Abstract
BubR1 kinase is essential for the mitotic checkpoint and also for kinetochores to establish microtubule attachments. In this study, we report that BubR1 is phosphorylated in mitosis on four residues that differ from sites recently reported to be phosphorylated by Plk1 (Elowe, S., S. Hummer, A. Uldschmid, X. Li, and E.A. Nigg. 2007. Genes Dev. 21:2205–2219; Matsumura, S., F. Toyoshima, and E. Nishida. 2007. J. Biol. Chem. 282:15217–15227). S670, the most conserved residue, is phosphorylated at kinetochores at the onset of mitosis and dephosphorylated before anaphase onset. Unlike the Plk1-dependent S676 phosphorylation, S670 phosphorylation is sensitive to microtubule attachments but not to kinetochore tension. Functionally, phosphorylation of S670 is essential for error correction and for kinetochores with end-on attachments to establish tension. Furthermore, in vitro data suggest that the phosphorylation status of BubR1 is important for checkpoint inhibition of the anaphase-promoting complex/cyclosome. Finally, RNA interference experiments show that Mps1 is a major but not the exclusive kinase that specifies BubR1 phosphorylation in vivo. The combined data suggest that BubR1 may be an effector of multiple kinases that are involved in discrete aspects of kinetochore attachments and checkpoint regulation.
Introduction
BubR1 protein kinase is an essential component of the mitotic checkpoint in metazoans (Cahill et al., 1998; Taylor et al., 1998), where one of its roles is to act as a mechanosensor that monitors the microtubule attachment status of kinetochores through its interaction with the kinesin-like motor centromere protein (CENP) E (Schaar et al., 1997; Chan et al., 1999; Yao et al., 2000; McEwen et al., 2001; Mao et al., 2003, 2005). In addition to its checkpoint roles at the kinetochore, BubR1 is part of the mitotic checkpoint complex (MCC) that acts downstream of the kinetochore by directly inhibiting the anaphase-promoting complex/cyclosome (APC/C; Sudakin et al., 2001; Braunstein et al., 2007; Musaro et al., 2008). Separate from its checkpoint functions, BubR1 has been shown to be essential for proper kinetochore microtubule attachments (Lampson and Kapoor, 2005). Recently, BubR1 was reported to associate with adenomatous polyposis coli, and its kinase activity regulated the ability of the adenomatous polyposis coli–EB1 complex to establish kinetochore/microtubule attachments (Kaplan et al., 2001; Zhang et al., 2007).
BubR1 is hyperphosphorylated in mitosis (Chan et al., 1999; Taylor et al., 2001), but its significance to kinetochore attachments and checkpoint regulation was not known. Recent studies have shown that human BubR1 can be phosphorylated by Plk1, and these modifications are critical for stable kinetochore attachments (Elowe et al., 2007; Matsumura et al., 2007). Similarly, Xenopus laevis BubR1 is a substrate of Plx1, and the phosphorylation generates the 3F3/2 phosphoepitope that is important for the checkpoint in egg extracts (Wong and Fang, 2007). We report the identification of four new mitosis-specific phosphorylation sites that are not targets of Plk1 or aurora B kinases. Their functional importance was examined with phosphospecific antibodies and various phosphomutants. We demonstrate that the phosphorylation of S670 and S1043 at kinetochores is sensitive to loss of microtubule attachments but not to tension. This contrasts with the response of the Plk1 S676 phosphorylation that is sensitive to tension (Elowe et al., 2007). Cells expressing phosphodefective (S to A) and phosphomimic (S to D) BubR1 mutants were delayed in metaphase because of defective kinetochore attachments that failed to generate proper levels of tension. Furthermore, analysis of these phosphomutants suggests that phosphorylation of S670 is critical for error correction at kinetochores. Injection of phospho-BubR1 antibodies also delayed cells at metaphase because kinetochores failed to generate proper levels of tension.
Using a cell-free system that recapitulated the checkpoint events that lie downstream of the kinetochore (Sudakin et al., 2001; Braunstein et al., 2007), we found that the addition of phospho-S670 (pS670) antibodies prolonged the inhibition of the APC/C. Thus, the phosphorylation status of BubR1 may be a critical determinant of checkpoint activity. Finally, we show that Mps1 is a major upstream kinase of all four phosphorylation sites in vivo. Combining our data with others suggests that multiple kinases regulate BubR1 to facilitate proper kinetochore attachments and checkpoint signaling.
Results
BubR1 is differentially phosphorylated at attached and unattached kinetochores
BubR1 was immunopurified from extracts prepared from asynchronous and nocodazole-blocked HeLa cells. Mass spectrometry (Fig. S1, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200805163/DC1) identified four major signals that corresponded to phosphoserines (S453, S543, S670, and S1043). A minor peak at S676 was also identified that was one of several sites (S676, T792, and T1008) that were recently reported to be phosphorylated by Plk1 (Elowe et al., 2007; Matsumura et al., 2007). Of the new phosphoresidues, S670 was conserved from Drosophila melanogaster to humans, whereas the others exhibited variable degrees of conservation among different species (Fig. S1 C). Phosphoantibodies were raised against the four phosphorylation sites. Western blots of mitotic lysates treated and untreated with λ protein phosphatase showed that all four antibodies were phosphospecific (Fig. S2 A). Phosphospecificity of the pS670 and pS1043 antibodies was further confirmed as the signals obtained with blots were eliminated with phosphopeptide but not with the unphosphorylated peptide (Fig. S2 B). In all subsequent experiments, unphosphopeptides were used to ensure phosphospecificity. Only the pS670 and pS1043 antibodies did not exhibit strong cross-reactivity with other phosphoproteins in Western blots of whole cell lysates, which allowed their use in immunocytochemistry.
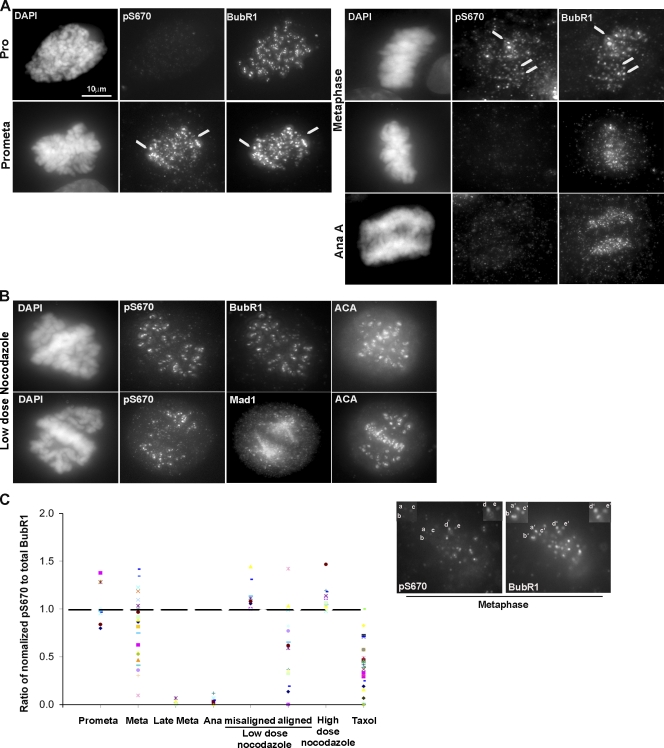
Immunofluorescence staining showed that both pS670 and pS1043 antibodies produced identical patterns (Fig. 1 A and Fig. S2 C). Staining was sensitive to phosphopeptide but not to the unphosphorylated peptide (Fig. S2 D). To assess the phosphorylation status of BubR1 at kinetochores during different stages of mitosis, cells were costained with antibodies to detect pS670 and S1043 (rabbit) and total BubR1 (rat; Fig. 1 A and Fig. S2 C). BubR1 was detected at kinetochores as early as prophase, but pS670 and pS1043 staining at kinetochores did not appear until after nuclear envelope breakdown (NEB) when cells entered mitosis. Phosphostaining remained detectable at kinetochores from prometaphase to metaphase. Some cells that were presumably more advanced in metaphase and in early anaphase lacked pS670 and pS1043 staining even though BubR1 was still detectable. Thus, S670 and S1043 are phosphorylated at kinetochores upon mitotic entry and are dephosphorylated at the onset of anaphase (Fig. 1 A and Fig. S2 C).
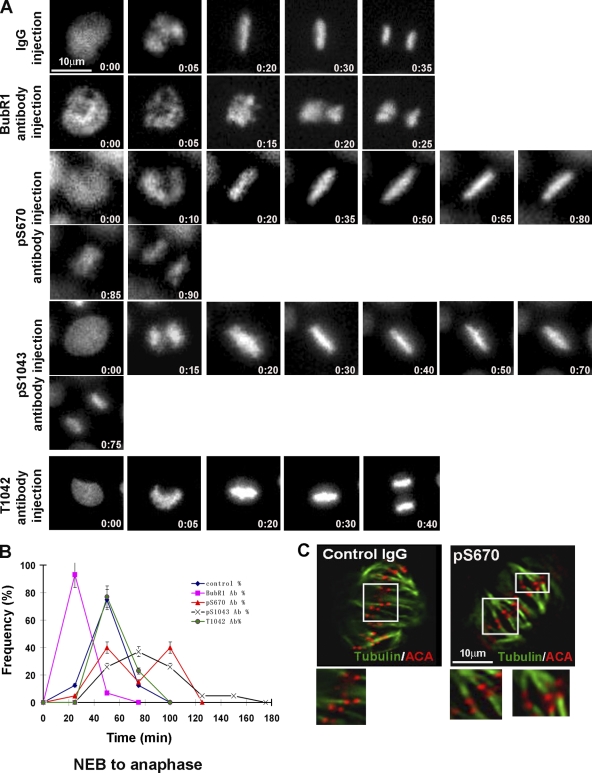
Figure 1.
BubR1 is differentially phosphorylated at attached and unattached kinetochores. (A) HeLa cells were stained for pS670, BubR1, and DAPI. The arrows indicate kinetochores. Background signal is caused by incomplete extraction of the cytosol. (B) HeLa cells were blocked in mitosis with a low dose of nocodazole (20 ng/ml) and stained for pS670, BubR1, Mad1, ACA, and DAPI. (C) Intensities of pS670 and BubR1 signals at individual kinetochores were quantitated, and the values were normalized to the kinetochore with the highest intensity (100%). The normalized values of pS670 and BubR1 for each kinetochore were used to obtain a ratio of pS670/total BubR1 for each kinetochore and plotted. An example of differential intensities of pS670 relative to BubR1 at metaphase is presented. Kinetochores a–c showed weaker signals relative to d and e for pS670, whereas the same kinetochores, a’–e’, showed equal signal intensities for BubR1. The dashed line indicates the position of the ratio of 1. Pro, prophase; Prometa, prometaphase; Meta, metaphase; Ana, anaphase; misaligned and aligned, monopolar and bipolar attachments, respectively.
Examination of the phosphorylation status of BubR1 in lysates that were harvested at various times after cells were released from a nocodazole block showed that S543, S670, and S1043 were gradually dephosphorylated as cells exited mitosis when compared with S435, which was completely dephosphorylated by the time cyclin B1 was degraded (Fig. S2 E). We noted that the kinetics of S670 and S1043 dephosphorylation, as determined by blots, differed from the abrupt loss of phosphosignal as determined by staining. This difference was attributed to the fact that the cytosolic pool of BubR1 was extracted from cells before fixing and staining. When extraction was omitted, pS670 signal was detected in the cytosol of early/midanaphase cells (Fig. S2 F). As chromosomes were absent from lysates used for blots, the phosphosignals likely reflect the cytosolic pool of BubR1.
We next tested whether the variable intensity of pS670 staining at kinetochores of prometaphase and early metaphase cells was caused simply by changes in the amount of BubR1 or reflected differences in the phosphorylation state of BubR1. We determined the relative BubR1 levels among individual kinetochores within a cell by normalizing the signals to the kinetochore with the highest intensity (100%) as described previously (Hoffman et al., 2001; Feng et al., 2006; Liu et al., 2006; H. Huang et al., 2007). The relative pS670 intensities among the identical set of kinetochores were determined by normalizing to the strongest phospho-BubR1 signal (this comparison was independent of the BubR1 normalization). By comparing the ratio of the normalized intensities of pS670 with BubR1, it was possible to determine whether the differences in BubR1 phosphorylation were caused simply by changes in total BubR1 levels or actual changes in its phosphorylation state (Fig. 1 C). A ratio of 1 indicates that the phosphorylation was directly related to the amount of BubR1 at the kinetochore. Ratios <1 would indicate that BubR1 was dephosphorylated at kinetochores, as shown in late metaphase and early anaphase. Kinetochores in prometaphase tend to exhibit stronger BubR1 phosphorylation (ratio >1). The distribution pattern for early metaphase kinetochores was broader, and there was a clear shift toward the dephosphorylated state. Thus, the changes in BubR1 phosphorylation in prometaphase and metaphase probably reflect differences in kinetochore attachments.
We next compared the pS670/BubR1 ratios in cells that were treated with nocodazole and taxol to suppress microtubule dynamics. This produced unattached kinetochores that are Mad1 positive as well as bipolar-attached kinetochores that lack detectable Mad1 staining but fail to generate full tension (Fig. 1 B). pS670/BubR1 ratios were increased at unattached kinetochores, as was seen at many of the kinetochores in normal prometaphase (Fig. 1 C) and when microtubules were completely depolymerized after treatment with a high dose of nocodazole. BubR1 phosphorylation was reduced at many of the attached but tensionless kinetochores. Thus, S670 is phosphorylated and dephosphorylated at kinetochores in response to the absence and presence of microtubule attachments, respectively (Fig. 1 C). These changes do not appear to be sensitive to the lack of tension as reported for the pS676 residue (Elowe et al., 2007).
In vivo characterization of BubR1 phosphomutants
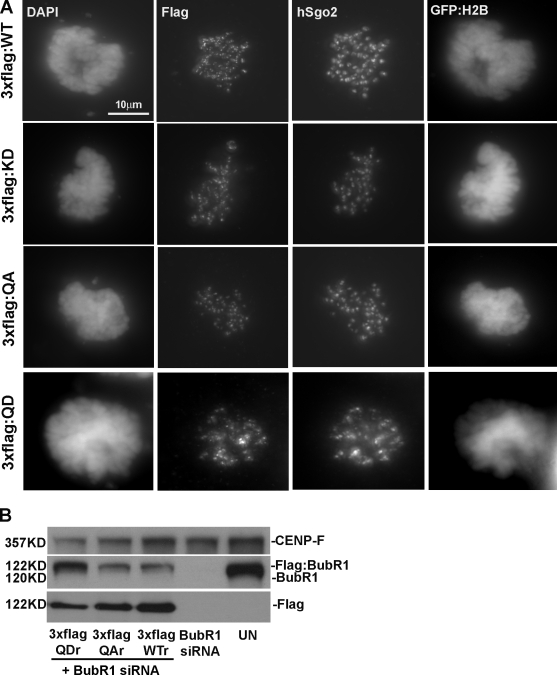
As BubR1 contributes to attachment as well as checkpoint-monitoring functions at the kinetochore, the changes in pS670 might reflect either or both of these activities. The functional significance of the mitotic phosphorylation sites in BubR1 was investigated by characterizing a series of phosphodefective (S to A) and phosphomimic (S to D) BubR1 mutants at a single or at all four sites. The BubR1 mutants were tagged with either 3×Flag or GST and cloned into vectors with an H2B-GFP expression cassette. RNAi-resistant alleles of the wild-type and BubR1 mutants were generated to facilitate experiments that included RNAi knockdown of endogenous BubR1 (Fig. 2 B and Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200805163/DC1). We first established that all of the mutants along with wild-type BubR1 were able to localize to kinetochores in mitotic cells (Fig. 2 A and Fig. S3, B and C). We used time-lapse videomicroscopy to track the fates of transfected cells that expressed H2B-GFP.
Figure 2.
Phospho-BubR1 mutants localize to kinetochores in cells depleted of endogenous BubR1. (A and B) HeLa cells depleted of endogenous BubR1 by siRNA were transfected with a vector that coexpressed 3×Flag BubR1RNAi (RNAi-resistant alleles) and H2B-GFP and were stained for Flag, hSgo2, and DAPI (A), or the lysates were probed for CENP-F, BubR1, and Flag (B). Western blots show siRNA-depleted endogenous BubR1 but not the 3×Flag-BubR1. UN, untransfected cell lysate; WT, wild type.
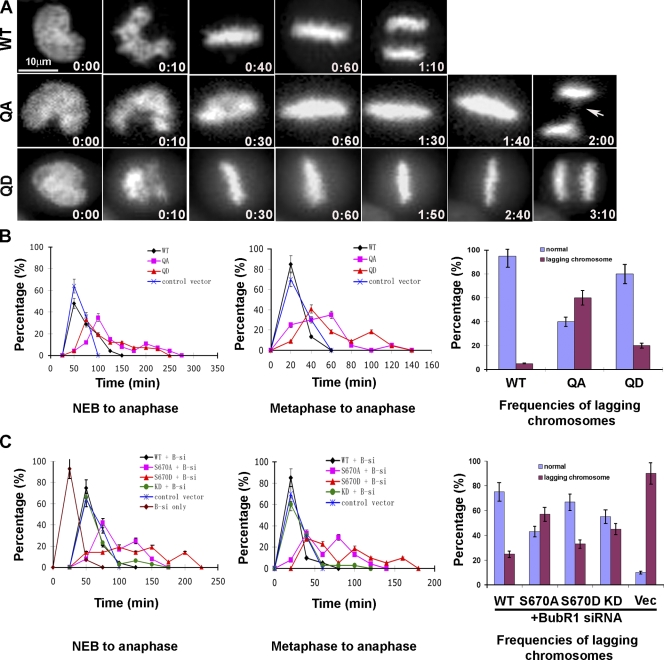
Initial experiments focused on testing the quadruple phosphodefective (QA) and phosphomimic (QD) mutants in the presence of endogenous BubR1 (Fig. 3 A). Cells expressing the wild-type BubR1 entered anaphase on average 50 min after NEB, but some took as long as 120 min (Fig. 3 B, left). The majority (80%) of the cells entered anaphase ∼20 min after reaching metaphase. These times are similar to cells transfected with H2B-GFP alone (Fig. 3 B, middle). The majority of the cells expressing the QA mutant took ∼100 min from NEB to anaphase, but cells that were delayed for >250 min were also seen (Fig. 3 B, left). The QA mutant took approximately the same amount of time as wild-type and QD cells to align their chromosomes even though the metaphase plates were qualitatively not as tight as cells expressing wild type or the QD mutant (Fig. 3 A). Compared with wild type, QA-expressing cells were delayed at metaphase for 20–100 min (Fig. 3 B, middle). The metaphase delay was likely caused by aberrant attachments, as >60% of the QA cells that entered anaphase exhibited lagging chromosomes as compared with ∼5% seen in wild-type BubR1-expressing cells (Fig. 3 B, right). The majority (70%) of the cells that expressed the QD mutant also exhibited a metaphase delay (Fig. 3 B, left and middle). However, the frequency of lagging chromosomes exhibited by the QD mutant was threefold lower than the QA mutant (Fig. 3 B, right).
Figure 3.
BubR1 phosphomutants delay the metaphase to anaphase transition. (A) HeLa cells released from a G1/S block were injected with 3×Flag constructs, and H2B-GFP-expressing cells were monitored by time-lapse microscopy (hours:minutes). Select frames from videos of cells injected with wild-type (WT) BubR1 and the quadruple phosphomutants (QA and QD) are shown. The arrow points to lagging chromosomes. (B) The videos were analyzed frame by frame, and the behavior of chromosomes was used to determine the percentage of cells from NEB to anaphase onset (left), from metaphase to anaphase onset (middle), and the frequencies of lagging chromosomes (right). For wild type, n = 20; QA, n = 27; QD, n = 44; vector, n = 22. (C) HeLa cells depleted of endogenous BubR1 by siRNA were released from a G1/S block and were injected with 3×Flag–wild-type BubR1, KD, S670A, and S670D phosphomutant and empty vector. The cells that expressed H2B-GFP were monitored by time lapse and were quantitated as in B. For wild type, n = 30; S670A, n = 24; S670D, n = 21; KD, n = 35. Vec, control vector. (B and C) Error bars indicate the highest and lowest values within a dataset.
We next characterized the single phosphodefective (S670A) and phosphomimic (S670D) mutants in cells whose endogenous BubR1 were depleted by siRNA. This residue was selected because it was conserved among all vertebrate species examined. Time-lapse experiments showed that depletion of BubR1 accelerated cells into anaphase as previously described (Meraldi et al., 2004). Transfected wild-type BubR1 rescued the BubR1-depleted cells, as the kinetics of mitotic progression was restored to that seen in cells transfected with vector alone (Fig. 3 C, left and middle). Cells expressing either the S670A or S670D mutants were able to achieve metaphase alignment with normal kinetics but were delayed by 20 to >150 min from entering anaphase (Fig. 3 C, left and middle). Over 90% of the cells depleted of BubR1 exhibited lagging chromosomes upon entry into anaphase (Fig. 3 C, right). The S670A mutant consistently failed to reduce the frequency of lagging chromosomes to that obtained with wild type and the S670D mutant. This suggests that the failure to phosphorylate S670 generates aberrant attachments that are not resolved when cells enter anaphase (Fig. 4).
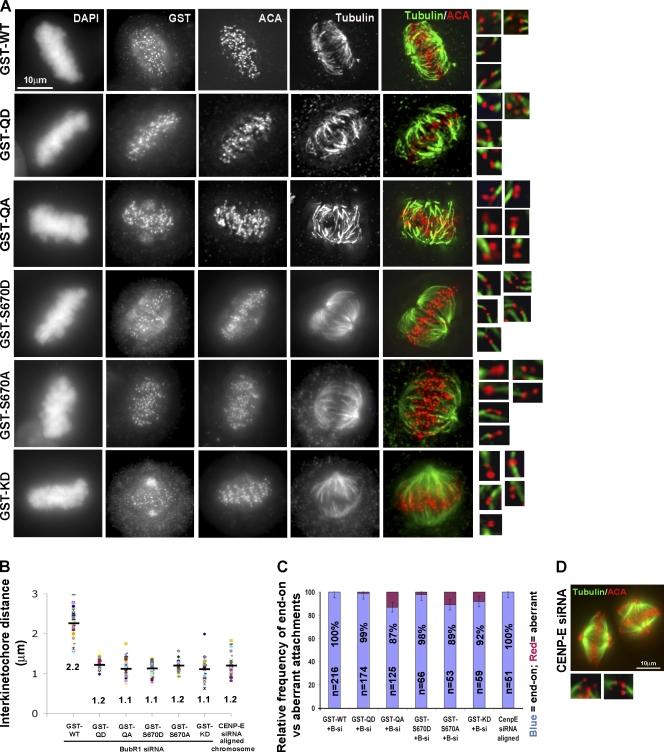
Figure 4.
Phosphorylation of S670 is required for error correction and kinetochore tension. (A) Synchronized HeLa cells depleted of BubR1 by siRNA were injected with various GST/BubR1 constructs and treated with MG132 to prevent mitotic exit. Coverslips were chilled on ice for 10 min, extracted, fixed, and stained for GST, ACA, tubulin, and DAPI. Tubulin and ACA signals were deconvolved to visualize microtubule attachments at individual bioriented kinetochores that are shown in the insets. (B) Interkinetochore distances were measured between pairs of ACA foci with end-on attachments (50 < n < 100). Black bars represent the mean. (C) Histogram depicting the relative frequency of end-on and aberrant attachments (n > 50) in cells expressing various BubR1 constructs. Error bars indicate the range for each dataset. (D) HeLa cells depleted of CENP-E were cold treated and stained for ACA and tubulin to depict end-on attachments and kinetochore tension. WT, wild type.
We discovered that the kinase-dead (KD) BubR1 mutant (Elowe et al., 2007; Matsumura et al., 2007) exhibited a mixed phenotype when compared with the phospho-BubR1 mutants (Fig. 3 C, left and middle). As with wild-type BubR1, the KD mutant restored mitotic progression to the BubR1-depleted cells, but unlike the phospho-BubR1 mutants, they did not exhibit a pronounced delay. Nevertheless, the high frequency of lagging chromosomes in anaphase indicated the presence of defective attachments that were not resolved at the time of exit. The mixed phenotype may be best explained if the KD mutant affected both kinetochore attachments and mitotic checkpoint control.
We used cold treatment to directly assess the integrity of the kinetochore attachments in BubR1-depleted cells that expressed the various transfected BubR1 mutants (Fig. 4). All of the attachments (100%) examined in wild-type–transfected cells exhibited proper bipolar end-on microtubule attachments that were under full tension (Fig. 4, A–C). As predicted, 13% and 11% of all attachments in the QA and the S670A mutants, respectively, exhibited defects that included lateral, monotelic, syntelic, and others (Fig. 4, A and C). These defects were very similar to those seen in cells expressing the KD BubR1 mutant. Although lack of tension generated by these defective attachments is likely responsible for the transient metaphase delay, it is noteworthy that kinetochores that established end-on connections also failed to generate tension.
In contrast to the QA and S670A mutants, virtually all (>98%) of the attached kinetochores examined in the QD and S670D mutants had end-on attachments (Fig. 4, A and C). Nevertheless, the attachments were also defective, as they failed to generate tension (Fig. 4 B). The failure of end-on attachments to generate tension is reminiscent of kinetochores depleted of CENP-E (Fig. 4, B–D; Yao et al., 2000; McEwen et al., 2001) or when microtubule dynamics are dampened pharmacologically. The combined data suggest that phosphorylation of S670 is critically important for kinetochores to resolve aberrant attachments. However, phosphorylation is also important for end-on attachments to generate tension.
Phospho-BubR1 antibodies delay anaphase onset in vivo and in vitro
The in vivo significance of the pS670 and pS1043 residues was independently investigated by injecting a panel of phospho- and nonphospho-BubR1 antibodies into cells. HeLa cells released from a double thymidine block were injected several hours before they were scheduled to enter mitosis. Cells were either fixed for staining or were monitored by time-lapse microscopy. We first determined that the various BubR1 antibodies were concentrated at kinetochores in the injected cells that had entered mitosis (Fig. S4 A, available at http://www.jcb.org/cgi/content/full/jcb.200805163/DC1). As shown previously (Chan et al., 1999; Shannon et al., 2002), cells injected with plain BubR1 antibodies prematurely exited mitosis before chromosomes achieved metaphase alignment. In support of our (Chan et al., 1999) and others' RNAi experiments (Meraldi et al., 2004), the BubR1 antibody–injected cells exited mitosis ∼10 min earlier than cells injected with nonimmune IgG. In contrast, cells injected with either the pS670 or pS1043 antibodies exhibited a metaphase delay of between 30 and 100 min before entering anaphase (Fig. 5, A and B).
Figure 5.
Phospho-BubR1 antibodies delay anaphase onset in vivo. (A) Select frames from time-lapse videos of HeLa H2B-GFP that were injected with the indicated antibodies. (B) Videos were analyzed frame by frame to determine the percentage of cells from NEB to anaphase onset. For pS670, n = 20; pS1043, n = 20; T1042, n = 30; BubR1, n = 12; control IgG, n = 23. Error bars represent the highest and lowest values for each timepoint. (C) Cells injected with nonimmune and pS670 IgG were cold treated and stained for ACA and tubulin to visualize kinetochore attachments (insets).
We were able to rule out nonspecific steric effects by the injected pS1043 antibodies as an explanation for the metaphase delay. We had fortuitously raised a nonphosphoantibody (T1042) that spanned the same epitope used to raise the pS1043 antibodies. Despite the fact that the injected T1042 antibodies were concentrated at kinetochores (Fig. S4 A), they did not interfere with chromosome alignment or with mitotic progression (Fig. 5, A and B). Given that the peptides used to raise antibodies between T1042 and pS1043 were virtually identical, the clear difference in their in vivo response strongly suggests that the effects of the pS1043 antibodies were phosphospecific.
We attempted to detect spindle or kinetochore defects in the phospho-BubR1 antibody–injected cells to identify a cause for the metaphase delay. Antibody injections did not interfere with the formation of a bipolar spindle or the accumulation of Mad1 and CENP-E at kinetochores (Fig. S4 C). Furthermore, the injected antibodies did not interfere with normal kinetochore attachments as determined by their stability to cold treatment and the end-on nature of the microtubule attachments (Fig. 5 C). Consistent with the behavior of the phosphomutants, the interkinetochore distances in the cells injected with pS670 antibody (1.0 ± 0.1 μm) were reduced relative to the control (2.0 ± 0.2 μm).
We tested whether the ability of the pS670 and pS1043 antibodies to delay anaphase onset might be caused by the preservation of these phosphoresidues. This possibility was confirmed in vitro, as addition of the phospho-BubR1 antibodies into mitotic HeLa lysates interfered with the ability of λ protein phosphatase to dephosphorylate endogenous BubR1 (Fig. 6 A). These experiments do not exclude other possibilities in which the phosphoantibody blocks the interaction of p670 BubR1 with critical factors important for generating kinetochore tension.
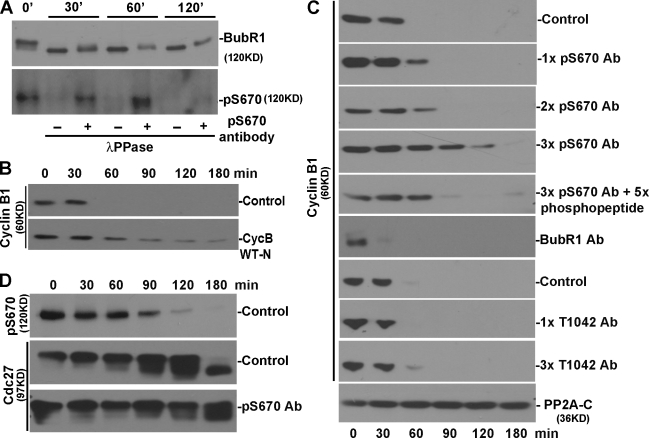
Figure 6.
Phospho-BubR1 antibodies prolong the lag in the APC/C activation in cell extracts. (A) Mitotic extracts were incubated with λ protein phosphatase (PPase) in the presence of pS670 or nonimmune IgG for 0, 30, 60, and 120 min. Membranes were probed for BubR1 to see changes in the mobility of BubR1 (top) and pS670 status (bottom). (B) Mitotic extracts were incubated with and without the N terminus of cyclin B1 (CycB), and samples were taken at various times and probed for endogenous cyclin B1. WT-N, wild-type cyclin B1 N-terminus fragment. (C) Extracts were incubated in the presence of the indicated antibodies (Abs) for various times, and the level of endogenous cyclin B1 was determined. 1× = 0.8 μM of antibody or phosphopeptide. (D) Cell extracts with and without pS670 antibody were probed to evaluate the phosphorylation status of BubR1 (pS670) and Cdc27 (based on mobility shifts).
The metaphase delay exhibited by the phospho-BubR1 mutants can be ascribed to defective kinetochore attachments. However, the delay may also be caused by the fact that BubR1 can act downstream of the kinetochore as an inhibitor of the APC/C. We and recently others (Sudakin et al., 2001; Braunstein et al., 2007) have shown that BubR1 is part of the MCC that binds to and potently inhibits the APC/C in mitotic HeLa cells. Using a cell-free system (extracts prepared from nocodazole-arrested HeLa cells) that recapitulates the mitotic checkpoint events that lie downstream of the kinetochore (Braunstein et al., 2007), we tested whether addition of the phospho-BubR1 antibodies altered the kinetics of checkpoint inhibition. As shown previously (Braunstein et al., 2007), control extracts incubated at 30°C remained in a checkpoint-arrested state for a brief period before inhibition of APC/C is relieved, as is evident by the degradation of endogenous cyclin B1 (or securin; Fig. 6 B and not depicted). As has been shown, the degradation was dependent on the APC/C, as the addition of peptide containing the wild-type destruction box of cyclin B1 effectively competed with the endogenous substrates for APC/C (Fig. 6 B). Addition of different amounts of pS670 antibodies to the extracts prolonged the repression of APC/C activity in a dose-dependent fashion (Fig. 6 C). Furthermore, the effects of the phospho-BubR1 antibodies were neutralized if the antibodies were preincubated briefly with a 1.7-fold excess of phosphopeptide. APC/C activation was not delayed when similar amounts of the T1042 BubR1 antibody were added to the extract. Furthermore, the addition of plain BubR1 antibodies accelerated the onset of APC/C activity (Fig. 6 C). Thus, the responses of the extracts to the pS670, T1042, and plain BubR1 antibodies were similar to the response of cells that were injected with the same set of antibodies.
The level of pS670 in the extracts was reduced by 30 min of incubation and continued to decline (Fig. 6 D). At 90 min, the level of Cdc27 phosphorylation that is specified in large part by Cdk1 (Rudner and Murray, 2000; Kraft et al., 2003; J.Y. Huang et al., 2007) was reduced. Addition of pS670 antibodies delayed the dephosphorylation rate of Cdc27 relative to controls. Based on these in vitro studies, one explanation for how the injected phospho-BubR1 antibodies delayed metaphase is that they preserved the phosphorylation of BubR1, and this prolonged its ability to inhibit the APC/C. Similarly, the phosphomimic BubR1 mutants could have contributed to the metaphase delay by extending the actions of the cytosolic inhibitor of the APC/C in addition to their effects on kinetochore attachments.
Mps1 is required to phosphorylate BubR1 in cells
Recent studies showed that BubR1 is phosphorylated by Plk1 (Elowe et al., 2007; Matsumura et al., 2007) and aurora B (Ditchfield et al., 2003; Hauf et al., 2003). Western blots using the four phosphopeptide antibodies showed that none of them were affected in mitotic cells treated with the aurora kinase inhibitor hesperadin. Furthermore, treatment of cells with roscovitine, a Cdk inhibitor, or staurosporine did not affect these phosphorylation sites (Fig. S5, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200805163/DC1). pS670 levels were unaffected in mitotic cells that were depleted of aurora B or Plk1 by siRNA (Fig. S5, C–E). In cells that were depleted of >90% of their Mps1 kinase, phosphorylation of S435, S543, S670, and S1043 was reduced to ∼25% of control levels (Fig. 7). Mps1 is a major but not the exclusive kinase that specifies BubR1 phosphorylation in vivo.
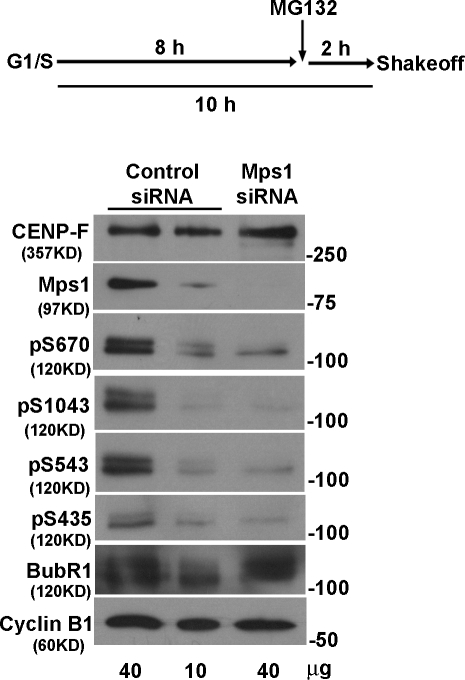
Figure 7.
Mps1 is required to phosphorylate BubR1 in cells. Cells transfected with control, and Mps1 siRNA were released from a double thymidine block. MG132 was added 8 h later, and mitotic cells were harvested 2 h later by shakeoff. Lysates were probed with all four phospho-BubR1 antibodies. Two different amounts of control lysates were used, so it was possible to directly compare the band intensities with Mps1-depleted lysate. CENP-F, BubR1, and cyclin B1 were used as loading controls.
Discussion
BubR1 is hyperphosphorylated in mitosis, and some of the sites depend on aurora B and Plk1 kinases (Ditchfield et al., 2003; Elowe et al., 2007; King et al., 2007; Lenart et al., 2007; Matsumura et al., 2007). Although the sites that are dependent on aurora B remain to be identified, three candidate Plk1 sites were recently reported in human BubR1 (Elowe et al., 2007; Matsumura et al., 2007; Wong and Fang, 2007). However, only one (S676) of the three sites was directly confirmed to exist in vivo. We have identified and confirmed with phosphoantibodies the existence of four additional mitosis-specific phosphorylation sites (S435, S543, S670, and S1043) that are not dependent on aurora B or Plk1 kinases. Instead, Mps1 appears to be a major, but not the only, kinase responsible for these phosphorylations in vivo. Whether it directly or indirectly phosphorylates BubR1 will require in vitro experiments. The contribution of Cdk1 is unclear despite the fact that the addition of Cdk inhibitor to mitotic cells failed to inhibit phosphorylation of BubR1. It is possible that Cdk1 is important for phosphorylating BubR1 at the onset of mitosis and is not essential for maintaining the phosphorylated state. Finally, it is also possible that some of the phosphorylation sites may be caused by BubR1 autokinase activity.
Immunofluorescence staining with pS670 and pS1043 antibodies showed that they first appeared at kinetochores at the onset of mitosis and were dephosphorylated at late metaphase and in early anaphase. Quantitative analysis showed that changes in the levels of pS670 at kinetochores were not caused solely by the fluctuations in BubR1 levels (Hoffman et al., 2001). Thus, phosphorylation of S670 (and pS1043) indeed increased in response to unoccupied microtubule-binding sites as seen during prometaphase. Upon microtubule attachment, S670 is dephosphorylated such that it is no longer detectable by late metaphase when kinetochores are fully saturated with microtubules. We found that dephosphorylation of pS670 was not directly sensitive to kinetochore tension. Interestingly, this is opposite of the increased phosphorylation of S676 that was recently reported to result from the loss of tension (Elowe et al., 2007). Given that the sites reported here are not targets of Plk1, the difference in the phosphorylation response to loss of tension suggests that BubR1 is differentially phosphorylated depending on the status of microtubule attachment and tension.
Phosphorylation of S670 appears to be important for error correction, as kinetochores containing the S670A mutant accumulated aberrant attachments at an ∼15-fold higher frequency than wild type. This conclusion is strengthened by the fact that the S670D phosphomimic mutant is able to prevent the accumulation of defective attachments as effectively as wild-type BubR1. The inability to correct these defective attachments by the S670A mutant explains the high incidence of lagging chromosomes in anaphase. As the attachment defects of the S670A mutant were similar to the KD mutant, the phosphorylation of S670 may act through its kinase domain (Kaplan et al., 2001; Matsumura et al., 2007; Zhang et al., 2007). The defective attachments reported here are similar to those reported for the Plk1 phosphodefective mutants (Elowe et al., 2007; Matsumura et al., 2007). Thus, it is possible that phosphorylation of BubR1 at S670 affects its kinase activity or the ability of the other sites to be phosphorylated by Plk1. However, it remains to be seen whether the phosphorylation sites identified in this study regulate BubR1 kinase activity.
Mechanistically, phosphorylation of BubR1 may be required for error detection and correction. As long as end-on attachments are not made, BubR1 remains phosphorylated so that the error correction system remains active. The kinetochore localization of MCAK (mitotic centromere–associated kinesin), aurora B, and hSgo2, proteins thought to partly contribute to the error correction system (Desai et al., 1999; Hauf et al., 2003; Andrews et al., 2004; Kline-Smith and Walczak, 2004; Lan et al., 2004; Cimini et al., 2006; H. Huang et al., 2007), were not noticeably affected by the phospho-BubR1 mutants (unpublished data). As the frequency of attachment defects exhibited by the phospho-BubR1 mutants seems to be higher than that reported for cells depleted of MCAK, other systems may also be affected. More recent data that link BubR1 with blinkin, a subunit of the Mis12 complex (Kline et al., 2006; Kiyomitsu et al., 2007), suggest another explanation for the origin of the defective attachments seen in the phosho-BubR1 mutants.
Our experiments also showed that BubR1 is required by kinetochores that have established end-on microtubule attachments to develop tension. This was made evident by the fact that both of the S670A and S670D mutants were unable to generate normal levels of tension at kinetochores that established end-on attachments. Whether phosphorylation of S670 is essential for generating tension is difficult to say, as the S670D mutant was as equally defective as the S670A mutant. One possibility is that the aspartic acid (D) cannot functionally substitute for a phosphoserine, as in a case where S670 has to undergo cycles of phosphorylation and dephosphorylation. Conceptually, the S670 residue may be critical for BubR1 to stimulate downstream kinetochore components to generate tension. As the tension defect exhibited by the S670 mutants is very similar to that seen when CENP-E is depleted from kinetochores (Yao et al., 2000; McEwen et al., 2001), it is possible that BubR1 regulates CENP-E's ability to generate tension after microtubules have established end-on connections. Although the frequency of lagging chromosomes exhibited by the S670D mutant is low, we cannot exclude the possibility that the failure to generate tension might result in chromosome nondisjunction, which would not be detected by conventional fluorescence microscopy.
Our results suggest that BubR1 phosphorylation at S670 acts in error correction and in promoting tension to end-on attachments. It is noteworthy that immuno-EM data showed that BubR1 is localized primarily to the outer kinetochore plate in metaphase cells, but in prometaphase cells it was also found to occupy a second zone that was situated near the inner plate (Jablonski et al., 1998). It is possible that the two functions ascribed for phospho-BubR1 reflect discrete functional domains that are spatially separated within the kinetochore. The loss of the inner BubR1 labeling in metaphase cells could also reflect the reduction of BubR1 that is seen at the light level when microtubules attach.
The fact that cells expressing the BubR1 phosphomutants are able to induce a transient metaphase delay in the presence of defective attachments suggests that these phosphorylation sites are not essential for BubR1's ability to delay mitotic exit. Indeed, the QA or QD mutants did not interfere with the cells' ability to become arrested in mitosis for a prolonged period (>6 h) in response to nocodazole (unpublished data). The reason why the phosphomutants were only able to mount a transient delay in response to the attachment defects may be because the amount of wait anaphase signal generated from these kinetochores is not as high as the level generated by unattached kinetochores. This is supported by the fact that Mad1 (and thus Mad2) checkpoint protein is no longer present at the kinetochores that have established microtubule attachments. In the case of nocodazole treatment, Mad1 and Mad2 are recruited back to the kinetochores, and the full complement of checkpoint proteins is available to generate a robust level of wait anaphase signal. Similar arguments were used to explain why cells depleted of CENP-F and hSgo2 only delay transiently despite the presence of numerous aberrant attachments (Feng et al., 2006; H. Huang et al., 2007).
The behavior of the BubR1 KD mutant was complex. As mentioned in the Results, we believe that kinase activity is required for error correction. However, unlike the phosphomutants that exhibited a transient metaphase delay, the KD mutant exited mitosis with kinetics similar to wild-type cells. As the anaphase cells exhibited a high frequency of lagging chromosomes, the KD mutant exited mitosis not because it had repaired its attachment defects but most likely because of its inability to maintain the delay. The possibilities are that the KD mutant prevented the defective kinetochores from generating a wait anaphase signal and that it may also have failed to sustain the checkpoint signaling events that are required to inhibit the APC/C. Others have reported that the kinase activity of BubR1 is not essential for checkpoint activity (Tang et al., 2001; Chen, 2002; Harris et al., 2005; Rancati et al., 2005; Kiyomitsu et al., 2007; Malmanche et al., 2007). Although this may be true for nocodazole treatment, the large number of unattached kinetochores could collectively generate sufficient amounts of signal to sustain a checkpoint delay. In contrast, subtle kinetochore defects (as seen here) that may not generate robust wait anaphase signals may depend more critically on BubR1 kinase activity. Indeed, our original claim that kinase activity was important for the mitotic checkpoint was based on the inability of a BubR1 KD mutant to delay cells that had only a few unattached kinetochores as a result of treatment with a low dose of nocodazole (Chan et al., 1999). The importance of BubR1 kinase activity in the spindle checkpoint was also reported in Xenopus, where mutants that lacked kinase activity failed to sustain a checkpoint arrest (Wong and Fang, 2007).
Analysis of cytosolic extracts prepared from cells released from a nocodazole block for different times revealed that only S435 was completely dephosphorylated with the same kinetics as cyclin B1 degradation. The significance of the S435 site to kinetochore attachments or checkpoint regulation remains to be investigated. The phosphorylation of the remaining three sites gradually declined as cells exited mitosis. By staining unextracted cells, we confirmed that phosphorylation of at least S670 was detectable in the cytosol of early anaphase cells. The reduced level of these phosphorylations in anaphase cells suggests that there may be a threshold that maintains the mitotic state.
We showed that the addition of phospho-BubR1 antibodies to checkpoint-arrested extracts delayed the activation of the APC/C. These extracts were made from HeLa cells that were arrested in mitosis with nocodazole. In the absence of chromosomes, APC/C is reactivated after a 30-min lag. During the lag, APC/C is inhibited by the checkpoint, as shown recently by association of the MCC with the APC/C (Braunstein et al., 2007). This was further reinforced in this study, as the addition of BubR1 antibodies, the same ones that abrogated the checkpoint in cells, accelerated the activation of APC/C. The ability of the pS670 antibodies to extend the lag in a dose-dependent manner suggests that the phosphorylation state of BubR1 in the extracts is a critical determinant of APC/C inhibitory activity. Although there are many explanations for how the phosphoantibodies act, the most straightforward explanation for which we have evidence is that they preserve the phosphorylation state of BubR1. As the majority of BubR1 in the extracts is associated with the MCC (Sudakin et al., 2001), we believe that phosphorylation of BubR1 is important for MCC inhibitory functions. Indeed, the pS670 levels are lowered when APC/C is reactivated. These in vitro findings can be used to explain how the injected pS670 antibodies and the S670D phosphomutant delayed cells in mitosis. In addition to their actions at the kinetochore, the antibodies and phosphomimic mutant can prolong the inhibition of the APC/C as part of the MCC.
The newly identified phosphorylation sites in BubR1 add to the complexity of its regulation. The fact that BubR1 is phosphorylated (directly or indirectly) by multiple kinases such as Plk1, aurora B, and Mps1 suggests that it is an effector of multiple upstream events. Therefore, BubR1 may integrate and coordinate many of the early events that are required to capture and establish end-on microtubule attachments to later events such as the generation of tension and silencing of the checkpoint signaling. In addition, phosphorylation of the cytosolic pool of BubR1 appears to be critically important for regulating mitotic exit.
Materials and Methods
Mass spectrometry
BubR1 was immunopurified from asynchronous and mitotically arrested HeLa cells with BubR1 antibodies that were coupled to protein A beads. Samples were separated on a precast 4–12% denaturing gel (Novex) and stained with Colloidal blue (Invitrogen). Bands corresponding to BubR1 were excised, reduced, alkylated, and digested with trypsin in situ as described previously (Joyal et al., 1997). An aliquot of each tryptic digest was concentrated on a pipette (C18 ZipTip; Millipore), eluted with 2:1 methanol/ammonium hydroxide (30% vol/vol), and loaded into a nanospray needle for analysis by precursor ion-scanning mass spectrometry. Precursor ion spectra for m/z 79, a selective marker for phosphopeptides, were recorded on a triple quadrupole mass spectrometer (API 3000; Sciex) equipped with a nanoelectrospray source and operated in the negative-ion mode (Gomez et al., 2007). Phosphopeptides identified by precursor ion scanning were sequenced by liquid chromatography tandem mass spectrometry. Peptides were loaded on a trap cartridge and backflushed at 300 nl/min to a 75-μm inner diameter 15-cm column (C18 Zorbax; Agilent Technologies) or to a 75-μm inner diameter 15-cm column (PepMap C18; Applied Biosystems) using an acetonitrile/water containing 0.1% formic acid gradient. The mass spectrometer was set to perform full-time tandem mass spectrometry on precursor ions selected for each target phosphopeptide.
DNA and antibodies
BubR1 was PCR amplified from a cDNA library (marathon-ready cDNA; Clontech Laboratories, Inc.) and confirmed by sequence analysis. The full-length BubR1 cDNA was cloned into pENTR (Gateway; Invitrogen) to facilitate transfer into Destination vectors by in vitro recombination reactions. A QuikChange Site-Directed Mutagenesis kit (Agilent Technologies) was used to mutate phosphorylation sites as well as to introduce RNAi-resistant alleles of BubR1. To make phosphospecific antibodies, phosphopeptides were coupled to keyhole limpet hemocyanin and used to immunize rabbits (Babco). Serum was passed through a phosphopeptide affinity column. The eluted antibodies that contain a mixture of phospho- and nonphosphoantibodies were passed through a nonphosphopeptide column. The flow through was tested for phosphospecificity.
Cell culture and RNAi
HeLa cells were grown in DME + 10% FBS in a humidified incubator at 37°C. Nocodazole was used at 20-nM (low) and 60-nM (high) final concentrations.
BubR1 siRNA, CAGGAACAACCTCATTCTAAA, was obtained from QIAGEN. siRNAs were diluted in serum-free OptiMEM and HiPerfect (QIAGEN) as per the manufacturer's instructions and added to cells so that the final concentration of siRNA was 20 nM. 24–36 h after transfection, cells were fixed and stained or lysed in SDS sample buffer.
Cell synchronization and microinjection
Cells were synchronized at the G1/S boundary by a double thymidine block. Cells were grown in the presence of 2 mM thymidine for 15 h, washed, and released into fresh medium for 9 h, and thymidine was added for another 15 h. 5–6 h after thymidine release, 2–4 mg/ml of antibodies in PBS was injected into the cytoplasm of cells. Cells were either fixed several hours later for immunofluorescence staining or monitored by time-lapse microscopy. To analyze BubR1 mutants in cells depleted of endogenous BubR1, siRNAs were transfected at the time of the first thymidine block. DNA was injected as described previously (Hagting et al., 2002; Di Fiore and Pines, 2007) into the nucleus of cells the next day, before the second round of thymidine was added. Plasmid DNA for injections was diluted in PBS into a final concentration of 50–75 ng/μl. Injections were performed with a semiautomated microinjector (model 5242; Eppendorf) attached to an inverted microscope (Eclipse TE300; Nikon). The cells were plated onto no. 1.5 coverslips (18 × 18 mm), and the injection area was scribed with a diamond pen. All cells (∼300–400) within the scribed loop were injected, and viability was >95% at 2–3 h after injection.
Microscopy
Cells were fixed for 7 min in freshly prepared 3.5% paraformaldehyde/PBS, pH 6.9, extracted in KB (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% BSA) with 0.2% Triton X-100 for 5 min at room temperature, and rinsed in KB. In some cases, cells were preextracted for 90 s before fixing. Primary and secondary antibodies were diluted in KB and added to coverslips for 30 min at 37°C in a humidified chamber. Commercial antibodies to tubulin (Sigma-Aldrich), GST (Cell Signaling Technology), Flag (Sigma-Aldrich), and Plk1 (Santa Cruz Biotechnology, Inc.) were used. Human anticentromere antibody (ACA) serum was provided by J.B. Rattner (University of Alberta, Calgary, Canada). Antibodies to human BubR1, CENP-E, and Mad1 were obtained from our laboratory (Liao et al., 1994; Chan et al., 1998; Jablonski et al., 1998; Campbell et al., 2001). Antibodies were used at a final concentration of 0.5–1.0 μg/ml. Secondary antibodies conjugated to Alexa Fluor 488, 555, and 647 (Invitrogen) were used at 1 μg/ml. Images were visualized with a 100×/1.4 NA objective attached to an inverted microscope (Eclipse TE2000S; Nikon), and 0.25–1-μm image stacks were captured with a charge-coupled device camera (Photometrics Cascade 512F; Roper Scientific). Raw images were analyzed with MetaMorph software (MDS Analytical Technologies). Images are presented as maximum projections and quantitated as previously described (Hoffman et al., 2001). Deconvolution was conducted with AutoQuant (Media Cybernetics). All image files were reformatted as TIFF files, and Photoshop (Adobe) was used to assemble the figures. For time-lapse studies, HeLa or HeLa–GFP-H2B was plated onto No. 1.5 coverslips (18 × 18 mm) in Hepes-buffered medium and imaged with either an Eclipse TE300 or TE2000S inverted microscope. Images were captured every 5–10 min overnight at 37°C and processed with ImagePro Plus software (Media Cybernetics).
Online supplemental material
Fig. S1 shows the identification of BubR1 phosphorylation sites by mass spectrometry. Fig. S2 shows the specificity of BubR1 phosphoantibodies and phosphorylation states of BubR1 in vivo. Fig. S3 shows that phospho-BubR1 mutants do not disrupt Mad1 and CENP-E localization at kinetochores. Fig. S4 shows that injected antibodies are concentrated at kinetochores, and antibody injections do not affect formation of the spindle and localization of Mad1 and CENP-E at kinetochores. Fig. S5 shows that aurora B and Plk1 are not required for S670 phosphorylation. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200805163/DC1.
Supplementary Material
Acknowledgments
We would like to acknowledge expert services provided by B. Connor, the core facilities at Fox Chase Cancer Center that include the Laboratory Animal Facility, Hybridoma facilities, and the DNA synthesis and sequencing facilities. We also gratefully acknowledge S.T. Liu for insightful discussions and J. Jackson and P. Huang (GlaxoSmithKline, Collegeville, PA) for providing support to raise the BubR1 phosphoantibodies.
This work was supported by grants from the Leukemia and Lymphoma Society, the National Institutes of Health (GM86877 and GM44762), a core grant (CA06927), an appropriation from the Commonwealth of Pennsylvania, and the Greenberg Fund. H. Huang is supported by the Plain and Fancy Fellowship from the Fox Chase Cancer Center Board of Associates.
Abbreviations used in this paper: ACA, anticentromere antibody; APC/C, anaphase-promoting complex/cyclosome; CENP, centromere protein; KD, kinase dead; MCC, mitotic checkpoint complex; NEB, nuclear envelope breakdown.
References
- Andrews, P.D., Y. Ovechkina, N. Morrice, M. Wagenbach, K. Duncan, L. Wordeman, and J.R. Swedlow. 2004. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 6:253–268. [DOI] [PubMed] [Google Scholar]
- Braunstein, I., S. Miniowitz, Y. Moshe, and A. Hershko. 2007. Inhibitory factors associated with anaphase-promoting complex/cyclosome in mitotic checkpoint. Proc. Natl. Acad. Sci. USA. 104:4870–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, D.P., C. Lengauer, J. Yu, G.J. Riggins, J.K. Willson, S.D. Markowitz, K.W. Kinzler, and B. Vogelstein. 1998. Mutations of mitotic checkpoint genes in human cancers. Nature. 392:300–303. [DOI] [PubMed] [Google Scholar]
- Campbell, M.S., G.K. Chan, and T.J. Yen. 2001. Mitotic checkpoint proteins HsMAD1 and HsMAD2 are associated with nuclear pore complexes in interphase. J. Cell Sci. 114:953–963. [DOI] [PubMed] [Google Scholar]
- Chan, G.K., B.T. Schaar, and T.J. Yen. 1998. Characterization of the kinetochore binding domain of CENP-E reveals interactions with the kinetochore proteins CENP-F and hBUBR1. J. Cell Biol. 143:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, G.K., S.A. Jablonski, V. Sudakin, J.C. Hittle, and T.J. Yen. 1999. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 146:941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R.H. 2002. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 158:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini, D., X. Wan, C.B. Hirel, and E.D. Salmon. 2006. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 16:1711–1718. [DOI] [PubMed] [Google Scholar]
- Desai, A., S. Verma, T.J. Mitchison, and C.E. Walczak. 1999. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 96:69–78. [DOI] [PubMed] [Google Scholar]
- Di Fiore, B., and J. Pines. 2007. Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J. Cell Biol. 177:425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield, C., V.L. Johnson, A. Tighe, R. Ellston, C. Haworth, T. Johnson, A. Mortlock, N. Keen, and S.S. Taylor. 2003. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowe, S., S. Hummer, A. Uldschmid, X. Li, and E.A. Nigg. 2007. Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev. 21:2205–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, J., H. Huang, and T.J. Yen. 2006. CENP-F is a novel microtubule-binding protein that is essential for kinetochore attachments and affects the duration of the mitotic checkpoint delay. Chromosoma. 115:320–329. [DOI] [PubMed] [Google Scholar]
- Gomez, R., A. Valdeolmillos, M.T. Parra, A. Viera, C. Carreiro, F. Roncal, J.S. Rufas, J.L. Barbero, and J.A. Suja. 2007. Mammalian SGO2 appears at the inner centromere domain and redistributes depending on tension across centromeres during meiosis II and mitosis. EMBO Rep. 8:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting, A., N. Den Elzen, H.C. Vodermaier, I.C. Waizenegger, J.M. Peters, and J. Pines. 2002. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157:1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, L., J. Davenport, G. Neale, and R. Goorha. 2005. The mitotic checkpoint gene BubR1 has two distinct functions in mitosis. Exp. Cell Res. 308:85–100. [DOI] [PubMed] [Google Scholar]
- Hauf, S., R.W. Cole, S. LaTerra, C. Zimmer, G. Schnapp, R. Walter, A. Heckel, J. van Meel, C.L. Rieder, and J.M. Peters. 2003. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore–microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, D.B., C.G. Pearson, T.J. Yen, B.J. Howell, and E.D. Salmon. 2001. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol. Biol. Cell. 12:1995–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H., J. Feng, J. Famulski, J.B. Rattner, S.T. Liu, G.D. Kao, R. Muschel, G.K. Chan, and T.J. Yen. 2007. Tripin/hSgo2 recruits MCAK to the inner centromere to correct defective kinetochore attachments. J. Cell Biol. 177:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J.Y., G. Morley, D. Li, and M. Whitaker. 2007. Cdk1 phosphorylation sites on Cdc27 are required for correct chromosomal localisation and APC/C function in syncytial Drosophila embryos. J. Cell Sci. 120:1990–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski, S.A., G.K. Chan, C.A. Cooke, W.C. Earnshaw, and T.J. Yen. 1998. The hBUB1 and hBUBR1 kinases sequentially assemble onto kinetochores during prophase with hBUBR1 concentrating at the kinetochore plates in mitosis. Chromosoma. 107:386–396. [DOI] [PubMed] [Google Scholar]
- Joyal, J.L., R.S. Annan, Y.D. Ho, M.E. Huddleston, S.A. Carr, M.J. Hart, and D.B. Sacks. 1997. Calmodulin modulates the interaction between IQGAP1 and Cdc42. Identification of IQGAP1 by nanoelectrospray tandem mass spectrometry. J. Biol. Chem. 272:15419–15425. [DOI] [PubMed] [Google Scholar]
- Kaplan, K.B., A.A. Burds, J.R. Swedlow, S.S. Bekir, P.K. Sorger, and I.S. Nathke. 2001. A role for the adenomatous polyposis coli protein in chromosome segregation. Nat. Cell Biol. 3:429–432. [DOI] [PubMed] [Google Scholar]
- King, E.M., N. Rachidi, N. Morrice, K.G. Hardwick, and M.J. Stark. 2007. Ipl1p-dependent phosphorylation of Mad3p is required for the spindle checkpoint response to lack of tension at kinetochores. Genes Dev. 21:1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyomitsu, T., C. Obuse, and M. Yanagida. 2007. Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev. Cell. 13:663–676. [DOI] [PubMed] [Google Scholar]
- Kline, S.L., I.M. Cheeseman, T. Hori, T. Fukagawa, and A. Desai. 2006. The human Mis12 complex is required for kinetochore assembly and proper chromosome segregation. J. Cell Biol. 173:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Smith, S.L., and C.E. Walczak. 2004. Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol. Cell. 15:317–327. [DOI] [PubMed] [Google Scholar]
- Kraft, C., F. Herzog, C. Gieffers, K. Mechtler, A. Hagting, J. Pines, and J.M. Peters. 2003. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 22:6598–6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson, M.A., and T.M. Kapoor. 2005. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nat. Cell Biol. 7:93–98. [DOI] [PubMed] [Google Scholar]
- Lan, W., X. Zhang, S.L. Kline-Smith, S.E. Rosasco, G.A. Barrett-Wilt, J. Shabanowitz, D.F. Hunt, C.E. Walczak, and P.T. Stukenberg. 2004. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 14:273–286. [DOI] [PubMed] [Google Scholar]
- Lenart, P., M. Petronczki, M. Steegmaier, B. Di Fiore, J.J. Lipp, M. Hoffmann, W.J. Rettig, N. Kraut, and J.M. Peters. 2007. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 17:304–315. [DOI] [PubMed] [Google Scholar]
- Liao, H., G. Li, and T.J. Yen. 1994. Mitotic regulation of microtubule cross-linking activity of CENP-E kinetochore protein. Science. 265:394–398. [DOI] [PubMed] [Google Scholar]
- Liu, S.T., J.B. Rattner, S.A. Jablonski, and T.J. Yen. 2006. Mapping the assembly pathways that specify formation of the trilaminar kinetochore plates in human cells. J. Cell Biol. 175:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmanche, N., S. Owen, S. Gegick, S. Steffensen, J.E. Tomkiel, and C.E. Sunkel. 2007. Drosophila BubR1 is essential for meiotic sister-chromatid cohesion and maintenance of synaptonemal complex. Curr. Biol. 17:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, Y., A. Abrieu, and D.W. Cleveland. 2003. Activating and silencing the mitotic checkpoint through CENP-E-dependent activation/inactivation of BubR1. Cell. 114:87–98. [DOI] [PubMed] [Google Scholar]
- Mao, Y., A. Desai, and D.W. Cleveland. 2005. Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. J. Cell Biol. 170:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura, S., F. Toyoshima, and E. Nishida. 2007. Polo-like kinase 1 facilitates chromosome alignment during prometaphase through BubR1. J. Biol. Chem. 282:15217–15227. [DOI] [PubMed] [Google Scholar]
- McEwen, B.F., G.K. Chan, B. Zubrowski, M.S. Savoian, M.T. Sauer, and T.J. Yen. 2001. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol. Biol. Cell. 12:2776–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi, P., V.M. Draviam, and P.K. Sorger. 2004. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell. 7:45–60. [DOI] [PubMed] [Google Scholar]
- Musaro, M., L. Ciapponi, B. Fasulo, M. Gatti, and G. Cenci. 2008. Unprotected Drosophila melanogaster telomeres activate the spindle assembly checkpoint. Nat. Genet. 40:362–366. [DOI] [PubMed] [Google Scholar]
- Rancati, G., V. Crispo, G. Lucchini, and S. Piatti. 2005. Mad3/BubR1 phosphorylation during spindle checkpoint activation depends on both Polo and Aurora kinases in budding yeast. Cell Cycle. 4:972–980. [DOI] [PubMed] [Google Scholar]
- Rudner, A.D., and A.W. Murray. 2000. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol. 149:1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar, B.T., G.K. Chan, P. Maddox, E.D. Salmon, and T.J. Yen. 1997. CENP-E function at kinetochores is essential for chromosome alignment. J. Cell Biol. 139:1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, K.B., J.C. Canman, and E.D. Salmon. 2002. Mad2 and BubR1 function in a single checkpoint pathway that responds to a loss of tension. Mol. Biol. Cell. 13:3706–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin, V., G.K. Chan, and T.J. Yen. 2001. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154:925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Z., R. Bharadwaj, B. Li, and H. Yu. 2001. Mad2-independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev. Cell. 1:227–237. [DOI] [PubMed] [Google Scholar]
- Taylor, S.S., E. Ha, and F. McKeon. 1998. The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. J. Cell Biol. 142:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, S.S., D. Hussein, Y. Wang, S. Elderkin, and C.J. Morrow. 2001. Kinetochore localisation and phosphorylation of the mitotic checkpoint components Bub1 and BubR1 are differentially regulated by spindle events in human cells. J. Cell Sci. 114:4385–4395. [DOI] [PubMed] [Google Scholar]
- Wong, O.K., and G. Fang. 2007. Cdk1 phosphorylation of BubR1 controls spindle checkpoint arrest and Plk1-mediated formation of the 3F3/2 epitope. J. Cell Biol. 179:611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, X., A. Abrieu, Y. Zheng, K.F. Sullivan, and D.W. Cleveland. 2000. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nat. Cell Biol. 2:484–491. [DOI] [PubMed] [Google Scholar]
- Zhang, J., S. Ahmad, and Y. Mao. 2007. BubR1 and APC/EB1 cooperate to maintain metaphase chromosome alignment. J. Cell Biol. 178:773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.