Abstract
In juvenile monkeys, precocious puberty can be induced by administration of gonadotropins resulting in testicular somatic cell maturation and germ cell differentiation. It is, however, unknown whether testicular maturation can also be induced in younger monkeys. Here we used testis tissue xenografting to investigate whether infant monkey testis tissue will undergo somatic cell maturation and/or spermatogenesis in response to endogenous adult mouse gonadotropins or exogenous gonadotropins. Testicular tissue pieces from 3- and 6-month-old rhesus monkeys were grafted to immunodeficient, castrated mice. Recipient mice were either left untreated or treated with pregnant mare serum gonadotropin and/or human chorionic gonadotropin twice weekly and were killed 28 weeks after grafting. Testicular maturation in grafted tissue was assessed based on morphology and the most advanced germ cell type present and by immunohistochemistry for expression of proliferating cell nuclear antigen, Mullerian-inhibiting substance, and androgen receptor. Testis grafts, irrespective of donor age or treatment, contained fewer germ cells than donor tissue. Grafts from 6-month-old donors showed tubular expansion with increased seminiferous tubule diameter and lumen formation, whereas those harvested from gonadotropin-treated mice contained elongated spermatids. Grafts from 3-month-old donors recovered from gonadotropin-treated mice contained pachytene spermatocytes, whereas those recovered from untreated mice showed only slight tubular expansion. Immunohistochemistry revealed that exposure to exogenous gonadotropins supported Sertoli cell maturation, irrespective of donor age. These results indicate that sustained gonadotropin stimulation of immature (<12 months old) monkey testis supports Sertoli cell maturation, thereby terminating the unresponsive phase of the germinal epithelium and allowing complete spermatogenesis in testis tissue from infant rhesus monkeys.
POSTNATAL SEXUAL development in male nonhuman primates can be categorized into four distinct phases: infantile, juvenile, pubertal, and adult (1). The infantile period ranges from birth until approximately 6 months of age in the rhesus monkey (Macaca mulatta) (1) and is characterized by release of adult-like levels of GnRH by the hypothalamus that stimulate release of adult-like levels of gonadotropins from the anterior pituitary gland. The testes in the infants respond to the high levels of gonadotropins by secreting testosterone, the androgen necessary for germ cell differentiation mediated by Sertoli cell stimulation (2,3). However, despite the elevated levels of gonadotropins and testosterone, spermatogenic differentiation does not occur during infancy (1). This has been attributed to immaturity of Sertoli cells and their failure to express androgen receptors (AR) during infancy (1).
During the juvenile phase of development, the hypothalamic GnRH pulse generator is suppressed, leading to reduced or absent gonadotropin release by the pituitary (1). This reduction in gonadotropin release is said to be responsible for maintaining the testes in the prepubertal state in juvenile primates, which lasts 3–4 yr in macaques. In support of this hypothesis, it has been reported that puberty in juvenile primates can be induced by hormonal/chemical stimulation of the hypothalamus (4).
Using the approach of testis tissue xenografting (5), we previously reported stimulation and acceleration of juvenile (13 months old) rhesus monkey testicular tissue development and germ cell differentiation after ectopic grafting into adult castrated, immunodeficient mice (6). In that study, gonadotropins secreted by the castrated adult mouse host appeared sufficient to stimulate the rhesus Leydig and Sertoli cells, leading to establishment of a functional hypothalamic-pituitary-graft axis as previously demonstrated for mouse testis allografts (7), testicular tissue development, and spermatogenesis.
Whereas studies on endocrine stimulation of the immature monkey testis have been performed in juvenile monkeys, relatively little has been reported regarding the control of testicular maturation in younger monkeys. Specifically, it is unknown whether precocious puberty can be induced experimentally in infant primates. Therefore, the objective of the present study was to test whether 3- or 6-month-old monkey testis tissue will respond to levels of gonadotropins provided by an adult mouse host and whether spermatogenic differentiation can be accelerated by stimulation with exogenous gonadotropins. We used xenografting as an accessible model to study development of rhesus monkey testis (6). This approach allowed replication of treatments within individual donors as well as analysis over time, thereby drastically reducing the number of primate donors required. Testis tissue fragments from 3- and 6-month-old rhesus monkeys were grafted under the back skin of castrated, immunodeficient mice and the grafted tissue was allowed to develop for a period of 28 wk in the host mice, after which it was recovered and analyzed. The recovered tissue was analyzed for the presence of germ cell differentiation based on morphology and immunocytochemical detection of ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), also known as Protein Gene Product 9.5 (8). Sertoli cell maturation was assessed based on expression of proliferating cell nuclear antigen (PCNA), Mullerian inhibiting substance (MIS), and androgen receptor (AR).
Materials and Methods
Donor testis tissue
Testes from 3-month-old (n = 5 donors) and 6-month-old (n = 6 donors) rhesus monkeys were used as donor tissue for xenografting. The animals were housed in single cages and were maintained under a controlled photoperiod (lights on 0600–1800 h) according to National Institutes of Health guidelines. After an initial sedation with ketamine hydrochloride (50 mg im, Ketaset; Fort Dodge Laboratories, Inc., Fort Dodge, IA), animals were killed with sodium pentobarbital (30 mg/kg body weight, iv) and the testes removed. Testes were kept in Dulbecco’s PBS on ice and shipped overnight to the University of Pennsylvania. All experimental procedures were approved by the University of California Institutional Animal Care and Use Committee.
Recipient mice and procedures for xenografting
Six to 8-wk-old (adult) male ICR/SCID (Taconic, Germantown, NY) mice were used as recipients. Testis tissue xenografting was performed as described previously (5). Briefly, after removal of the capsule and overt connective tissue, donor testes were cut into small fragments (∼0.5–1 mm in diameter). Testis tissue fragments were kept in DMEM on ice until grafting. Some fragments of the donor testes were fixed in Bouin’s solution for histology. At least four recipient mice per donor rhesus macaque were anesthetized and castrated. During the same surgery, each mouse received four incisions (∼5 mm each) on each side of the back and one fragment of testis tissue was inserted through each skin incision. Animals were handled and treated in accordance with the University of Pennsylvania Institutional Animal Care and Use Committee.
Gonadotropin treatment
In a subset of two donors each from both age groups (3 and 6 month old rhesus macaques), the recipient mice were assigned to three groups (n = 3 mice per group and donor). Mice in one group were treated with pregnant mare serum gonadotropin (PMSG; 10 IU sc; Sigma, St. Louis, MO) and human chorionic gonadotropin (hCG; 10 IU sc; Chorulon, Intervet, Millsboro, DE), those in the second group were treated with hCG alone, and mice in group 3 were left untreated. The mice with xenografts from remaining donors, i.e. three 3-month-old donors and four 6-month-old donors, were divided in two groups each (n = minimum of two mice per group, per donor), with mice in one group being treated with hCG alone and those in the second group left untreated. Treatment with PMSG started 1 wk after grafting and with hCG 4 wk after grafting and continued twice weekly until the recipient mice were killed 28 wk after grafting.
Recovery and analysis of xenografts
The host mice were killed by CO2 inhalation at 28 wk after grafting. Seminal vesicles from all recipient mice were removed and weighed as an indication of secretion of bioactive testosterone by the xenografts (5,6,7). Grafts were recovered from under the back skin of mice, weighed, and fixed overnight in Bouin’s solution followed by three changes of 70% ethanol before being embedded in paraffin and sectioned at 5 μm using standard procedures. Sections were deparaffinized and processed for hematoxylin and eosin staining and for immunohistochemistry. A graft was classified as healthy if it was found to contain even a single seminiferous tubule with Sertoli cells on histologic evaluation. It was classified as degenerated if it could either not be recovered or if it contained tubules that were collapsed and did not contain distinct cell types. Only the data from healthy grafts were subsequently analyzed. In the histologic sections of each graft, all seminiferous tubule cross-sections were examined for the status of testicular tissue maturation (assessment of proliferating cells and Sertoli cell maturation) and spermatogenesis (most advanced germ cell type). Spermatogonia were identified using UCH-L1 immunostaining, and all other germ cell types were identified by their morphology and location in the seminiferous tubules. The maturity of Sertoli cells was assessed based on expression of PCNA, MIS, and AR.
Immunohistochemistry of PCNA, MIS, AR, and UCH-L1
Immunohistochemical procedures for all antibodies were the same, except as noted. Specificity of primary antibodies was confirmed by observation of expected expression patterns in tissue sections from sexually mature (6 and 13 yr of age) and immature monkeys, respectively. After deparaffinization, antigen retrieval was performed by heating the sections in antigen unmasking solution (Vector Laboratories, Burlingame, CA) for 10 min (20 min in case of AR immunostaining) over boiling (95 C) water. The sections were then allowed to stand in the solution for 1 h at room temperature to cool down before being treated with 3% hydrogen peroxide in methanol for 10 min to block the endogenous peroxidase activity. Nonspecific binding was blocked using avidin and biotin blocking (Vector) for 10 min each, followed by CAS block (Zymed, San Francisco, CA) for 10 min, all at room temperature. Primary antibodies against UCH-L1 (rabbit polyclonal; Biogenesis, Kingston, NH; 1:1000), PCNA (clone PC10, mouse monoclonal; DakoCytomation, Carpinteria, CA; 1:100), MIS (C-20, goat polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA; 1:200), and AR (N-20, rabbit polyclonal; Santa Cruz; 1:250) were diluted in PBS (pH 7.2, without calcium and magnesium), added to the slides and incubated overnight at 4 C in a humidified chamber. Samples were then incubated with the biotinylated secondary antibodies at a dilution of 1:400 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 30 min at room temperature. Subsequently the slides were exposed to horseradish peroxidase streptavidin (Vector) at a concentration of 3 μg/ml in PBS for 30 min, and the peroxidase activity was detected with VIP (Vector) as per the manufacturer’s instructions. Between each step throughout the procedure, other than after the CAS block or unless otherwise stated, the sample slides were rinsed twice in PBS for 5 min each. The sections were analyzed under a light microscope at ×200 and ×400. The resulting VIP staining was visually evaluated based on intensity and the number of cells or nuclei expressing the staining and was subsequently scored on a scale of 0–4 as absent, faintly, moderately, strongly, or very strongly present.
Data collection and statistical analysis
Data were obtained from all seminiferous tubules per graft, and data from all grafts per mouse were pooled. Mouse was considered the experimental unit. Student’s t test was performed to compare two groups. Data were analyzed using SigmaStat 3.0 (SPSS Inc., Chicago, IL). Data were expressed as means ± sem and P < 0.05 was considered significant.
Results
Gross evaluation of recovered grafts and seminal vesicles
Overall, 408 of 520 grafts (78%) were recovered from 65 recipient mice. Xenografts recovered from mice treated with hCG alone or in combination with PMSG did not show any significant difference in size and weights within donor age (graft weight 10.9 ± 1.4 mg (n = 12) vs. 13.6 ± 2.1 mg (n = 7) for grafts recovered from 3-month-old donors, and 21.2 ± 2.4 mg (n = 14) vs. 20.4 ± 3.1 mg (n = 6) for grafts from 6-month-old donors recovered from recipients treated with hCG or PMSG and hCG, respectively; P > 0.05) Thus, the data from both groups was combined and presented under one category of gonadotropin treated mice.
The recovered testis tissue xenografts showed a marked increase in weight, compared with the tissue fragments before grafting, which weighed around 2 mg. The testis tissue grafts from the 6-month-old monkey donors recovered from gonadotropin-treated mice (n = 20) tended to be larger (20.9 ± 1.9 mg) than those recovered from untreated mice (14.8 ± 2.3 mg; n = 14). A similar trend was observed in the testis tissue grafts from the 3-month-old monkey donors (12.3 ± 1.1 mg; from gonadotropin-treated mice, n = 19 vs. 11.2 ± 1.4 mg recovered from untreated mice, n = 12). However, these differences were not statistically significant.
The weight of the seminal vesicles in the recipient mice at the time of graft recovery reflected whether the mice were subjected to gonadotropin treatment. The average weight of the seminal vesicles recovered from gonadotropin-treated mice (254.4 ± 10.5 mg; n = 39) was significantly higher (P < 0.05) than those recovered from untreated mice (12.2 ± 1.1 mg; n = 26). In the gonadotropin-treated mice without healthy graft development, the weight of the seminal vesicles was low (10.6 mg ± 1.2; n = 3). These findings indicate that infant monkey testis xenografts were functional endocrinologically, secreting androgen. In the small number of mice in which grafts did not develop, exogenous gonadotropin administration did not result in significant growth of the seminal vesicles.
Histological evaluation of the donor tissue and recovered grafts
Histological evaluation of xenografts showed no difference in the development of grafts recovered from mice-treated with hCG alone or in combination with PMSG (3.7 ± 1.2% vs. 3.5 ± 1.3% of tubules contained pachytene spermatocytes in grafts from hCG vs. hCG/PMSG-treated recipients, n = 14 and 6, respectively; P > 0.05). Thus, the data from these two groups were combined.
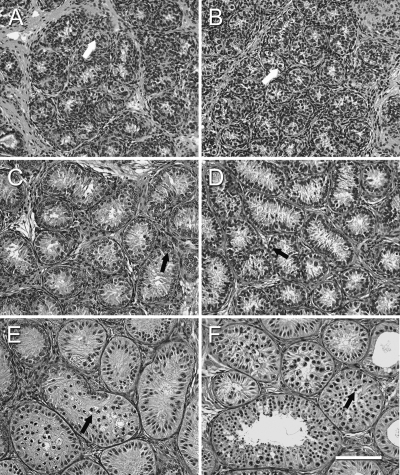
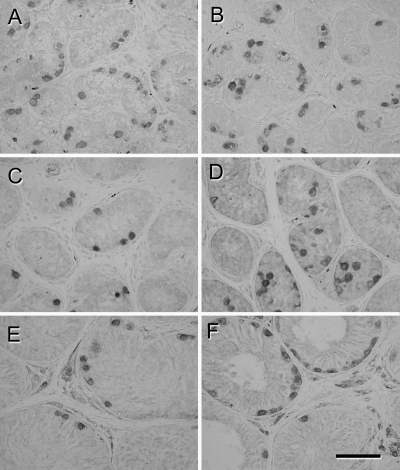
Histology of the donor tissue at the time of grafting and that of recovered grafts 28 wk after grafting is illustrated in Fig. 1. Donor tissue consisted of seminiferous cords containing Sertoli cells and spermatogonia (Fig. 1, A and B). Testis tissue xenografts showed varying degrees of somatic cell maturation and germ cell differentiation. The degree of germ cell development within individual grafts ranged from tubules containing only spermatogonia to tubules containing pachytene spermatocytes or elongated spermatids as the most advanced germ cell type present (Fig. 1, E and F). In individual grafts recovered from single recipients, percentage of tubules containing meiotic germ cells ranged from 0 to 40%. Differentiation of the seminiferous epithelium was most pronounced in the testis tissue grafts from 6-month-old monkeys recovered from the gonadotropin-treated mice (Fig. 1F). On average, 0.6 ± 0.3% (range 0.0–2.8%) of seminiferous tubules in the grafts from three donors (n = 10 mice) showed complete spermatogenesis with elongated spermatids as the most advanced germ cell type and 3.2 ± 1.0% (range 0.0–20.0%) of seminiferous tubules in grafts from all six donors (n = 20 mice) showed germ cell differentiation at least up to pachytene spermatocytes (Fig. 1F).
Figure 1.
Histological appearance of donor and grafted testis tissue. A, Three-month-old donor tissue. B, Six-month-old donor tissue. C, Graft from 3-month-old donor recovered from untreated mouse. D, Graft from 6-month-old donor recovered from untreated mouse. E, Graft from 3-month-old donor recovered from gonadotropin-treated mouse. F, Graft from 6-month-old donor recovered from gonadotropin-treated mouse. Arrows indicate spermatogonia in A–D, pachytene spermatocytes in E, and spermatids in F. Hematoxylin and eosin staining. Bar, 50 μm.
Grafts from one 3-month-old monkey recovered from treated mice (n = 6 mice) also showed germ cell differentiation, with pachytene spermatocytes as the most advanced germ cell type present in 0.4 ± 0.2% of seminiferous tubules per mouse (range 0.0–1.5%; Fig. 1E).
The grafts recovered from untreated mice, irrespective of the donor age, showed little germ cell differentiation with spermatogonia being the most advanced germ cell type. Nevertheless, marked tissue maturation was observed (Fig. 1, C and D), compared with the donor tissue (Fig. 1, A and B).
Immunolocalization of PCNA, MIS, AR, and UCH-L1
All tubules (493 ± 39 tubules per graft, range 33–1050) in all grafts (408 grafts total; 64 and 89 grafts from untreated recipients and 120 and 135 grafts recovered from gonadotropin treated recipients for the 3- and 6-month-old donors, respectively) were evaluated and data for each mouse were pooled for analysis. Analysis of immunohistochemistry results for PCNA, MIS, and AR are illustrated in Table 1. Expression pattern of all proteins varied between the grafts recovered from individual mice and also within grafts, suggesting uneven development of the grafted tissue between grafts within recipient mice and also within a single graft.
Table 1.
Immunolocalization of PCNA, MIS, and AR in 3- and 6-month-old donor testes tissue and grafted tissue recovered 28 wk after grafting
| PCNA
|
MIS
|
AR
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue | GC | SC | LC | GC | SC | LC | GC | SC | LC |
| 3 months old | |||||||||
| Donors (n = 5) | 0.8 ± 0.5 (0–1) | 0.8 ± 0.5 (0–1) | 0.3 ± 0.5 (0–1) | 0 | 4 | 0 | 0 | 0 1 | |
| Grafts, untreated (n = 12) | 1.5 ± 0.5 (1–2) | 1.3 ± 0.7 (0–2) | 0.6 ± 0.5 (0–1) | 0 | 3.3 ± 0.6 (3–4)a | 0 | 0 | 0.6 ± 0.5 (0–1) | 2.7 ± 0.6 (2–3) |
| Grafts, treated (n = 19) | 1.4 ± 0.6 (0–2) | 0.8 ± 0.4 (0–1) | 1.0 ± 0.4 (0–2) | 0 | 1.3 ± 0.9 (0–3)a | 0 | 0 | 0 1.3 ± 0.8 (0–2) | 2.9 ± 0.4 (2–3) |
| 6 months old | |||||||||
| Donors (n = 6) | 3.3 ± 1.5 (1–4) | 0.8 ± 0.5 (0–1) | 0 | 0 | 3 | 0 | 0 | 0 | 1 |
| Grafts, untreated (n = 14) | 2.1 ± 1.2 (0–4) | 1.6 ± 0.7 (0–3)a | 1.5 ± 0.5 (0–3) | 0 | 2.6 ± 0.9 (2–4)a | 0 | 0 | 0.2 ± 0.4 (0–1)a | 1 ± 1 (0–2) |
| Grafts, treated (n = 20) | 1.5 ± 1.0 (0–3) | 0.2 ± 0.4 (0–1)a | 1.5 ± 0.5 (0–3) | 0 | 1.2 ± 0.4 (0–3)a | 0 | 0 | 2.9 ± 0.4 (2–3)a | 3 |
Staining intensity was scored as very strong (4), strong (3), moderate (2), faint (1), or absent (0). Data are presented as mean ± sd and range(in parentheses).GC, Germ cells; SC, Sertoli cells; LC, Leydig cells.
Average scores are different (P < 0.05) between treatment groups within donor age.
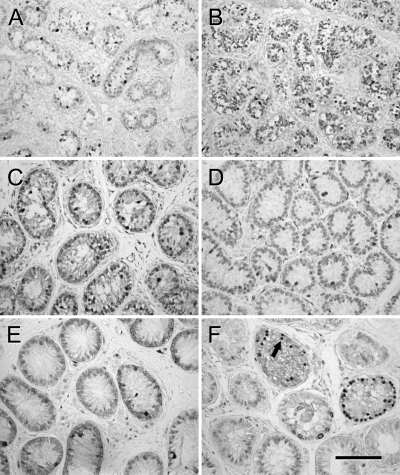
PCNA is localized in the nuclei of all dividing cells. Immunolocalization of PCNA in the donor tissue from both age groups showed that germ cells and Sertoli cells were dividing as expected for immature growing testis tissue (Fig. 2, A and B). The testis tissue grafts from 6-month-old monkeys recovered 28 wk after grafting from the gonadotropin-treated mice showed strong PCNA expression in germ cells, whereas only faint staining was evident in the Sertoli cells (Fig. 2F and Table 1), suggesting that the Sertoli cells had ceased replication at the time of graft recovery. In contrast, in testis tissue grafts from the same donors recovered from untreated mice, PCNA was still strongly expressed in Sertoli cells and germ cells (Fig. 2D and Table 1). The testis tissue grafts from 3-month-old monkeys recovered from untreated mice showed slightly stronger expression of PCNA with respect to the grafts recovered from gonadotropin-treated mice; however, the difference was not substantial (Fig. 2, C and E, and Table 1).
Figure 2.
Expression of PCNA in donor and grafted tissue. A, Three-month-old donor tissue. B, Six-month-old donor tissue. C, Graft from 3-month-old donor recovered from untreated mouse. D, Graft from 6-month-old donor recovered from untreated mouse. E, Graft from 3-month-old donor recovered from gonadotropin-treated mouse. F, Graft from 6-month-old donor recovered from gonadotropin-treated mouse. Strong expression of PCNA was apparent in germ cells (arrow) in F. Bar, 50 μm.
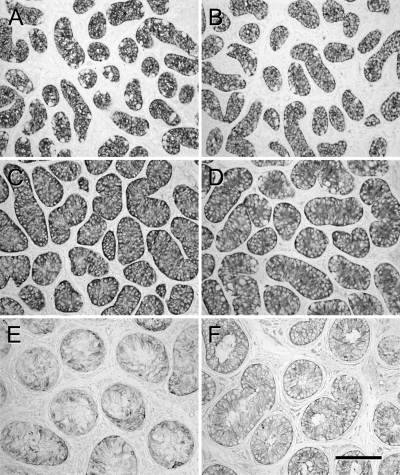
Immunolocalization of MIS in the Sertoli cell cytoplasm showed strong expression in 100% of tubules in the donor tissues from 3- and 6-month-old monkeys as expected for immature Sertoli cells (Fig. 3, A and B, and Table 1). Testis tissue grafts from 6-month-old monkeys recovered from treated mice showed very faint MIS expression in the Sertoli cell cytoplasm in 98% of tubules with no detectable staining in 2% of tubules (Fig. 3F). The grafts recovered from the untreated mice, however, displayed more intense staining in 100% of tubules (Fig. 3D and Table 1). MIS expression observed in the testis tissue xenografts from 3-month-old monkeys recovered from treated and untreated mice was lower in grafts with germ cell differentiation recovered from treated mice than in those recovered from untreated mice (Fig. 3, C and E). Whereas staining intensity was significantly different between grafts recovered from treated and untreated mice in both donor age groups (Table 1), the percentage of tubules showing any degree of MIS expression was not different between groups (98% vs. 100%).
Figure 3.
Expression of MIS in donor and grafted tissue. A, Three-month-old donor tissue. B, Six-month-old donor tissue C, Graft from 3-month-old donor recovered from untreated mouse. D, Graft from 6-month-old donor recovered from untreated mouse. E, Graft from 3-month-old donor recovered from gonadotropin-treated mouse. F, Graft from 6-month-old donor recovered from gonadotropin-treated mouse. Expression is stronger in donor tissue and grafts recovered from untreated mice (A–D), compared with expression in grafts recovered from treated mice (E and F). Bar, 50 μm.
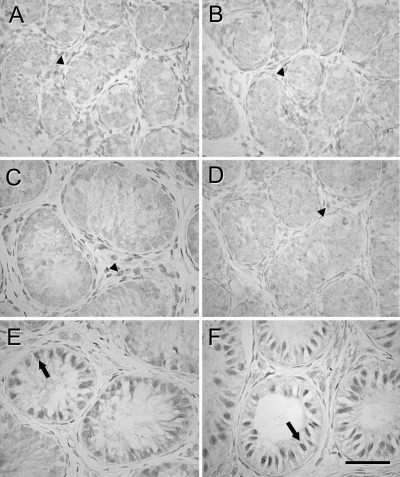
AR was strongly expressed in the nuclei of Sertoli cells in 100% of tubules in the testis tissue grafts from 6-month-old monkeys recovered from the treated mice (Fig. 4F and Table 1). The testis tissue grafts from 6-month-old monkeys recovered from untreated mice and grafts from 3-month-old monkey, irrespective of the treatment, mainly showed no or faint AR expression (Fig. 4, C–E), although in grafts recovered from treated mice in which germ cell differentiation was evident, AR expression was observed in Sertoli cell nuclei (Fig. 4E). In grafts in which germ cell differentiation had occurred, AR expression in Sertoli cells was detected predominantly in tubules containing differentiated germ cells. No AR immunolocalization was observed in Sertoli cells in the donor tissues (Fig. 4, A and B). Staining intensity was significantly different between grafts recovered from treated and untreated mice from 6-month-old donors (Table 1). The percentage of tubules showing strong nuclear AR expression in Sertoli cells was different between grafts recovered from treated vs. untreated mice in both donor age groups (0.1 vs. 100%; P < 0.05).
Figure 4.
Expression of AR in donor and grafted tissue. A, Three-month-old donor tissue. B, Six-month-old donor tissue. C, Graft from 3-month-old donor recovered from untreated mouse. D, Graft from 6-month-old donor recovered from untreated mouse. E, Graft from 3-month-old donor recovered from gonadotropin-treated mouse. F, Graft from 6-month-old donor recovered from gonadotropin-treated mouse. AR expression was apparent in Leydig cells in donor tissue and grafts recovered from untreated mice (A-D, arrowheads) whereas AR was expressed strongly in Sertoli cells in grafts recovered from treated mice (E and F, arrows). Bar, 50 μm.
Immunohistochemistry for UCH-L1 revealed that there was a significant decrease in percentage of seminiferous tubules containing spermatogonia in the recovered grafts, compared with the donor tissue (seminiferous cords) before grafting (Fig. 5). The percentage of tubules with germ cells in the recovered grafts from 6-month-old donors decreased by 45.3 ± 4.9% (n = 34 mice) and that from the 3-month-old donors decreased by 55.8 ± 2.7% (n = 31 mice). This was independent of whether the grafts were recovered from treated or untreated mice, suggesting that exogenous gonadotropins supported tissue maturation but did not result in increased germ cell numbers. Donor tissue from 3- and 6-month-old donors contained 9.3 ± 0.8 and 9.8 ± 1.3 germ cells per 100 Sertoli cells. For 3-month-old donors, grafts contained 0.8 ± 1.0 and 3.8 ± 2.0 germ cells per 100 Sertoli cells in treated vs. untreated mice. In grafts from 6-month-old donors, there were 13.2 ± 2.7 germ cells per 100 Sertoli cells in grafts recovered from gonadotropin treated mice and 10.0 ± 3.3 germ cells per 100 Sertoli cells in grafts recovered from untreated mice.
Figure 5.
Spermatogonia identified by expression of UCH-L1 in donor and grafted tissue. A, Three-month-old donor tissue. B, Six-month-old donor tissue. C, Graft from 3-month-old donor recovered from untreated mouse. D, Graft from 6-month-old donor recovered from untreated mouse. E, Graft from 3-month-old donor recovered from gonadotropin-treated mouse. F, Graft from 6-month-old donor recovered from gonadotropin-treated mouse. Fewer spermatogonia were present in grafted tissue recovered from treated and untreated mice, compared with donor tissue. Bar, 50 μm.
Discussion
In the present study, we used the approach of testis tissue xenografting (5,6,7) to demonstrate for the first time that maturation of the somatic and germ cell component of the infant monkey testis can be induced precociously by stimulation with gonadotropins. Complete germ cell differentiation occurred within 28 wk when the host mice were supplemented with exogenous gonadotropins.
In the infant primate, gonadotropin levels are comparable with those in adults. However, gonadotropin secretion in infants is transient and is followed by minimal gonadotropin release during the juvenile phase of development. During the juvenile phase, Sertoli cell proliferation and maturation is slowed (1), but germ cell proliferation does not appear to be affected. At the onset of puberty, gonadotropin levels increase again, leading to Sertoli cell maturation and an increase in the rate of germ cell proliferation and differentiation beyond spermatogonia (1).
The importance of the juvenile phase, with low levels of gonadotropin secretion, for the development of the primate testis is not known. It was also unclear whether the infant primate testicular tissue could respond to exogenous gonadotropins mimicking continued adult levels of gonadotropins, leading to onset of precocious testicular maturation. Recombinant human FSH or recombinant single-chain human LH administered independently could precociously induce Sertoli cell proliferation in juvenile rhesus monkeys (9), and Arslan et al. (10) reported a significant increase in testicular volume in juvenile monkeys in response to gonadotropin treatment. Moreover, hCG has also been shown to stimulate morphological differentiation of Sertoli cells (11). In the present study, PMSG and hCG were used as alternatives to FSH and LH, respectively.
After 28 wk, the recovered tissue had increased in size and weight, irrespective of whether the mice were treated with gonadotropins, suggesting an increase in length of the seminiferous tubules as a result of Sertoli cell proliferation (12). Although the average weight of the grafts recovered from exogenously treated mice was not significantly higher than the average weight of those recovered from untreated mice, an effect of exogenous gonadotropins on maturation was observed. The tissue recovered from gonadotropin-treated mice showed more tissue maturation, compared with tissue recovered from untreated mice based on Sertoli cell expression of MIS and AR and the most advanced germ cell types observed. Complete spermatogenesis with appearance of elongated spermatids occurred in testis tissue from 6-month-old monkeys, whereas tissue from 3-month-old monkeys displayed germ cell differentiation with entry into meiosis as evidenced by the appearance of pachytene spermatocytes. In comparison, the testis tissue recovered from untreated mice did not show any germ cell differentiation beyond the spermatogonia stage, although tissue maturation was evident. This effect of exogenous gonadotropin treatment can be attributed to increased production of testosterone by the Leydig cells in the grafts. It has previously been shown that treatment of primates with gonadotropins leads to increased production of testosterone and an increase in testis volume and rate of spermatogenesis (1,10,11). In the present study, the exogenous gonadotropin treatment led to increased androgen production by the testicular tissue graft Leydig cells, as suggested by the higher weight of the seminal vesicles (6) recovered from the gonadotropin-treated mice, compared with seminal vesicles from untreated mice. This observation also underscores that Leydig cells are functional in the testis tissue grafts because seminal vesicle weight did not increase in mice in which no grafts were recovered. Seminal vesicle weight also remained low in untreated recipients, indicating that stimulation of infant monkey Leydig cells by endogenous mouse gonadotropins was not sufficient to elicit an increase in testosterone production. This is in contrast to our previous observations that mouse gonadotropins stimulated physiologic levels of testosterone production supporting complete spermatogenesis in testis grafts from 1-yr-old monkeys (6). Therefore, the infant monkey testis appears less sensitive to mouse gonadotropins than the 1-yr-old monkey testis, resulting in lower spermatogenic differentiation; however, this low responsiveness to endogenous gonadotropins in the host can at least partially be overcome by supplementation of exogenous gonadotropins as demonstrated in the current study.
There was a notable donor effect on tissue maturation. Complete germ cell differentiation was observed in three of the six 6-month-old donors, whereas germ cells in grafts from the remaining donors matured only to pachytene spermatocytes. Similarly, grafts from one of the five 3-month-old donors showed germ cell differentiation into meiosis with tissue from the remaining donors showing spermatogonia as the most advanced germ cell type. This marked effect of donor on testicular maturation was also observed in bovine and equine testis tissue when xenografted into mice (13,14). Tissue maturation within a graft was also variable in the current study, with some areas of the graft more mature than others. The reasons for this variation in the maturation of grafts (and tubules) are not apparent. It is possible that variable delivery of nutrients and hormones due to deficiencies in vascularization could lead to variable tissue development; however, this phenomenon is not observed in tissue grafts from all donor species (5).
To evaluate the effect of endogenous gonadotropins provided by the host mice and exogenous gonadotropins on somatic cell maturation, expression of proteins associated with Sertoli cell maturation was monitored. Sertoli cells cease to divide once they reach maturity, whereas immature Sertoli cells continue to divide (15). Immunolocalization of PCNA has been shown to be a valid approach for the study of cell proliferation in developing tissue (16) including the testes (17). As expected, PCNA was detected in most of the cell types including germ cells, Sertoli cells, and Leydig cells in the donor monkey tissue before grafting. Cells continued to divide, thus indicating the presence of immature Sertoli cells, in the grafted tissues obtained from the untreated mice. The grafts obtained from the treated mice showed decreased expression of PCNA in Sertoli cells; however, the germ cells were still strongly stained. This indicates that the Sertoli cells had ceased to divide in response to exogenous gonadotropins, supporting the interpretation that gonadotropin stimulation promoted Sertoli cell maturation. In grafts in which Sertoli cells did not express PCNA the germ cells (spermatogonia and spermatocytes) were actively dividing, indicating that Sertoli cell maturation was supporting spermatogenic proliferation and meiotic differentiation.
Sertoli cell maturation in the grafts was further confirmed by the presence or absence of MIS and AR expression. MIS is responsible for regression of the Mullerian duct in the male (18) and is mainly produced by immature Sertoli cells (19). Grafts recovered 28 wk after grafting from untreated mice showed lower expression of MIS, compared with the donor tissue, suggesting that Sertoli cells were progressing toward maturation. Most grafts recovered from treated mice showed no expression of MIS in the Sertoli cells. Therefore, the pattern of MIS expression indicated maturation of the Sertoli cells in the grafted tissue. Expression of AR in Sertoli cell nuclei could be detected only in the grafted tissues from the 6-month-old monkeys recovered from gonadotropin-treated mice and in the few grafts from the 3-month-old monkeys that showed germ cell differentiation, also recovered from treated mice. Thus AR was expressed only in grafts that were recovered from treated mice, and this appears to be the best indicator of Sertoli cell maturity among those used here. Germ cell differentiation occurred only in grafts where AR was expressed in Sertoli cells, whereas germ cell differentiation was not supported where AR expression was not detected despite the absence of PCNA and MIS expression.
As expected based on the observed variability of germ cell differentiation, there was variability among grafts and among tubules within grafts in MIS and AR immunolocalization, indicating that not all the Sertoli cells matured synchronously. We hypothesize that variability in Sertoli cell maturation is responsible for the variable degree of germ cell differentiation observed between and within individual grafted tissue fragments. In addition to variability in Sertoli cell maturation, a decrease in the number of tubules containing germ cells, compared with donor tissue, also contributes to the variable degree of germ cell differentiation. This initial loss of germ cells in the grafted tissue appears to be inherent to the technique of xenografting as a similar decrease in the number of germ cells in the grafted tissue has also been observed for other donor species (13,14). It is possible, however, that incomplete Sertoli cell maturation could also contribute to germ cell loss over time. Conversely, Sertoli cell maturation could be impaired in tubules without germ cells.
We reported previously that exposure to gonadotropins in the adult mouse host accelerates testicular maturation in testes from juvenile (13 months old) rhesus monkeys (6). The results of the present study suggest that continued exposure to sustained gonadotropic stimulation in castrated recipient mice can induce testicular maturation and germ cell differentiation in even younger, infantile primate testis. These maturational changes can be further accelerated with supplemental exposure to exogenous gonadotropins. Therefore, under the experimental conditions used in this study, a prolonged phase of testis development in the absence of gonadotropin stimulation, as it occurs under physiological conditions in the juvenile monkey, does not appear to be essential for testicular somatic cell maturation and spermatogenesis. The xenograft approach used in the present study enabled replication of treatments within donors for the investigation of gonadotropin stimulation on testicular maturation in tissue from infant primates. The results reported here are expected to be representative of the situation in vivo and can aid in designing further studies in intact monkeys.
Acknowledgments
The authors thank Terry Jordan for help with animal care; James Hayden, R.B.P., for help with figure preparation; and Dr. Tony Plant for critical review.
Footnotes
This work was supported by Grant RR17359 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 19, 2008
Abbreviations: AR, Androgen receptor; hCG, human chorionic gonadotropin; MIS, Mullerian inhibiting substance; PCNA, proliferating cell nuclear antigen; PMSG, pregnant mare serum gonadotropin; UCH-L1, ubiquitin carboxy-terminal hydrolase L1.
References
- Plant TM, Ramaswamy S, Simorangkir D, Marshall G 2005 Postnatal and pubertal development of the rhesus monkey (Macaca mulatta) testis. Ann NY Acad Sci 1061:149–162 [DOI] [PubMed] [Google Scholar]
- Li L-H, Donald JM, Golub MS 2005 Review on testicular development, structure, function, and regulation in common marmoset. Birth Defects Res B Dev Reprod Toxicol 74:450–469 [DOI] [PubMed] [Google Scholar]
- Handelsman DJ 2006 Aging in the hypothalamus-pituitary-testicular axis. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. 3rd ed. St. Louis: Elsevier Academic Press; 2697–2728 [Google Scholar]
- Plant TM, Gay VL, Marshall GR, Arslan M 1989 Puberty in monkeys is triggered by chemical stimulation of the hypothalamus. Proc Natl Acad Sci USA 86:206–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A, Snedaker A, Boiani M, Scholar H, Dobrinski I, Sclatt S 2002 Sperm from neonatal mammalian testes grafted in mice. Nature 418:778–781 [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I 2004 Accelerated maturation of primate testis by xenografting into mice. Biol Reprod 70:1500–1503 [DOI] [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Boiani M, Scholer HR, Dobrinski I 2003 Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol Reprod 68:2331–2335 [DOI] [PubMed] [Google Scholar]
- Wrobel KH, Bickel D, Kujat R, Schimmel M 1995 Configuration and distribution of bovine spermatogonia. Cell Tissue Res 279:277–289 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Plant TM, Marshall GR 2000 Pulsatile stimulation with recombinant single chain human luteinizing hormone elicits precocious Sertoli cell proliferation in the juvenile male rhesus monkey (Macaca mulatta). Biol Reprod 63:82–88 [DOI] [PubMed] [Google Scholar]
- Arslan M, Weinbauer GF, Schlatt S, Shahab M, Nieschlag E 2000 FSH and testosterone, alone or in combination, initiate testicular growth and increase the number of spermatogonia and Sertoli cells in a juvenile non-human primate (Macaca mulatta). J Endocrinol 136:235–243 [DOI] [PubMed] [Google Scholar]
- Schlatt S, Arslan M, Weinbauer GF, Behre HM, Nieschlag E 1995 Endocrine control of testicular somatic and premeiotic germ cell development in the immature testis of the primate Macaca mulatta. Eur J Endocrinol 133:235–247 [DOI] [PubMed] [Google Scholar]
- Chemes HE 2001 Infancy is not a quiescent period of testicular development. Int J Androl 24:2–7 [DOI] [PubMed] [Google Scholar]
- Rathi R, Honaramooz A, Zeng W, Schlatt S, Dobrinski I 2005 Germ cell fate and seminiferous tubule development in bovine testis xenografts. Reproduction 130:923–924 [DOI] [PubMed] [Google Scholar]
- Rathi R, Honaramooz A, Zeng W, Turner R, Dobrinski I 2006 Germ cell development in equine testis tissue xenografted into mice. Reproduction 131:1091–1098 [DOI] [PubMed] [Google Scholar]
- Griswold MD, McLean D 2006 The Sertoli cell. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. 3rd ed. St. Louis: Elsevier Academic Press; 949–975. [Google Scholar]
- Casasco A, Giordano M, Danova M, Casasco M, Icaro Cornaglia A, Calligaro A 1993 PC10 monoclonal antibody to proliferating cell nuclear antigen as probe for cycling cell detection in developing tissues. A combined immunocytochemical and flow cytometric study. Histochemistry 99:191–199 [DOI] [PubMed] [Google Scholar]
- Schlatt S, Weinbauer GF 1994 Immunohistochemical localization of proliferating cell nuclear antigen as a tool to study cell proliferation in rodent and primate testes. Int J Androl 17:214–222 [DOI] [PubMed] [Google Scholar]
- Lee MM, Kuroda T, Donahue PK 1993 Mullerian inhibiting substance. In: Desjardins C, Ewing LL, eds. Cell and molecular biology of the testis. New York: Oxford University Press; 108–126 [Google Scholar]
- Tran D, Josso N 1982 Localization of anti-Mullerian hormone in the rough endoplasmic reticulum of the developing bovine Sertoli cell using immunocytochemistry with a monoclonal antibody. Endocrinology 111:1562–1567 [DOI] [PubMed] [Google Scholar]