Abstract
The active form of vitamin D, 1,25-dihydroxyvitamin D3, [1,25(OH)2D3] has potent actions on innate and adaptive immunity. Although endocrine synthesis of 1,25(OH)2D3 takes place in the kidney, the enzyme that catalyzes this, 25-hydroxyvitamin D-1α-hydroxylase (CYP27b1 in humans, Cyp27b1 in mice), is expressed at many extra-renal sites including the colon. We have shown previously that colonic expression of CYP27b1 may act to protect against the onset of colitis. To investigate this further, we firstly characterized changes in Cyp27b1 expression in a mouse model of colitis. Mice treated with dextran sodium sulfate (DSS) showed weight loss, histological evidence of colitis, and increased expression of inflammatory cytokines. This was associated with decreased renal expression of Cyp27b1 (5-fold, P = 0.013) and lower serum 1,25(OH)2D3 (51.8 ± 5.9 pg/nl vs. 65.1 ± 1.6 in controls, P < 0.001). However, expression of CYP27b1 was increased in the proximal colon of DSS mice (4-fold compared with controls, P < 0.001). Further studies were carried out using Cyp27b1 null (−/−) mice. Compared with +/− controls the Cyp27b1 −/− mice showed increased weight loss (4.9% vs. 22.8%, P < 0.001) and colitis. This was associated with raised IL-1 in the distal colon and IL-17 in the proximal and distal colon. Conversely, DSS-treated Cyp27b1−/− mice exhibited lower IL-10 in the proximal colon and toll-like receptors 2 and 4 in the distal colon. These data indicate that both local and endocrine synthesis of 1,25(OH)2D3 affect colitis in DSS-treated mice. Lack of Cyp27b1 exacerbates disease in this model, suggesting that similar effects may occur with vitamin D deficiency.
THE ACTIVE FORM of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], is capable of inducing biological responses above and beyond its accepted role as a regulator of calcium homeostasis and bone metabolism. Notably, much recent attention has focused on the ability of 1,25(OH)2D3 to regulate both innate and adaptive immunity (1,2). In the case of the former, 1,25(OH)2D3 has been shown to act as a potent stimulator of the antibiotic protein cathelicidin (LL37) and thus actively promotes bacterial killing by both macrophages and epithelial cells (3,4,5,6). Expression of pattern recognition receptors such as the toll-like receptors (TLRs) is also regulated by 1,25(OH)2D3. In the case of macrophages, 1,25(OH)2D3 induces the TLR4 coreceptor CD14 (7), but it also suppresses expression of TLR2 and TLR4 resulting in decreased sensitivity to pathogen-associated molecular patterns, possibly as part of a feedback control mechanism to limit innate immune responsiveness (8). By contrast, in keratinocytes 1,25(OH)2D3 is a potent inducer of TLR2, providing a mechanism by which these cells can respond to a bacterial challenge, particularly after epidermal wounding (9).
The immunomodulatory actions of 1,25(OH)2D3 also span the link between innate and adaptive immunity. In vitro, 1,25(OH)2D3 potently suppresses the maturation of dendritic cells, leading to profound effects on T-cell function (10,11,12,13). As well as inhibiting generalized activation and proliferation of T cells via inhibition of costimulatory molecules and MHC class II (12,13), 1,25(OH)2D3 has also been shown to promote DC-mediated tolerogenicity by supporting the generation of IL-10-secreting regulatory T cells (13,14). Other studies have shown that 1,25(OH)2D3 promotes a shift in T-cell phenotype by suppressing Th1 cytokines such as IL-12 and interferon-γ (14). Finally, recent data indicate that 1,25(OH)2D3, acting via DCs, influences T-cell localization by enhancing T-cell expression of chemokine receptor 10 (CCR10) (15). As this binds the epidermis-specific cytokine CCL27, it would appear that an additional attribute of 1,25(OH)2D3 is its ability to act as a T-cell homing factor (15). Although DCs are an important conduit for 1,25(OH)2D3, it is also likely that some of its effects on T cells are mediated directly as activated T cells express abundant levels of the vitamin D receptor (VDR) (16). In a similar fashion, 1,25(OH)2D3 can act directly to inhibit B-cell proliferation, as well as plasma and memory cell development (17).
From the studies outlined above, it is clear that 1,25(OH)2D3 can exert effects on both innate and adaptive immunity through either direct or indirect pathways. An additional crucial consideration concerns the origin of 1,25(OH)2D3 itself. In classical vitamin D endocrinology, 1,25(OH)2D3 is synthesized from precursor 25-hydroxyvitamin D3 (25OHD3) in the kidneys that express abundant levels of the activating enzyme 25-hydroxyvitamin D-1α-hydroxylase (CYP27b1 in humans, Cyp27b1 in mice) (18). However, it is now clear that CYP27b1 is detectable in many other cell types, most notably in macrophages and DCs, and in epithelial cells at barrier sites such as the skin, placenta and gastrointestinal tract (19,20). The consequence of this is that localized, autocrine/paracrine activity may be the pivotal mechanism by which 1,25(OH)2D3 influences the human immune system (15,17,21,22). To assess the impact of renal and extra-renal production of 1,25(OH)2D3 on immune homeostasis at a specific barrier site, the colon, we have used a mouse model of gastrointestinal colitis [dextran sodium sulfate (DSS)-induced injury]) in which changes in innate and adaptive immunity were assessed in the context of altered Cyp27b1 expression and activity. Data indicate that induction of colitis in mice with DSS enhanced expression of CYP27b1 in the colon but suppressed the enzyme in the kidney. Importantly, mice lacking the Cyp27b1 gene were more susceptible to DSS-induced colitis, underlining the importance of 1,25(OH)2D3 as a regulator of colonic immunity and tissue homeostasis.
Materials and Methods
Mice
Wild-type C57BL/6 mice and C57BL/6 mice heterozygous for the Cyp27b1 gene (23) (gift from Dr. H. F. DeLuca, University of Wisconsin, WI) were bred for use at Cedars-Sinai Medical Center. Breeding and genotyping of mice heterozygous (+/−) or homozygous (−/−) for the Cyp27b1 gene was carried out using previously documented protocols (23). All animals, including wild-type, +/− and −/− mice were maintained on LabDiet 5015 formula chow containing 0.8% calcium and 3.3 IU/g vitamin D3 (Purina, Richmond, IN). All procedures were reviewed and approved by Cedars-Sinai Medical Center Institutional Animal Care and Use Committee (IACUC No. 1369). In preliminary experiments, n five male and five female wild-type C57BL/6 mice were assessed for changes in weight and serum vitamin D metabolites. For all subsequent experiments and treatments, five male or female mice were used.
Induction of colitis using dextran sodium sulfate (DSS)
Eight-week-old mice (20–25 g in weight) were administered regular filter-purified and sterilized water (controls) or water containing 2.5% dextran sodium sulfate (DSS) ad libitum for 7 d, after which all the mice were given regular water for a further 3 d. After the 10-d experiment, all animals were euthanized by carbon dioxide asphyxiation and cervical dislocation, and tissue collected as outlined below. During the experiment, animals were weighed daily and monitored for rectal bleeding, diarrhea, and general signs of ill health. To minimize unnecessary suffering in DSS-treated animals, a limit of no greater than 25% loss in body weight was set before animals would be euthanized and listed as dead.
Tissue collection and analysis
Immediately following euthanasia, blood was removed from mice by cardiac puncture and the resulting serum stored at −80 C before analysis of circulating levels of 25OHD3 and 1,25(OH)2D3 (Dr. B. Hollis, Medical University of South Carolina, Charleston, SC). Kidneys and spleen were also removed from the euthanized mice and snap-frozen before RNA extraction. The small and large intestines were also removed from euthanized mice, washed to remove fecal matter and then either: 1) preserved in 10% formaldehyde before embedding in paraffin blocks; 2) washed to remove fecal material and then divided into sections corresponding to small intestine, proximal colon (caecum to mid-transverse colon) and distal colon (mid-transverse colon to anus), then snap frozen before RNA extraction.
Histological analysis of colitis
Paraffin-embedded sections of colon tissue from euthanized mice (n = 5) were stained with hematoxylin/eosin and the histologic severity of colitis graded in a blinded fashion by an independent pathologist according to previously described methods which evaluate severity of inflammation, extent of injury, crypt damage and percentage tissue involvement (24).
Extraction of RNA and RT
RNA was extracted from mouse tissues using the RNeasy Total RNA extraction kit as detailed by the manufacturer (QIAGEN, Valencia, CA). RNA was eluted in ribonuclease-free elution solution and aliquots (1.5 μg) were reverse-transcribed using Powerscript Moloney murine leukemia virus reverse transcriptase as described by the manufacturer (ABI, Foster City, CA).
Quantitative real-time RT-PCR amplification of cDNAs
Expression of mRNAs for mouse VDR, Cyp27b1, IL-1, IL-10, IL-17, toll-like receptor (TLR) 2 and TLR4 was quantified using an ABI 7700 sequence detection system (ABI) as described previously (25). Approximately 50 ng of cDNA were used per reaction. All reactions were multiplexed with the housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, provided as an optimized control probe labeled with VIC fluorochrome (ABI), enabling data to be expressed in relation to an internal reference to allow for differences in sampling. Data were obtained as cycle threshold (Ct) values (the cycle number at which logarithmic PCR plots cross a calculated threshold line), and used to determine δCt values (Ct of target gene-Ct of housekeeping GAPDH gene). PCR amplification of target gene cDNA was carried out using the following Taqman mouse gene expression assays: CYP27b1 (Mm01165922); VDR (Mn004337297); IL-1α (00439620); IL-10 (Mn00439616); IL-17 (Mn00439619); toll-like receptor 2 (TLR2) (Mn00442346); TLR4 (Mn00445274); GAPDH (4352339E) (ABI). cDNAs were amplified under the following conditions: 50 C for 2 min; 95 C for 10 min; followed by 40 cycles of 95 C for 15 sec and 60 C for 1 min. All reactions were performed in triplicate and initially expressed as mean ± sd δCt values for n = 5 mouse tissue samples that were employed in statistical comparisons. Visual representation of data were carried out by converting δCt values to fold-change data relative to δCt values for control (regular water)-treated mice using the equation 2δδCt.
Immunohistochemical analysis of Cyp27b1
Immunohistochemical analysis of Cyp27b1 protein expression in paraffin-embedded tissue sections was carried out using methods as described previously (26). Briefly, sections were dewaxed through a series of xylene and graded ethanol washes, and antigen retrieval was carried out in 0.01 m sodium citrate buffer in a pressure cooker at 103 kPa for 2 min. Slides were then incubated in 3% methanol-hydrogen peroxide for 15 min to block endogenous peroxidase activity, washed in Tris-buffered saline (TBS), pH 7.6, and incubated with sheep antimouse CYP27b1 antiserum in 10% normal swine serum in TBS at dilutions between 1/100 and 1/300 for 45 min at room temperature. After a 15-min TBS wash, donkey antisheep IgG peroxidase conjugate with 10% normal swine serum in TBS at 1/100 dilution was added to sections for 45 min. 3,3′-Diaminobenzidine was used to visualize the secondary antibody. Mayers hematoxylin was used to counter stain sections. Control staining was carried out as shown above but with Cyp27b1 antiserum preincubated with a 100-fold excess of immunizing peptide.
Statistics
Experimental means were compared statistically using an unpaired Student’s t test. However, where indicated, multifactorial data involving Cyp27b1 +/− and −/− treatments were compared using one way ANOVA with the Holm-Sidak method used as a post hoc multiple comparison procedure. Statistical analyses were carried out using raw ΔCt values.
Results
Treatment with 2.5% DSS induces colitis in wild-type C57BL/6 mice
Preliminary studies indicated that C57BL/6 mice exposed to 2.5% DSS in drinking water experienced a steady weight loss compared with control mice receiving regular drinking water (Fig. 1A). After the 10-d treatment regime, DSS-treated mice had average weights of 22.1 ± 1.9 g (n = 5 mice) compared with a starting weights of 23.7 ± 2.0 g (n = 5), a 6.9% weight loss. By contrast control mice showed a 7.1% weight gain from 23.5 ± 1.0 g on d 0 to 25.2 ± 1.3 on d 10 (P = 0.008 compared with d 10 DSS-treated mice). These data were obtained with 8-wk-old male C57BL/6 mice, but similar observations were also made with C57BL/6 female mice and balb/c male mice (data not shown). Histological analysis of colonic tissue revealed increased colitis scores in DSS-treated mice (Fig. 1B) and enhanced expression of mRNA for the inflammatory cytokine, IL-1β in both the proximal and distal colon (Fig. 1C). By contrast, colonic expression of mRNA for the VDR was unaffected by DSS treatment (data not shown).
Figure 1.
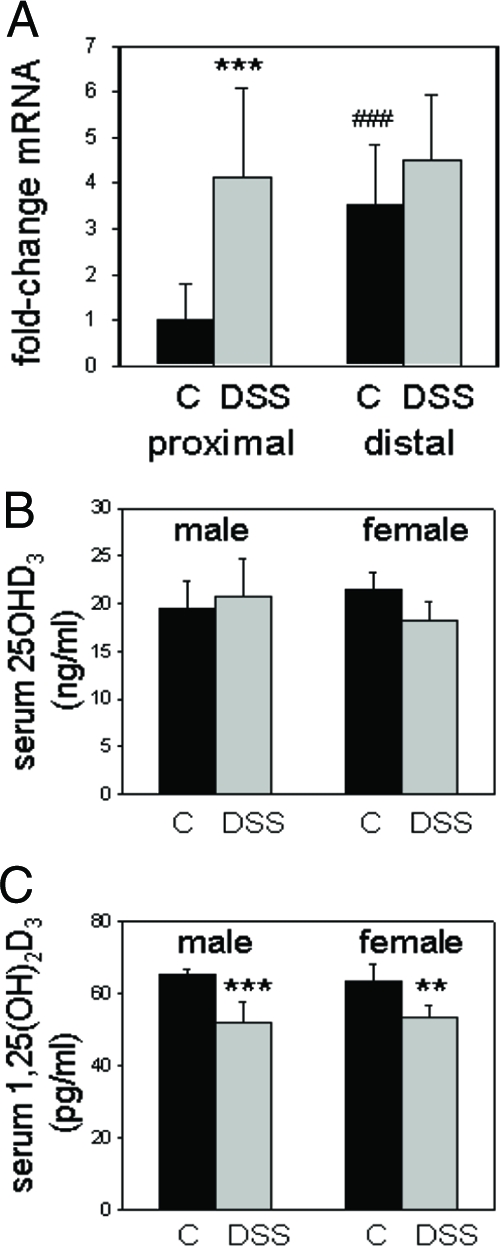
DSS-induced colitis in C57BL/6 mice. Panel A, Weight changes (g) in 8-wk-old mice receiving regular water (control) or water with 2.5% DSS (DSS) (7 d treatment followed by 3 d recovery). Each symbol (•, ▾, ○, ▵, ▪) represents weights for individual mice. Panel B, Histological colitis scoring in control or DSS mice. Data are the mean ± sd from n = 5 mice. Panel C, Real-time RT-PCR analysis of mRNA for IL-1β in tissue from the proximal and distal colon of control and +DSS mice. Data are shown as mean fold-change in expression in +DSS mice compared with control mice (n = 5 in both cases, ± sd). Statistical analysis was carried out using raw δCt values. **, Statistically different from water control, P < 0.01; ***, statistically different from water control, P < 0.001. ###, Statistically different from DSS-treated proximal colon, P < 0.001.
DSS-induced colitis stimulates colonic expression of Cyp27b1 but suppresses circulating levels of 1,25(OH)2D3
Unlike the VDR, expression of mRNA for Cyp27b1 increased 4-fold in the proximal colon of wild-type C57BL/6 mice following DSS treatment (Fig. 2A). Serum levels of 25OHD3 were unaffected by treatment with DSS (Fig. 2B). However, circulating levels of 1,25(OH)2D3 were significantly lower in DSS-treated mice (51.8 ± 5.9 pg/nl for males and 53.3 ± 3.2 pg/ml for females) compared with untreated controls (65.1 ± 1.6 and 63.6 ± 4.6, P < 0.001 and 0.01, respectively) (Fig. 2C).
Figure 2.
Altered expression of Cyp27b1 and synthesis of 1,25(OH)2D3 in DSS-treated mice. Panel A, Real-time RT-PCR analysis of Cyp27b1 mRNA in the proximal and distal colon of 8-wk-old mice (n = 5 for each treatment) receiving regular water (control, C) or water with 2.5% DSS (DSS) (7 d treatment followed by 3 d recovery). Data are shown as mean fold-change in expression relative to proximal colon from control mice (arbitrary mean value = 1) ± sd. Statistical analysis was carried out using raw δCt values. Panel B, Levels of serum 25OHD3 in male (n = 5) and female (n = 5) wild-type C57BL/6 mice receiving regular control water (C) or water with DSS (DSS). Panel C, Levels of serum 1,25(OH)2D3 in male (n = 5) and female (n = 5) wild-type C57BL/6 mice receiving regular control water (C) or water with DSS. **, Statistically different from water control, P < 0.01; ***, statistically different from water control, P < 0.001. ###, Statistically different from proximal colon control, P < 0.001.
The presence of protein for Cyp27b1 was confirmed by immunohistochemistry (Fig. 3), which showed predominant apical staining for the enzyme along with non-specific staining in mucus globules. Levels of Cyp27b1 expression were qualitatively higher in the distal colon but did not appear to be significantly enhanced in this region of the gastrointestinal tract in mice treated with DSS. In the proximal colon, apical expression of Cyp27b1 also appeared to be unaffected by DSS, but enhanced levels of the enzyme were clearly visible in lymphoid patches (see arrow in Fig. 3).
Figure 3.
The effects of DSS-treatment on colonic expression of Cyp27b1 protein. Immunohistochemical analysis of Cyp27b1 protein expression in panel A, the proximal and distal colon of control or DSS-treated mice (DSS); in panel B, control immunohistochemistry using Cyp27b1 antiserum preabsorbed with immunizing peptide. All pictures show ×200 magnification; arrow indicates a lymphoid patch.
Cyp27b1 KO mice are more susceptible to DSS-induced colitis
In view of the fact that wild-type C57BL/6 mice treated with 2.5% DSS exhibited changes in both systemic and localized activation of vitamin D (in the form of serum 1,25(OH)2D3 levels and colonic Cyp27b1 expression, respectively), we carried out further studies using Cyp27b1 KO mice on a C57BL/6 background. Consistent with data in Fig. 1, mice heterozygous for the Cyp27b1 gene (+/−) showed a 4.9% decrease in weight over the 10-d period of DSS treatment, similar to that observed with +/+ wild-type mice (6.6%) (Fig. 4A). However, DSS-treated mice homozygous for Cyp27b1 gene ablation (−/−) lost significantly more weight (22.8%) when compared with +/+ or +/− mice (both P < 0.001). All control mice gained weight over the same period. The increased weight loss in DSS-treated Cyp27b1 −/− mice was also associated with a further increase in scoring for colitis based on histological analysis of colonic tissue (Fig. 4B). DSS increased levels of colitis in the proximal colon of both Cyp27b1 +/− and −/− mice but the score for the DSS-treated −/− mice was statistically higher than that observed in +/− mice. DSS-induced colitis was most pronounced in the distal colon of both Cyp27b1 +/− and −/− mice but, in a similar fashion to the proximal colon, this score was statistically higher in tissue from Cyp27b1 −/− mice.
Figure 4.

Cyp27b1 knockout mice are more susceptible to DSS-induce colitis. Panel A, Weights of wild-type (+/+), Cyp27b1 heterozygous (+/−) and Cyp27b1 knockout (−/−) mice receiving regular water (control, C) or water with 2.5% DSS (DSS) (7 d treatment followed by 3 d recovery). Data shown are mouse weights (g) on d 0 and 10. Panel B, Histological colitis scoring in Cyp27b1 heterozygous (+/−) and Cyp27b1 knockout (−/−) mice receiving regular water (control, C) or water with 2.5% DSS (DSS). Data are the mean ± sd from n = 5 mice. ***, Statistically different from water control, P < 0.001. ###, Statistically different from DSS +/− mouse, P < 0.001. ##, Statistically different from DSS +/− mouse, P < 0.01. ###, Statistically different from DSS +/− mouse, P < 0.001. Data analyzed by ANOVA.
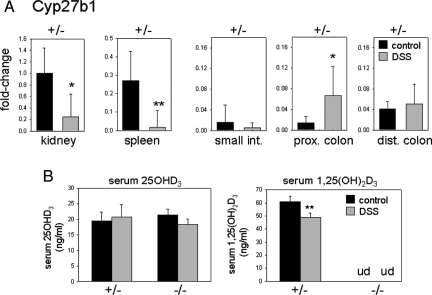
Although mRNA for Cyp27b1 was undetectable in the −/− mice (data not shown), we investigated further the expression of this enzyme in +/− control mice. The expression of mRNA for Cyp27b1 in kidney, spleen, small intestine, as well as proximal and distal colon, was assessed using kidneys from regular water control mice as a reference tissue (Fig. 5A). Treatment with DSS stimulated expression of Cyp27b1 in the proximal colon of Cyp27b1 +/− mice in a similar fashion to that observed in +/+ wild-type mice. Although there was no change in Cyp27b1 expression in other regions of the gastrointestinal tract, it was noted that basal expression of the enzyme in untreated control distal colon was 4-fold higher than in the proximal colon and not statistically different from DSS-treated proximal colon tissue. It was also noted that, in contrast to the proximal colon, expression of Cyp27b1 was strikingly suppressed in kidneys and spleen from DSS-treated mice. As observed with wild-type mice, treatment with DSS resulted in lower circulating levels of 1,25(OH)2D3 in the Cyp27b1 +/− mice (Fig. 5B).
Figure 5.
Tissue-specific alterations in Cyp27b1 expression and activity in DSS-treated mice. Panel A, Real-time RT-PCR analysis of Cyp27b1 mRNA in kidney, spleen, small intestine (small int.), proximal colon (prox. colon), and distal colon (dist. colon) from Cyp27b1 +/− mice treated with regular water (control, dark bars) or water with 2.5% DSS (DSS, gray bars). Data shown are the mean fold-change in Cyp27b1 expression relative to control kidney tissue (mean value = 1) ± sd. Statistical analysis of real-time RT-PCR data were carried out using raw δCt values (not shown), which were used to generate p values for statistically significant differences compared with water control mRNA expression (shown for each tissue). Panel B, Levels of serum 25OHD3 and 1,25(OH)2D3 in Cyp27b1 +/− and −/− mice receiving regular control water (C) or water with DSS. n = 5 mice for all treatments. **, Statistically different from water control, P < 0.01; *, statistically different from water control, P < 0.05. Data analyzed by ANOVA.
Further real-time RT-PCR was carried out to assess expression of other key genes associated with vitamin D function, namely VDR and the vitamin d-inactivating enzyme 24-hydroxylase (Cyp24). Data in Fig. 6A indicate that in the kidneys VDR expression was decreased in Cyp27b1 −/− mice but was unaffected by treatment with DSS. In the spleen, VDR expression was the same for Cyp27b1 +/− and −/− mice but was significantly suppressed by DSS in both types of animals. In the small intestine, VDR expression was decreased in DSS-treated Cyp27b1 +/− mice and in control −/− mice vs. +/− mice. DSS-treatment of Cyp27b1−/− mice increased VDR expression in the proximal colon but had no effect in the distal colon.
Figure 6.

Tissue-specific alterations in the expression of VDR and Cyp24 in DSS-treated mice. Real-time RT-PCR analysis of (panel A) VDR and (panel B) Cyp24 mRNA in kidney, spleen, small intestine, proximal colon (prox. colon), and distal colon from Cyp27b1 +/− and −/− mice treated with regular water (control, dark bars) or water with 2.5% DSS (DSS, gray bars). Data shown are the mean fold-change in gene expression relative to water control for each specific tissue (mean value = 1) ± sd. Statistical analysis of real-time RT-PCR data were carried out using raw δCt values (not shown). n = 5 mice for all treatments. Statistically significant difference in mRNA expression between equivalent samples from water control and DSS-treated animals is shown as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistically significant difference in mRNA expression between equivalent samples from Cyp27b1 +/− and −/− mice is shown as follows: #, P < 0.05; ##, P < 0.01; ###, P < 0.001. Statistically significant difference in mRNA expression between DSS-treated Cyp27b1 −/− mice and control +/− mice is shown as follows: a, P < 0.05; b, P < 0.01; c, P < 0.001. Data analyzed by ANOVA.
Analysis of Cyp24 expression (Fig. 6B) showed that in Cyp27b1 +/− mice mRNA for this enzyme was most abundant in the kidney, with levels being approximately 1000-fold higher than any of the other tissues (data not shown). However, in kidneys from Cyp27b1 −/− mice expression of Cyp24 was profoundly suppressed (3.2 × 105 and 5.5 × 105-fold, respectively, in control and DSS-treated mice). By contrast, expression of Cyp24 in the spleen was similar for Cyp27b1 −/− and +/− mice, and this was significantly increased in both groups of animals following treatment with DSS. Expression of Cyp24 in the gastrointestinal tract was variable but was highest in the small intestine of control mice. At this site and in the proximal and distal colon, Cyp27b1 −/− animals showed significantly lower levels of Cyp24 compared with equivalent tissues in +/− mice. Treatment with DSS increased levels of Cyp24 in the proximal colon (both control and DSS-treated animals) but was without effect in the small intestine and distal colon.
Increased colitis in Cyp27b1 KO mice is associated with dysregulation of cytokine and TLR expression
Vitamin D is now recognized as a potent modulator of innate and adaptive immunity (1,2). Effects on the former include the induction of antibacterial peptides (21), and either the stimulation (9) or suppression (8) of TLR expression. Active 1,25(OH)2D3 is also a powerful modulator of adaptive immune responses, including effects on T-cell proliferation and phenotype (27,28). With these observations in mind, we carried out further RT-PCR analyses to assess the possible involvement of innate and/or adaptive immunity genes in the pathophysiology of colitis associated with DSS treatment of Cyp27b1 KO mice.
As demonstrated in Fig. 1, induction of the inflammatory cytokine IL-1β is a key marker of DSS-induced colitis in the proximal and distal colon. Further studies using Cyp27b1 +/− and −/− mice confirmed this observation and showed that in the distal colon induction of IL-1β was significantly higher in Cyp27b1 −/− mice than +/− mice (P < 0.001) (Fig. 7A). In contrast to the colon, IL-1β levels in the small intestine were only affected by combined Cyp27b1 knockout and DSS-treatment, which potently suppressed mRNA expression for this cytokine. It was also interesting to note that DSS treatment induced expression of IL-1β in the kidney. Basal expression of IL-1β was relatively weak (20- to 25-fold lower) in this tissue compared with the colon, and DSS-induction of kidney IL-1β was unaffected by Cyp27b1 genotype. Predictably, levels of IL-1β were highest in the spleen. This was unaffected by Cyp27b1 knockout but was decreased in Cyp27b1 +/− mice following DSS treatment in contrast to −/− mice, which showed no change in expression.
Figure 7.
Tissue-specific alterations in cytokine expression in DSS-treated mice. Real-time RT-PCR analysis of (panel A) IL-1β, (panel B) IL-10, (panel C) IL-17 mRNA in kidney, spleen, small intestine (small int.), proximal colon (prox. colon), and distal colon (dist. colon) from Cyp27b1 +/− and −/− mice treated with regular water (control, dark bars) or water with 2.5% DSS (DSS, gray bars). Data shown are the mean fold-change in gene expression relative to water control for each specific tissue (mean value = 1). Statistical analysis of real-time RT-PCR data were carried out using raw δCt values (not shown). n = 5 mice for all treatments. Statistically significant difference in mRNA expression between equivalent samples from water control and DSS-treated animals is shown as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistically significant difference in mRNA expression between equivalent samples from Cyp27b1 +/− and −/− mice is shown as follows: #, P < 0.05; ##, P < 0.01; ###, P < 0.001. Statistically significant difference in mRNA expression between DSS-treated Cyp27b1 −/− mice and control +/− mice is shown as follows: a, P < 0.05; b, P < 0.01; c, P < 0.001. Data analyzed by ANOVA.
IL-10, a cytokine associated with suppressor or regulatory T-cell function (13,14), was strongly expressed in the spleen but was also detectable in the small intestine and proximal and distal colon (Fig. 7B). Like IL-1β, basal levels of IL-10 in these tissues were unaffected by Cyp27b1 knockout. Furthermore, like IL-1β, DSS-treatment potently induced IL-10 levels in the proximal and distal colon of Cyp27b1 +/− mice (5- and 10-fold, respectively), with a similar level of induction being observed in the kidneys of Cyp27b1 +/− mice (6-fold). By contrast, DSS treatment had no significant effect on IL-10 expression in the kidney or proximal colon of Cyp27b1 −/− mice, but in the distal colon of these mice there was a greater induction of IL-10 with DSS. Consistent with observations for IL-1β, expression of IL-10 in the small intestine was unaffected by either Cyp27b1 knockout or DSS-treatment but was potently suppressed by a combination of the two. Treatment with DSS also suppressed expression of IL-10 in the spleen and this was unaffected by Cyp27b1 genotype.
IL-17 is another cytokine that has been implicated in the pathophysiology of inflammatory bowel disease (29) and, consistent with previous studies (30), its expression was potently induced in the distal colon of DSS-treated mice (Fig. 7C). However, this effect was much stronger in Cyp27b1 −/− mice compared with +/− mice. Likewise, in the proximal colon of Cyp27b1 +/− mice, DSS treatment resulted in no significant increase in IL-17 expression, whereas −/− mice showed a significant induction. In the kidneys and spleen of +/− and −/− mice, a different trend in IL-17 expression was observed, with the cytokine being suppressed following DSS treatment. It was also interesting to note that, while differences in expression of IL-1β and IL-10 were only noted following treatment with DSS, levels of IL-17 in the small intestine were enhanced in control as well as DSS-treated Cyp27b1 −/− mice (Fig. 7C).
Expression of TLR2 was relatively weak in the small intestine and proximal colon when compared with the spleen (30- to 60-fold lower, data not shown), and this was unaffected by DSS-treatment or ablation of the CYP27b1 gene (Fig. 8A). However, expression of TLR2 was stronger in the distal colon (10- to 20-fold higher, data not shown) and this was further enhanced in Cyp27b1 +/− mice following DSS treatment (2.5-fold, P = 0.012) (Fig. 8A). By contrast, the Cyp27b1 −/− mice did not exhibit DSS-induction of TLR2 in the distal colon. Similar data were also obtained for TLR4, with low levels of expression for this gene in the kidney, small intestine and proximal colon relative to the spleen (data not shown). TLR4 levels were higher in the distal colon of Cyp27b1 +/− and −/− mice (20- to 30-fold, data not shown) and, as with TLR2, treatment with DSS further induced TLR4 in the distal colon of +/− mice but not −/− mice (Fig. 8B).
Figure 8.

Tissue-specific alterations in toll-like receptor expression in DSS-treated mice. Real-time RT-PCR analysis of (A) toll-like receptor 2 (TLR2), (B) toll-like receptor 4 (TLR4) mRNA in kidney, spleen, small intestine (small int.), proximal colon (prox. colon), and distal colon (dist. colon) from Cyp27b1 +/− and −/− mice treated with regular water (control, dark bars) or water with 2.5% DSS (DSS, gray bars). Data shown are the mean fold-change in gene expression relative to water control for each specific tissue (mean value = 1). Statistical analysis of real-time RT-PCR data were carried out using raw δ Ct values (not shown). n = 5 mice for all treatments. Statistically significant difference in mRNA expression between equivalent samples from water control and DSS-treated animals is shown as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistically significant difference in mRNA expression between equivalent samples from Cyp27b1 +/− and −/− mice is shown as follows: #, P < 0.05; ##, P < 0.01; ###, P < 0.001. Statistically significant difference in mRNA expression between DSS-treated Cyp27b1 −/− mice and control +/− mice is shown as follows: a, P < 0.05; b, P < 0.01; c, P < 0.001. Data analyzed by ANOVA.
Conclusions
Several strands of evidence have implicated vitamin D in the dysregulated immune responses that are characteristic of inflammatory bowel disease (IBD). Firstly, recent epidemiology suggests that patients with IBD have an impaired vitamin D status, as represented by serum levels of the major circulating form of vitamin D, 25OHD3 (31,32,33,34,35,36). Secondly, studies using various animal models indicate that 1,25(OH)2D3 signaling via the VDR plays a crucial role in the pathophysiology of IBD (37,38,39). Finally, in addition to its expression by macrophages and DCs (19,20), CYP27b1 has been detected in the human colon (26), with the vitamin D-activating enzyme being up-regulated in disease-affected tissue from patients with Crohn’s disease (40). In the case of the latter, dysregulated colonic expression of CYP27b1 was associated with increased circulating levels of 1,25(OH)2D3 indicating that localized synthesis of this vitamin D metabolite can spill over into the general circulation under conditions of persistent disease (40). Collectively these observations suggest: 1) That there is local conversion of 25OHD3 to 1,25(OH)2D3 in the colon, possibly as protective mechanism for this barrier site; 2) In patients with low vitamin D (25OHD3) status, this protective mechanism maybe compromised leading to IBD; 3) Under conditions of persistent colonic inflammation local metabolism of 25OHD3 becomes dysregulated, leading to ancillary clinical problems. To investigate further the role of renal and extra-renal synthesis of 1,25(OH)2D3 in the pathophysiology of IBD, we studied the effect of experimentally induced colitis on local and systemic vitamin D metabolism in both wild-type and Cyp27b1 knockout mice.
The induction of Cyp27b1 in the colon of DSS-treated mice was consistent with the elevated expression of this enzyme reported previously in patients with Crohn’s disease (40). In both cases, elevated expression of CYP27b1/Cyp27b1 was particularly evident in granulomatous or lymphoid tissue, suggesting that increased levels of the enzyme are due to the presence of cells from the immune system, such as macrophages. However, in contrast to the situation in humans, DSS-induced expression of colonic Cyp27b1 in mice was associated with decreased serum levels of 1,25(OH)2D3 (see Figs. 2C and 5B), presumably as a consequence of the lower levels of renal Cyp27b1 in these animals (Fig. 5A). This dichotomy between renal and extra-renal CYP27b1 with inflammatory disease has been demonstrated previously. In patients with rheumatoid arthritis, serum levels of 1,25(OH)2D3 are negatively correlated with disease activity (41), and further studies have highlighted the capacity for suppression of CYP27b1 in renal cells by the inflammatory mediator nuclear factor-κB (42). By contrast, macrophages from the affected synovial fluid of rheumatoid arthritis patients have profoundly elevated levels of CYP27b1 activity (43), underlining the similarity between this disorder and the local vs. endocrine metabolism of vitamin D in DSS-treated mice. The systemic inhibitor of renal Cyp27b1 associated with DSS-treatment remains to be elucidated. However, the presence of elevated levels of IL-1β in the kidneys of these mice (see Fig. 7A) coupled with the previously reported renal cell suppressive effects of a downstream mediator of IL-1β, nuclear factor-κB (42), indicates that inflammatory cytokines are potential candidates.
The functional significance of DSS-induced expression of Cyp27b1 in the proximal colon remains unclear. In particular, it was interesting to note that this effect on Cyp27b1 expression was restricted to the proximal colon where DSS-induced colitis was relatively low (see Fig. 4B). By contrast, higher levels of colitis were observed in the distal colon but this was not associated with increased expression of Cyp27b1. A possible explanation for these observations is that higher basal levels of Cyp27b1 in the distal colon provide a constitutive, localized source of 1,25(OH)2D3 even in the absence of any disease. This is clearly different to the innate immunity mechanisms recently described for human macrophages (21), and keratinocytes (9) where CYP27b1 is specifically induced by bacterial TLR agonists and tissue injury, respectively. In view of the fact that the gastrointestinal tract is constantly exposed to enteric bacteria, constitutive expression of Cyp27b1 in the colon may provide a more effective mechanism for maintaining vitamin D-regulated innate immune surveillance at this barrier site. Because knockout of Cyp27b1 was associated with increased colitis in both the proximal and distal colon, constitutive production of 1,25(OH)2D3 may contribute to barrier function at both sites.
Another scenario is that the effects of 1,25(OH)2D3 on DSS-induced colitis in mice involve autocrine and/or endocrine responses. However, although circulating levels of 1,25(OH)2D3 are suppressed following treatment with DSS, it is difficult to determine the relative tissue function of local vs. systemic production of 1,25(OH)2D3. Analysis of the key vitamin D target gene Cyp24 showed that expression was significantly decreased in the kidney and all parts of the colon in control Cyp27b1−/− mice. This confirms that all of these tissues are targets for 1,25(OH)2D3 but does not clarify the source of Cyp27b1 activity. Interestingly, expression of Cyp24 was significantly induced by DSS in the proximal but not distal colon (Fig. 5B). Given that Cyp27b1 was also induced at this site, it is possible that induction of Cyp24 occurs in response to enhanced localized production of 1,25(OH)2D3. However, the fact that a similar induction of Cyp24 was also observed in the proximal colon of DSS-treated Cyp27b1 −/− mice suggests that a 1,25(OH)2D3-independent mechanism is more likely.
In contrast to the VDR, which showed relatively modest changes in expression after DSS treatment, the alterations in Cyp27b1 levels appear complex. As outlined above, we have postulated that the suppression of renal Cyp27b1 during DSS-induced colitis may be due to the effects of systemic inflammation and this mechanism may also explain the striking suppression of both Cyp27b1 and VDR in spleens from DSS-treated mice (see Figs. 5A and 6A). Previous studies have shown that DSS-induced colitis is associated with increased spleen size (44), as well as profound changes in splenic protein metabolism (45). Despite this, the spleen does not appear to be important for the progression of DSS-induced colitis (46), or translocation of enteric bacteria (47). It is unclear what role, if any, splenic Cyp27b1 plays in the pathophysiology of DSS-induced colitis in mice. However, in view of the fact that most of the target genes assessed in this study showed decreased expression in spleens after DSS treatment, a likely scenario is that the effects on Cyp27b1 and VDR are not specific.
The mechanism for increased expression of Cyp27b1 in the proximal colon also remains to be clarified. In macrophages (21,48) and epithelial cells (9,49), the enzyme is potently induced by ligands to TLR2 and TLR4. Both of these receptors along with several other TLRs have been detected in the human and mouse intestine where they play an integral role in initiating immune responses following colonic barrier challenge (50,51). However, the heterogeneity of cell types expressing TLRs within the gastrointestinal tract (51), means that it is difficult to define precisely which receptor is involved in stimulating Cyp27b1 expression. Immunohistochemical analyses presented here (Fig. 3) indicate that under basal conditions, the enzyme is located predominantly within intestinal epithelial cells that are known to express relatively weak levels of TLR2 and TLR4 (52). Moreover, levels of mRNA for TLR2 and TLR4 were potently up-regulated in the distal colon of DSS-treated mice (Fig. 8) without any apparent effect on Cyp27b1 expression (Fig. 5A). Thus, it is possible that DSS-induced enhancement of colonic Cyp27b1 occurs via an alternative pathogen recognition receptor, such as TLR5, which is functionally active in intestinal epithelial cells (53). Another possibility is that induction of colonic Cyp27b1 occurs via a TLR-independent mechanism. Recent elegant studies using keratinocytes have shown that Cyp27b1 expression by these cells is up-regulated following epidermal injury via a transforming growth factor-β-mediated pathway (9), and it is tempting to speculate that a similar mechanism is involved in stimulating colonic expression of Cyp27b1 following DSS-induced injury. However, immunohistochemistry (see Fig. 3) shows that DSS induction of Cyp27b1 in the proximal colon occurs primarily in lymphoid patches. Although inconsistent with the epithelial wounding model, this is reminiscent of the enhanced levels of CYP27b1 in granulomata from patients with Crohn’s disease (40).
Previous studies using VDR knockout mice indicate that the increased susceptibility of these mice to DSS-induced colitis involves dysregulated expression of colonic cytokines (38) and proteins linked to the integrity of tight junctions (37). Here we show that ablation of the Cyp27b1 gene in mice also results in enhanced sensitivity to DSS-induced colitis. In common with the VDR knockout mouse, the increased sensitivity of Cyp27b1 knockout mice to DSS was associated with dysregulation of key cytokines involved in adaptive immunity. Of particular note was the divergent regulation of IL-10 and IL-17 in the proximal colon. Knockout mice for IL-10 are known to have increased susceptibility to colitis (54), whereas transgenic overexpression of IL-10 protects against colitis (55). Other studies have shown that IL-10 is enhanced in the colon of DSS-treated mice (56), suggesting that the cytokine may be induced as part of a mechanism to protect against colitis. As such, the absence of this mechanism in the proximal colon of DSS-treated Cyp27b1 knockout mice may be crucial in sensitizing these animals to colitis, particularly as vitamin D is known to promote the generation of IL-10 secreting regulatory T cells (13,14). Recent reports have also implicated vitamin D as an attenuator of the inflammatory cytokine IL-17 in another mouse model of colitis (57), suggesting that suppression of TH17 cells may be another pivotal target for vitamin D in protecting against colitis. It was therefore interesting to note that levels of IL-17 mRNA were significantly enhanced in the proximal colon of DSS-treated Cyp27b1 knockout mice but not heterozygous mice (see Fig. 7C).
One of the key recent developments in our understanding of inflammatory bowel disease has been the recognition that microbial-innate immune interactions are crucial to the transition from normal commensal interaction with enteric bacteria to pathological inflammation (58). The recognition of, and response to, pathogens by TLRs has been shown to be a pivotal step in controlling gastrointestinal innate immunity, with TLR2 and TLR4 being particularly important (51). Both receptors were induced in the distal colon of Cyp27b1 heterozygous mice following treatment with DSS (see Fig. 8), consistent with previous studies using wild-type mice (59), but this effect was not observed in Cyp27b1 knockout mice. In view of the fact that expression of TLR4 appears to be essential for normal innate immune responsiveness in DSS-induced colitis (60), it seems likely that the dysregulation of TLR expression in Cyp27b1 mice knockout will play a significant role in the increased susceptibility of these animals to colitis. As outlined above, these data are consistent with the proposed role of local synthesis of 1,25(OH)2D3 as a regulator of TLR expression in the skin following epidermal injury (9). As yet, it is uncertain whether similar patterns of TLR regulation are associated with mouse models of IBD and this will be a key objective of future studies.
In conclusion, data presented here provide further evidence of a role for vitamin D in protecting against inflammatory bowel disease via the barrier actions of 1,25(OH)2D3. Inability to produce this active form of vitamin D either locally, systemically or both, compromises both innate and adaptive immune responses in the gastrointestinal tract with resulting susceptibility to colitis. Several key questions remain to be answered, in particular whether similar results will also be obtained using vitamin D-deficient animals rather than VDR or Cyp27b1 knockouts. Likewise, it will be important to determine whether other mouse models of inflammatory bowel disease are more reflective of patients with Crohn’s disease. In contrast to the human disease, DSS colitis does not appear to involve cells from the adaptive immune (61), raising the possibility that T or B cells are required to manifest the pathological over-production of 1,25(OH)2D3 that is frequently observed with Crohn’s patients (40). Finally, by using established TLR knockout mice, it will be possible to assess whether or not these receptors are central to the regulation of Cyp27b1 in the colon or whether other injury mechanisms are involved.
Acknowledgments
We thank Gloria Kiel for help in preparing the manuscript and Dr. Bing Hu for support in processing and analyzing pathological specimens.
Footnotes
Disclosure Statement: B.H. is a consultant for Diasorin Corp. N.L., L.N., R.F.C, V.L., S.R., S.W., H.F.D., J.S.A., and M.H. have nothing to disclose.
This work was supported by National Institutes of Health Grant RO1AR050626 (to M.H.).
First Published Online June 5, 2008
Abbreviations: DSS, Dextran sodium sulfate; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IBD, inflammatory bowel disease; TLRs, toll-like receptors; VDR, vitamin D receptor.
References
- Adams JS, Hewison M 2008 Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 4:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JS, Liu P, Chun R, Modlin RL, Hewison M 2007 Vitamin D in defense of the human immune response. Ann NY Acad Sci 1117:94–105 [DOI] [PubMed] [Google Scholar]
- Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH 2004 Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912 [DOI] [PubMed] [Google Scholar]
- Gombart AF, Borregaard N, Koeffler HP 2005 Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19:1067–1077 [DOI] [PubMed] [Google Scholar]
- Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G 2007 Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros 6:404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sorensen OE, Kampmann B, Griffiths CJ, Wilkinson RJ 2007 IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol 178:7190–7198 [DOI] [PubMed] [Google Scholar]
- Hewison M, Barker S, Brennan A, Katz DR, O'Riordan JL 1989 Modulation of myelomonocytic U937 cells by vitamin D metabolites. Bone Miner 5:323–333 [DOI] [PubMed] [Google Scholar]
- Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zugel U, Steinmeyer A, Pollak A, Roth E, Boltz-Nitulescu G, Spittler A 2006 Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol 36:361–370 [DOI] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL 2007 Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest 117:803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R 2001 Dendritic cell modulation by 1α,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA 98:6800–6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorini L, Penna G, Giarratana N, Roncari A, Amuchastegui S, Daniel KC, Uskokovic M 2004 Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J Steroid Biochem Mol Biol 89- 90:437–441 [DOI] [PubMed] [Google Scholar]
- Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V 2000 Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol 164:4443–4451 [DOI] [PubMed] [Google Scholar]
- Penna G, Adorini L 2000 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 164:2405–2411 [DOI] [PubMed] [Google Scholar]
- van Halteren AG, van Etten E, de Jong EC, Bouillon R, Roep BO, Mathieu C 2002 Redirection of human autoreactive T-cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D(3). Diabetes 51:2119–2125 [DOI] [PubMed] [Google Scholar]
- Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC 2007 DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol 8:285–293 [DOI] [PubMed] [Google Scholar]
- Karmali R, Hewison M, Rayment N, Farrow SM, Brennan A, Katz DR, O'Riordan JL 1991 1,25(OH)2D3 regulates c-myc mRNA levels in tonsillar T lymphocytes. Immunology 74:589–593 [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE 2007 Modulatory effects of 1,25-dihydroxyvitamin d3 on human B cell differentiation. J Immunol 179:1634–1647 [DOI] [PubMed] [Google Scholar]
- Sakaki T, Kagawa N, Yamamoto K, Inouye K 2005 Metabolism of vitamin D3 by cytochromes P450. Front Biosci 10:119–134 [DOI] [PubMed] [Google Scholar]
- Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS 2007 Extra-renal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. J Steroid Biochem Mol Biol 103:316–321 [DOI] [PubMed] [Google Scholar]
- Townsend K, Evans KN, Campbell MJ, Colston KW, Adams JS, Hewison M 2005 Biological actions of extra-renal 25-hydroxyvitamin D-1α-hydroxylase and implications for chemoprevention and treatment. J Steroid Biochem Mol Biol 97:103–109 [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL 2006 Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R 2003 Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol 170:5382–5390 [DOI] [PubMed] [Google Scholar]
- Vanhooke JL, Prahl JM, Kimmel-Jehan C, Mendelsohn M, Danielson EW, Healy KD, Deluca HF 2006 CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3–1α-hydroxylase promoter activity in the skin. Proc Natl Acad Sci USA 103:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowinkel T, Mori M, Krieglstein CF, Russell J, Saijo F, Bharwani S, Turnage RH, Davidson WS, Tso P, Granger DN, Kalogeris TJ 2004 Apolipoprotein A-IV inhibits experimental colitis. J Clin Invest 114:260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M 2006 Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod 75:816–822 [DOI] [PubMed] [Google Scholar]
- Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M 2001 Extrarenal expression of 25-hydroxyvitamin d(3)-1α-hydroxylase. J Clin Endocrinol Metab 86:888–894 [DOI] [PubMed] [Google Scholar]
- Mathieu C, van Etten E, Decallonne B, Guilietti A, Gysemans C, Bouillon R, Overbergh L 2004 Vitamin D and 1,25-dihydroxyvitamin D3 as modulators in the immune system. J Steroid Biochem Mol Biol 89–90:449–452 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Zhu Y, Froicu M, Wittke A 2004 Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr 80:1717S–1720S [DOI] [PubMed] [Google Scholar]
- Young Y, Abreu MT 2006 Advances in the pathogenesis of inflammatory bowel disease. Curr Gastroenterol Rep 8:470–477 [DOI] [PubMed] [Google Scholar]
- Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y 2003 Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagianos K, Bector S, McConnell J, Bernstein CN 2007 Nutrition assessment of patients with inflammatory bowel disease. J Parenter Enteral Nutr 31:311–319 [DOI] [PubMed] [Google Scholar]
- Pappa HM, Grand RJ, Gordon CM 2006 Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis 12:1162–1174 [DOI] [PubMed] [Google Scholar]
- Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, Grand RJ 2006 Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics 118:1950–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Mahon BD 2004 Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 229:1136–1142 [DOI] [PubMed] [Google Scholar]
- Jahnsen J, Falch JA, Mowinckel P, Aadland E 2002 Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand J Gastroenterol 37:192–199 [DOI] [PubMed] [Google Scholar]
- Gilman J, Shanahan F, Cashman KD 2006 Determinants of vitamin D status in adult Crohn’s disease patients, with particular emphasis on supplemental vitamin D use. Eur J Clin Nutr 60:889–896 [DOI] [PubMed] [Google Scholar]
- Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC 2007 Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294:G208–G216 [DOI] [PubMed] [Google Scholar]
- Froicu M, Cantorna MT 2007 Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol 8:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT 2003 A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 17:2386–2392 [DOI] [PubMed] [Google Scholar]
- Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, Chen S, Zehnder D, Lin YC, Yang H, Hewison M, Adams JS 2004 Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut 53:1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelzner P, Muller A, Deschner F, Huller M, Abendroth K, Hein G, Stein G 1998 Relationship between disease activity and serum levels of vitamin D metabolites and PTH in rheumatoid arthritis. Calcif Tissue Int 62:193–198 [DOI] [PubMed] [Google Scholar]
- Ebert R, Jovanovic M, Ulmer M, Schneider D, Meissner-Weigl J, Adamski J, Jakob F 2004 Down-regulation by nuclear factor κB of human 25-hydroxyvitamin D3 1α-hydroxylase promoter. Mol Endocrinol 18:2440–2450 [DOI] [PubMed] [Google Scholar]
- Hayes ME, Denton J, Freemont AJ, Mawer EB 1989 Synthesis of the active metabolite of vitamin D, 1,25(OH)2D3, by synovial fluid macrophages in arthritic diseases. Ann Rheum Dis 48:723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morteau O, Morham SG, Sellon R, Dieleman LA, Langenbach R, Smithies O, Sartor RB 2000 Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest 105:469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier S, Breuille D, Mosoni L, Obled C, Patureau Mirand P 2002 Chronic inflammation alters protein metabolism in several organs of adult rats. J Nutr 132:1921–1928 [DOI] [PubMed] [Google Scholar]
- Krieglstein CF, Cerwinka WH, Laroux FS, Grisham MB, Schurmann G, Bruwer M, Granger DN 2001 Role of appendix and spleen in experimental colitis. J Surg Res 101:166–175 [DOI] [PubMed] [Google Scholar]
- Guo W, Andersson R, Willen R, Ljungh A, Wang X, Liu X, Bengmark S 1994 Bacterial translocation after intraperitoneal implantation of rubber fragments in the splenectomized rat. J Surg Res 57:408–415 [DOI] [PubMed] [Google Scholar]
- Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C 2006 Immune regulation of 25-hydroxyvitamin-d(3)-1α-hydroxylase in human monocytes. J Bone Miner Res 21:37–47 [DOI] [PubMed] [Google Scholar]
- Bland R, Zehnder D, Hughes SV, Ronco PM, Stewart PM, Hewison M 2001 Regulation of vitamin D-1α-hydroxylase in a human cortical collecting duct cell line. Kidney Int 60:1277–1286 [DOI] [PubMed] [Google Scholar]
- Fukata M, Abreu MT 2007 TLR4 signalling in the intestine in health and disease. Biochem Soc Trans 35:1473–1478 [DOI] [PubMed] [Google Scholar]
- Abreu MT, Fukata M, Arditi M 2005 TLR signaling in the gut in health and disease. J Immunol 174:4453–4460 [DOI] [PubMed] [Google Scholar]
- Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M 2001 Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol 167:1609–1616 [DOI] [PubMed] [Google Scholar]
- Sun J, Fegan PE, Desai AS, Madara JL, Hobert ME 2007 Flagellin-induced tolerance of the Toll-like receptor 5 signaling pathway in polarized intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 292:G767–G778 [DOI] [PubMed] [Google Scholar]
- Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, Berg DJ, Rennick DM 1996 T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med 184:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbaugh A, Sharma S, Dubinett SM, Wei SH, Aranda R, Cheroutre H, Fowell DJ, Binder S, Tsao B, Locksley RM, Moore KW, Kronenberg M 1997 Altered immune responses in interleukin 10 transgenic mice. J Exp Med 185:2101–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW 2000 Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion 62:240–248 [DOI] [PubMed] [Google Scholar]
- Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM 2008 Immune modulatory treatment of TNBS colitis with calcitriol is associated with a change of a Th1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther 324:23–33 [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK 2007 Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434 [DOI] [PubMed] [Google Scholar]
- Ortega-Cava CF, Ishihara S, Rumi MA, Kawashima K, Ishimura N, Kazumori H, Udagawa J, Kadowaki Y, Kinoshita Y 2003 Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol 170:3977–3985 [DOI] [PubMed] [Google Scholar]
- Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu MT 2005 Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol 288:G1055–G1065 [DOI] [PubMed] [Google Scholar]
- Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO 1994 Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107:1643–1652 [DOI] [PubMed] [Google Scholar]