Abstract
p120 catenin regulates the activity of the Rho family guanosine triphosphatases (including RhoA and Rac1) in an adhesion-dependent manner. Through this action, p120 promotes a sessile cellular phenotype when associated with epithelial cadherin (E-cadherin) or a motile phenotype when associated with mesenchymal cadherins. In this study, we show that p120 also exerts significant and diametrically opposing effects on tumor cell growth depending on E-cadherin expression. Endogenous p120 acts to stabilize E-cadherin complexes and to actively promote the tumor-suppressive function of E-cadherin, potently inhibiting Ras activation. Upon E-cadherin loss during tumor progression, the negative regulation of Ras is relieved; under these conditions, endogenous p120 promotes transformed cell growth both in vitro and in vivo by activating a Rac1–mitogen-activated protein kinase signaling pathway normally activated by the adhesion of cells to the extracellular matrix. These data indicate that both E-cadherin and p120 are important regulators of tumor cell growth and imply roles for both proteins in chemoresistance and targeted therapeutics.
Introduction
Traditionally, cadherins have been studied for their central role in cell–cell adhesion. They comprise a superfamily of transmembrane proteins that link adjacent cells via calcium-dependent homophilic interactions (Yagi and Takeichi, 2000). A group of cytoplasmic proteins, the catenins, interact with and modulate cadherins at two highly conserved intracellular domains. β-Catenin associates with the most C-terminal conserved domain and is responsible for indirectly inducing the reorganization of the actin cytoskeleton (Gottardi and Gumbiner, 2001). p120 catenin (herein, p120), however, binds to the juxtamembrane domain (Yap et al., 1998; Thoreson et al., 2000) and is critical for cadherin stability and turnover (Ireton et al., 2002; Davis et al., 2003; Xiao et al., 2003). Although cadherins have been heavily studied for their vital role in cell adhesion, several studies have also shown them to be involved in signaling events that modulate other cellular processes, including cell migration and invasiveness, cell growth, and terminal differentiation (Behrens et al., 1989; Vleminckx et al., 1991; Perl et al., 1998; St. Croix et al., 1998; Gottardi et al., 2001).
Epithelial cadherin (E-cadherin) loss is thought to promote tumor invasion and metastasis via a mechanism that involves both β-catenin and p120 (Wong and Gumbiner, 2003; Yanagisawa and Anastasiadis, 2006). p120 plays a pivotal role in these events by either promoting E-cadherin stability and a sessile cellular phenotype or by inducing cell migration and invasiveness of E-cadherin–deficient cells through its effects on Rho GTPase activities (Yanagisawa and Anastasiadis, 2006; Yanagisawa et al., 2008). Rho GTPases are important molecular switches that regulate cytoskeletal dynamics, cell migration, adhesion, and growth (Hall, 2005). Several studies indicate that p120 regulates the activity of Rho family GTPases (including RhoA, Rac1, and Cdc42) in a cadherin/adhesion-dependent manner (for review see Anastasiadis, 2007). p120 inhibits RhoA activity by suppressing GDP dissociation and therefore activation in the cadherin-unbound state (Anastasiadis et al., 2000; Castano et al., 2007; Yanagisawa et al., 2008) or by recruiting the negative regulator p190 Rho GTPase-activating protein to cadherin complexes (Wildenberg et al., 2006). In addition, p120 overexpression promotes Rac1 activation in fibroblasts (Noren et al., 2000; Grosheva et al., 2001), whereas the recruitment and activation of Rac1 upon cadherin ligation requires p120 catenin binding to the cadherin complexes (Goodwin et al., 2003; Gavard et al., 2004). As Rho GTPases play critical roles in the formation and maintenance of both cadherin-mediated adherens junctions and integrin-mediated focal adhesions, the data argue that p120 is a critical intermediary in the cross talk between these cellular structures and their respective roles in regulating the sessile versus motile behavior of cells (for review see Anastasiadis, 2007).
The effects of either E-cadherin or p120 on cell growth are far less understood. Available data show that E-cadherin has both positive and negative effects on cell growth. Early studies argued that E-cadherin suppresses cell proliferation by inhibiting nuclear β-catenin signaling (Gottardi et al., 2001; Stockinger et al., 2001). More recently, E-cadherin expression was shown to inhibit cell growth via a mechanism that involves β-catenin association but not signaling and likely the subsequent suppression of receptor tyrosine kinase (RTK) signaling (Perrais et al., 2007). Cadherins associate closely with RTKs (including the EGF receptor [EGFR], c-erbB2, c-Met, IGFR1, VEGF receptor, and FGF receptor), and several studies have uncovered a complex and intimate relationship in which E-cadherin–mediated cell–cell adhesion is either promoted or disrupted by RTK signaling or, conversely, in which RTK signaling to downstream effectors is either promoted or inhibited by cadherin clustering (Pece and Gutkind, 2000; Comoglio et al., 2003; Qian et al., 2004; Lilien and Balsamo, 2005). Finally, early studies suggested that the inhibitory effect of E-cadherin–mediated adhesion on cell proliferation is mediated by the Cdk inhibitor p27 (KIP1; St. Croix et al., 1998; Motti et al., 2005). However, despite a general agreement that E-cadherin suppresses tumor cell migration, the role of E-cadherin as a more classical suppressor of tumor growth has not been studied extensively. Confounding this effort, recent data indicate that E-cadherin–mediated cell–cell adhesion can promote cell proliferation under conditions of low confluence via a mechanism that requires p120 and Rac1 activation (Liu et al., 2006). Furthermore, increased expression of E-cadherin induces Akt and MAPK signaling and promotes the growth of ovarian carcinomas (Reddy et al., 2005).
p120 is often mislocalized or lost altogether in human tumors (Thoreson and Reynolds, 2002). These p120 phenotypes have been correlated to altered Rho GTPase signaling and increased invasiveness in vitro (Yanagisawa and Anastasiadis, 2006). The ability of p120 to stabilize cadherin complexes on the plasma membrane, to mediate signaling downstream of RTKs (c-Met and the PDGF receptor), and to regulate the activity of Rho family GTPases suggests that the mislocalization or loss of p120 in human cancer may have important implications on tumor cell growth. Interestingly, conditional p120 knockout in either the salivary glands or skin of mice induces a hyperproliferative response (Davis and Reynolds, 2006; Perez-Moreno et al., 2006). In this study, we consider p120's role in regulating tumor cell growth through its effects on Rho GTPases and E-cadherin. Our data reveal significant and diametrically opposing effects of endogenous p120 on tumor cell growth depending on the presence of E-cadherin. Endogenous p120 promotes the transformed growth of E-cadherin–deficient tumors by promoting Rac1 activation and inducing MAPK signaling. In contrast, p120 potently suppresses the growth of tumor cells expressing endogenous or exogenous E-cadherin. Furthermore, E-cadherin suppresses tumor cell growth in a p120 and Ras-MAPK–dependent manner. These data provide important insights into the roles of cadherin complexes in general, and p120 in particular, in cellular proliferation, anchorage-independent growth, and tumor progression.
Results
Endogenous p120 induces MDA-MB-231 tumor growth
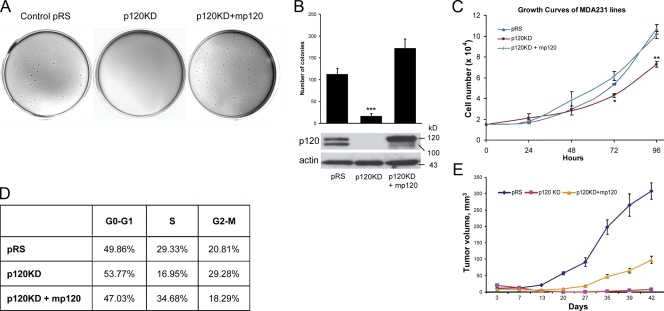
Previously, we reported that depletion of endogenous p120 in E-cadherin–deficient MDA-MB-231 cells significantly reduces cell migration and invasiveness and that reexpression of full-length murine p120 rescues these effects (Yanagisawa and Anastasiadis, 2006). We have now used the same retrovirally infected stable cell lines to test the hypothesis that endogenous p120 affects tumor cell growth. Initially, we tested the ability of p120 to mediate anchorage-independent growth by plating MDA-MB-231 cells in soft agar. Control pRS/LZRS-neo–infected MDA-MB-231 cells (pRS) grew well under these conditions (Fig. 1, A and B). In contrast, p120-depleted cells (p120 knockdown [KD]) failed to grow under conditions of anchorage independence, and this effect was reversed by the expression of exogenous murine p120 isoform 1A (p120KD + mp120). Fig. 1 C shows that when p120-depleted MDA-MB-231 cells were plated on plastic, they exhibited a slower growth rate than control cells over time, which was significant at 72 and 96 h. This effect was also reversed by ectopic p120 expression, indicating that it is not caused by “off-target” effects of the p120 short hairpin RNA (shRNA). Furthermore, cell cycle analysis using flow cytometry indicated that p120-depleted cells exhibit greater distribution during either G0–G1 or G2–M phases of the cell cycle and a significant reduction of cells in the S phase. Fig. 1 D shows that these effects of p120 depletion on the cell cycle were also completely reversed by the ectopic expression of murine p120 (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200805113/DC1). These data indicate that endogenous p120 regulates MDA-MB-231 cell growth in vitro.
Figure 1.
p120 promotes the transformed growth of MDA-MB-231 cells. (A and B) MDA-MB-231 cells were infected with control pRS and LZRS-neo retroviruses (pRS), p120 shRNA virus together with LZRS-neo (p120KD), or p120 shRNA virus together with a retrovirus expressing murine p120 isoform 1A (p120KD + mp120). Stable polyclonal cell populations were generated, and the ability of the cells to grow in soft agar was tested. p120-depleted cells (p120KD) were less able to grow in soft agar when compared with control (pRS) cells or cells reexpressing p120 (***, P < 0.0001; analysis of variance [ANOVA]). Data represent the mean ± SEM (error bars) of three independent experiments performed in triplicate. (C) The ability of the same cell lines to grow on plastic was also determined over time. 1.5 × 104 cells per well were plated in 24-well plates, and their growth was examined over time by cell counting. p120-depleted cells grew slower on plastic (**, P < 0.001 at 96 h; ANOVA). Mean ± SD (error bars; n = 9). (D) Cells were fixed in ethanol, treated with RNase, stained with propidium iodide, and analyzed with flow cytometry for their distribution in the cell cycle. Cells without p120 exhibit a G1 and G2 arrest that is rescued by mp120 expression. (E) 106 cells were injected into each flank of nude mice, and tumor growth was monitored over time by measuring tumor volume. p120 depletion inhibited MDA-MB-231 tumor growth in nude mice. Results represent the mean ± SD (error bars) of 10 independent determinations.
To test the possibility that p120 also regulates the growth of MDA-MB-231 cells in vivo, we injected tumor cells subcutaneously in the flanks of nude mice (10 injections per cell line) and determined tumor growth over a period of 42 d (Fig. 1 E). The mean tumor volume increased rapidly in control pRS tumors. In contrast, flanks injected with p120-depleted cells exhibited an initial decline in tumor volume, which was followed by a very slow tumor growth in only 2 of the 10 injection sites (compared with 10 out of 10 for control). Reexpression of p120 in these cells by the ectopic addition of murine p120 significantly increased tumor volume as well as tumor take (seven tumors for 10 injected flanks). Collectively, these data indicate that endogenous p120 promotes the transformed growth of MDA-MB-231 breast cancer cells.
p120 promotes MDA-MB-231 tumor growth via activation of Rac1
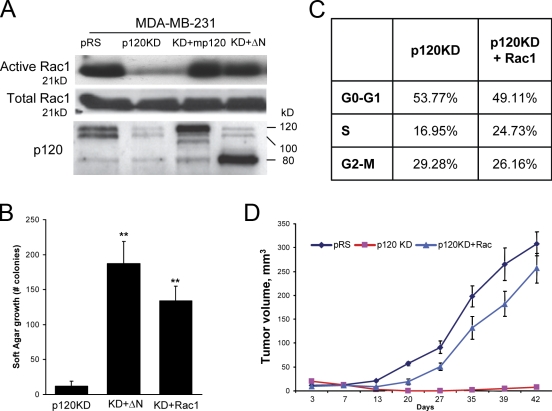
We have previously shown that the depletion of endogenous p120 in MDA-MB-231 cells results in increased RhoA and reduced Rac1 basal activities (Yanagisawa and Anastasiadis, 2006). The reexpression of full-length murine p120 isoform 1A (mp120) rescues both Rac1 and RhoA activities. In contrast, the expression of a p120 truncation mutant lacking 323 N-terminal amino acids (ΔN) and resembling endogenous p120 isoform 4 can rescue Rac1 activation (Fig. 2 A) but has no effect on RhoA (Yanagisawa et al., 2008). To test the hypothesis that p120 promotes anchorage-independent growth via its effects toward Rac1, we initially expressed the p120 ΔN truncation mutant in endogenous p120-depleted cells and determined its effect on soft agar growth. As can be seen in Fig. 2 B, the expression of p120 ΔN rescued the anchorage-independent growth of p120-depleted MDA-MB-231 cells, suggesting that the ability of p120 to affect Rac1 but not RhoA is important for its effects on MDA-MB-231 cell growth.
Figure 2.
p120 promotes MDA-MB-231 tumor growth via activation of Rac1. (A) Levels of total and GTP-bound (active) Rac1 were determined in MDA-MB-231 cells infected with control pRS and LZRS-neo viruses (pRS), p120-depleted cells (p120KD) or cells reexpressing full-length murine p120 (KD + mp120), or a RhoA-uncoupled p120 mutant (Yanagisawa et al., 2008) lacking 323 N-terminal amino acids (KD + ΔN). The bottom panel shows levels of endogenously expressed and exogenous p120. p120-depleted cells exhibited low levels of basal Rac1 activity. Expression of either murine p120 or the ΔN p120 mutant rescued Rac1 activation. (B) The ability of p120-depleted cells (p120KD) or p120-depleted cells stably expressing either the RhoA-uncoupled ΔN p120 mutant (KD + ΔN) or constitutively active V12-Rac1 (KD + Rac1) to grow in soft agar was tested. Data represent the mean ± SEM (error bars) of three independent determinations performed in triplicate (**, P < 0.001 as compared with p120KD control; Student's t test). (C) The ability of constitutively active Rac1 (p120KD + Rac1) to rescue the cell cycle distribution of p120-depleted cells (p120KD) was also examined using flow cytometry, as described for Fig. 1 D. (D) The ability of p120-depleted cells ectopically expressing constitutively active Rac1 (p120KD + Rac1) to grow tumors was examined in nude mice. Results represent the mean ± SD (error bars) of 10 independent determinations. Note that activated Rac1 is able to rescue the growth of p120-depleted MDA-MB-231 cells both in vitro and in mice.
To directly test this hypothesis, we expressed a constitutively active (V12) mutant of Rac1 in p120-depleted MDA-MB-231 cells (KD + Rac1) and tested its effects on growth in soft agar, cell cycle progression, and tumor growth in mice. Fig. 2 B shows that the expression of constitutively active Rac1 can rescue the anchorage-independent growth of p120-depleted cells. Furthermore, Rac1 activation significantly reversed cell cycle defects induced by p120 depletion (Figs. 1 D and 2 C). Finally, activation of Rac1 completely rescued the ability of p120-depleted MDA-MB-231 cells to grow tumors when injected in mice (Fig. 2 D). Combined, these data strongly argue that endogenous p120 promotes the growth of MDA-MB-231 cells by inducing Rac1 activation.
The MAPK pathway is essential for p120-mediated growth effects
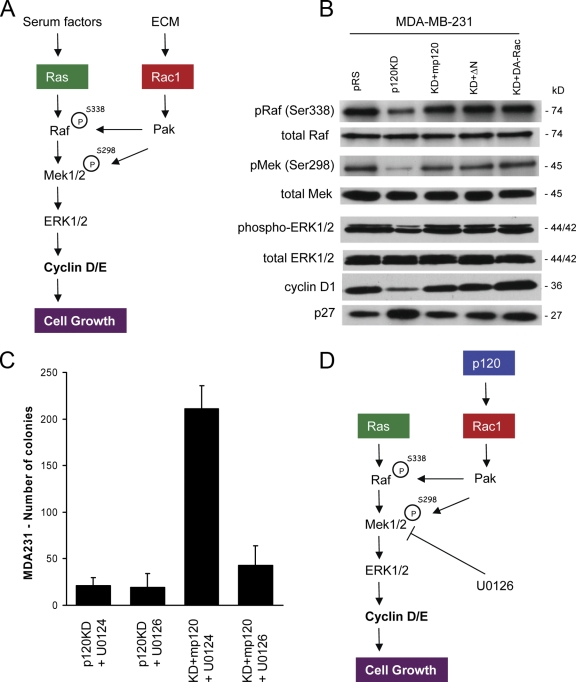
Recent data have shown that significant cross talk exists between growth factor signaling and integrin-mediated adhesion to the extracellular matrix (ECM). More specifically, fibronectin-mediated activation of Rac1 and its effector p21-activated kinase (PAK) have been shown to promote the phosphorylation, increased association, and constitutive activation of the Ras–Raf–MAPK/ERK kinase (MEK)–extracellular signal-regulated kinase (ERK) signaling pathway (Fig. 3 A; Frost et al., 1997; Coles and Shaw, 2002; Eblen et al., 2002; Slack-Davis et al., 2003; Beeser et al., 2005). MDA-MB-231 cells, like many tumor cells, can grow under conditions of anchorage independence and exhibit high levels of activated Rac1 (Fig. 2 A). The observation that p120 depletion blocks both Rac1 activation and growth in soft agar suggested the possibility that p120 promotes the anchorage-independent growth of MDA-MB-231 cells through the induction of a Rac1–PAK–MEK–ERK signaling pathway.
Figure 3.
The MAPK pathway is essential for p120-mediated growth effects. (A) One of the proposed mechanisms by which the ECM is thought to promote cell growth is via the Rac1/PAK-mediated constitutive activation of the MAPK pathway. Phosphorylation of Raf (S338) and MEK (S298) at specific serines by the Rac1 effector PAK induces complex formation and promotes the constitutive activation of the MAPK–ERK pathway in mitogen-stimulated cells, leading to the increased expression of cyclin D. (B) Western blots of phosphorylated and total Raf, MEK, and ERK, cyclin D1, and p27 levels in control MDA-MB-231 cells (pRS), p120-depleted cells (p120KD), p120-depleted cells expressing murine p120 (KD + mp120), N-terminally deleted p120 (KD + ΔN), or constitutively active Rac1 (KD + DA-Rac). p120 depletion in MDA-MB-231 cells results in reduced phosphorylation of activating sites in Raf (Ser338), MEK (Ser298), and ERK1/2 (Thr202/Tyr204), reduced cyclin D1, and increased p27 levels. These effects are reversed by p120 expression or expression of activated Rac1. (C) p120-depleted cells (p120KD) and cells reexpressing p120 (KD + mp120) were grown in soft agar in the presence of either 10 μM of the MEK inhibitor U0126 or 10 μM of its inactive isomer U0124. The inhibition of MEK by U0126 blocks p120-mediated growth in soft agar. Mean ± SEM (error bars; n = 9). (D) Proposed model of p120 action in MDA-MB-231 cells. In MDA-MB-231 cells, p120 promotes growth by activating a Rac1–MAPK signaling cascade.
To test this hypothesis, we initially determined the effects of p120 depletion on PAK-specific phosphorylation sites of Raf (Ser338) and MEK1/2 (Ser298) as well as on the activation of ERK1/2 and the levels of cyclins and Cdk inhibitors. Fig. 3 B shows that depletion of endogenous p120 results in reduced phosphorylation of Raf-Ser338 and MEK-Ser298. Consistent with this, p120-depleted cells exhibit reduced ERK phosphorylation, reduced levels of cyclin D1, and increased levels of p27/KIP1. Consistent with their ability to activate Rac1, expression of either full-length murine p120 or of the N-terminally truncated p120 mutant (ΔN) rescued both Raf and MEK phosphorylation at these sites, induced activation of ERK1/2, increased expression of cyclin D1, and inhibited p27. Furthermore, direct activation of Rac1 in p120-depleted MDA-MB-231 cells by expression of constitutively active Rac1 induced the same phosphorylation events and activated MAPK signaling (Fig. 3 B). These data show that the MAPK signaling pathway is severely impaired in p120-depleted MDA-MB-231 cells and that activation of Rac1 rescues MAPK signaling.
Next, we examined whether the activation of MAPK signaling by p120 is required for the ability of MDA-MB-231 cells to grow in soft agar. To test this, we treated p120-depleted MDA-MB-231 cells (p120KD) or p120-depleted cells expressing murine p120 (KD + mp120) with 10 μM of the MEK inhibitor U0126 or with 10 μM of its inactive isomer U0124. Fig. 3 C shows that neither compound affected the growth of p120-depleted cells in soft agar. However, U0126 selectively and potently inhibited the soft agar growth of cells expressing murine p120. These data strongly argue that endogenous p120 promotes the growth of MDA-MB-231 cells by activating Rac1 and inducing the Rac1-mediated activation of MAPK signaling (Fig. 3 D).
E-cadherin expression reverses p120 oncogenic effects and suppresses growth, Rac1, and Ras activity in a p120-dependent manner
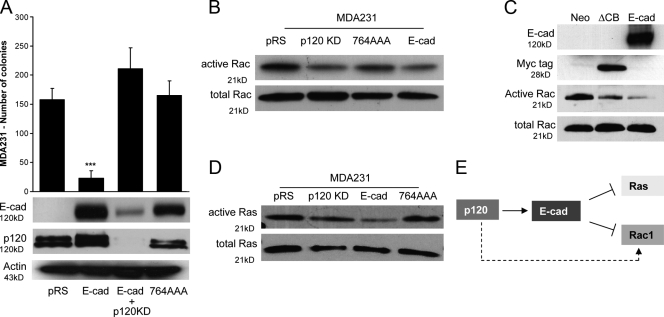
Previous data show that ectopic expression of E-cadherin potently antagonizes the ability of endogenous p120 to promote the migration and invasiveness of MDA-MB-231 cells (Yanagisawa and Anastasiadis, 2006). Therefore, we next examined whether E-cadherin expression can block the oncogenic effects of p120. Fig. 4 A shows that ectopic expression of E-cadherin potently blocks the growth of MDA-MB-231 cells in soft agar. To test the potential involvement of p120, we examined the effect of p120 depletion on the growth of cells expressing E-cadherin as well as the ability of a p120-uncoupled E-cadherin mutant (764AAA) to suppress MDA-MB-231 growth in soft agar. These data show that association with endogenous p120 is necessary for E-cadherin's ability to suppress the growth of MDA-MB-231 cells (Fig. 4 A).
Figure 4.
E-cadherin expression reverses p120 oncogenic effects and suppresses growth, Rac1, and Ras activity in a p120-dependent manner. (A) MDA-MB-231 cells expressing control pRS virus (pRS) or p120-specific shRNA were infected with an LZRS retrovirus expressing wild-type E-cadherin (E-cad and E-cad + p120KD, respectively). Control pRS cells were also infected with a virus expressing a p120-uncoupled E-cadherin mutant (764AAA). Polyclonal cell populations stably expressing these cadherin constructs were generated after G418 selection. The ability of these cell lines to grow in soft agar was tested. E-cadherin expression potently inhibits growth (***, P < 0.0001; ANOVA; n = 9). p120 is required for this because depletion of endogenous p120 reversed the E-cadherin phenotype, whereas p120-uncoupled E-cadherin failed to inhibit the growth of MDA-MB-231 cells. Mean ± SEM (error bars; n = 9). (B) Levels of total and GTP-bound (active) Rac1 were determined in MDA-MB-231 cells infected with control pRS and LZRS-neo viruses (pRS), p120-depleted cells (p120KD), or cells reexpressing wild-type (E-cad) or p120-uncoupled E-cadherin (764AAA). E-cadherin–expressing cells exhibited lower levels of Rac1 activation when compared with control (pRS) or 764AAA-expressing cells. (C) Levels of total and active Rac1 were also tested in MDA-MB-231 cells infected with control LZRS-neo virus (neo), a virus expressing the ΔCB E-cadherin mutant, or a virus expressing wild-type E-cadherin (E-cad). ΔCB is a Myc-tagged truncation mutant of the E-cadherin cytoplasmic domain, which binds p120 but not β-catenin. Cells expressing ΔCB exhibited reduced levels of Rac1 activation when compared with LZRS-neo virus control. (D) Levels of total and GTP-bound (active) Ras were also determined in the same cells. E-cadherin–expressing cells (E-cad) exhibit lower Ras activity than control cells (pRS), p120KD cells, or cells expressing the p120-uncoupled E-cadherin mutant (764AAA). (E) Proposed model of action. In the absence of E-cadherin (dotted arrow), p120 activates Rac1. In MDA-MB-231 cells expressing exogenous E-cadherin (solid arrow), p120 promotes the suppressive function of E-cadherin on both Rac1 and Ras activities.
Next, we examined whether E-cadherin expression affects Rac1 activity by performing Rac1 pull-down assays for activated GTP-bound Rac1. Previous studies have indicated that Rac1 is activated by E-cadherin–mediated cell–cell adhesion of epithelial cells (Nakagawa et al., 2001; Kovacs et al., 2002; Fukuyama et al., 2006; Perez et al., 2008). However, the expression of E-cadherin in MDA-MB-231 cells results in an overall suppression of basal Rac1 activity (Fig. 4 B). Furthermore, in repeated experiments, the ability of E-cadherin to suppress Rac1 required p120 binding because a p120-uncoupled E-cadherin mutant failed to significantly inhibit Rac1 (Fig. 4 B). In addition, the expression of a nonmembrane-targeted E-cadherin tail mutant that can bind p120 but not β-catenin (ΔCB; Yanagisawa and Anastasiadis, 2006) also inhibits Rac1 activity in MDA-MB-231 cells (Fig. 4 C), suggesting that the adhesive function of E-cadherin is not required for inhibiting Rac1 activity. These data are consistent with our previous observation that p120-mediated Rac1 activation in control MDA-MB-231 cells requires mesenchymal cadherin association and that E-cadherin expression competes p120 away from mesenchymal cadherins, leading to their destabilization (Yanagisawa and Anastasiadis, 2006).
Combined, our data support a model in which p120-mediated Rac1 activation cooperates with Ras-MAPK signaling to induce the transformed growth of MDA-MB-231 cells. Consistent with this hypothesis, activated Ras is readily detectable in both control (pRS) and p120-depleted cells (p120KD) under normal tissue culture conditions (Fig. 4 D). In contrast, ectopic expression of wild-type but not p120-uncoupled E-cadherin potently inhibits Ras activation (Fig. 4 D). The data argue that E-cadherin suppresses the growth of MDA-MB-231 cells by affecting Ras-MAPK signaling at two distinct levels: it suppresses activation of Ras, and it blocks the cross talk of Rac1–MAPK signaling by inhibiting Rac1. In the absence of E-cadherin (control MDA-MB-231 cells), p120 promotes Rac1 activation and tumor growth. However, upon E-cadherin expression, p120 mediates the stabilization of E-cadherin complexes, resulting in reduced activation of Ras and Rac1 (Fig. 4 E).
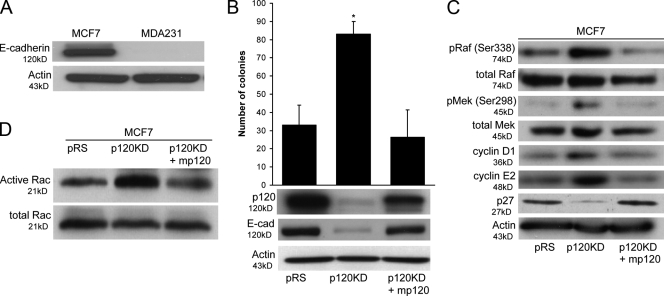
p120 maintains endogenous E-cadherin levels and suppresses the growth of MCF7 cells
Experiments in MDA-MB-231 cells suggested that endogenous p120 can either promote or inhibit tumor growth, depending on E-cadherin expression. To further examine this possibility, we used MCF7 breast cancer cells, which express endogenous E-cadherin (Fig. 5 A). Fig. 5 B shows that depletion of endogenous p120 reduces E-cadherin levels and increases the anchorage-independent growth of MCF7 cells. This effect of the p120 shRNA is specific to p120 depletion because the expression of murine p120 (p120KD + mp120) inhibits soft agar growth to control (pRS) levels. Therefore, p120 suppresses the growth of cells expressing either endogenous (MCF7) or exogenous (MDA-MB-231) E-cadherin.
Figure 5.
p120 increases E-cadherin levels and suppresses growth in MCF7 cells. (A) Total lysates of MCF7 and MDA-MB-231 cells were immunoblotted for E-cadherin and actin as a loading control. MCF7 cells express endogenous E-cadherin. (B) MCF7 cells were infected with control pRS and LZRS-neo retroviruses (pRS), p120 shRNA virus together with LZRS-neo (p120KD), or p120 shRNA virus together with a retrovirus expressing murine p120 isoform 1A (p120KD + mp120). Stable cell populations were generated, and the ability of the cells to grow in soft agar was tested. p120-depleted MCF7 cells (p120KD) exhibited low levels of endogenous E-cadherin (bottom) and grew better than control cells (pRS) or cells expressing murine p120 (p120KD + mp120). Data are mean ± SEM (error bars; *, P < 0.01; ANOVA; n = 6). (C) Lysates of the same cell lines were also subjected to immunoblots for Raf, MEK, cyclins D1 and E2, and actin (control). p120-depleted MCF7 cells exhibited higher levels of known PAK1 phosphorylation sites in Raf (Ser338) and MEK (Ser298) than in control (pRS) or murine p120 reconstituted cells (p120KD + mp120). In accordance with this finding, the levels of cyclins D1 and E2 were also elevated in these cells. (D) Finally, the levels of total and GTP-bound (active) Rac1 activity were also determined using a specific pull-down assay. Consistent with the previous data, p120-depleted cells have higher Rac1 activity than control cells or cells expressing murine p120.
To determine the mechanism of this p120 effect, we then examined the effect of p120 depletion on Rac1/PAK-mediated phosphorylation of Raf and MEK as well as levels of cyclins and Cdk inhibitors. These data show that p120 depletion induces the phosphorylation of Raf-Ser338 and MEK-Ser298, increases levels of cyclins D1 and E2, and decreases p27/KIP1 (Fig. 5 C). Consistent with these data, p120 depletion results in the activation of Rac1 in MCF7 cells (Fig. 5 D).
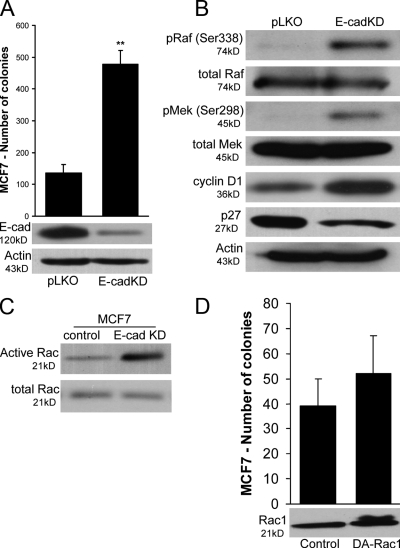
Depletion of endogenous E-cadherin increases the anchorage-independent growth of MCF7 cells in a Rac1-independent manner
Given the growth-suppressive effect of E-cadherin in MDA-MB-231 cells, we postulated that depletion of the endogenous E-cadherin in MCF7 cells would result in increased Rac1–MAPK signaling and increased cell growth. As can be seen in Fig. 6 A, depletion of E-cadherin (E-cadKD) by lentiviral infection of MCF7 cells with E-cadherin–specific shRNA (TRCN0000039666) increases colony formation in soft agar (identical results were also obtained with TRCN0000039664; not depicted) when compared with infected control cells (pLKO). Furthermore, Fig. 6 B shows that E-cadherin–depleted cells exhibit increased phosphorylation of Raf-Ser338 and MEK-Ser298, indicating that both the increase in soft agar growth and the increase in MAPK signaling downstream of p120 depletion in MCF7 cells are mediated by the destabilization of E-cadherin levels (Fig. 5 B). Finally, in agreement with the MDA-MB-231 data, the depletion of E-cadherin in MCF7 cells increased Rac1 activity (Fig. 6 C). A similar increase in the overall Rac1 activity was also observed upon E-cadherin depletion in A431 or Caco-2 cells (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200805113/DC1), suggesting that this effect of E-cadherin is not restricted to MCF7 and MDA-MB-231 cells.
Figure 6.
Depletion of endogenous E-cadherin in MCF7 cells increases anchorage-independent growth. (A) Control MCF7 cells were infected with control pLKO lentivirus or lentivirus expressing E-cadherin–specific shRNA. After the selection of stable polyclonal cell lines in puromycin, the ability of cell lines to form colonies in soft agar was tested. Cells expressing low levels of E-cadherin (E-cadKD) grow better in soft agar than control cells (pLKO). Data are mean ± SEM (error bars; **, P < 0.001; Student's t test; n = 6). Identical results were also obtained with a separate shRNA construct targeting E-cadherin (not depicted). (B) Lysates of control and E-cadherin–depleted cells (E-cadKD) were immunoblotted for E-cadherin, active and total Raf and MEK, and cyclin D1 as well as actin as a loading control. E-cadherin–depleted cells exhibited increased phosphorylation of activating sites on both Raf (Ser338) and MEK (Ser298). In agreement with these results, cyclin D1 levels were also higher. (C) Levels of GTP-bound (active) and total Rac1 were also determined. Consistent with the previous data, E-cadherin–depleted cells exhibited higher levels of activated Rac1. (D) To test the hypothesis that increased Rac1 activation is responsible for the increased ability of E-cadherin–deficient cells to grow in soft agar, we infected control MCF7 cells with a retrovirus expressing a constitutively active V12-Rac1 mutant (DA-Rac1). The data show that Rac1 activation is not sufficient to increase the ability of MCF7 cells to grow in soft agar. Mean ± SEM (error bars; n = 9).
Our data raise the possibility that the increased activity of Rac1 in p120- or E-cadherin–depleted MCF7 cells is responsible for the increased growth of these cells in soft agar. To test this, we stably expressed constitutively active Rac1 in control MCF7 cells and tested their ability to grow in soft agar. As can be seen in Fig. 6 D, the expression of activated Rac1 is not sufficient to increase MCF7 anchorage-independent growth.
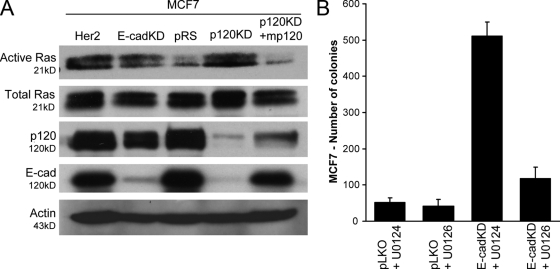
p120 and E-cadherin suppress MCF7 cell growth by blocking Ras-MAPK activation
The expression of E-cadherin in MDA-MB-231 cells resulted in the inhibition of both Rac1 and Ras signaling (Fig. 4, B and D). To test the possibility that Ras activation is responsible for the increased growth of E-cadherin–depleted MCF7 cells, we initially determined the levels of active Ras in control cells as well as cells depleted of endogenous E-cadherin or p120. The depletion of E-cadherin increased Ras activation to a level comparable with that of MCF7 cells overexpressing the RTK Her2, which is used here as a positive control (Fig. 7 A). Importantly, Her2 overexpression induced anchorage-independent growth of MCF7 cells to a level comparable with that of cells depleted of endogenous E-cadherin (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200805113/DC1). The depletion of endogenous p120 strongly inhibited the cellular levels of E-cadherin and induced Ras activation, which was readily inhibited by the reexpression of murine p120 (Fig. 7 A). These data argue that endogenous E-cadherin suppresses MCF7 cell growth by inhibiting Ras signaling and that p120 inhibits the growth of these cells by promoting the stabilization of E-cadherin complexes.
Figure 7.
E-cadherin suppresses growth by blocking Ras-MAPK activation in a p120-dependent manner. (A) Levels of total and GTP-bound (active) Ras were determined in MCF7 cells infected with control pRS and LZRS-neo viruses (pRS), p120-depleted cells (p120KD), p120-depleted cells expressing murine p120 (p120KD + mp120), E-cadherin–depleted cells (E-cadKD), or cells overexpressing the RTK Her2 (as a positive control). Both E-cadherin– and p120-depleted cells exhibited high levels of Ras activation, which are comparable with the levels induced by overexpression of Her2. The expression of murine p120 in p120-depleted cells potently blocks Ras activation. Levels of p120 and E-cadherin as well as actin loading controls are shown in the bottom panels. Note that upon p120 depletion, E-cadherin levels are drastically reduced but reinstated by exogenous expression of murine p120. (B) E-cadherin–depleted cells (E-cadKD) and cells expressing control pLKO lentivirus (pLKO) were grown in soft agar in the presence of either 10 μM of the MEK inhibitor U0126 or 10 μM of its inactive isomer U0124. Inhibition of MEK by U0126 blocks the ability of E-cadherin–deficient MCF7 cells to grow in soft agar, suggesting the involvement of the MAPK pathway. Mean ± SEM (error bars; n = 9).
Finally, we used the MEK inhibitor U0126 to test the involvement of the Ras–MAPK–ERK signaling pathway on the growth of E-cadherin–depleted MCF7 cells in soft agar. As can be seen in Fig. 7 B, inhibition of MEK completely abrogates the ability of E-cadherin–depleted MCF7 cells to grow colonies in soft agar. Combined, the data indicate that p120 cooperates with E-cadherin to suppress Ras signaling events and inhibit the growth of epithelial cells (Fig. 8). The loss of E-cadherin expression or function results in increased Ras activation, p120-mediated stabilization of mesenchymal cadherins and activation of Rac1, and, subsequently, Rac1-mediated constitutive activation of Ras-MAPK signaling and increased tumor growth.
Figure 8.
Proposed model of p120 action. The presence of E-cadherin causes the potent inhibition of Ras and Rac1, inhibits MAPK/ERK signaling, reduces cyclin D1, and promotes increased levels of p27, thus blocking cell growth. Under these conditions, p120 promotes the stabilization of E-cadherin complexes and their ability to suppress Ras. p120 recruitment by E-cadherin also decreases mesenchymal cadherin stability and function, resulting in reduced Rac1 activation. However, upon E-cadherin loss during tumor progression, the negative regulation of Ras is relieved. Under these conditions, endogenous p120 induces transformed cell growth by activating Rac1 and inducing the Rac1-dependent, PAK-mediated phosphorylation of Raf and MEK, which result in the constitutive, anchorage-independent activation of the Ras–MAPK–ERK signaling pathway. Dotted arrows denote potential Ras-mediated signaling events leading to anchorage-independent growth.
Discussion
p120 catenin is known to regulate the stabilization of cadherins at the plasma membrane and the activation of Rho family GTPases. Several recent studies indicate that p120 regulates the sessile versus motile behavior of cells by reorganizing the cytoskeleton and promoting either cell–cell adhesion or cell migration (for review see Anastasiadis, 2007). Nonetheless, both cadherin-mediated cell–cell contact and Rho GTPase signaling can also regulate cell growth, suggesting a potential growth regulatory role for p120. This notion was further supported by the observation that conditional p120 knockout in either the salivary gland or the skin induces hyperproliferation (Davis and Reynolds, 2006; Perez-Moreno et al., 2006). Given that misregulation of both E-cadherin and p120 are common events in tumor progression, we examined the potential effects of p120 on tumor cell growth in the presence or absence of E-cadherin. Our data reveal that endogenous p120 induces the anchorage-independent transformed growth of E-cadherin–deficient cells, whereas it promotes cadherin-mediated growth suppression in cells expressing endogenous or exogenous E-cadherin.
The loss of E-cadherin expression or function is a common event during tumor progression (Yap, 1998; Nollet et al., 1999). E-cadherin is widely considered to be a suppressor of tumor cell invasion, and several studies have indicated that its reexpression in E-cadherin–deficient carcinomas reverts cells to a less invasive, less aggressive phenotype (Behrens et al., 1989; Frixen et al., 1991; Vleminckx et al., 1991; Perl et al., 1998; St. Croix et al., 1998; Gottardi et al., 2001; Yanagisawa and Anastasiadis, 2006). Furthermore, a more traditional tumor-suppressing function for E-cadherin has also been suggested (St. Croix et al., 1998; Gottardi et al., 2001; Stockinger et al., 2001). The loss of E-cadherin function results in tissue dysfunction and often cell death (Ohsugi et al., 1997; Boussadia et al., 2002; Tinkle et al., 2004). However, several modifications, including reduced p53 function, activation of Ras, or SV40 T antigen expression, rescue this phenotype and cooperate with E-cadherin loss to induce both the growth and dissemination of epithelial tumors (Perl et al., 1998; Yamaguchi et al., 2004; Derksen et al., 2006; Igaki et al., 2006). In this study, we show that in the absence of E-cadherin, p120 promotes tumor cell growth on plastic, in soft agar, as well as after tumor cell injection into nude mice. The reduced growth of p120-depleted MDA-MB-231 cells is not related to decreased Ras activation (Fig. 4 D) but to reduced activity of Rac1 and reduced cross talk between Rac1 and Ras-MAPK signaling. Indeed, the expression of an activated Rac1 mutant rescues both the signaling and the growth of p120-depleted cells in vitro and in vivo.
Previous experiments on the mechanism by which anchorage to the ECM induces cell growth have implicated integrin-mediated Rac1 activation (Frost et al., 1997; Coles and Shaw, 2002; Eblen et al., 2002; Slack-Davis et al., 2003; Beeser et al., 2005). Through its downstream effector kinase PAK, Rac1 induces the phosphorylation of critical serines on Raf and MEK, promoting complex formation and recruitment of ERK. Thus, Rac1 cooperates with other growth signals in the propagation of Ras-MAPK signaling. Transformed cells that can grow anchorage independently would be expected to have developed mechanisms to overcome the need for this integrin-mediated signaling. Our data show that p120-mediated activation of Rac1 is one of the mechanisms that E-cadherin–deficient tumor cells use to grow under conditions of anchorage independence. We have shown previously that p120 induces Rac1 in these cells via its association with mesenchymal cadherins (Yanagisawa and Anastasiadis, 2006). We have also shown that reexpression of E-cadherin results in the preferential association of p120 with E-cadherin complexes and the destabilization of mesenchymal cadherins (Yanagisawa and Anastasiadis, 2006). Consistent with these observations, ectopic expression of E-cadherin in MDA-MB-231 cells results in a marked reduction of Rac1 activation and potently inhibits anchorage-independent cell growth. Remarkably, the growth-suppressive function of E-cadherin requires p120 because either p120 depletion or mutation of the p120-binding site of E-cadherin abrogates E-cadherin's ability to inhibit the growth of cells in soft agar (Fig. 4 A). Therefore, p120 induces the transformed growth of E-cadherin–deficient cells by activating a Rac1–MAPK signaling pathway normally activated by the adhesion of cells to the ECM, whereas E-cadherin (stabilized by p120 association) selectively and potently inhibits the transformed growth of epithelial cells.
The aforementioned interpretation is also in agreement with experiments in MCF7 cells, which express endogenous E-cadherin. The depletion of p120 in these cells sharply reduces the levels of E-cadherin and induces anchorage-independent growth, phenocopying the effect of p120 depletion in MDA-MB-231 cells that ectopically express wild-type E-cadherin. Furthermore, the depletion of endogenous E-cadherin mimics the effects of p120 depletion on intracellular signaling and anchorage-independent growth. Therefore, in E-cadherin–expressing cells, p120 affects growth by promoting the tumor-suppressive function of E-cadherin complexes. The exact mechanism by which E-cadherin suppresses cell growth is unclear. However, several recent studies implicate β-catenin and either its nuclear signaling or the negative regulation of RTK signaling (Gottardi et al., 2001; Stockinger et al., 2001; Qian et al., 2004; Perrais et al., 2007). Although we have not specifically addressed this question, our data show that E-cadherin exerts a potent inhibitory effect on Ras activation, which is consistent with an inhibitory effect on cadherin-associated RTKs. Interestingly, our experiments in MDA-MB-231 cells show that p120 does not affect Ras activation in the absence of E-cadherin; however, it is essential for E-cadherin–mediated suppression of Ras.
In addition to binding cadherins, p120 can also associate with microtubules and centrosomes (Chen et al., 2003; Franz and Ridley, 2004; Yanagisawa et al., 2004). p120 overexpression experiments have suggested that this association can induce the stabilization of cyclin E–Cdk2 complexes during mitosis, inhibit cell growth, and increase polyploidy (Chartier et al., 2007). The observation that the microtubule-uncoupled (Yanagisawa et al., 2004) p120 ΔN mutant can induce anchorage-independent growth in our cells suggests that this mechanism of growth regulation by p120 cannot account for our effects. Finally, using a Rho-uncoupled p120 mutant (Fig. 2 B) or constitutively active and dominant-negative RhoA mutants (not depicted), we found no evidence that either p120 or E-cadherin–mediated MDA-MB-231 growth effects were mediated by changes in RhoA signaling. However, several studies indicate that RhoA and its downstream effector Rho kinase can also promote sustained ERK/MAPK activation and cell cycle progression of mitogen-stimulated cells, especially fibroblasts (for review see Coleman et al., 2004). Therefore, it is possible that, as with Rac1 and RhoA, the ability of p120 to affect growth is context dependent.
Cellular context is also likely critical for the ability of p120 and E-cadherin to regulate Rac1 activity. Previous studies using primarily MDCK cells and calcium switch to induce cell–cell adhesion have shown that E-cadherin trans-interaction can induce Rac1 activation (Nakagawa et al., 2001; Kovacs et al., 2002; Fukuyama et al., 2006; Perez et al., 2008). Increased cell confluence was shown to activate Rac1 in MDCK and 293 cells (Noren et al., 2001). Surprisingly, in this study, we show that the overall Rac1 activity is significantly reduced in E-cadherin–expressing MDA-MB-231 cells. Consistent with this, the depletion of endogenous E-cadherin increases Rac1 activity in MCF7 (breast), A431 (squamous), and Caco-2 (intestinal epithelial) cells. These data suggest the existence of cell-specific differences in cadherin signaling, which are likely important for normal cell function and tumor progression. Previous data have argued that E-cadherin–dependent activation of Rac1 requires the EGFR (Betson et al., 2002). However, E-cadherin clustering can also potently inhibit several RTKs, including EGFR (Qian et al., 2004), suggesting that the interplay between cadherins and RTKs is critical for E-cadherin effects on Rac1. In the case of MDA-MB-231 cells, Rac1 activation is induced by p120 binding to mesenchymal cadherins and is inhibited by the recruitment of p120 away from these complexes. We postulate that the binding of mesenchymal cadherins to a diverse group of RTKs and their reduced ability to cluster into adhesive complexes when compared with E-cadherin may result in an increased overall activation of Rac1. Therefore, the effects of E-cadherin on Rac1 may depend on the complement of classical cadherins and associated RTKs expressed in a given cell. Future experiments using cadherin cytoplasmic domain mutants or chimeras of E-cadherin and mesenchymal cadherin will be important for elucidating the differential mechanisms by which E-cadherin or mesenchymal cadherin signals toward Rac1.
In summary, our data support an unexpected dual role of p120 on the transformed growth of tumor cells (Fig. 8). In the presence of E-cadherin expression, p120 acts to stabilize E-cadherin complexes and to promote their tumor-suppressive function, potently inhibiting Ras activation. Upon E-cadherin loss during tumor progression, the negative regulation of Ras is relieved; under these conditions, endogenous p120 induces transformed cell growth by activating Rac1 and the subsequent phosphorylation of Raf and MEK, which result in the constitutive, anchorage-independent activation of the Ras–MAPK–ERK signaling pathway. These data have significant implications for tumor biology and cancer treatment. The status of E-cadherin has long been associated with a worse prognosis and aggressive disease. We have recently shown that targeting p120 function prevents the invasive behavior of E-cadherin–deficient carcinomas (Yanagisawa and Anastasiadis, 2006; Yanagisawa et al., 2008). The current data suggest that understanding and targeting the ability of p120 to activate Rac1 may also block the transformed growth of these E-cadherin–deficient cells. Recent studies have shown that tumors exhibiting an epithelial to mesenchymal transition, and thus lacking E-cadherin expression, are more resistant to treatment with EGFR inhibitors (Yauch et al., 2005; Witta et al., 2006; Black et al., 2008). These data raise the possibility that upon E-cadherin loss, multiple mechanisms leading to Ras activation are induced. This possibility is supported by the observation that multiple RTKs can be inhibited by E-cadherin expression (Qian et al., 2004). Therefore, in E-cadherin–deficient cells, specific RTK inhibitors may fail to inhibit Ras activation, whereas endogenous p120 could still promote the constitutive activation of MAPK signaling and transformed growth via Rac1 activation.
Materials and Methods
Cell cultures, infections, and transfections
Cells were cultured in DME supplemented with 10% heat-inactivated FBS (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Mediatech). shRNA expression was achieved by retroviral (pRS) or lentiviral (pLKO) infection, and polyclonal stable cell lines were generated after selection in 5 μg/ml puromycin. Cell lines reexpressing the indicated proteins were infected with the LZRS-neo retrovirus and selected with 1 mg/ml G418. pRS and LZRS amphotropic retroviruses were produced as described previously (Davis et al., 2003). Lentivirus was generated using the standard protocols set forth by the Mayo Clinic Comprehensive Cancer Center RNA Interference Technology Resource. Confluence for all experiments was between 30 and 50%, unless otherwise noted.
Constructs
LZRS–mp120 isoform 1A-neo, LZRS–mp120 isoform ΔN-neo, LZRS-DA-Rac1-neo, LZRS–wild type–E-cadherin–neo, and LZRS-764AAA-neo were described previously (Davis et al., 2003; Yanagisawa and Anastasiadis, 2006). The pRS vector was a gift from R. Agami (The Netherlands Cancer Institute, Amsterdam, Netherlands). pRS human p120 shRNA was also described previously (Davis et al., 2003). The pLKO and pLenti-human E-cadherin shRNA (TRCN0000039663–67) were purchased from the Mission RNAi Consortium shRNA collection (Sigma-Aldrich) and obtained from the Mayo Clinic Comprehensive Cancer Center RNA Interference Technology Resource. TRCN0000039666 achieved the best E-cadherin KD and was used in most experiments followed by TRCN0000039664, which was used to validate results.
Western blotting
Western blotting procedures were conducted as described previously (Ireton et al., 2002). Primary antibodies used were as follows: anti–c-Raf, cyclins D1 and E2, MEK1, p44/42 MAPK, phospho-p44/42 MAPK (Thr202/Tyr204), phospho-MEK1 (Ser298), phospho–c-Raf (Ser338), Ras, and p27/KIP1 antibodies were all purchased from Cell Signaling Technology; anti-p120 mAb 15D2 was purchased from Invitrogen; actin (C-11), RhoA (26C4), and the HRP-conjugated secondary antibodies (mouse, rabbit, and goat) were purchased from Santa Cruz Biotechnology, Inc.; anti-Rac1 and anti–E-cadherin were purchased from BD.
Cell growth assay
Cells growing under standard conditions were harvested using Cell Stripper (Mediatech) to prevent the proteolytic degradation of cadherins, counted, and resuspended in complete media at 1.5 × 104 cells per well in 24-well plates. At the indicated times after plating, cells were trypsinized, mixed with trypan blue at a 1:1 dilution, and counted using a hemacytometer. All experiments were performed in triplicate. Data are expressed as mean ± SEM.
Cell cycle analysis
2 × 106 cells were fixed with cold ethanol for 1 h at 4°C. Samples were washed with PBS, treated with RNase A solution (1 mg/ml in 0.1% sodium citrate), and stained with propidium iodide before flow cytometry analysis was conducted at the Mayo Clinic Flow Cytometry/Optical Morphology Resource.
Soft agar growth assays
Soft agar growth assays were performed as previously described (Murray et al., 2004). In brief, 104 cells were suspended in DME supplemented with 10% heat-inactivated FBS, penicillin/streptomycin, and 1.5% low melting point agarose (SeaKem LE Agarose). Suspensions were plated on 60-mm culture dishes containing a layer of DME supplemented with 10% heat-inactivated FBS, penicillin/streptomycin, and 1.5% low melting point agarose. After 14–21 d in culture, colonies were stained with a 0.005% crystal violet solution and counted. When indicated, 10 μM of control (U0124) or 10 μM of MEK inhibitor (U0126) was added to the suspended cells and fed every other day with media containing the respective compounds.
Rac, Rho, and Ras assays
Cells were plated on 100-mm dishes under normal culture conditions (DME supplemented with 10% heat-inactivated FBS and penicillin/streptomycin). RhoA, Rac1, and Ras activity was assessed as previously described (Anastasiadis et al., 2000). Lysates were collected by adding 1 ml of lysis buffer (20 mM Hepes, pH 7.5, 0.5% NP-40, 100 mM NaCl, 0.2% deoxycholic acid, 10% glycerol, and 10 mM MgCl2) supplemented with protease and phosphatase inhibitors (Halt's protease inhibitor cocktail and Halt's phosphatase inhibitor cocktail). Cell lysates were cleared with a 5-min microcentrifugation. The supernatant was transferred to a fresh tube and subsequently aliquoted to tubes containing 30 μg Rac1, RhoA, or Ras-GTP affinity-binding beads (Rac/cdc42 assay reagent, Rho assay reagent, and Ras assay reagent; Millipore). A 40-μl aliquot of the lysate was retained before this step for the determination of total Rac1, RhoA, or Ras levels. After a 45-min incubation at 4°C, beads were washed in wash buffer (20 mM Hepes, pH 7.5, 0.5% NP-40, 100 mM NaCl, 10% glycerol, and 10 mM MgCl2), and bound Rac1-, RhoA-, or Ras-GTP was visualized by Western blotting using Rac1 (BD), RhoA (Santa Cruz Biotechnology, Inc.), or Ras antibodies (Cell Signaling Technology).
Tumor growth in mice
Suspensions of 106/0.1 ml of stable polyclonal cell populations of MDA-MB-231 cells infected with control pRS and LZRS-neo retroviruses (pRS), p120 shRNA virus together with LZRS-neo (p120KD), or p120 shRNA virus together with a retrovirus expressing murine p120 isoform 1A (p120KD + mp120) in DME were injected subcutaneously in each flank of 5–6-wk-old athymic female nu/nu mice (Harlan). Tumors were measured over 42 d using calipers. Tumor volumes were calculated by the formula 0.5236 × (a × b × c), where a is the shortest diameter, b is the diameter perpendicular to a, and c is the diameter height. 10 tumors were used per cell line with two injection sites per mouse. Results represent the mean ± SD of 10 independent determinations.
Online supplemental material
Fig. S1 contains individual FACS graphs corresponding to the data in Figs. 1 D and 2 C. Fig. S2 shows the effect of E-cadherin depletion on the Rac1 activity of Caco-2, A431, or H1437 cells. Fig. S3 shows the effect of E-cadherin depletion or Her2 overexpression on MCF7 soft agar growth. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200805113/DC1.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants R01CA100467 (to P.Z. Anastasiadis) and R01CA104505 (to J.A. Copland) and by a grant from the Breast Cancer Research Foundation (to E.A. Perez and P.Z. Anastasiadis).
Abbreviations used in this paper: ANOVA, analysis of variance; E-cadherin, epithelial cadherin; ECM, extracellular matrix; EGFR, EGF receptor; ERK, extracellular signal-regulated kinase; KD, knockdown; MEK, MAPK/ERK kinase; PAK, p21-activated kinase; RTK, receptor tyrosine kinase; shRNA, short hairpin RNA.
References
- Anastasiadis, P.Z. 2007. p120-ctn: a nexus for contextual signaling via Rho GTPases. Biochim. Biophys. Acta. 1773:34–46. [DOI] [PubMed] [Google Scholar]
- Anastasiadis, P.Z., S.Y. Moon, M.A. Thoreson, D.J. Mariner, H.C. Crawford, Y. Zheng, and A.B. Reynolds. 2000. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2:637–644. [DOI] [PubMed] [Google Scholar]
- Beeser, A., Z.M. Jaffer, C. Hofmann, and J. Chernoff. 2005. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J. Biol. Chem. 280:36609–36615. [DOI] [PubMed] [Google Scholar]
- Behrens, J., M.M. Mareel, F.V. Roy, and W. Birchmeier. 1989. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J. Cell Biol. 108:2435–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betson, M., E. Lozano, J. Zhang, and V.M. Braga. 2002. Rac activation upon cell-cell contact formation is dependent on signaling from the epidermal growth factor receptor. J. Biol. Chem. 277:36962–36969. [DOI] [PubMed] [Google Scholar]
- Black, P.C., G.A. Brown, T. Inamoto, M. Shrader, A. Arora, A.O. Siefker-Radtke, L. Adam, D. Theodorescu, X. Wu, M.F. Munsell, et al. 2008. Sensitivity to epidermal growth factor receptor inhibitor requires E-cadherin expression in urothelial carcinoma cells. Clin. Cancer Res. 14:1478–1486. [DOI] [PubMed] [Google Scholar]
- Boussadia, O., S. Kutsch, A. Hierholzer, V. Delmas, and R. Kemler. 2002. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev. 115:53–62. [DOI] [PubMed] [Google Scholar]
- Castano, J., G. Solanas, D. Casagolda, I. Raurell, P. Villagrasa, X.R. Bustelo, A. Garcia de Herreros, and M. Dunach. 2007. Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA. Mol. Cell. Biol. 27:1745–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier, N.T., C.I. Oddou, M.G. Laine, B. Ducarouge, C.A. Marie, M.R. Block, and M.R. Jacquier-Sarlin. 2007. Cyclin-dependent kinase 2/cyclin E complex is involved in p120 catenin (p120ctn)-dependent cell growth control: a new role for p120ctn in cancer. Cancer Res. 67:9781–9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., S. Kojima, G.G. Borisy, and K.J. Green. 2003. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J. Cell Biol. 163:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, M.L., C.J. Marshall, and M.F. Olson. 2004. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat. Rev. Mol. Cell Biol. 5:355–366. [DOI] [PubMed] [Google Scholar]
- Coles, L.C., and P.E. Shaw. 2002. PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene. 21:2236–2244. [DOI] [PubMed] [Google Scholar]
- Comoglio, P.M., C. Boccaccio, and L. Trusolino. 2003. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 15:565–571. [DOI] [PubMed] [Google Scholar]
- Davis, M.A., and A.B. Reynolds. 2006. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev. Cell. 10:21–31. [DOI] [PubMed] [Google Scholar]
- Davis, M.A., R.C. Ireton, and A.B. Reynolds. 2003. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 163:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen, P.W., X. Liu, F. Saridin, H. van der Gulden, J. Zevenhoven, B. Evers, J.R. van Beijnum, A.W. Griffioen, J. Vink, P. Krimpenfort, et al. 2006. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 10:437–449. [DOI] [PubMed] [Google Scholar]
- Eblen, S.T., J.K. Slack, M.J. Weber, and A.D. Catling. 2002. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol. Cell. Biol. 22:6023–6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz, C.M., and A.J. Ridley. 2004. p120 catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J. Biol. Chem. 279:6588–6594. [DOI] [PubMed] [Google Scholar]
- Frixen, U.H., J. Behrens, M. Sachs, G. Eberle, B. Voss, A. Warda, D. Lochner, and W. Birchmeier. 1991. E-cadherin-mediated cell–cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 113:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, J.A., H. Steen, P. Shapiro, T. Lewis, N. Ahn, P.E. Shaw, and M.H. Cobb. 1997. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 16:6426–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama, T., H. Ogita, T. Kawakatsu, M. Inagaki, and Y. Takai. 2006. Activation of Rac by cadherin through the c-Src-Rap1-phosphatidylinositol 3-kinase-Vav2 pathway. Oncogene. 25:8–19. [DOI] [PubMed] [Google Scholar]
- Gavard, J., M. Lambert, I. Grosheva, V. Marthiens, T. Irinopoulou, J.F. Riou, A. Bershadsky, and R.M. Mege. 2004. Lamellipodium extension and cadherin adhesion: two cell responses to cadherin activation relying on distinct signalling pathways. J. Cell Sci. 117:257–270. [DOI] [PubMed] [Google Scholar]
- Goodwin, M., E.M. Kovacs, M.A. Thoreson, A.B. Reynolds, and A.S. Yap. 2003. Minimal mutation of the cytoplasmic tail inhibits the ability of E-cadherin to activate Rac but not phosphatidylinositol 3-kinase: direct evidence of a role for cadherin-activated Rac signaling in adhesion and contact formation. J. Biol. Chem. 278:20533–20539. [DOI] [PubMed] [Google Scholar]
- Gottardi, C.J., and B.M. Gumbiner. 2001. Adhesion signaling: how beta-catenin interacts with its partners. Curr. Biol. 11:R792–R794. [DOI] [PubMed] [Google Scholar]
- Gottardi, C.J., E. Wong, and B.M. Gumbiner. 2001. E-cadherin suppresses cellular transformation by inhibiting β-catenin signaling in an adhesion-independent manner. J. Cell Biol. 153:1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosheva, I., M. Shtutman, M. Elbaum, and A.D. Bershadsky. 2001. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J. Cell Sci. 114:695–707. [DOI] [PubMed] [Google Scholar]
- Hall, A. 2005. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 33:891–895. [DOI] [PubMed] [Google Scholar]
- Igaki, T., R.A. Pagliarini, and T. Xu. 2006. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 16:1139–1146. [DOI] [PubMed] [Google Scholar]
- Ireton, R.C., M.A. Davis, J. van Hengel, D.J. Mariner, K. Barnes, M.A. Thoreson, P.Z. Anastasiadis, L. Matrisian, L.M. Bundy, L. Sealy, et al. 2002. A novel role for p120 catenin in E-cadherin function. J. Cell Biol. 159:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs, E.M., R.G. Ali, A.J. McCormack, and A.S. Yap. 2002. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 277:6708–6718. [DOI] [PubMed] [Google Scholar]
- Lilien, J., and J. Balsamo. 2005. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr. Opin. Cell Biol. 17:459–465. [DOI] [PubMed] [Google Scholar]
- Liu, W.F., C.M. Nelson, D.M. Pirone, and C.S. Chen. 2006. E-cadherin engagement stimulates proliferation via Rac1. J. Cell Biol. 173:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motti, M.L., D. Califano, G. Baldassarre, A. Celetti, F. Merolla, F. Forzati, M. Napolitano, B. Tavernise, A. Fusco, and G. Viglietto. 2005. Reduced E-cadherin expression contributes to the loss of p27kip1-mediated mechanism of contact inhibition in thyroid anaplastic carcinomas. Carcinogenesis. 26:1021–1034. [DOI] [PubMed] [Google Scholar]
- Murray, N.R., L. Jamieson, W. Yu, J. Zhang, Y. Gokmen-Polar, D. Sier, P. Anastasiadis, Z. Gatalica, E.A. Thompson, and A.P. Fields. 2004. Protein kinase Cι is required for Ras transformation and colon carcinogenesis in vivo. J. Cell Biol. 164:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, M., M. Fukata, M. Yamaga, N. Itoh, and K. Kaibuchi. 2001. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J. Cell Sci. 114:1829–1838. [DOI] [PubMed] [Google Scholar]
- Nollet, F., G. Berx, and F. v. Roy. 1999. The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol. Cell Biol. Res. Commun. 2:77–85. [DOI] [PubMed] [Google Scholar]
- Noren, N.K., B.P. Liu, K. Burridge, and B. Kreft. 2000. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren, N.K., C.M. Niessen, B.M. Gumbiner, and K. Burridge. 2001. Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276:33305–33308. [DOI] [PubMed] [Google Scholar]
- Ohsugi, M., L. Larue, H. Schwarz, and R. Kemler. 1997. Cell-junctional and cytoskeletal organization in mouse blastocysts lacking E-cadherin. Dev. Biol. 185:261–271. [DOI] [PubMed] [Google Scholar]
- Pece, S., and J.S. Gutkind. 2000. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J. Biol. Chem. 275:41227–41233. [DOI] [PubMed] [Google Scholar]
- Perez, T.D., M. Tamada, M.P. Sheetz, and W.J. Nelson. 2008. Immediate-early signaling induced by E-cadherin engagement and adhesion. J. Biol. Chem. 283:5014–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno, M., M.A. Davis, E. Wong, H.A. Pasolli, A.B. Reynolds, and E. Fuchs. 2006. p120-catenin mediates inflammatory responses in the skin. Cell. 124:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl, A.K., P. Wilgenbus, U. Dahl, H. Semb, and G. Christofori. 1998. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 392:190–193. [DOI] [PubMed] [Google Scholar]
- Perrais, M., X. Chen, M. Perez-Moreno, and B.M. Gumbiner. 2007. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol. Biol. Cell. 18:2013–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, X., T. Karpova, A.M. Sheppard, J. McNally, and D.R. Lowy. 2004. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 23:1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, P., L. Liu, C. Ren, P. Lindgren, K. Boman, Y. Shen, E. Lundin, U. Ottander, M. Rytinki, and K. Liu. 2005. Formation of E-cadherin-mediated cell-cell adhesion activates AKT and mitogen activated protein kinase via phosphatidylinositol 3 kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Mol. Endocrinol. 19:2564–2578. [DOI] [PubMed] [Google Scholar]
- Slack-Davis, J.K., S.T. Eblen, M. Zecevic, S.A. Boerner, A. Tarcsafalvi, H.B. Diaz, M.S. Marshall, M.J. Weber, J.T. Parsons, and A.D. Catling. 2003. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J. Cell Biol. 162:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Croix, B., C. Sheehan, J.W. Rak, V.A. Florenes, J.M. Slingerland, and R.S. Kerbel. 1998. E-cadherin–dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1). J. Cell Biol. 142:557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger, A., A. Eger, J. Wolf, H. Beug, and R. Foisner. 2001. E-cadherin regulates cell growth by modulating proliferation-dependent β-catenin transcriptional activity. J. Cell Biol. 154:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson, M.A., and A.B. Reynolds. 2002. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation. 70:583–589. [DOI] [PubMed] [Google Scholar]
- Thoreson, M.A., P.Z. Anastasiadis, J.M. Daniel, R.C. Ireton, M.J. Wheelock, K.R. Johnson, D.K. Hummingbird, and A.B. Reynolds. 2000. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J. Cell Biol. 148:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle, C.L., T. Lechler, H.A. Pasolli, and E. Fuchs. 2004. Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc. Natl. Acad. Sci. USA. 101:552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleminckx, K., L. Vakaet Jr., M. Mareel, W. Fiers, and F. van Roy. 1991. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 66:107–119. [DOI] [PubMed] [Google Scholar]
- Wildenberg, G.A., M.R. Dohn, R.H. Carnahan, M.A. Davis, N.A. Lobdell, J. Settleman, and A.B. Reynolds. 2006. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 127:1027–1039. [DOI] [PubMed] [Google Scholar]
- Witta, S.E., R.M. Gemmill, F.R. Hirsch, C.D. Coldren, K. Hedman, L. Ravdel, B. Helfrich, R. Dziadziuszko, D.C. Chan, M. Sugita, et al. 2006. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 66:944–950. [DOI] [PubMed] [Google Scholar]
- Wong, A.S., and B.M. Gumbiner. 2003. Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J. Cell Biol. 161:1191–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, K., D.F. Allison, K.M. Buckley, M.D. Kottke, P.A. Vincent, V. Faundez, and A.P. Kowalczyk. 2003. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 163:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi, T., and M. Takeichi. 2000. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 14:1169–1180. [PubMed] [Google Scholar]
- Yamaguchi, M., F. Hirose, Y.H. Inoue, K. Ohno, H. Yoshida, Y. Hayashi, P. Deak, and A. Matsukage. 2004. Genetic link between p53 and genes required for formation of the zonula adherens junction. Cancer Sci. 95:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa, M., and P.Z. Anastasiadis. 2006. p120 catenin is essential for mesenchymal cadherin–mediated regulation of cell motility and invasiveness. J. Cell Biol. 174:1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa, M., I.N. Kaverina, A. Wang, Y. Fujita, A.B. Reynolds, and P.Z. Anastasiadis. 2004. A novel interaction between kinesin and p120 modulates p120 localization and function. J. Biol. Chem. 279:9512–9521. [DOI] [PubMed] [Google Scholar]
- Yanagisawa, M., D. Huveldt, P. Kreinest, C.M. Lohse, J.C. Cheville, A.S. Parker, J.A. Copland, and P.Z. Anastasiadis. 2008. A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion and predicts metastatic disease. J. Biol. Chem. 283:18344–18354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, A.S. 1998. The morphogenetic role of cadherin cell adhesion molecules in human cancer: a thematic review. Cancer Invest. 16:252–261. [DOI] [PubMed] [Google Scholar]
- Yap, A.S., C.M. Niessen, and B.M. Gumbiner. 1998. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J. Cell Biol. 141:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch, R.L., T. Januario, D.A. Eberhard, G. Cavet, W. Zhu, L. Fu, T.Q. Pham, R. Soriano, J. Stinson, S. Seshagiri, et al. 2005. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin. Cancer Res. 11:8686–8698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.