Abstract
Neuronal activity underlying the pulsatile secretion of GnRH remains poorly understood, as does the endogenous generation of such activity. It is clear that changes at the level of the hypothalamus are taking place during reproductive aging, yet virtually nothing is known about GnRH neuronal physiology in aging and postreproductive animals. In these studies, we performed cell-attached and whole-cell recordings in GnRH-enhanced green fluorescent protein neurons dissociated from young (3 months), middle-aged (10 months), and old (15–18 months) female mice. All mice were ovariectomized; half were estradiol replaced. Neurons from all ages fired spontaneously, most in a short-burst pattern that is characteristic of GnRH neuronal firing. Membrane characteristics were not affected by age. However, firing frequency was significantly reduced in neurons from old animals, as was spike patterning. The amplitude of the depolarizing afterpotential, evoked by a 200-msec current pulse, was significantly smaller in aged animals. In addition, inward whole-cell currents were reduced in estradiol-treated animals, although they were not significantly affected by age. Because depolarizing afterpotentials have been shown to contribute to prolonged discharges of activity after a very brief excitatory input, a decreased depolarizing afterpotential could lead to attenuated pulses in older animals. In addition, decreases in frequency and pattern generation could lead to improper information coding. Therefore, changes in the GnRH neuron during aging could lead to dysregulated activity, potentially resulting in the attenuated LH pulses observed in the transition to reproductive senescence.
REPRODUCTION IN MAMMALS is critically dependent on the appropriate neurosecretion of GnRH. The pulsatile release of GnRH into the portal bloodstream is necessary for the maintenance of normal reproductive function. Numerous recent studies involving GnRH neurons have advanced our knowledge of the endogenous properties of GnRH neurons (1,2) and their regulation by fast neurotransmitters (3,4,5,6) and steroids (5,7,8,9,10,11). Still, many questions about the endogenous generation and exogenous regulation of GnRH pulsatile activity remain unanswered.
In recent years, it has become clear that a number of changes are occurring in the hypothalamus (12) during reproductive aging. Both women and female rodents experience a preovulatory rise in FSH (12,13,14) before the transition to reproductive senescence. This is followed by a decreased LH pulse frequency (12,13,15), variable cycle length (12), and an attenuated LH response (12) to estradiol. Therefore, to understand the transition to reproductive senescence, we must understand how changes in the aging brain contribute to reproductive aging.
Using push-pull perfusion protocols in vivo, it has been shown that the number of GnRH pulses decreases in middle-aged female rats (13,16) during a steroid-induced LH surge, but it is not clear why this decrease occurs. Although decreases in GnRH neuronal number with age have been reported (17,18), other reports refute these studies (12,19,20). However, it has been shown that the number of neurons activated (as indicated by Fos expression) in conjunction with an LH surge does decrease (21,22,23,24). The pattern of GnRH secretion may reflect a balance of excitatory and inhibitory influences (13), superimposed on an endogenous pattern of activity (1,8,10,25,26,27). To determine whether decreases in GnRH pulsatile activity during the aging process are endogenous in nature and how reproductive status affects activity during aging, we examined how aging and hormone status affect individual GnRH neurons. Neurons isolated from young, middle-aged, and old mice were examined for changes in endogenous activity, membrane properties, and current and voltage responses to a variety of inputs.
Materials and Methods
Animals
Adult virgin female GnRH-enhanced green fluorescent protein (eGFP) transgenic mice (28,29) were used for all experiments. Animals were maintained in a colony at the University of Missouri. The original mice for the colony were a gift from Dr. Sue Moenter (University of Virginia, Charlottesville, VA). Mice were maintained under a 12-h light, 12-h dark cycle. Food (LabDiet 5008; PMI Nutrition International, Richmond, IN) and water were available at all times. Three ages of mice were used: 3 months (young adult), 10 months [middle aged (MA)], and 15–18 months (old). All animal experimentation was conducted in accord with accepted standards of humane animal care, and the University of Missouri Animal Care and Use Committee approved all procedures. All mice were bilaterally ovariectomized (OVX) under isoflurane anesthesia 5–7 d before they were killed. Banamine (0.025 mg per 10 g body weight) was administered preoperatively as an analgesic. At the time of surgery, a SILASTIC brand capsule (0.052 in. inner diameter, 0.125 in. outer diameter; Dow Corning, Midland, MI) was placed sc. The capsule contained either 0.625 μg β-estradiol (E2; OVX+E; Sigma, St. Louis, MO) in 20 μl of tocopherol-stripped corn oil (MP Biomedicals, Solon, OH) or corn oil only (OVX). This amount of E2 has been shown previously to maintain plasma estradiol at physiological levels in young adult GnRH-eGFP mice (29). Mice were killed 5–7 d after steroid replacement to allow sufficient time for the endocrine adjustment to hormone manipulation while avoiding long-term steroid withdrawal (26).
Vaginal cytology was performed on virgin animals of 3 months (young adult), 10 months (MA), and 15–17 months (old) of age. Vaginal washes were taken in the afternoon for 10 consecutive days on three to four animals of each age. Briefly, 20 μl of sterile saline were introduced into the vagina of each mouse and withdrawn. The fluid was smeared on a microscope slide, air dried, stained with a modified Wright’s stain, and viewed at ×20 on an inverted Diaphot-TMD microscope (Nikon, Melville, NY). Slides were examined for the presence of polymorphonuclear cells and the percentage of nucleated and nonnucleated, cornified epithelial cells. Estrus was defined by a predominance of cornified epithelial cells and the absence of polymorphonuclear cells.
Blood was obtained from a limited number of OVX and OVX+E animals via cardiac puncture at the time they were killed. Whole blood was spun down and serum was removed and stored at −20 C. The RIA for serum estradiol was performed as described previously (30,31). Briefly, [125-I]estradiol and antiserum were obtained from MP Diagnostics (Costa Mesa, CA), and unlabeled estradiol was obtained from Steraloids (Wilton, NH). Sensitivity of the assay was 0.5 pg/tube. Samples were analyzed in one assay, and the intraassay coefficient of variation was 3.95%. We determined the percent cross-reactivity of the estradiol antiserum with estrone to be 0.6%. Cross-reactivity with other steroids was reported by MP Diagnostics to be negligible.
Acute dissociation of neurons
Cells were acutely isolated as described previously (25). This protocol has been designed specifically to allow the physiological analysis of neurons from aged brains (32) because the preparation of neurons suitable for electrophysiological studies becomes very difficult with increased age. Briefly, brains were quickly removed and sliced at 400 μm in low-calcium artificial cerebrospinal fluid [in millimoles: 124 NaCl, 3 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 10 dextrose, 0.1 CaCl2 (pH 7.4); bubbled with 95% O2, 5% CO2]. Isolated regions were enzymatically treated with proteinase K (0.2 mg/ml; Sigma) followed by trypsin (type XI, 1 mg/ml; Sigma) at 30 C for 24–31 min, depending on the lot of trypsin and the age of the animal. Because older brains contain more connective tissue, slices from the older animals (10–18 months old) were trypsinized for 2–3 min longer than the young animals to produce healthy isolated neurons at all ages (32). Slices were triturated with flame-polished Pasteur pipettes, and dispersed cells were incubated overnight in Neurobasal A/B-27 (Life Technologies, Inc., Carlsbad, CA) before electrophysiological recordings. Unless stated, no estradiol was added to the dish.
Electrophysiology
Electrophysiology was performed 16–24 h after dissociation, as described previously (25). Fluorescent cells were viewed on a Nikon Diaphot-TMD inverted microscope with a green fluorescent protein filter. Cells were perfused with normal-calcium artificial cerebrospinal fluid (2.5 mm CaCl2) at 22 C. Cell-attached and whole-cell recordings were obtained with thin-walled borosilicate glass micropipettes (World Precision Instruments; 2–3.5 mΩ) filled with a potassium gluconate intracellular solution [in millimoles: 120 potassium gluconate, 1 CaCl2, 1 MgCl2, 10 HEPES, 1 NaCl, 5 EGTA, 2 ATP, 0.2 GTP (pH 7.2–7.4)]. A sodium-based ICS (in millimoles: 150 NaCl, 10 HEPES, 10 glucose, 2.5 CaCl2, 1.3 MgCl2, 3.5 KCl) was compared for cell-attached recordings, but there were no differences in firing frequency between the two intracellular solutions (P = 0.13, n = 5–12). All recordings were performed using a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA), digitized with a Digidata 1322A (Molecular Devices), and stored on a Dell computer (Dell, Round Rock, TX) using pClamp 9.2 software (Molecular Devices).
Cell-attached recordings were performed to examine the frequency and pattern of spontaneous firing in cells isolated from aged animals. Recordings were initiated with a seal greater than 300 mΩ, although most seals were greater than 1 GΩ by the end of the recording. Recordings were initiated about 30 sec after the establishment of the seal. Signals were acquired at 5 kHz and low-pass filtered at 1 kHz. The duration of the recordings lasted for 5–40 min. In many recordings, only the first 5–10 min of the recording were analyzed for the purpose of comparing different ages and hormone treatments because a compound was subsequently applied to the cell for other studies. However, an analysis of long-term recordings revealed no significant change in frequency with time (P = 0.75, frequency of firing over first 5 min vs. frequency of firing over the remaining recording, n = 5 each).
Whole-cell configuration was initiated only on the establishment of a gigaohm seal. Only stable recordings with a series resistance of less than 10 mΩ and a stable membrane capacitance were included. Series resistance was compensated 50–90%. In current clamp, bridge balance and pipette capacitance neutralization were not used. A liquid junction potential of approximately −10 mV was not corrected.
Whole-cell current-clamp recordings were initiated to examine action potential responses and resulting depolarizing afterpotentials [DAPs (1,2,25)] in cells isolated from animals at different ages under varying hormone treatments. The majority of cells were firing (resting membrane potential <−50 to −55 mV) when whole-cell, current-clamp mode was achieved. Cells were hyperpolarized to −55 mV to prevent spontaneous activity; cells requiring greater than 20 pA of current to hyperpolarize below threshold were discarded. Depolarizing current pulses were delivered at 3.5 msec, 200–220 pA, to evoke a single-action potential, or 200 msec, 15–30 pA, to evoke a string of action potentials. Data were digitized at 20 kHz and low-pass filtered at 4 kHz. All amplitude measurements were taken in reference to the prepulse baseline. Latency and duration measurements were taken at the time of pulse initiation.
Whole-cell voltage-clamp recordings were made to examine the currents underlying the DAP [IADP) (2) in animals of different ages. Cells were clamped at −60 mV. Single-action potentials were simulated with an 80-mV, 2-msec square voltage pulse (2,33). The stimulus was repeated 35 times at 1-sec intervals, and responses were averaged. Amplitude responses were measured from the prepulse baseline. In addition, whole-cell inward and outward currents were evoked in cells from animals at different ages and hormone treatments with a 12-msec, 10-mV per step depolarizing voltage protocol.
Immunohistochemistry
Three young (3 months) and six old (13–15 months) intact female mice were anesthetized with isoflurane. Animals were perfused with heparinized 0.1 m PBS (pH 7.4) for 15 min with a peristaltic pump. This was followed by 4% paraformaldehyde-0.1 m PBS (pH 7.4) for an additional 15 min. The forebrain was removed and postfixed in 4% paraformaldehyde-0.1 m PBS for 2 h at 4 C. Tissue encompassing the septum, diagonal band of Broca, and medial preoptic area was washed in PBS and cut on a vibratome (VT1000S; Leica, Bannockburn, IL) at 40 μm. Every section taken from each animal was processed for immunohistochemistry. Free-floating tissue sections were washed in PBS and blocked in 10% normal donkey serum (NDS)-0.3% Triton X-100-PBS for 2 h. Sections were incubated at room temperature overnight on an orbital shaker in anti-LHRH (1:500, catalog no. 20075; Immunostar, Hudson, WI) in 1% NDS-0.3% Triton X-100-PBS. In preliminary experiments, sections were incubated without the primary antibody to confirm specificity. After 3 × 15-min PBS wash, sections were incubated for 120 min with Cy3-conjugated donkey antirabbit IgG (1:200; Vector Laboratories, Burlingame, CA) in 1% NDS-0.3% Triton X-100-PBS at room temperature. Sections were washed in PBS (2 × 15 min), mounted on Gelatin-coated slides, air dried for 2 h, and coverslipped using Prolong Gold (Invitrogen, Carlsbad, CA) mounting medium. Tissue slides containing eGFP and Cy3-conjugated LHRH were examined using an epifluorescent microscope (BX60; Olympus, Center Valley, PA) mounted with appropriate filter sets and camera. Pictures encompassing all labeling were taken of all slices. Green (eGFP) and red (Cy3) cells were counted for each tissue slice by an individual blind to the age of the animal. Only bright, distinctly positive cells were counted. Data are presented as means ± sem of positively stained eGFP and Cy3 [GnRH-immunoreactive (IR)] cells.
Data analysis
Spontaneous activity was analyzed with Spike2 (Cambridge Electronic Design, Cambridge, UK) for spike discrimination and Interlab (34); Cambridge Electronic Design contributed software; this software can be downloaded (http://www.pdn.cam.ac.uk/staff/dyball/index. html) for analysis of interval patterns. Using Interlab, the entropy of the log interspike interval distribution was used as a measure of variability, or the ability of a cell to encode information, and mutual information (MI), generated by plotting each interspike interval against its predecessor, was used as a measurement of spike patterning. Data were generated for spikes, DAPs, IADPs, and whole-cell currents with Clampfit 9.2 (Molecular Devices). Comparisons between ages and hormone treatments were made with one- and two-way ANOVAs and unpaired t tests (as appropriate) for normally distributed data and by Kruskal-Wallis ANOVA on ranks and Mann-Whitney rank sum tests for nonparametric data. The box plot was used to represent data that were not normally distributed, with the boundary of the box indicating the 25th and 75th percentile, and error bars above and below the box indicating the 90th and 10th percentiles. Diamonds indicated the fifth and 95th percentile, if applicable. Post hoc analyses (Holm-Sidak, Dunn’s) were performed when appropriate. All values are expressed as mean ± sem.
Results
Animals
Female breeders in this colony are bred at 8 wk and retired at 9–10 months of age due to a decline in pup production. Mice in this study were taken at 3 months of age to represent young reproductive females, 10 months of age, expected to represent the peripostreproductive phase, and 15–18 months of age, assumed to be postreproductive animals. Vaginal cytology confirmed reproductive status in the three age groups. All young animals were cycling with a distinctive transition from diestrus through proestrus to estrus. Young animals exhibited a predictable, 4- to 5-d cycle and reached estrus two to three times in a 10-d period. The MA animals cycled irregularly, with multiday stretches of cytology consistent with proestrus or metestrus. Only 66% of MA animals reached estrus (once) in a 10-d period. None of the four old animals reached estrus in a 10-d period. Thus, it appears that the MA group is indeed peripostreproductive, with longer, irregular cycles, and the old animals in our studies represent late peripostreproductive to truly postreproductive animals.
To confirm the presence or absence of estradiol after OVX, uterine weights were taken from each animal at the time they were killed. Uterine weights were significantly greater in OVX+E animals (mean OVX = 62.84 ± 4.47 mg, mean OVX+E = 159.43 ± 8.62 mg; P < 0.001; n = 59–66; Table 1) and were also significantly higher in old animals when compared with MA or young animals (P = 0.006; Table 1). Body weight also significantly increased with age [F(2, 13) = 5.98; P = 0.027; n = 5–6 per age], but there was no difference in body weight between MA and old animals (P = 0.925). Estradiol appeared to have a similar stimulatory effect on uterine growth at all ages (percent increase: young = 313%; MA = 228%, old = 226%).
Table 1.
Effect of estradiol on uterine weights
| Age | OVX | OVX+E |
|---|---|---|
| Young | 46.27 ± 2.98 mg | 143.83 ± 5.81 mga |
| n = 34 | n = 41 | |
| Middle aged | 57.19 ± 8.12 mg | 130.42 ± 12.53 mga |
| n = 10 | n = 12 | |
| Old | 104.17 ± 9.07 mgb | 235.42 ± 30.73 mga,b |
| n = 15 | n = 13 |
Estradiol significantly increases uterine weight at all ages.
Significantly different from OVX animals (P < 0.001, Mann-Whitney rank sum test).
Significantly different from middle-aged or young animals (P = 0.006, one-way ANOVA on ranks).
Serum estradiol
Serum from three young OVX, three young OVX+E, and three old OVX+E mice was assayed for estradiol. As expected, very low estradiol was found in the serum of OVX animals (6.67 ± 1.61 pg/ml). Serum estradiol was significantly higher in young (14.32 ± 1.79 pg/ml; P = 0.024) and old (22.93 ± 4.01 pg/ml; P = 0.02) OVX+E animals but was lower than concentrations reported previously (29).
Slices and cells
GnRH-eGFP neurons were easily identified (Fig. 1) in 400-μm slices before dissociation. Because variability in the expression of fluorescence was noted within and between age groups, a score (0–4) was assigned to each animal based on the number and relative brightness of cells (fluorescent score based on the average number of brightly fluorescent cells per slice: <1 = 0; 1–8 = 1; 9–15 = 2; 16–22 = 3, ≥23 = 4). There was a significant decrease in average score in MA (Fig. 1B) and old animals (Fig. 1C), compared with young animals (Fig. 1A; P < 0.01; Table 2). In addition, an increase in score was noted with estradiol treatment in the young age group (Table 2). No significant differences were seen between hormone treatments in MA or old animals. In agreement with fluorescent scores, isolated eGFP neurons from older animals were typically fewer in number and often appeared dim, although overall level of fluorescence was not quantified. The number of isolated eGFP neurons obtained from an animal of a given fluorescent score did not vary with age.
Figure 1.
GnRH-eGFP neurons decrease with age in the slice. A–C, Representative images of 400-μm-thick coronal brain slices taken through the septum and diagonal band of Broca, demonstrating eGFP-labeled GnRH neurons. Slices were taken from a 3-month-old OVX animal (A); a 10-month-old OVX+E animal (B); and a 16-month-old OVX+E animal (C). Fluorescent GnRH neurons, with processes, are evident at each age. However, the number of fluorescent cells and processes decreases with age. Scale bar in C applies to all images. D, The mean number of GnRH-IR cells counted per animal decreases insignificantly with age. E, The percentage of GnRH-IR neurons that are eGFP positive decreases significantly with age (a, P = 0.035 when compared with young). All animals were intact females (n = 3 young, six old animals).
Table 2.
Effect of age and estradiol on the qualitative score of eGFP in the 400-μm slice
| Age | OVX | OVX+E |
|---|---|---|
| Young | 1.83 ± 0.27 | 2.82 ± 0.30a |
| n = 14 | n = 11 | |
| Middle aged | 1.53 ± 0.34b | 0.87 ± 0.48b |
| n = 8 | n = 4 | |
| Old | 1.00 ± 0.43b | 1.46 ± 0.39b |
| n = 5 | n = 6 |
The qualitative score significantly decreases with increasing age.
Significantly different from young OVX animals (P = 0.02, t test).
Significantly different from young animals (P < 0.01, one-way ANOVA).
To further explore the qualitative decrease in eGFP expression with age, immunohistochemistry for GnRH was performed on a limited number of young and old intact female mice. Mean numbers were not significantly different between young and old animals for eGFP or GnRH expression (between young and old: eGFP, P = 0.13; GnRH-IR, P = 0.33; n = 3–6/group; Fig. 1D). However, the percent of GnRH-positive neurons expressing eGFP was significantly decreased in old animals (P = 0.035; n = 3–6/group; Fig 1E).
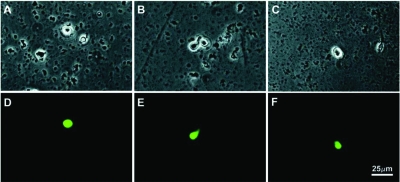
Isolated cells from all ages (Fig. 2) did not appear to differ in size or morphology and were typical of neurons cultured less than 24 h (25). Cells from all ages of animal appeared compact and phase bright and had membrane properties comparable with previous studies of isolated GnRH neurons from younger animals (25). Membrane capacitance was typical for isolated GnRH neurons (25) and did not vary with age or hormone status, although there was a trend toward decreased capacitance with increasing age (Table 3). Input resistance also did not vary with age (P = 0.33; Table 3) but was affected by hormone status (P = 0.003, Table 4). This was an unexpected finding because estradiol does not affect input resistance in GnRH neurons from slice recordings (29).
Figure 2.
Fluorescent GnRH neurons can be isolated from old, as well as young, animals. Phase-contrast (A–C) and corresponding fluorescent (D–F) images of isolated GnRH-eGFP neurons are shown. A/D are from a 3-month-old OVX animal; B/D are from a 10-month-old OVX animal; C/F are from a 15-month-old OVX animal. Scale bar in F applies to all images.
Table 3.
Effect of age on isolated GnRH neurons
| Property | Young | Middle aged | Old |
|---|---|---|---|
| Membrane properties | |||
| Capacitance | 9.48 ± 0.27 pF | 8.87 ± 0.37 pF | 8.52 ± 0.43 pF |
| n = 57 | n = 31 | n = 24 | |
| Input resistance | 2.22 ± 0.14 GΩ | 1.86 ± 0.18 GΩ | 2.23 ± 0.25 GΩ |
| n = 11 | n = 7 | n = 4 | |
| Action potential | |||
| Amplitude | 123.3 ± 2.7 mV | 115.4 ± 5.3 mV | 115.6 ± 3.2 mV |
| n = 13 | n = 10 | n = 13 | |
| Duration (at 1/2 amplitude) | 1.56 ± 0.08 ms | 1.60 ± 0.13 ms | 1.55 ± 0.42 ms |
| n = 10 | n = 13 | ||
| Spontaneous firing | |||
| Frequency | 3.73 ± 0.31 Hza | 2.79 ± 0.46 Hz | 2.50 ± 0.35 Hz |
| n = 28 | n = 12 | n = 14 | |
| Entropy | 7.04 ± 0.07 bits | 7.26 ± 0.11 bits | 7.27 ± 0.10 bits |
| Mutual information | 0.12 ± 0.02 bitsb | 0.07 ± 0.03 bits | 0.05 ± 0.03 bits |
| n = 28 | n = 12 | n = 14 | |
| Depolarizing afterpotentials | |||
| 3.5-msec pulse | 5.32 ± 0.51 mV | 3.57 ± 0.6 mV | 4.09 ± 0.53 mV |
| n = 14 | n = 10 | n = 14 | |
| 200-msec pulse | 8.12 ± 0.41 mVc | 5.17 ± 0.62 mV | 5.23 ± 0.59 mV |
| n = 26 | n = 12 | n = 12 |
Age does not affect typical membrane measurements of health but does affect spontaneous firing and DAPs.
Significantly different from middle aged and old (P = 0.031, AVOVA on ranks).
Significantly different from middle aged and old (P = 0.046, Spearman correlation).
Significantly different from middle aged and old (P < 0.001, two-way ANOVA).
Table 4.
Effect of estradiol in the animal on isolated GnRH neurons
| Property | OVX | OVX+E |
|---|---|---|
| Membrane properties | ||
| Capacitance | 8.90 ± 0.30 pF | 9.00 ± 0.29 pF |
| n = 58 | n = 54 | |
| Input resistance | 1.73 ± 0.12 GΩa | 2.48 ± 0.19 GΩ |
| n = 13 | n = 9 | |
| Action potential | ||
| Amplitude | 119.34 ± 3.1 mV | 116.73 ± 3.1 mV |
| n = 18 | n = 18 | |
| Duration (at 1/2 amplitude) | 1.57 ± 0.07 msec | 1.56 ± 0.07 msec |
| n = 18 | n = 18 | |
| Whole-cell current density | ||
| Inward (picoampere per picofarad) | −751.9 ± 37.3b | −593.0 ± 33.6 |
| n = 31 | n = 36 |
Estradiol affects input resistance and whole-cell inward current density in the isolated cell preparation.
Significantly different from OVX+E (P = 0.003, two-way ANOVA).
Significantly different from OVX+E (P = 0.002, two-way ANOVA).
Spontaneous firing
The measurement of spike coding in neurons is a challenge. Recently it has been shown that the entropy of the log interval distribution of interspike interval, and the mutual information between adjacent log intervals, can provide objective measures of the capacity of a cell to encode information (34,35,36,37,38). To determine whether spontaneous activity in isolated neurons varies with age, cell-attached recordings of greater than 5 min in length were analyzed for firing frequency, entropy (a measure of interspike interval variability), and MI (a measure of firing pattern). Spike patterning, or interval coding, was quantified by plotting each interspike interval against its predecessor. Isolated neurons fired spontaneously at all ages studied; no GnRH neurons studied were silent. Variability in rate and pattern of firing was noted between neurons from a single mouse as well as between mice, as reported previously in isolated neurons (25). However, most recordings exhibited the short burst pattern that is characteristic of GnRH neurons (Fig. 3, A and B) (25). Contrary to data obtained from GnRH neurons in slices (26,39,40), hormone treatment had no significant effect on overall spike frequency (P = 0.76, n = 25–29), entropy (P = 0.76, n = 25–29), or MI (P = 0.40, n = 25–29), so data were pooled for the two treatments. A significant decrease in firing rate (Fig. 3C and Table 3) and mutual information (Fig. 3, E and F, and Table 3) were noted with increasing age. Entropy was not significantly affected by age, although there was a trend toward increasing variability with increasing age (Fig. 3D and Table 3). Data comparing spike frequency in neurons from OVX+E animals incubated overnight in 1 nm estradiol + 0.1% EtOH with those exposed to 0.1% EtOH only also showed no differences in spike frequency (P = 0.46, n = 3–9).
Figure 3.
Spontaneous firing patterns during aging in GnRH neurons. Four-minute cell-attached recordings from a 3-month-old (A1) and 18-month-old (B1) animal demonstrate that cells fire spontaneously at all ages studied. Each trace is expanded to demonstrate a 24-sec (A2, B2,) and 10-sec (A3, B3, of the first burst of firing in A2/B2) section. Although the mean spike frequency was similar between the two traces (young = 2.68 Hz, old = 2.56 Hz), the trace from the old animal demonstrates increased entropy and decreased mutual information. C, A comparison of mean spike frequency reveals a significant decrease during aging (a, P < 0.031 when compared with young animals). D, A comparison of mean entropy, a measure of variability, reveals no significant differences, although there is a trend toward increasing variability with age. E, MI, a measure of spike patterning, shows nonsignificant decreases with age when compared with an ANOVA on ranks. F, A scatter plot of MI, analyzed with Spearman’s rank moment, reveals a significant decrease with age (a, P = 0.046). Because there was no difference between OVX and OVX+E animals, data from the two treatments were pooled in C–F (n = 12–28 neurons/group).
Recordings from GnRH neurons in slice preparations have demonstrated that activity can be dependent on time of day and hormone treatment (10). To further address the effect of time of day on spontaneous activity, measures of activity were compared between morning (0900–1300 h) recordings and afternoon (1400–1800 h) recordings in young adult OVX and OVX+E animals. All animals were killed between 1100 and 1200 h on the previous day. A significant difference was observed between overall morning and evening spike frequency [morning = 3.31 ± 0.42 Hz; evening = 5.29 ± 0.62 Hz; F (1, 18) = 6.94; P = 0.017; n = 7–15] but not between either treatment group [OVX = 4.41 ± 0.54 Hz; OVX+E = 4.19 ± 0.52 Hz; F (1, 18) = 0.09; P = 0.77; n = 10–12; two-way ANOVA, no interaction].
Response to current pulses
Single action potential responses to a 3.5-msec, 200-pA current pulse (Fig. 4A) were compared between age and hormone treatments. No significant differences were observed in action potential amplitude (Fig. 4B and Tables 3 and 4), duration at half-amplitude (Fig. 4C and Tables 3 and 4), or afterhyperpolarization amplitude (Fig. 4D; n = 34) when examining either age or hormone treatment. The mean number of spikes generated by a 200-msec, 15–25 pA current pulse from a membrane potential of −55 mV (Fig. 5A) varied both with age and hormone treatment. The number of spikes per 200-msec pulse was significantly larger in neurons from old animals [F (2,33) = 13.04; P < 0.001; n = 12–16] and OVX+E animals [Fig. 5B; F (1, 33) = 6.33; P = 0.02; n = 20; two-way ANOVA, no interaction].
Figure 4.
Analysis of action potentials during aging in GnRH neurons. A, Representative whole-cell current-clamp traces (averaged from three to five records) recorded from GnRH neurons isolated from young, MA, and old animals are overlaid. Action potentials were evoked by a 3.5-msec, 200-pA current pulse. No significant differences in action potential amplitude (B), mean duration at half-amplitude (C), or AHP; (D) were observed. Data from OVX and OVX+E animals were pooled for demonstration because no significant differences were observed (n = 10–13/group).
Figure 5.
Response to a 200-msec current pulse. A, A 200-msec, 15-pA current pulse evoked multiple spikes and a DAP at every age. Representative whole-cell current-clamp traces (averaged from two records), recorded from GnRH neurons isolated from young, MA, and old animals, are overlaid. Note the decrease in DAP amplitude with age. B, A comparison of mean spike number per 200-msec current pulse demonstrates a significant increase in spike number with age (a, P < 0.001, compared with young and MA) and estradiol treatment (b, P = 0.02, compared with control animals; n = 6–8/group. C, A comparison of mean DAP amplitude after a 200-msec current pulse reveals a significant decrease with age (a, P < 0.001, compared with MA and old animals; n = 6–15/group). co, OVX; e2, OVX+E. D, A comparison of mean DAP amplitude after a single action potential reveals a decrease with age that is nonsignificant (P = 0.26; n = 5–8/group).
DAPs and IADPs
DAPs were evoked by a 200-msec current pulse (Fig. 5A) or a 3.5-msec current pulse that evoked a single action potential (Fig. 4A). Mean DAP amplitude was determined from data generated at −55 to −60 mV. DAP amplitude after a 200-ms current pulse was significantly decreased in cells from MA and old animals, compared with neurons from young adults (Fig. 5C and Table 3). No significant age differences were found in DAP amplitude after a single action potential (Fig. 5D and Table 3), although there was a trend toward decreased DAP amplitude with increased age. There was no significant effect of hormone treatment on DAP amplitude. An analysis of latency to peak DAP after a 200-msec depolarizing current pulse revealed no significant differences with hormone treatment (P = 0.14; n = 25–27) or age (ANOVA on ranks; P = 0.38; n = 11–28). Estradiol in the dish had no significant effect on DAP amplitude (P = 0.38, n = 7/group), so no further studies included estradiol in the dish.
To examine the currents underlying the DAP (IADP), a 2-msec stimulus protocol was used in voltage clamp to simulate a single action potential (2) (Fig. 6A). This stimulus protocol in GnRH neurons evoked a very brief outward current (IOUT), followed by a longer inward current (IADP). The amplitude of IOUT, although reduced in older animals, was not significantly different between cells from different ages (Fig. 6B) or hormone treatments (P = 0.19; n = 26–33), nor did IADP vary significantly with age (Fig. 6C) or hormone treatment (P = 0.08; n = 26–32). These findings were in concordance with the current-clamp data after a single action potential but do not address the changes with age seen after a 200-msec current pulse.
Figure 6.
Current response to a simulated action potential. A, A representative current response to a 2-msec, 80-mV voltage pulse (trace = average of 35 records), recorded from a GnRH neuron isolated from a 10-month-old OVX animal. A fast IOUT is followed by a slower IADP. B, Mean IOUT after a single action potential stimulus for each treatment group. There were no significant differences between ages or hormone treatment (n = 8–11/group). C, Mean IADP after a single action potential stimulus for each treatment group. There were no significant differences between ages or hormone treatment (n = 8–11/group). co, OVX; e2, OVX+E.
Whole-cell currents
A series of depolarizing steps (12 msec) was applied to neurons in voltage clamp to establish whole-cell current-voltage relationships (Fig. 7, A and B). An analysis of inward whole-cell currents at 0 mV demonstrated that increasing age resulted in significantly decreased currents [F (2, 61) = 4.63; P = 0.01; n = 20–26], an effect that was no longer apparent (Fig. 7C) when currents were converted to current density (current/capacitance). However, current density was significantly decreased in cells from animals treated with estradiol (Fig. 7C and Table 4). Mean outward currents were analyzed at +50 mV. Similar to the results of inward current analysis, outward currents decreased significantly with age [F (2, 61) = 3.82; P = 0.03; n = 20–26], but current density was not significantly affected by age or hormone status (Fig. 7D).
Figure 7.
Whole-cell currents in GnRH neurons. Whole-cell inward and outward currents were recorded in GnRH neurons isolated from young and aged mice. A, Representative trace of the current response to a series of voltage steps in a neuron from a young animal. B, Current density-voltage curve of mean inward currents. There is a trend toward decreased current with age. An analysis of mean current density at 0 mV (C) shows that neurons isolated from OVX+E animals demonstrate significantly smaller currents (a, P = 0.002; n = 8–14/group). Analysis of mean outward current density measured at +50 mV (D) demonstrates no significant differences with age or hormone treatment (n = 8–14/group). co, OVX; e2, OVX+E.
Discussion
A number of significant differences were found between GnRH-eGFP neurons isolated from young and older animals. Isolated cells from all ages studied exhibited characteristic, endogenously generated activity (25), yet neurons isolated from old animals demonstrated a reduced frequency of spontaneous spikes and decreased patterning of activity. In addition, DAPs after a 200-msec stimulus were reduced in old cells, even though the stimulus evoked more spikes in aged animals.
Results from these studies demonstrate that GnRH neurons from aged female mice retain the ability to generate spontaneous spikes and maintain the characteristic bursting pattern seen in recordings from isolated cells and slices (25,26,39,40). Although the intrinsic generator of GnRH activity is still intact, there is a significant decrease in spontaneous spike frequency with advancing age, accompanied by increased variability in activity and decreased pattern generation. Measures of spike irregularity and patterns in spike activity represent the ability of the cell to encode information (34,35,38). Our data suggest that aged GnRH neurons lose the ability to precisely encode information, which could lead to the attenuated GnRH pulses seen in aging animals.
GnRH neurons tend to fire in bursts of several action potentials (25,26,28,40). GnRH neurons from aged animals demonstrated a significant decrease in the amplitude of the DAP after a brief burst. It has been shown that very small changes in DAP amplitude can affect subsequent activity (2), and, conversely, that bursting activity can modulate DAP amplitude (2). Thus, smaller DAPs after a burst could lead to briefer bursts yet smaller DAPs, a decreased frequency of firing and the loss of patterned bursting seen in cell-attached experiments. In addition, we have shown that DAPs uniquely position the neuron to fire a prolonged episode of activity in response to a brief excitatory input (one or a few excitatory postsynaptic potentials) (1). Thus, a decrease in DAP amplitude during aging could produce a blunted response to excitatory inputs and reduced spike patterning, resulting in smaller, irregular discharges that could lead to dysregulated GnRH release.
Significant differences were seen in properties of cells from animals maintained 5–7 d with the constant presence (OVX+E) or absence (OVX) of estradiol. As seen for age, the presence of estradiol in the animal increased the number of spikes evoked by a 200-msec current pulse. Input resistance also increased in GnRH neurons from estradiol-treated animals, which could account for the increased spike response. The inward whole-cell current was significantly reduced in OVX+E animals, although the whole-cell outward current was not affected. We found no significant effects of estradiol on DAP amplitude, DAP latency to peak after a 200-msec current pulse, or IADP amplitude at any age studied. This is in contrast to results from experiments using the slice preparation (2) that demonstrated an increase in DAP and IADP amplitude in GnRH neurons from young OVX+E animals. This could be due to a lower serum estradiol in our animals (29) but could also suggest that DAP amplitude may be modulated indirectly because our neurons were removed from inputs for many hours before recording.
This is the first report of an inward current being modulated by estradiol in GnRH neurons. We currently do not know the mechanism of this modulation or whether acute estrogen withdrawal in the dish impacts inward currents. Chu and Moenter (2) demonstrated that a tetrodotoxin-sensitive sodium current contributes to the IADP. Under our experimental conditions, this current was not modulated by estradiol. Further studies are needed to determine the inward currents modulated by chronic estradiol, the mechanisms involved, and their putative role in GnRH neurons.
It is interesting that even hours after neurons are isolated from the animal, characteristic spontaneous bursts of activity are present. However, the influence of hormones on spontaneous activity appears to be lost in this preparation. A number of studies using the slice preparation have shown that estradiol increases the time between episodes, although it does not modulate short-term bursts (26,39,40). In contrast, we saw no effect of estradiol on spike frequency, variability, or patterning at any age. There may be a number of reasons for this discrepancy. Our serum estradiol concentration may have been too low to impact activity. Perhaps the effects of estradiol are not evident within the short time (5 min) of activity analyzed. Also, the effects of in vivo estradiol on activity regulation may be lost during the overnight culture of our neurons, although adding estradiol to the dish did not have an impact on firing characteristics. In addition, some effects of estradiol appear to be modulated by inputs that are no longer present in our preparation (26). The loss of external (hormonal or synaptic) regulation of activity may explain the significant increase in spike frequency seen in afternoon recordings. Although increases in afternoon GnRH activity are hormone related in the slice (10), we saw increases in activity that were not hormone specific. This increase is possibly due to a decreased regulation of spike generation as time in culture increased. However, it could reflect the ability of the cell, even after hours in culture, to respond to internal time-of-day cues. Future studies will be performed to address this question.
There are some obvious differences between dissociated neurons and GnRH neurons recorded from brain slices. Notably, dissociated neurons lose or retract their processes. However, the dissociated preparation offers some distinct advantages, including the ability to record from neurons from any age of animal, a very difficult undertaking in slice preparations. In addition, tetrodotoxin is not used to artificially isolate cells from the network, and space clamp issues are avoided during physiological experiments (41). The neurons isolated in this study, whether from young or old animals, were phase bright and had membrane and action potential characteristics indicative of healthy adult isolated GnRH neurons (25). However, GnRH neurons in general tend to be a heterogeneous group (25,42,43). It is possible that our dissociation procedure selects for a particularly hardy population. We have not characterized the neurons in the present study for known variable molecular markers, such as estrogen receptors (44,45,46), although we do see heterogeneity in glutamate current amplitude responses in neurons from both young and old animals (personal observation). The fact that membrane characteristics did not change with age supports the strength of this procedure for aging studies.
Because rodents do not have menstrual cycles, they do not undergo menopause in the strictest sense, yet reproductive senescence in female rodents is well documented. Ovarian follicular depletion has been reported in rodents as well as humans (14). However, a number of changes taking place at the level of the hypothalamus (12) before the loss of reproductive cyclicity suggest alterations in the integrity of the GnRH pulse generator independent of ovarian aging. Numerous recent studies have advanced our knowledge of hypothalamic regulation during aging (47,48,49,50,51). Undoubtedly, some changes seen with increasing age are due to changes in the many neurotransmitter systems that regulate GnRH neurons (51). However, it is now clear that there are changes in GnRH neurons themselves during the aging process. It had been shown previously that there is an altered pattern of diurnal GnRH gene expression in middle-aged rats (52) as well as decreased Fos expression during the LH surge (21,22,23,24). Now we have been able to demonstrate changes in the physiology of GnRH neurons isolated from old mice, including decreases in firing frequency and DAP amplitude. This is the first time that changes in GnRH function at the level of the individual neuron during aging have been documented. Alterations in the ability to respond to inputs and generate coordinated activity could lead to attenuated pulses of hormone release, blunted responses to hormones, and decreased reproductive function. Studies are underway to determine specific channel alterations with age that may lead to our observed changes.
Acknowledgments
We thank S. Moenter (University of Virginia, Charlottesville, VA) for providing the original GnRH-eGFP transgenic mice and G. S. Bhumbra and R. E. J. Dyball (University of Cambridge, Cambridge, UK) for advice on the analysis of spontaneous activity. We also thank Ms. Michelle Mooney for her technical assistance.
Footnotes
This work was supported by National Institutes of Health Grant AG023139 (to M.C.K.-K.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online June 26, 2008
Abbreviations: DAP, Depolarizing afterpotential; E2, β-estradiol; eGFP, enhanced green fluorescent protein; IADP, current underlying the DAP; IOUT, outward current; IR, immunoreactive; MA, middle aged; MI, mutual information; NDS, normal donkey serum; OVX, ovariectomized.
References
- Kuehl-Kovarik MC, Partin KM, Handa RJ, Dudek FE 2005 Spike-dependent depolarizing afterpotentials contribute to endogenous bursting in gonadotropin releasing hormone neurons. Neuroscience 134:295–300 [DOI] [PubMed] [Google Scholar]
- Chu Z, Moenter SM 2006 Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci 26:11961–11973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Moenter SM 2005 Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci 25:5740–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA 2005 Endogenous γ-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 146:5374–5379 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM 2007 Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 27:1913–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol Cell Endocrinol 254–255:32–38 [DOI] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Ronnekleiv OK, Kelly MJ 2007 Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27:10153–10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka J, Moenter SM 2006 Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod 74:931–937 [DOI] [PubMed] [Google Scholar]
- Pielecka J, Quaynor SD, Moenter SM 2006 Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 147:1474–1479 [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM 2005 Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102:15682–15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Terasawa E 2005 Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology 146:4312–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, Bottner M, Rosewell KL 2002 Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res 57:235–256 [DOI] [PubMed] [Google Scholar]
- Rubin BS 2000 Hypothalamic alterations and reproductive aging in female rats: evidence of altered luteinizing hormone-releasing hormone neuronal function. Biol Reprod 63:968–976 [DOI] [PubMed] [Google Scholar]
- Wise PM 1999 Neuroendocrine modulation of the “menopause”: insights into the aging brain. Am J Physiol 277:E965–E970 [DOI] [PubMed] [Google Scholar]
- Hall JE, Lavoie HB, Marsh EE, Martin KA 2000 Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J Clin Endocrinol Metab 85:1794–1800 [DOI] [PubMed] [Google Scholar]
- Rubin BS, Bridges RS 1989 Alterations in luteinizing hormone-releasing hormone release from the mediobasal hypothalamus of ovariectomized, steroid-primed middle-aged rats as measured by push-pull perfusion. Neuroendocrinology 49:225–232 [DOI] [PubMed] [Google Scholar]
- Miller MM, Joshi D, Billiar RB, Nelson JF 1990 Loss of LH-RH neurons in the rostral forebrain of old female C57BL/6J mice. Neurobiol Aging 11:217–221 [DOI] [PubMed] [Google Scholar]
- Funabashi T, Kimura F 1995 The number of luteinizing hormone-releasing hormone immunoreactive neurons is significantly decreased in the forebrain of old-aged female rats. Neurosci Lett 189:85–88 [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Finch CE 1986 LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: no loss up to middle age. Neurobiol Aging 7:45–48 [DOI] [PubMed] [Google Scholar]
- Miller BH, Gore AC 2002 N-Methyl-d-aspartate receptor subunit expression in GnRH neurons changes during reproductive senescence in the female rat. Endocrinology 143:3568–3574 [DOI] [PubMed] [Google Scholar]
- Gore AC, Oung T, Yung S, Flagg RA, Woller MJ 2000 Neuroendocrine mechanisms for reproductive senescence in the female rat: gonadotropin-releasing hormone neurons. Endocrine 13:315–323 [DOI] [PubMed] [Google Scholar]
- Le WW, Wise PM, Murphy AZ, Coolen LM, Hoffman GE 2001 Parallel declines in Fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology 142:4976–4982 [DOI] [PubMed] [Google Scholar]
- Lloyd JM, Hoffman GE, Wise PM 1994 Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology 134:1800–1805 [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lee CE, King JC 1994 A reduced proportion of luteinizing hormone (LH)-releasing hormone neurons express Fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biol Reprod 51:1264–1272 [DOI] [PubMed] [Google Scholar]
- Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM 2002 Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci 22:2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM 2002 Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 143:2284–2292 [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Geusz ME, Herzog ED, Pitts GR, Moenter SM 2001 Long-term recordings of networks of immortalized GnRH neurons reveal episodic patterns of electrical activity. J Neurophysiol 86:86–93 [DOI] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM 2000 Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Moenter SM 2002 Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2255–2265 [DOI] [PubMed] [Google Scholar]
- Ruhlen RL, Howdeshell KL, Mao J, Taylor JA, Bronson FH, Newbold RR, Welshons WV, vom Saal FS 2008 Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the “fetal estrogenization syndrome” and obesity in CD-1 mice. Environ Health Perspect 116:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Quadagno DM, Even MD, Keisler LW, Keisler DH, Khan S 1990 Paradoxical effects of maternal stress on fetal steroids and postnatal reproductive traits in female mice from different intrauterine positions. Biol Reprod 43:751–761 [DOI] [PubMed] [Google Scholar]
- Kuehl-Kovarik MC, Partin KM, Magnusson KR 2003 Acute dissociation for analyses of NMDA receptor function in cortical neurons during aging. J Neurosci Methods 129:11–17 [DOI] [PubMed] [Google Scholar]
- Mitterdorfer J, Bean BP 2002 Potassium currents during the action potential of hippocampal CA3 neurons. J Neurosci 22:10106–10115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumbra GS, Dyball RE 2004 Measuring spike coding in the rat supraoptic nucleus. J Physiol 555:281–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumbra GS, Inyushkin AN, Dyball RE 2004 Assessment of spike activity in the supraoptic nucleus. J Neuroendocrinol 16:390–397 [DOI] [PubMed] [Google Scholar]
- Bhumbra GS, Inyushkin AN, Saeb-Parsy K, Hon A, Dyball RE 2005 Rhythmic changes in spike coding in the rat suprachiasmatic nucleus. J Physiol 563:291–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumbra GS, Inyushkin AN, Syrimi M, Dyball RE 2005 Spike coding during osmotic stimulation of the rat supraoptic nucleus. J Physiol 569:257–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inyushkin AN, Bhumbra GS, Gonzalez JA, Dyball RE 2007 Melatonin modulates spike coding in the rat suprachiasmatic nucleus. J Neuroendocrinol 19:671–681 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Defazio RA, Straume M, Nunemaker CS 2003 Steroid regulation of GnRH neurons. Ann NY Acad Sci 1007:143–152 [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, Straume M, DeFazio RA, Moenter SM 2003 Gonadotropin-releasing hormone neurons generate interacting rhythms in multiple time domains. Endocrinology 144:823–831 [DOI] [PubMed] [Google Scholar]
- Bean BP 2007 The action potential in mammalian central neurons. Nat Rev Neurosci 8:451–465 [DOI] [PubMed] [Google Scholar]
- Herbison AE 2006 Physiology of the GnRH neuronal network. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. 3rd ed. San Diego: Academic Press; 1415–1482 [Google Scholar]
- Liu X, Lee K, Herbison AE 15 May 2008 Kisspeptin excites gonadotropin-releasing hormone (GnRH) neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 10.1210/en.2008-0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner DC, Dufourny L 2005 Oestrogen receptor β-immunoreactive neurones in the ovine hypothalamus: distribution and colocalisation with gonadotropin-releasing hormone. J Neuroendocrinol 17:29–39 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Szlavik N, Keller E, Merchenthaler I, Liposits Z 2007 Gonadotropin-releasing hormone neurons express estrogen receptor-β. J Clin Endocrinol Metab 92:2827–2830 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z 2001 Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142:3261–3264 [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Rosewell KL, Wise PM 2005 Suppression of vasoactive intestinal polypeptide in the suprachiasmatic nucleus leads to aging-like alterations in cAMP rhythms and activation of gonadotropin-releasing hormone neurons. J Neurosci 25:62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhold LM, Wise PM 2006 Vasoactive intestinal polypeptide regulates dynamic changes in astrocyte morphometry: impact on gonadotropin-releasing hormone neurons. Endocrinology 147:2197–2202 [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB 2005 The aging reproductive neuroendocrine axis. Steroids 70:273–283 [DOI] [PubMed] [Google Scholar]
- Gore AC 2007 Is reproductive ageing controlled by the brain? J Neuroendocrinol 19:667–668 [DOI] [PubMed] [Google Scholar]
- Yin W, Gore AC 2006 Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reproduction 131:403–414 [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lee CE, Ohtomo M, King JC 1997 Luteinizing hormone-releasing hormone gene expression differs in young and middle-aged females on the day of a steroid-induced LH surge. Brain Res 770:267–276 [DOI] [PubMed] [Google Scholar]