Abstract
The axon initial segment (AIS) functions as both a physiological and physical bridge between somatodendritic and axonal domains. Given its unique molecular composition, location, and physiology, the AIS is thought to maintain neuronal polarity. To identify the molecular basis of this AIS property, we used adenovirus-mediated RNA interference to silence AIS protein expression in polarized neurons. Some AIS proteins are remarkably stable with half-lives of at least 2 wk. However, silencing the expression of the cytoskeletal scaffold ankyrinG (ankG) dismantles the AIS and causes axons to acquire the molecular characteristics of dendrites. Both cytoplasmic- and membrane-associated proteins, which are normally restricted to somatodendritic domains, redistribute into the former axon. Furthermore, spines and postsynaptic densities of excitatory synapses assemble on former axons. Our results demonstrate that the loss of ankG causes axons to acquire the molecular characteristics of dendrites; thus, ankG is required for the maintenance of neuronal polarity and molecular organization of the AIS.
Introduction
Axon and dendrite specification during development (i.e., neuronal polarity) depends on positive and negative signals that regulate protein trafficking and cytoskeletal dynamics (Arimura and Kaibuchi, 2007). However, the molecular mechanisms maintaining neuronal polarity throughout life are unknown. The axon initial segment (AIS) functions as both a physical and physiological bridge between the somatodendritic and axonal domains. It is responsible for action potential initiation and modulation (Kole et al., 2007, 2008) and is highly enriched in ion channels, cell adhesion molecules (CAMs), and cytoskeletal and scaffolding proteins (Hedstrom et al., 2007). Given its unique molecular composition, location, and physiology, the AIS is thought to maintain neuronal polarity. Winckler et al. (1999) demonstrated that membrane proteins have different lateral mobilities in the AIS as compared with the distal axon. Furthermore, they demonstrated that disruption of the actin-based cytoskeleton removed the AIS diffusion barrier and concluded that the AIS cytoskeleton regulates neuronal polarity.
Among the known AIS proteins, ankyrinG (ankG) is essential for AIS assembly (Jenkins and Bennett, 2001) and may be important for its long-term maintenance. Alternatively, neurofascin (NF)-186, an AIS CAM that binds ankG, could function independently of ankG to retain voltage-gated Na+ (Nav) channels at the AIS because it interacts with both the unique AIS extracellular matrix (Hedstrom et al., 2007) and Na+ channel β subunits (Ratcliffe et al., 2001). To test the hypothesis that the AIS contributes to the maintenance of neuronal polarity and to determine whether NF-186, ankG, or other AIS proteins mediate this function, we silenced AIS protein expression in fully polarized 10-d in vitro (DIV) neurons (Yang et al., 2007) using adenoviruses to transduce neurons with NF-186, Nav channel, βIV spectrin, or ankG short hairpin RNA (shRNA) expression constructs.
Results and discussion
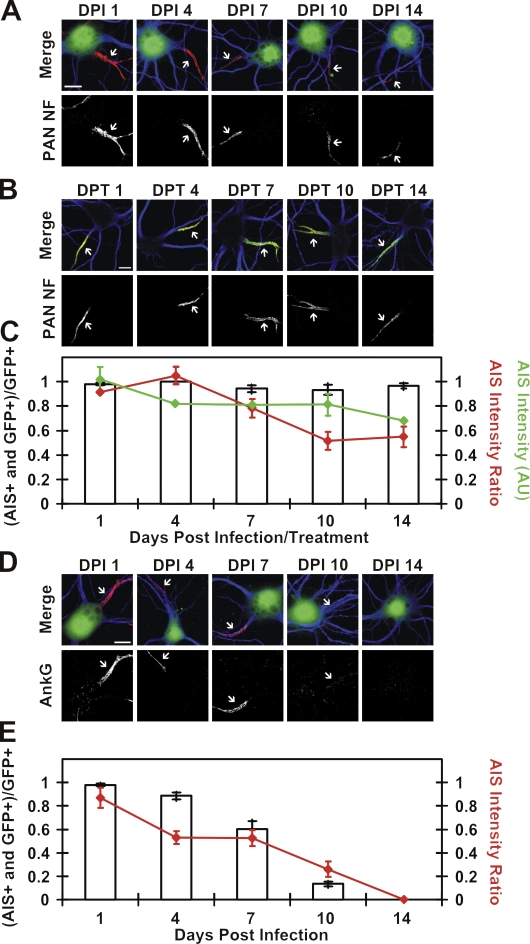
The silencing of NF-186 did not reduce the number of neurons with NF-186 at the AIS (Fig. 1, A and C, bars), and a control GFP adenovirus did not affect the AIS (Fig. S1, A–C, available at http://www.jcb.org/cgi/content/full/jcb.200806112/DC1). However, by 14 d post infection (DPI; 14 DPI = 24 DIV), the mean NF-186 fluorescence intensity of infected (GFP+) neurons decreased by 45% (Fig. 1, A and C, red lines). Failure to eliminate NF-186 from the AIS was not a result of poor shRNA efficacy because the silencing of NF-186 expression during development blocked its AIS accumulation (Hedstrom et al., 2007). We confirmed that NF-186 protein is very stable at the AIS by labeling 10 DIV hippocampal neurons live for 30 min using a mAb (A12/18.1) we generated that recognizes NF-186's extracellular domain. Consistent with the NF-186 shRNA results, we found this antibody was retained at every hippocampal neuron's AIS for at least 14 d post treatment (DPT) with antibodies (Fig. 1 B). We observed only a gradual decrease in fluorescence intensity during this time that was similar to shRNA-treated neurons (33% decrease at 14 DPT; Fig. 1 C, green line). These results, using two independent methods, suggest that NF-186 is a long-lived AIS component with a half-life of >2 wk. Similar to NF-186, Nav channels and βIV spectrin are long-lived components of the AIS because silencing their expression by adenoviral-delivered shRNAs resulted in only an ∼50% decrease in the targeted protein after 14 DPI (Fig. S1, D–G).
Figure 1.
Stability of NF-186 and ankG at the AIS. (A) NF-186 immunoreactivity (PAN NF, L11A/41.6) in NF-186 shRNA adenovirus–infected (i.e., GFP+) cells at various DPI. (B) Live labeling of neurons using the A12/18.1 mAb (PAN NF) at various DPT. βIV spectrin (green) marks the AIS (merge). (C) Bars indicate the ratio of AIS NF-186–labeled GFP+ neurons to the total number of GFP+ neurons. The mean intensity ratio of a GFP+ cell's AIS to a GFP− cell's AIS was calculated per DPI. The red line indicates the mean of the average NF-186 intensity ratio. The green line indicates the mean fluorescence intensity per AIS in A12/18.1 live labeled neurons. AU, arbitrary units. (D) AnkG immunoreactivity in ankG shRNA adenovirus–infected (i.e., GFP+) cells. (E) Bars indicate the ratio of AIS ankG–labeled GFP+ neurons to the total number of GFP+ neurons, and the red line indicates the mean of the average ankG intensity ratio. In A, B, and D, MAP2 (blue) marks somatodendritic domains, and arrows point to the AIS. Error bars represent ±SEM. Bars, 10 μm.
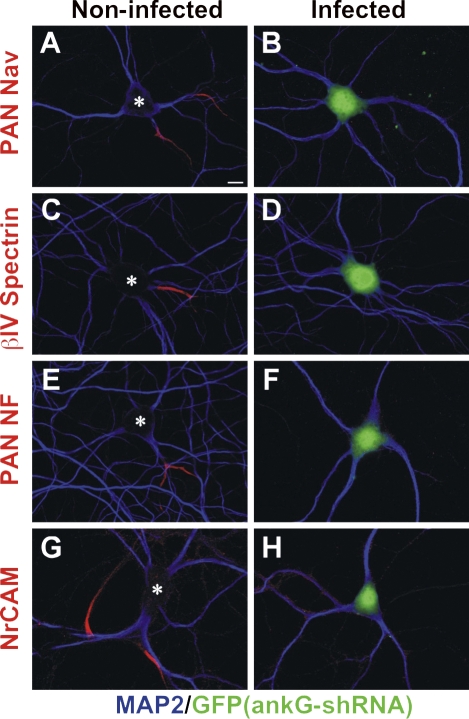
In contrast to NF-186, Nav channels, and βIV spectrin, the silencing of ankG expression resulted in its loss from the AIS. By 7 DPI, ankG immunoreactivity was undetectable in 40% of GFP+ neurons; in the remaining 60%, the mean AIS ankG fluorescence intensity decreased by 47% compared with noninfected controls (Fig. 1, D and E). By 14 DPI, ankG immunoreactivity could not be detected in any infected neurons. The loss of ankG caused dismantling of the AIS; at 10 DPI (20 DIV), 99% of GFP+ (i.e., ankG shRNA transduced) neurons lacked immunostaining for Nav channels, 90% lacked βIV spectrin, 90% lacked NF-186, and 98% lacked neuronal CAM (NrCAM) immunoreactivity (Fig. 2). Thus, ankG is necessary for both the initial recruitment of AIS proteins (Hedstrom et al., 2007) and for their long-term stability and retention. Furthermore, long-lived AIS proteins like NF-186 are rapidly lost in ankG's absence, indicating that their interactions with other AIS proteins (e.g., NF-186's interactions with the extracellular matrix; Hedstrom et al., 2007) cannot stabilize them within this domain.
Figure 2.
AnkG is required for AIS maintenance. Control (A, C, E, and G) and ankG shRNA–infected (B, D, F, and H) neurons immunolabeled for various AIS components: Nav channels (PAN Nav; A and B), βIV spectrin (C and D), NF (PAN NF; E and F), and NrCAM (G and H). GFP fluorescence (green) indicates infected cells, whereas MAP2 (blue) defines somatodendritic domains. Asterisks indicate noninfected neurons. Bar, 10 μm.
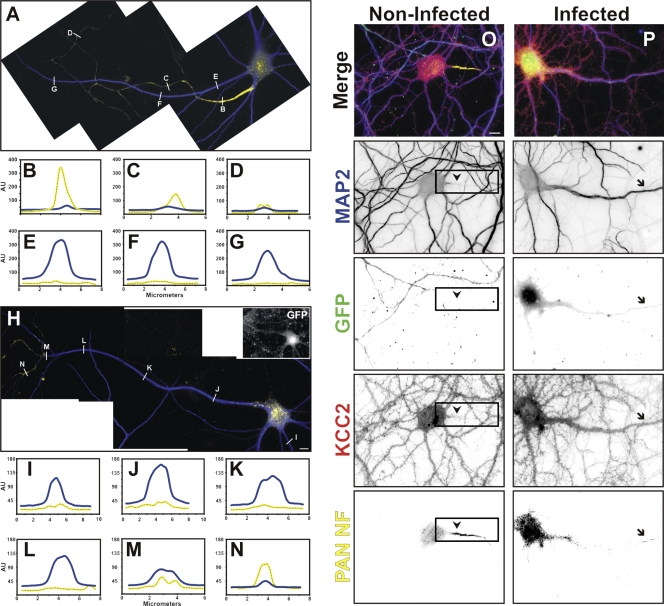
To determine whether ankG contributes to the maintenance of neuronal polarity, we examined axonal and somatodendritic markers 10 d after ankG silencing (20 DIV). In 20 DIV control neurons, NF-186 immunoreactivity is highly enriched at the AIS with decreasing amounts in distal axonal locations (Fig. 3 A, yellow). In contrast, cytoplasmic MAP2 defines the somatodendritic domain (Fig. 3 A, blue). Line scans at three locations along the axon demonstrate exclusion of MAP2, high levels of NF-186 near the cell body, and decreasing NF-186 immunoreactivity at progressively more distal locations (Fig. 3, B–D). In contrast, line scans through dendrites demonstrate uniform MAP2 immunoreactivity and a lack of NF-186 (Fig. 3, E–G). In GFP+ neurons without ankG, all processes emanating from the soma were MAP2+ (Fig. 3 H and Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200806112/DC1). Remarkably, one of these had features characteristic of both axons and dendrites: at sites far from the cell body, there was a transition from somatodendritic MAP2 to axonal NF-186 (Fig. 3 H and Fig. S3). In some neurons, we also used GFP immunostaining to identify the axon as the longest process; these too had increased levels of MAP2 immunoreactivity (Fig. S2). Similar to other dendrites (Fig. 3 I), line scans show a thick MAP2+ shaft (Fig. 3, J–L). However, this process, with the molecular characteristics of a dendrite, then transitions (Fig. 3 M) from MAP2+ immunoreactivity to NF-186+ immunoreactivity (Fig. 3 N), comparable with that seen at the distal axons of noninfected neurons (Fig. 3, C and D). Of the 103 GFP+ neurons we examined, 41 had MAP2+ processes that also had distal NF-186 immunoreactivity (Fig. 3 H and Fig. S3). The remaining 62 had no detectable NF-186 immunoreactivity, and all processes were MAP2+. Thus, the loss of AIS ankG permits cytoplasmic MAP2 to enter the axon.
Figure 3.
AnkG is required for maintenance of neuronal polarity. (A) In 20 DIV control neurons, MAP2 (blue) defines somatodendritic domains, whereas PAN NF (yellow) defines the AIS and distal axon. (B–G) Line scans of MAP2 and PAN NF immunofluorescence in A; each line scan shown corresponds to the white lines on the axon (B–D) or dendrite (E–G). (H) In 10 DPI ankG shRNA adenovirus–infected neurons, MAP2 is in all neuronal processes, including the former axon. (I–N) Line scans of MAP2 and PAN NF immunofluorescence shown in H; each line scan shown corresponds to the white lines on the former axon. Distal PAN NF immunofluorescence defines the former axon (N). (O) In 20 DIV control neurons, MAP2 and KCC2 (red) define somatodendritic domains, whereas PAN NF (yellow) defines the axon. Neither MAP2 nor KCC2 is detected in the axon (arrowheads and boxes). (P) In 20 DIV GFP+ ankG shRNA adenovirus–infected neurons, KCC2 and MAP2 immunofluorescence extends into the former axon identified by distal PAN NF immunoreactivity (arrows). AU, arbitrary units. Bars, 10 μm.
The loss of ankG also altered the distribution of somatodendritic integral membrane proteins. In control GFP− neurons, the K+/Cl− cotransporter KCC2 and MAP2 were both excluded from axons (Fig. 3 O, arrowheads and boxes). In contrast, GFP+ neurons had both MAP2 and KCC2 immunoreactivity that extended into the process identified as the former axon based on distal NF-186 immunoreactivity (Fig. 3 P, arrows). Thus, ankG and the AIS are required to exclude somatodendritic membrane proteins from the axon.
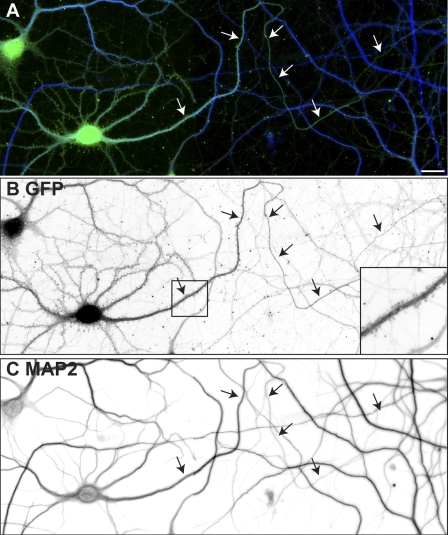
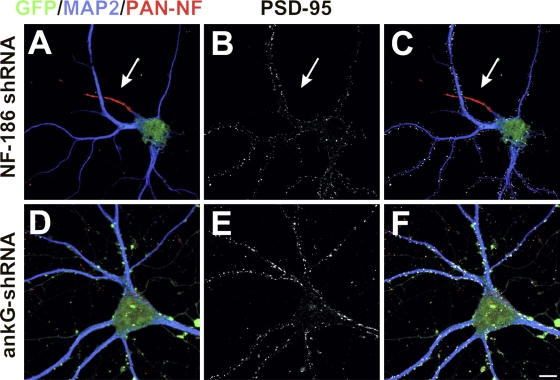
After silencing ankG expression in some anti-GFP immunolabeled cells where the identity of the axon was clearly determined based on morphology of the distal processes (i.e., longest and thinnest process; Fig. 4 A, arrows), we also observed dendritic spines in the proximal region of the former axon (Fig. 4 B, inset). The presence or absence of spines correlated well with MAP2 immunoreactivity along the former axon: regions with high levels of MAP2 had spines, whereas distal segments with low levels of MAP2 did not (Fig. 4 C). To further confirm that loss of ankG from the AIS resulted in the development of excitatory synapses, we used antibodies against the postsynaptic scaffolding protein PSD-95. In neurons infected with adenovirus to deliver NF-186 shRNA, the AIS remained intact (Fig. 1, A–C; and Fig. 5 A), and PSD-95 immunoreactivity could only be detected on dendrites; the axon and AIS never had punctate PSD-95 immunoreactivity (Fig. 5, B and C, arrows). In contrast, the silencing of ankG and dismantling of the AIS (Fig. 5 D) resulted in all GFP+ neuronal processes having PSD-95+ postsynaptic densities (Fig. 5, E and F). Thus, loss of ankG permits the formation of spines and excitatory postsynaptic densities on the proximal region of the former axon.
Figure 4.
Loss of ankG causes axons to develop spines. (A) A neuron infected with shRNA to silence the expression of ankG was double labeled for GFP (green) and MAP2 (blue). (B) GFP immunofluorescence shows the axon and the presence of dendritic spines along the axon near the cell body (inset). (C) MAP2 immunoreactivity illustrates high levels of MAP2 along the former axon. In A–C, two adjacent fields were merged to show the distal axon. Arrows point to axons. Bar, 20 μm.
Figure 5.
Loss of ankG causes axons to acquire excitatory postsynaptic densities. (A–F) Neurons infected with shRNA to silence the expression of NF-186 (A–C) or ankG (D–F) were quadruple labeled for GFP (green), PAN NF (red), MAP2 (blue), and PSD-95 (white). PSD-95 labels excitatory postsynaptic densities and is never found on the axon of NF-186 shRNA adenovirus–infected cells (A–C, arrows). In contrast, all processes of ankG shRNA adenovirus–infected neurons had PSD-95+ puncta, indicating postsynaptic densities on every single process, one of which is a former axon. MAP2 (blue) defines somatodendritic domains, whereas GFP (green) indicates infected neurons. Bar, 10 μm.
Although several experimental manipulations, including the overexpression of CRMP-2, Dishevelled, and α-PKC, can promote the differentiation of neurites that normally would become dendrites into axons (Inagaki et al., 2001; Zhang et al., 2007), our results demonstrate the first manipulation that causes an axon to acquire the molecular and structural properties of a dendrite (Jiang et al., 2005). Thus, besides the clustering of ion channels (Garrido et al., 2003; Pan et al., 2006; Dzhashiashvili et al., 2007), ankG is required for maintenance of the AIS and axon identity.
The AIS has previously unappreciated degrees of plasticity in the kinds of ion channels expressed, their densities, and their position in the axon (Grieco et al., 2005; Kuba et al., 2006; Ogiwara et al., 2007). Because most AIS proteins are long lived, we speculate that modulation of the action potential threshold might be regulated at the level of ankG transcription or through increasing/decreasing ankG turnover rates rather than the expression of ion channels.
Previous studies showed that the lateral mobility of lipids and axonal CAMs in the AIS plasma membrane was limited (Winckler et al., 1999; Nakada et al., 2003), suggesting a diffusion barrier at the AIS. The treatment of cultured neurons with actin-destabilizing agents disrupts the diffusion barrier (Winckler et al., 1999; Kole et al., 2008), although the mechanisms underlying this phenomenon are not known. Our results indicate that this barrier role extends to somatodendritic membrane and cytoplasmic proteins and suggest that it is ankG based. Thus, the ankG-based AIS functions as a spatial barrier to maintain neuronal polarity by restricting the types of proteins that can enter the axon.
Axon injury has been associated with changes in neuronal polarity, conversion of dendrites to axons, and the development of supernumerary axons (Havton and Kellerth, 1987). One recent study showed that transection of the axon at sites distal to the soma did not affect polarity, but transection of the axon close to the cell body (∼35 μm from the soma and about the same location as the AIS) caused a fate switch and conversion of a dendrite into an axon (Gomis-Ruth et al., 2008). Although this was attributed to altered microtubule stability in the distal axon, an alternative explanation consistent with the results presented here is that the AIS maintains neuronal polarity. We propose that diseases or injuries that disrupt ankG at the AIS may contribute to nervous system dysfunction through loss of neuronal polarity, loss of clustered ion channels, and inappropriate synaptic innervation of proximal segments of axons.
Materials and methods
Antibodies
The PAN NF (L11A/41.6), PAN Nav channel, and βIV spectrin antibodies were described previously (Schafer et al., 2004; Ogawa et al., 2006). The A12/18.1 antibody recognizes the NF-186 extracellular domain. AnkG antibody was provided by V. Bennett (Duke University, Durham, NC). NrCAM antibodies were obtained from Abcam. KCC2 antibody was purchased from the University of California, Davis, National Institute of Neurological Disorders and Stroke/National Institute of Mental Health NeuroMab Hybridoma Facility. Anti-MAP2 antibodies were purchased from EnCor Biotechnology, Inc. Anti-GFP and fluorescent secondary antibodies were obtained from Invitrogen or Accurate Chemical.
Adenovirus
Sense sequences for ankG, PAN NF, Nav1.x, and βIV spectrin shRNA were as follows: ankG, 5′-GCCGTCAGTACCATCTTCT-3′; PAN NF, 5′-TGCCTTCGTCAGCGTATTA-3′; Nav1.x, 5′-GTTCGACCCTGACGCCACT-3′; and βIV spectrin, 5′-CACTGGATAGCCGAGAAGG-3′. Efficacy and specificity of these shRNA sequences were demonstrated previously (Hedstrom et al., 2007). For adenovirus, the H1 promoter driving shRNA expression and the shRNA sequence were inserted into pENTR 11 (Invitrogen) via the EcoRI and HindIII sites. A CAG promoter driving the expression of EGFP was also inserted using the HindIII and XhoI sites. Plasmids were recombined with pAd vector (Invitrogen) using the ViraPower Adenoviral Gateway Expression kit (Invitrogen).
Hippocampal neuron culture, infections, and immunostaining
Primary hippocampal neurons were prepared and immunostained as described previously (Ogawa et al., 2006). Neurons were infected using the adenovirus at 10 DIV. The virus was introduced for 3 h before the culture media was exchanged. For live labeling, neurons were treated for 30 min with A12/18.1 antibodies at 4°C. Cells were washed to remove unbound antibody, growth media were replaced in each culture well, and neurons were returned back to 37°C. Neurons were fixed and immunolabeled at the indicated time points.
Image acquisition and analysis
Fluorescence images were collected using an inverted microscope (Axiovert 200M; Carl Zeiss, Inc.) fitted with an apotome for optical sectioning. Images were collected using 20×, 40×, and 63× NA 1.4 Plan-Apochromat objectives, a camera (Axiocam; Carl Zeiss, Inc.), and Axiovision software (Carl Zeiss, Inc.). Montage images were assembled using Photoshop (Adobe Systems, Inc.). All experiments were performed in triplicate with independent dissections. Infected GFP+ cells with detectable AIS immunoreactivity were counted as AIS positive (AIS+). Infected GFP+ cells without detectable AIS immunoreactivity were counted as negatives. AIS fluorescence intensity measurements were made using ImageJ (National Institutes of Health). 10 images per experimental condition were analyzed in three separate experiments. The mean pixel intensity per unit area was measured for each AIS. We calculated the ratio of the mean infected neuron's AIS fluorescence to the mean fluorescence of noninfected neurons on the same coverslip. All camera exposure times were below saturation for noninfected neurons on the same coverslip. For antibody-labeled live neurons, measurements shown are the mean of the raw AIS fluorescence intensity over three experiments. All images were acquired using a subsaturating exposure time determined in 1 DPT neurons.
Online supplemental material
Fig. S1 shows that control GFP adenovirus–infected neurons have a normal AIS even at 10 DPI and that Na+ channels and βIV spectrin are very stable after adenoviral-delivered shRNA knockdown. Fig. S2 shows a GFP-labeled axon that has high levels of MAP2 immunoreactivity after the silencing of ankG expression. Fig. S3 shows another example of distal PAN NF immunoreactivity and the invasion of MAP2 into the axon. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200806112/DC1.
Supplementary Material
Acknowledgments
We thank P. Shrager for helpful discussions.
This work was funded by National Institutes of Health grant NS044916, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and Mission Connect. Y. Ogawa was supported by a National Multiple Sclerosis Society postdoctoral fellowship. M.N. Rasband is a Harry Weaver Neuroscience Scholar of the National Multiple Sclerosis Society.
K.L. Hedstrom's present address is Dept. of Neurology, Center for Neuroscience and Regeneration Research, Yale University School of Medicine, New Haven, CT 06510.
Y. Ogawa's present address is Dept. of Pharmacology, Meiji Pharmaceutical University, Tokyo 204-8588, Japan.
Abbreviations used in this paper: AIS, axon initial segment; ankG, ankyrinG; CAM, cell adhesion molecule; DIV, day in vitro; DPI, day post infection; DPT, day post treatment; NF, neurofascin; NrCAM, neuronal CAM; shRNA, short hairpin RNA.
References
- Arimura, N., and K. Kaibuchi. 2007. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 8:194–205. [DOI] [PubMed] [Google Scholar]
- Dzhashiashvili, Y., Y. Zhang, J. Galinska, I. Lam, M. Grumet, and J.L. Salzer. 2007. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J. Cell Biol. 177:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, J.J., P. Giraud, E. Carlier, F. Fernandes, A. Moussif, M.P. Fache, D. Debanne, and B. Dargent. 2003. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 300:2091–2094. [DOI] [PubMed] [Google Scholar]
- Gomis-Ruth, S., C.J. Wierenga, and F. Bradke. 2008. Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr. Biol. 18:992–1000. [DOI] [PubMed] [Google Scholar]
- Grieco, T.M., J.D. Malhotra, C. Chen, L.L. Isom, and I.M. Raman. 2005. Open-channel block by the cytoplasmic tail of sodium channel β4 as a mechanism for resurgent sodium current. Neuron. 45:233–244. [DOI] [PubMed] [Google Scholar]
- Havton, L., and J.O. Kellerth. 1987. Regeneration by supernumerary axons with synaptic terminals in spinal motoneurons of cats. Nature. 325:711–714. [DOI] [PubMed] [Google Scholar]
- Hedstrom, K.L., X. Xu, Y. Ogawa, R. Frischknecht, C.I. Seidenbecher, P. Shrager, and M.N. Rasband. 2007. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J. Cell Biol. 178:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki, N., K. Chihara, N. Arimura, C. Menager, Y. Kawano, N. Matsuo, T. Nishimura, M. Amano, and K. Kaibuchi. 2001. CRMP-2 induces axons in cultured hippocampal neurons. Nat. Neurosci. 4:781–782. [DOI] [PubMed] [Google Scholar]
- Jenkins, S.M., and V. Bennett. 2001. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J. Cell Biol. 155:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H., W. Guo, X. Liang, and Y. Rao. 2005. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 120:123–135. [DOI] [PubMed] [Google Scholar]
- Kole, M.H., J.J. Letzkus, and G.J. Stuart. 2007. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 55:633–647. [DOI] [PubMed] [Google Scholar]
- Kole, M.H., S.U. Ilschner, B.M. Kampa, S.R. Williams, P.C. Ruben, and G.J. Stuart. 2008. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 11:178–186. [DOI] [PubMed] [Google Scholar]
- Kuba, H., T.M. Ishii, and H. Ohmori. 2006. Axonal site of spike initiation enhances auditory coincidence detection. Nature. 444:1069–1072. [DOI] [PubMed] [Google Scholar]
- Nakada, C., K. Ritchie, Y. Oba, M. Nakamura, Y. Hotta, R. Iino, R.S. Kasai, K. Yamaguchi, T. Fujiwara, and A. Kusumi. 2003. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat. Cell Biol. 5:626–632. [DOI] [PubMed] [Google Scholar]
- Ogawa, Y., D.P. Schafer, I. Horresh, V. Bar, K. Hales, Y. Yang, K. Susuki, E. Peles, M.C. Stankewich, and M.N. Rasband. 2006. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J. Neurosci. 26:5230–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara, I., H. Miyamoto, N. Morita, N. Atapour, E. Mazaki, I. Inoue, T. Takeuchi, S. Itohara, Y. Yanagawa, K. Obata, et al. 2007. Na(v)1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci. 27:5903–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Z., T. Kao, Z. Horvath, J. Lemos, J.-Y. Sul, S.D. Cranstoun, M.V. Bennett, S.S. Scherer, and E.C. Cooper. 2006. A common ankyrin-G-based mechanism retains KCNQ and Nav channels at electrically active domains of the axon. J. Neurosci. 26:2599–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe, C.F., R.E. Westenbroek, R. Curtis, and W.A. Catterall. 2001. Sodium channel β1 and β3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J. Cell Biol. 154:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, D.P., R. Bansal, K.L. Hedstrom, S.E. Pfeiffer, and M.N. Rasband. 2004. Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts? J. Neurosci. 24:3176–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler, B., P. Forscher, and I. Mellman. 1999. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 397:698–701. [DOI] [PubMed] [Google Scholar]
- Yang, Y., Y. Ogawa, K.L. Hedstrom, and M.N. Rasband. 2007. βIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J. Cell Biol. 176:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., J. Zhu, G.Y. Yang, Q.J. Wang, L. Qian, Y.M. Chen, F. Chen, Y. Tao, H.S. Hu, T. Wang, and Z.G. Luo. 2007. Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat. Cell Biol. 9:743–754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.