Abstract
In certain cell types, apoptosis in response to extracellular stimuli like Fas depends on a mitochondrial amplificatory loop: the apical caspase-8 cleaves and activates the BH3-only member of the Bcl-2 family BID. In turn, BID induces the release of cytochrome c from mitochondria to the cytoplasm, where it is required to fully activate effector caspases. In this issue of The Journal of Cell Biology, Gonzalvez et al. (see p. 681) show that when caspase-8 activation and production of functional BID is required, it is performed on mitochondrial platforms provided by the mitochondrion-specific lipid cardiolipin. Cardiolipin anchors caspase-8 at contact sites between inner and outer mitochondrial membranes, facilitating its self activation. These findings suggests that like other second messengers such as Ca2+ and cAMP, production of apoptotic messengers can be compartmentalized in close proximity to their intracellular target.
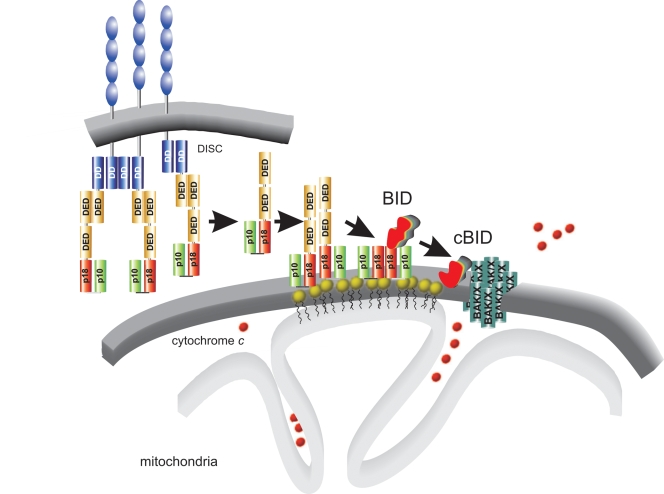
The developmental and homeostatic control over cell number and type in tissues and organs is accomplished by the tight equilibrium between proliferation and apoptosis. Old, damaged, unnecessary cells are eliminated by a genetically defined program of self destruction that, under many circumstances, ensues from an extracellular stimulus that engages a death receptor on the surface of the target cell and triggers an intracellular “killing” cascade (Peter and Krammer, 2003). Death caused from within as well as from the outside share the executioners, the effector caspases. However, how efficient activation of these proteases is accomplished differs between the two cases. In response to the activation of a death receptor, a platform (the so called death-induced signaling complex [DISC]) is assembled under the plasma membrane where the proenzymes of the apical caspases, like procaspase-8, are recruited and undergo activation by proximity. Their self cleavage is autoamplificatory in nature, and when enough DISCs are formed, the concentration of active apical caspases in the cell reaches levels high enough to directly process and turn effector caspases on (Peter and Krammer, 2003). When, conversely, the amount of active caspase-8 is lower, additional amplificatory mechanisms are required. In these cell types, called type II, caspase-8 cleaves and activates a BH3-only member of the Bcl-2 family of proteins, triggering its translocation to mitochondria, where it causes the release of cytochrome c (Scaffidi et al., 1998). In this issue, Gonzalvez et al. (see p. 681) show a novel, unexpected role for mitochondria and for a mitochondrial lipid in particular, cardiolipin (CL), in the activation of the apical caspase-8 in cells that require the mitochondrial amplificatory loop. When type II cells are triggered to die by extrinsic stimuli, they end up using mitochondria as their favorite surface to generate cleaved BID. Caspase-8 translocates to mitochondria, where it binds to CL to become activated by proximity, leading to the proteolytically active p43 and p10 fragments. These are inserted into the outer mitochondrial membrane and form an oligomer that can be stabilized on the membrane. Gonzalvez et al. (2008) demonstrate that this association with mitochondria depends on the presence of mature CL, which proves essential in situ and in vitro for the insertion of caspase-8 into natural and artificial membranes. The final outcome is that the production of active, cleaved BID is accomplished where it is needed, i.e., on the surface of its target organelle (Fig. 1).
Figure 1.
Localized production of active, cleaved BID on cardiolipin platforms that are used for the assembly of active caspase 8. The diagram depicts the sequence of events that occur in type II cells according to Gonzalvez et al. (2008). DD, death domain; DED, death effector domain; p10 and p18 are the catalytic core of the caspase. The p43/p10 caspase 8 isoform comprises two DEDs, one p10, and one p18 domain. The cardiolipin (yellow heads) platform at the contact sites between inner and outer mitochondrial membranes is used to produce active BID where it is needed. This in turn causes BAK/BAX oligomerization and cytochrome c release.
The BH3-only subset of the Bcl-2 family comprises a large number of molecules (like BAD, BIM, NOXA, PUMA, and BIK) that, in response to intrinsic or extrinsic death stimuli, transduce the signal to the multidomain proapoptotics, like BAK and BAX, whose activation results in the release of proapoptotic factors, like cytochrome c, from mitochondria (Scorrano and Korsmeyer, 2003). Cytochrome c is the only soluble component of the mitochondrial respiratory chain, and, once in the cytoplasm, it complexes with the adaptor Apaf-1 and the pro–caspase 9 to cause activation and self cleavage of the latter, an extremely efficient process (Wang, 2001). Normally, the vast majority of cytochrome c is located in the cristae, where it can be bound to CL (Ott et al., 2002). CL, also known as diphosphatidylglycerol, is an anionic phospholipid predominantly located in the inner membrane, but is also found at the contact sites formed between the mitochondrial inner and outer membranes (Ardail et al., 1990). CL is unique in that it contains four acyl chains, most of which highly unsaturated, and two negative charges on the head group, and is found almost exclusively in mitochondrial membranes. Mature CL is produced by the combined action of phospholipase A, which removes one saturated acyl chain to generate monolysocardiolipin (MLCL), and by Tafazzin, a mitochondrial enzyme that catalyzes the addition of an unsaturated chain to MLCL (Xu et al., 2006). Mutations in the Tafazzin gene in humans are associated with the X-linked Barth's syndrome characterized by neutropenia, skeletal myopathy, and growth delay (Barth et al., 1999).
The involvement of CL in the control of apoptosis is not new: CL acts as the mitochondrial receptor for BID (Lutter et al., 2000) and regulates the oligomerization of the multidomain proapoptotics BAK and BAX (Kuwana et al., 2002). Furthermore, it probably retains cytochrome c in the cristae, from where it is freed as a consequence of their remodeling, controlled by Opa1 (Ott et al., 2002; Frezza et al., 2006). However, so far, CL has never been found to be essential for the initiating steps of the apoptotic cascade, nor was evidence ever put forward for a localized production/accumulation of an active proapoptotic BH3-only member of the Bcl-2 family. The other BH3-only protein BAD has been previously associated with mitochondria in nonapoptotic cells, residing in a large complex that comprises the glycolysis enzyme hexokinase; however, it is likely that the main aim of this compartmentalization is to integrate apoptosis with glycolysis (Danial et al., 2003). The unexpected findings of Gonzalvez et al. (2008) indicate that the activation BID is performed where it should: on the surface of its target organelle, the mitochondrion. Mitochondria are not strangers to compartmentalized signaling. Perhaps the best characterized paradigm is that of Ca2+ signaling, where microdomains of high Ca2+ are produced at the interface between mitochondria and Ca2+-releasing channels of the ER in order to activate mitochondrial Ca2+ uptake (Rizzuto et al., 1993). Moreover, localization of PKA on the surface of mitochondria via specific AKAP binding proteins suggests the existence of cAMP microdomains on the surface of the organelle (Chen et al., 1997). Finally, biosynthesis of mitochondrial lipids requires a structural compartmentalization provided by the mitochondria-associated membranes, areas of close juxtaposition with the endoplasmic reticulum where several steps of the biosynthesis occur (Daum and Vance, 1997). What could be the functional consequences of a compartmentalized production of BID on the surface of mitochondria? This could avoid BID being “mistargeted” to other intracellular membranes, such as endoplasmic reticulum (Gajkowska et al., 2004); alternatively, the production of high locales of BID at contact sites allows it to insert in the inner membrane and trigger cristae remodeling (Kim et al., 2004). Finally, compartmentalization could be required to place BID in proximity to specific mitochondrial ubiquitin ligases (like MARCH-5, for example, Karbowski et al., 2007) because BID activation requires degradation of the N terminus after cleavage by caspase-8 (Tait et al., 2007).
The work of Gonzalvez et al. (2008) leaves the question of the pathogenesis of Barth's syndrome open. Mutations in Tafazzin that alter the total amount of mature CL in mitochondria cause a resistance in Fas-induced apoptosis and basically do not affect the death induced by intrinsic stimuli. So, what is the underlying mechanism for the observed myopathy and neutropenia? One simple possibility is that cells lacking mature CL display mitochondrial dysfunction, as it was observed in yeast (Ma et al., 2004). Alternatively, the block in apoptosis caused by the lack of mature CL might trigger an excess of autophagy that ultimately causes the clinical phenotype. Autophagy has indeed been described in neutropenia and cardiomyopathy, and it is known that blockage of the mitochondrial death pathway, in the long run, causes death by autophagy (Shimizu et al., 2004). Future research on the pathogenesis of Barth syndrome will surely capitalize on the work by Gonzalvez et al. (2008) and look into these possible new directions.
Acknowledgments
L. Scorrano is a senior scientist of the Dulbecco-Telethon Institute and the European Molecular Biology Organization Young Investigator Programme. Work in his laboratory is supported by Telethon Italy, Associazione Italiana Ricerca sul Cancro, Schweizerischer Nationalfonds (grant no. 3100A0-118171), Telethon Suisse, Oncosuisse, and Association Française contre les Myopathies.
References
- Ardail, D., J.P. Privat, M. Egret-Charlier, C. Levrat, F. Lerme, and P. Louisot. 1990. Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 265:18797–18802. [PubMed] [Google Scholar]
- Barth, P.G., R.J. Wanders, P. Vreken, E.A. Janssen, J. Lam, and F. Baas. 1999. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome) (MIM 302060). J. Inherit. Metab. Dis. 22:555–567. [DOI] [PubMed] [Google Scholar]
- Chen, Q., R.Y. Lin, and C.S. Rubin. 1997. Organelle-specific targeting of protein kinase AII (PKAII). Molecular and in situ characterization of murine A kinase anchor proteins that recruit regulatory subunits of PKAII to the cytoplasmic surface of mitochondria. J. Biol. Chem. 272:15247–15257. [DOI] [PubMed] [Google Scholar]
- Danial, N.N., C.F. Gramm, L. Scorrano, C.Y. Zhang, S. Krauss, A.M. Ranger, S.R. Datta, M.E. Greenberg, L.J. Licklider, B.B. Lowell, et al. 2003. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 424:952–956. [DOI] [PubMed] [Google Scholar]
- Daum, G., and J.E. Vance. 1997. Import of lipids into mitochondria. Prog. Lipid Res. 36:103–130. [DOI] [PubMed] [Google Scholar]
- Frezza, C., S. Cipolat, O. Martins de Brito, M. Micaroni, G.V. Beznoussenko, T. Rudka, D. Bartoli, R.S. Polishuck, N.N. Danial, B. De Strooper, and L. Scorrano. 2006. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 126:177–189. [DOI] [PubMed] [Google Scholar]
- Gajkowska, B., U. Wojewodzka, and J. Gajda. 2004. Translocation of Bax and Bid to mitochondria, endoplasmic reticulum and nuclear envelope: possible control points in apoptosis. J. Mol. Histol. 35:11–19. [DOI] [PubMed] [Google Scholar]
- Gonzalvez, F., Z.T. Schug, R.H. Houtkooper, E.D. MacKenzie, D.G. Brooks, R.J.A. Wanders, P.X. Petit, F.M. Vaz, and E. Gottlieb. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J. Cell Biol. 183:681–696. [DOI] [PMC free article] [PubMed]
- Karbowski, M., A. Neutzner, and R.J. Youle. 2007. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1-dependent mitochondrial division. J. Cell Biol. 178:71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T.H., Y. Zhao, W.X. Ding, J.N. Shin, X. He, Y.W. Seo, J. Chen, H. Rabinowich, A.A. Amoscato, and X.M. Yin. 2004. Bid-cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome C release. Mol. Biol. Cell. 15:3061–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana, T., M.R. Mackey, G. Perkins, M.H. Ellisman, M. Latterich, R. Schneiter, D.R. Green, and D.D. Newmeyer. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 111:331–342. [DOI] [PubMed] [Google Scholar]
- Lutter, M., M. Fang, X. Luo, M. Nishijima, X. Xie, and X. Wang. 2000. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2:754–761. [DOI] [PubMed] [Google Scholar]
- Ma, L., F.M. Vaz, Z. Gu, R.J. Wanders, and M.L. Greenberg. 2004. The human TAZ gene complements mitochondrial dysfunction in the yeast taz1Delta mutant. Implications for Barth syndrome. J. Biol. Chem. 279:44394–44399. [DOI] [PubMed] [Google Scholar]
- Ott, M., J.D. Robertson, V. Gogvadze, B. Zhivotovsky, and S. Orrenius. 2002. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. USA. 99:1259–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter, M.E., and P.H. Krammer. 2003. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26–35. [DOI] [PubMed] [Google Scholar]
- Rizzuto, R., M. Brini, M. Murgia, and T. Pozzan. 1993. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 262:744–747. [DOI] [PubMed] [Google Scholar]
- Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K.J. Tomaselli, K.M. Debatin, P.H. Krammer, and M.E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano, L., and S.J. Korsmeyer. 2003. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem. Biophys. Res. Commun. 304:437–444. [DOI] [PubMed] [Google Scholar]
- Shimizu, S., T. Kanaseki, N. Mizushima, T. Mizuta, S. Arakawa-Kobayashi, C.B. Thompson, and Y. Tsujimoto. 2004. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat. Cell Biol. 6:1221–1228. [DOI] [PubMed] [Google Scholar]
- Tait, S.W., E. de Vries, C. Maas, A.M. Keller, C.S. D'Santos, and J. Borst. 2007. Apoptosis induction by Bid requires unconventional ubiquitination and degradation of its N-terminal fragment. J. Cell Biol. 179:1453–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922–2933. [PubMed] [Google Scholar]
- Xu, Y., A. Malhotra, M. Ren, and M. Schlame. 2006. The enzymatic function of tafazzin. J. Biol. Chem. 281:39217–39224. [DOI] [PubMed] [Google Scholar]