonic effects of progesterone on hippocampal-memory are specific to non-spatial object memory. Alternatively, the lack of effect on spatial memory may indicate that the effects of progesterone in the water maze are disrupted by the greater stress involved in water maze testing (e.g. immersion in water) compared to object recognition testing (e.g., exposure to an open field). Indeed, a previous report from our laboratory has demonstrated that the stress of water maze testing can disrupt estradiol-enhanced CA1 synaptic spine density in ovariectomized rats [15]. As such, a higher dose of progesterone may be necessary to counteract any negative effects of stress on water maze performance, and allow progesterone to facilitate spatial memory consolidation.
The present data suggesting that progesterone treatment can beneficially affect memory may have implications for cognitive function in young women taking contraceptives consisting of progesterone only (i.e., the minipill and Depo-Provera). Future studies should assess whether these treatments also confer a benefit to memory function. Results of this study may also have implications for cognitive function in older women taking hormone therapy (HT). This study, and others in mice, have demonstrated that the cognitive effects of estrogen and progesterone vary by dose [20, 24] and, therefore, identifying optimal doses for combination of these hormones to be used in HT may be particularly important. Because some clinical studies have reported beneficial cognitive effects of HT [10, 25], whereas others report no effect of HT on cognitive function [23, 33, 40], the particular doses used in combination treatment may have influenced the success of various treatments in clinical research. Thus, future work should investigate whether it is possible to identify dose combinations of estrogen and progesterone that consistently enhance memory.
In conclusion, the present study demonstrates that a single post-training injection of 10 or 20 mg/kg progesterone, but not 5 mg/kg progesterone, can enhance object recognition memory consolidation in young female mice. Importantly, these effects were seen with a water-soluble form of progesterone tested in an object recognition task in which total exploration time was fixed. This design, therefore, permitted observation of specific effects of progesterone on object memory in the absence of confounds due to circulating progesterone and differential object exposure. In contrast, progesterone treatment did not facilitate spatial memory consolidation in the Morris water maze, although this may have resulted from a relative lack of forgetting in vehicle-treated mice. Together, these findings may suggest that object memory consolidation in young female mice is more sensitive to the modulatory effects of progesterone than spatial memory consolidation, at least at the doses and delays tested. These data may have important implications for memory in young women taking progesterone-based contraceptives and for the design of hormone therapies intended to reduce age-related memory decline in menopausal women.
Acknowledgments
We would like to thank Dr. Michael Lewis and Patrick Orr for their thoughtful comments on the manuscript. This work was supported by Yale University and NIH grant AG022525 to K.M.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Mem. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. Ovarian hormones and cognition in the aged female rat: II progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci. 2004;118:707–14. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- 3.Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–61. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- 4.Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987;231:359–69. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- 5.Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial Morris water maze in ovariectomized rats. Horm Behav. 2000;38:234–42. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- 6.Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS. Estradiol increases pre- and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta) Endocrinology. 2003;144:4734–8. doi: 10.1210/en.2003-0216. [DOI] [PubMed] [Google Scholar]
- 7.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–60. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–95. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- 9.Driscoll I, Sutherland RJ. The aging hippocampus: navigating between rat and human experiments. Rev Neurosci. 2005;16:87–121. doi: 10.1515/revneuro.2005.16.2.87. [DOI] [PubMed] [Google Scholar]
- 10.Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav. 2000;38:262–76. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- 11.El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. J Cell Mol Med. 2004;8:537–44. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–19. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 13.Farr SA, Flood JF, Scherrer JF, Kaiser FE, Taylor GT, Morley JE. Effect of ovarian steroids on footshock avoidance learning and retention in female mice. Physiol Behav. 1995;58:715–23. doi: 10.1016/0031-9384(95)00124-2. [DOI] [PubMed] [Google Scholar]
- 14.Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–91. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- 15.Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur J Neurosci. 2004;19:3026–32. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frye CA, Duncan JE. Progesterone metabolites, effective at the GABAA receptor complex, attenuate pain sensitivity in rats. Brain Res. 1994;643:194–203. doi: 10.1016/0006-8993(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 17.Frye CA, Lacey EH. Progestins influence performance on cognitive tasks independent of changes in affective behavior. Psychobiology. 2000;28:550–63. [Google Scholar]
- 18.Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–44. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 19.Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res. 2006;1115:135–47. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 20.Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–9. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Gresack JE, Kerr KM, Frick KM. Short-term environmental enrichment decreases the mnemonic response to estrogen in young, but not aged, female mice. Brain Res. 2007;1160:91–101. doi: 10.1016/j.brainres.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 22.Gresack JE, Kerr KM, Frick KM. Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol Learn Mem. 2007;88:393–408. doi: 10.1016/j.nlm.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grigorova M, Sherwin BB. No differences in performance on test of working memory and executive functioning between healthy elderly postmenopausal women using or not using hormone therapy. Climacteric. 2006;9:181–94. doi: 10.1080/13697130600727107. [DOI] [PubMed] [Google Scholar]
- 24.Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–10. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psychiatry. 2001;158:227–33. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- 26.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 27.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–12. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 28.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–11. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis MC, Orr PT, Frick KM. Differential effects of acute progesterone administration on spatial and object memory in middle-aged and aged female C57BL/6 mice. Horm Behav. doi: 10.1016/j.yhbeh.2008.05.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–88. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- 31.Pitha J. Amorphous water-soluble derivatives of cyclodextrins: nontoxic dissolution enhancing excipients. J Pharm Sci. 1985;74:987–90. doi: 10.1002/jps.2600740916. [DOI] [PubMed] [Google Scholar]
- 32.Pitha J, Harman SM, Michel ME. Hydrophilic cyclodextrin derivatives enable effective oral administration of steroidal hormones. J Pharm Sci. 1986;75:165–7. doi: 10.1002/jps.2600750213. [DOI] [PubMed] [Google Scholar]
- 33.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 34.Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–10. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 35.Sato T, Tanaka K, Ohnishi Y, Teramoto T, Irifune M, Nishikawa T. Effects of estradiol and progesterone on radial maze performance in middle-aged female rats fed a low-calcium diet. Behav Brain Res. 2004;150:33–42. doi: 10.1016/S0166-4328(03)00249-3. [DOI] [PubMed] [Google Scholar]
- 36.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor GT, Weiss J, Pitha J. Testosterone in a cyclodextrin-containing formulation: behavioral and physiological effects of episode-like pulses in rats. Pharm Res. 1989;6:641–6. doi: 10.1023/a:1015922019038. [DOI] [PubMed] [Google Scholar]
- 38.van Wingen G, van Broekhoven F, Verkes R, Petersson K, Backstrom T, Buitelaar J, Fernandez G. How progesterone impairs memory for biologically salient stimuli in healthy young women. J Neurosci. 2007;27:11416–23. doi: 10.1523/JNEUROSCI.1715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf OT, Heinrich AB, Hanstein B, Kirschbaum C. Estradiol or estradiol/progesterone treatment in older women: no strong effects on cognition. Neurobiol Aging. 2005;26:1029–33. doi: 10.1016/j.neurobiolaging.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 42.Woolley CS. Acute effects of estrogen on neuronal physiology. Ann Rev Pharmacol Toxicol. 2007;47:657–80. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]