Abstract
In vitro studies reveal that nuclear receptor coactivators enhance the transcriptional activity of steroid receptors, including estrogen (ER) and progestin receptors (PR), through ligand-dependent interactions. Whereas work from our laboratory and others shows that steroid receptor coactivator-1 (SRC-1) is essential for efficient ER and PR action in brain, very little is known about receptor-coactivator interactions in brain. In the present studies, pull-down assays were used to test the hypotheses that SRC-1 from hypothalamic and hippocampal tissue physically associate with recombinant PR or ER in a ligand-dependent manner. SRC-1, from hypothalamus or hippocampus, interacted with PR-A and PR-B in the presence of an agonist, but not in the absence of ligand or in the presence of a selective PR modulator, RU486. Interestingly, SRC-1 from brain associated more with PR-B, the stronger transcriptional activator, than with PR-A. In addition, SRC-1 from brain, which was confirmed by mass spectrometry, interacted with ERα and ERβ in the presence of agonist but not when unliganded or in the presence of the selective ER modulator, tamoxifen. Furthermore, SRC-1 from hypothalamus, but not hippocampus, interacted more with ERα than ERβ, suggesting distinct expression patterns of other cofactors in these brain regions. These findings suggest that interactions of SRC-1 from brain with PR and ER are dependent on ligand, receptor subtype, and brain region to manifest the pleiotropic functional consequences that underlie steroid-regulated behaviors. The present findings reveal distinct contrasts with previous cell culture studies and emphasize the importance of studying receptor-coactivator interactions using biologically relevant tissue.
THE STEROID HORMONES, estradiol and progesterone, exert many of their effects on reproductive behavior and physiology by binding to their respective intracellular receptors in specific brain regions (1,2,3). Intracellular estrogen receptors (ER) exist in two forms, ERα and ERβ, which are transcribed from different genes (4,5,6). These subtypes differ in their functions (7), abilities to bind different ligands (8,9,10,11), and distribution in brain (12,13,14,15,16). In addition, cell culture experiments indicate that ERα is a stronger transcriptional activator than ERβ due to differences in the activation function (AF)-1 region of the amino terminus (17). In most species, progestin receptors (PR) are expressed in two forms; the full-length PR-B and the truncated PR-A, which are encoded by the same gene but are under the regulation of different promoters (18,19). In vitro studies indicate that human PR-B is a stronger transcriptional activator than PR-A (20,21,22,23,24), due to an additional AF domain in the N terminus of PR-B (25,26). These two PR isoforms appear to have distinct functions in reproductive behavior and physiology (27,28,29,30).
Nuclear receptor coactivators dramatically enhance the transcriptional activity of steroid receptors in vitro, including ER and PR (31,32,33). In addition to early models of nuclear receptor coactivators functioning as a bridge between receptors and the general transcriptional machinery, nuclear receptor coactivators are thought to contribute to nuclear receptor transcription through a variety of processes, including phosphorylation, methylation, acetylation, and chromatin remodeling (32,34,35,36). The first steroid receptor coactivator to be cloned was steroid receptor coactivator (SRC)-1 [also known as nuclear receptor coactivator (NCoA)-1] (33), which was later found to be a member of a larger family of p160 proteins that includes SRC-2 (glucocorticoid receptor-interacting protein, transcription intermediary factor, and NCoA-2) (37) and SRC-3 (amplified in breast cancer 1, TRAM-1, p/CIP, activator of thyroid and retinoic acid receptor, and RAC3) (38). Under most conditions, the p160 family and other coactivators physically interact with steroid receptors, including ER and PR, in the presence of an agonist, but not in the absence of ligand or the presence of an antagonist or selective receptor modulators (33,39,40,41,42,43,44) (but cf. Refs. 45,46). It is well established that selective estrogen receptor modulators (SERMs) regulate ER activity in a tissue-specific manner (47). For example, tamoxifen can block ER action through competitive binding or can activate ER, depending on the cellular environment, including the ratio of coactivators and corepressors (48). Using this same rationale, it has been suggested that RU486 is a selective PR modulator (SPRM) (49,50).
A variety of studies have begun to investigate nuclear receptor coactivator function in hormone action in brain. SRC-1 mRNA and protein are expressed at high levels in the rodent hypothalamus, hippocampus, cerebellum, paraventricular nucleus, thalamus, and amygdala (51,52,53,54,55,56,57) (for review see Ref. 58). Moreover, recent work revealed that hypothalamic neurons coexpress ovarian steroid receptors (ER and PR) and SRC-1 (59). In addition, we and others have found that SRC-1 is important for ER and PR action in brain, including regulation of ER transcriptional activity (55,60), hormone-dependent sexual differentiation of the brain (61), and sexual behavior (55,60,61,62,63,64). Finally, the p160 coactivators appear to function in glucocorticoid receptor action in glial cells (65).
Whereas cell culture studies indicate that receptor-coactivator interactions occur in a ligand-dependent manner, it is not known whether coactivators from brain physically associate with receptors. Therefore, we tested the hypothesis that SRC-1, from brain regions rich in steroid receptors, physically associates with steroid receptors in a ligand-dependent manner. To test this hypothesis, we developed pull-down assays using recombinant PR and ER subtypes and SRC-1 from female rat hypothalamus and hippocampus. The present findings are in contrast with those of previous cell culture receptor-coactivator interaction studies and reveal the importance of investigating these interactions using biologically relevant brain tissue. In addition, such studies may lead to the discovery of new cofactors that modulate steroid receptor action in brain.
Materials and Methods
Experimental animals
Adult female (175–200 g) Sprague Dawley rats from Charles River Breeding Laboratories, Inc. (Wilmington, MA) were housed singly in a 14-h light, 10-h dark cycle, with lights off at 1100 h. Animals were given food and water ad libitum. Female rats were anesthetized with ketamine/xylazine cocktail (100 mg ketamine and 18 mg xylazine per 0.75 ml/kg in saline) and ovariectomized. A 1-wk recovery period followed to allow clearing of endogenous hormones. All animals were overdosed with sodium pentobarbitol (89 mg/kg) and chloral hydrate (425 mg/kg) and then decapitated. Hypothalamic and hippocampal (containing a small portion of the cortex dorsal to the Hipp) tissues were dissected out and flash frozen on dry ice. Tissue was then stored at −80 C. All animal procedures were approved by the Institutional Animal Care and Use Committees of Skidmore College and Wellesley College.
Recombinant glutathione-S-transferase (GST)- and Flag-tagged steroid receptors
Recombinant ER and PR fusion proteins were expressed in Spodoptera frugiperda (Sf9) insect cells by the Tissue Culture CORE Facility of the University of Colorado Cancer Center and the Baculovirus/Monoclonal Antibody Facility of the Baylor College of Medicine as described previously (66,67). Briefly, full-length human PR-A or PR-B was fused to a GST tag. Insect cell cultures for PR-GST (viruses kindly provided by David Bain, University of Colorado Health Science Center) were incubated with 200 nm of the PR agonist R5020, 200 nm of the SPRM RU486, or in the absence of PR ligand. Full-length human ERα or ERβ was fused to a Flag tag (viruses kindly provided by Lee Kraus, Cornell University, Ithaca, NY) (67,68). Sf9 cell cultures for ER-Flag were incubated with 200 nm estradiol, 200 nm 4-hydroxytamoxifen, or no ligand.
Tissue preparation
Brain tissue from female rats (n = 54) was pooled in groups of three for each sample and homogenized in buffer [10 mm Tris, 10% glycerol, 400 mm NaCl, 1 mm DTT, 1 mm EDTA (pH 7.4)] with protease inhibitors (1:10 dilution, P2714; Sigma, St. Louis, MO). Samples were incubated on ice for 30 min and then centrifuged for 30 min at 4 C at 12,000 rpm, and supernatants were aliquoted and frozen at −80 C.
PR GST pull-down assay procedure
All procedures were carried out at 4 C. Twenty-five microliters of glutathione Sepharose 4B packed resins (Amersham Biosciences, Uppsala, Sweden) were added to siliconized centrifuged tubes and washed with TG buffer [20 mm Tris-HCl (pH 8.0), plus 10% glycerol] containing 100 mm NaCl (TG + NaCl). The resin was then pretreated with ovalbumin (1 mg/ml; Fisher Scientific, Hampton, NH) for 15 min on an end-over-end rotator. After three rinses with TG + NaCl, equal amounts of recombinant human PR-GST in 100 mm salt were added to resins and incubated on a rotator for 1 h. The resins were washed with TG + NaCl. Equal amounts of pooled hypothalamic or hippocampal whole-cell extracts were added to the immobilized PR-GST, or GST alone as a control, and incubated on a rotator for 1 h. Resins were washed with TG + NaCl to eliminate nonspecific binding, and then samples were eluted with 2% sodium dodecyl sulfate sample buffer by boiling samples for 5 min and stored at −80 C until use.
Samples were analyzed by Western blot as described previously (62) for detection of SRC-1 interactions with PR. Briefly, SRC-1 from brain was probed for using a mouse monoclonal antibody generated against amino acids 477–947 of human SRC-1 (1135-H4, 0.5 μg/ml, kindly provided by Dean Edwards, Bert O’Malley, Ming Tsai, and Sergio Oñate, Baylor College of Medicine, Houston, TX) (43) or a rabbit polyclonal antibody generated against aa 350–690 of mouse SRC-1 (M-341, 1:750; Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were incubated in a sheep antimouse secondary (1:6000; Amersham) or a donkey antirabbit secondary (1:10,000; Amersham) antibody. Immunoreactive bands were detected with an enhanced chemiluminescence kit (New England Biolabs, Beverly, MA) and membranes exposed to film (Blue Sensitive x-ray film; Laboratory Products Sales, Rochester, NY). Membranes were stripped for 3 h at 70 C in stripping buffer [2% sodium laurel sulfate, 62.5 mm Tris-HCl, 100 mm 2-mercaptoethanol, H2O (pH 6.7)] and then reprobed for PR using a mouse monoclonal antibody that recognizes N-terminal amino acids 165–534 of both PR-A and PR-B (PR 1294, 0.1 μg/ml, kindly provided by Dean Edwards). Films were placed on a light box (Fotodyne, New Berlin, WI) and photographed with an Olympus Camedia digital camera (Melville, NY). Images were imported into the NIH Image analysis program (version 1.62, National Institutes of Health, Bethesda, MD) on a Power Macintosh G3 computer (Cupertino, CA) and analyzed for immunoreactive band area as measured by number of pixels, which has been found to be consistent with OD data (62).
ER Flag-tagged pull-down assay procedure
All steps were conducted at 4 C. Twenty-five microliters of packed anti-Flag M2 affinity gel resin (Sigma) was added to each siliconized centrifuge tube and prewashed three times with Tris-buffered saline and two times with 100 mm glycine HCl [100 mm glycine, water (pH 3.5)]. Resins were next washed three times with wash buffer + NaCl [50 mm Tris-HCl, 100 mm NaCl, 1% glycerol, 50 mm Na fluoride, water (pH = 7.4)] + 0.1% Triton X-100. Equal amounts of recombinant Flag-tagged ER were added to the resin column and rotated on an end-over-end rotator for 1 h. The resins with immobilized ER were washed three times with wash buffer + NaCl. Equal amounts of pooled hypothalamic, or hippocampal, whole-cell extracts were added to the immobilized ER-Flag and incubated on a rotator for 1 h. The resins were washed three times with wash buffer + NaCl to eliminate nonspecific binding, and then samples were eluted with 2% sodium dodecyl sulfate sample buffer as described above and stored at −80 C.
Samples were analyzed by Western blot, as described above, for detection of SRC-1 interactions with ER. After probing for SRC-1, membranes were stripped and reprobed for Flag-tagged ERα and ERβ using a mouse monoclonal antibody generated against the Flag tag (0.25 μg/ml, anti-Flag M2; Sigma) and a horseradish peroxidase-linked sheep antimouse secondary antibody (1:80,000 dilution; Amersham Biosciences).
Mass spectrometry
Rat hypothalamic extracts (approximately 40 mg of tissue per condition) were exposed to immobilized ERα in the presence of 200 nm estradiol or no ligand. Eluted samples were resolved in adjacent lanes by SDS-PAGE, and the region of the gel corresponding to SRC-1 was excised, digested with trypsin, and desalted as described previously (69,70). The peptide mixture was injected onto a C18 trap and then separated on a reversed phase nano-HPLC column (PicoFrrtTM, 75 μm ×10 cm; tip inner diameter 15 μm) with a linear gradient of 0–50% mobile phase B (0.1% formic acid-90% acetonitrile) in mobile phase A (0.1% formic acid) over 120 min at 200 nl/min. Liquid chromatography and tandem mass spectrometry experiments were performed with an LTQ linear ion trap mass spectrometer (ThermoFinnigan, San Jose, CA) equipped with a nanospray source; the mass spectrometer was coupled on-line to a ProteomX nano-HPLC system (ThermoFinnigan). The mass spectrometer was operated in the data-dependent mode using Xcalibur software. The most intense seven ions in each MS survey scan were automatically selected for tandem mass spectrometry. This approach allows the detection of individual proteins in the nanogram range and has been used to identify proteins in complexes using immunoaffinity purification as well as low abundance transcription factors such as RelA/p65 nuclear factor-κB (69,70). The acquired tandem mass spectrometry spectra were searched with SEQUEST algorithm from the SWISSPROT Protein Database on the Bioworks 3.2 platform (ThermoFinnigan).
Statistical analysis
Films from Western blots were analyzed as described previously (62). Data were analyzed as a ratio of area of SRC-1 immunoreactive band to area of PR-A or PR-B band, or area of ERα or ERβ band. Unless stated otherwise, the area of immunoreactive bands was analyzed using a two-way ANOVA in StatView version 5.0.1 (SAS Institute Inc., Cary, NC) to determine differences between receptor subtypes and ligand conditions. Differences were considered significant at a probability of less than 0.05.
Results
PR interacts with neural SRC-1 in a ligand-dependent and subtype-specific manner
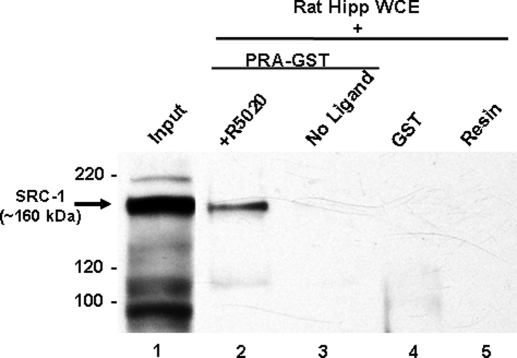
GST pull-down assays were used to investigate whether SRC-1 from brain physically associates with PR-A and PR-B and whether these interactions depend on the ligand condition. SRC-1 from the hippocampus interacted with PR-A in the presence of the agonist R5020 (Fig. 1, lane 2) but not in the absence of ligand (Fig. 1, lane 3). SRC-1 did not interact with the GST tag bound to resin (Fig. 1, lane 4) or the resin alone (lane 5), indicating that there was no nonspecific binding of SRC-1 to the GST tag or resin alone.
Figure 1.
SRC-1 from hippocampal whole-cell extracts associates with PR-A in a ligand-dependent manner. SRC-1 from hippocampal whole-cell extracts (WCE) associates with PR-A in the presence of the agonist R5020 (lane 2) but not the absence of ligand (lane 3). SRC-1 does not interact with GST tag alone (lane 4) or with the glutathione resin (lane 5). Input (1% of total) of SRC-1 from hippocampal extract is shown in lane 1.
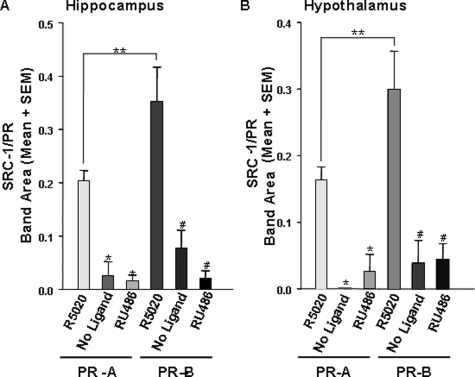
SRC-1 from brain associated with both PR-A and PR-B when bound to agonist (Figs. 2, lanes 2 and 5, and 3). In dramatic contrast, little to no SRC-1 from the hippocampus (Fig. 2, lanes 4 and 7) or hypothalamus associated with PR-A and PR-B in the absence of ligand or in the presence of the SPRM RU486 [F(5,32) = 17.08, P <0.0001; Fig. 3, A and B]. In confirmation of these results using the 1135-H4 monoclonal antibody to human SRC-1, similar findings were observed using the rabbit polyclonal antibody to mouse SRC-1 (data not shown). These findings indicate that SRC-1 from brain interacts with PR in a ligand-dependent manner. Figure 2 reveals lower molecular weight bands labeled with the SRC-1 monoclonal antibody that appear to interact with PR-A and PR-B in a manner that is not dependent on the ligand condition because they are present in all three ligand conditions. However, these same immunoreactive bands were observed using the polyclonal SRC-1 antibody (data not shown), suggesting that these bands are fragments of SRC-1 from brain.
Figure 2.
SRC-1 from hippocampal whole-cell extracts associates with PR-A and PR-B in a ligand-dependent manner. SRC-1 from the hippocampus associates with PR-A and PR-B in the presence of the agonist R5020 (lanes 2 and 5) but not the absence of ligand (lanes 3 and 6) or in the presence of the SPRM, RU486 (lanes 4 and 7). Input (1% of total) of SRC-1 from hippocampal extract is shown in lane 1.
Figure 3.
SRC-1 from the hippocampus and hypothalamus associates with PR in a ligand-dependent and receptor subtype-specific manner. A, SRC-1 from hippocampal extracts interacted with both PR-A and PR-B in the presence of R5020 but not the absence of ligand or the presence of RU486. *, P < 0.0001, significantly different from PR-A + R5020; #, P < 0.01, significantly different from PR-B + R5020, and SRC-1 from hippocampus interacted more with PR-B than PR-A when bound to R5020. **, P < 0.05, t test. B, Hypothalamic SRC-1 interacts with PR-A and PR-B when bound to R5020, but little to no interactions were detected in the absence of ligand or when receptors were bound to RU486, *, P < 0.01, significantly different from PR-A + R5020; #, P < 0.001, significantly different from PR-B + R5020, and SRC-1 from the hypothalamus interacted more with PR-B, than PR-A when bound to R5020; **, P < 0.05, t test, n = 5–7 per treatment group.
Initial findings suggested that SRC-1 associated more strongly with PR-B than PR-A in the presence of agonist (Fig. 2, lanes 2 and 5). Indeed, SRC-1 from hippocampus (Fig. 3A) and hypothalamus (Fig. 3B) interacted more with PR-B than with PR-A in the presence of agonist [F(5,32) = 11.75, P <0.0001].
ER associates with SRC-1 from the hypothalamus in a receptor subtype-specific manner
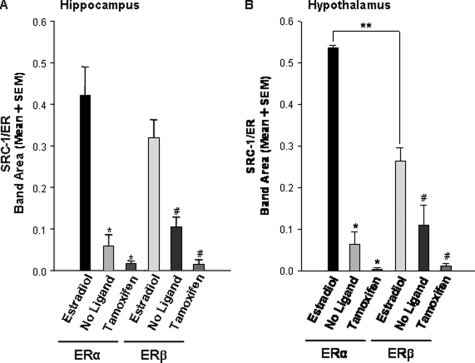
Flag-tagged pull-down assays were used to investigate whether ERα and ERβ physically associate with SRC-1 from brain and whether these interactions occur in a ligand-dependent manner. Hypothalamic SRC-1 interacted with ER in a ligand-dependent manner (Figs. 4 and 5B). Estradiol promoted the interactions of hypothalamic SRC-1 with ERα and ERβ (Figs. 4, lanes 2 and 5, and 5B). In contrast, in the absence of ligand or the presence of the SERM, tamoxifen, ERα and ERβ had little to no association with hypothalamic SRC-1 [F(5,18) = 28.86, P <0.0001; Fig. 4, lanes 3 and 6 and 4 and 7, and Fig. 5B]. SRC-1 from the hippocampus interacted strongly with both hippocampal ERα and ERβ in the presence of estradiol (Fig. 5A). In the absence of ligand or the presence of the SERM, tamoxifen [F(5,24) = 22.10, P < 0.0001], there was little interaction between hippocampal SRC-1 with either ERα or ERβ (Fig. 5A). Similar SRC-1 and ER interactions were observed using the polyclonal antibody to SRC-1 (data not shown).
Figure 4.
SRC-1 from hypothalamic whole-cell extracts associates with ERα and ERβ in a ligand-dependent manner. SRC-1 from the hypothalamus associates with ERα and ERβ in the presence of estradiol (lanes 2 and 5) but not the absence of ligand (lanes 3 and 6) or in the presence of the SERM, tamoxifen (lanes 4 and 7). Input (1% of total) of SRC-1 from hypothalamic extract is shown in lane 1.
Figure 5.
ER recruits SRC-1 from the hypothalamus in a receptor subtype-specific manner. A, In the presence of estradiol, both ERα and ERβ interacted with hippocampal SRC-1, but little to no interactions were detected in the absence of ligand or when receptors were bound to tamoxifen. *, P < 0.0001, significantly different from ERα + estradiol; #, P < 0.0001, significantly different from ERβ + estradiol. B, Hypothalamic SRC-1 interacted more strongly with both ERα and ERβ in the presence of estradiol than in the absence of ligand or when receptors were bound to tamoxifen. *, P < 0.0001, significantly different from ERα + estradiol; #, P < 0.01, significantly different from ERβ + estradiol, and SRC-1 interacted more with ERα than ERβ when bound to estradiol; **, P < 0.05, t test, n = 4–5 per treatment group.
Interestingly, SRC-1 from the hypothalamus physically associated more with ERα than with ERβ in the presence of estradiol (Figs. 4, lanes 2 and 5, and Fig. 5B). In contrast, we did not observe this differential interaction between SRC-1 from the hippocampus and ERα (0.42 ± 0.07) and ERβ (0.32 ± 0.04; P = 0.24, two-tailed t test) (Fig. 5A). Taken together, these data suggest that that ER subtypes interact with SRC-1 in a brain region-specific manner.
Mass spectrometry confirms hypothalamic SRC-1 interacts with ERα
To independently confirm the Western blot data for estradiol-dependent binding of ER to SRC-1 from rat brain, we used an unbiased mass spectrometry approach. Rat hypothalamic extracts were exposed to immobilized ERα in the presence of estradiol or no ligand, and eluted samples were resolved by SDS-PAGE. Gel slices corresponding to the putative SRC-1 region of the two lanes were digested with trypsin and peptides analyzed by liquid chromatography and tandem mass spectrometry. Database searching identified an abundant, doubly charged peptide with MH+ of 1336.65907. Whereas no matches were found in the rat SwissProt database, a search of the far more completely annotated human database matched the amino acid sequence SDISSSSQGVIEK with highly significant scores of XCorr = 3.62 and DeltaCn = 0.45. Furthermore, 18 of 24 of the observed fragment ions matched the predicted fragment ions. This peptide corresponds to amino acids 97–109 of the human nuclear receptor coactivator 1 (EC 2.3.1.48) with gene name of NCoA-1 (SRC-1) and SwissProt accession no. Q15788. It is important to note a match in the rat database was not found because, despite 100% identity of this human peptide with mouse, chicken, pig, and many other species, the rat NCoA-1 sequence is not currently in the SwissProt database. Interestingly, this peptide was found in the slice from the lane eluted from estradiol-bound ERα and not in the slice eluted from unliganded ERα, confirming our findings from the Western blot analysis.
Discussion
To test the hypotheses that SRC-1 from brain physically associates with PR and ER subtypes in a ligand-dependent manner, we developed pull-down assays with brain tissue from female rats. We found that SRC-1 from hypothalamic or hippocampal extracts interacted with both GST-tagged PR-A and PR-B when bound to the agonist R5020. In contrast, very little to no SRC-1 from brain associated with PR-A or PR-B in the absence of ligand or the presence of the SPRM, RU486. These findings are consistent with previous studies using recombinant SRC-1 and the concept that SRC-1 and PR interactions are agonist dependent (33,71). The present findings support our previous work indicating a role for SRC-1 action in the hypothalamus in PR-dependent female sexual behavior (62) and suggest that SRC-1 may contribute to the effects of progestins on memory in the hippocampus (72).
Interestingly, we found that SRC-1 from hypothalamus or hippocampus interacts more with PR-B than with PR-A in the presence of agonist (Fig. 3). The present results are in contrast to other pull-down assays using recombinant SRC-1. In one study, full-length recombinant SRC-1 interacted equally with PR-A and PR-B when bound to agonist (43). In another pull-down study, an SRC-1 fragment interacted with PR-B but not PR-A (71). Taken together, the present findings suggest the importance of using biologically relevant tissue, in contrast to the use of cell lines alone, in these pull-down assays. It may be that other cofactors and proteins, that are present in brain, are important for appropriate SRC-1 and PR interactions.
In vitro studies indicate that human PR-B is a stronger transcriptional activator than PR-A (20,22,23,24) due to the additional AF-3 region of PR-B (25,26). It is likely that this additional AF domain in PR-B allows for enhanced recruitment of coactivators, thus augmenting the transcriptional activity of PR-B (24,25,73). Interestingly, a recent study indicated that both PR isoforms are required for the complete expression of female sexual behavior in mice (27). Whereas it is not known whether PR-B is a stronger transcriptional activator than PR-A in brain, our findings suggest that PR-B is a stronger activator of SRC-1-dependent progesterone signaling pathways in brain than PR-A.
SRC-1 from hypothalamus or hippocampus interacted with ERα and ERβ when bound to estradiol (Figs. 4 and 5). In contrast, very little to no association of SRC-1 from brain was detected with ERα or ERβ in the absence of ligand or the presence of the SERM, tamoxifen (Figs. 4 and 5). Our findings are consistent with a variety of studies using cell lines demonstrating that estradiol facilitates the association of SRC-1 with ER, whereas antagonists prevent this association (40,48,74,75). In contrast to the present findings, under certain phosphorylation conditions, cell culture studies suggest that both ERα and ERβ can recruit coactivators to AF-1 in the absence of ligand (45,46). Whereas we detected little to no interactions between receptor and SRC-1 from brain in the absence of ligand, it will be important to investigate whether physiologically relevant events that modulate ligand-independent activation impact on receptor-coactivator interactions in brain. Furthermore, under the present experimental conditions, it appears that the selective receptor modulators, tamoxifen and RU486, function as antagonists to prevent receptor-coactivator interactions.
In the present studies, SRC-1 from the hippocampus appears to interact equally with ERα and ERβ (Fig. 5A). Association of SRC-1 with ligand-bound ERα and ERβ in the hippocampus may be an integral component of estrogen’s effects on cognition and memory (76,77). Interestingly, in contrast to the hippocampus, SRC-1 obtained from hypothalamic extracts interacted more with ERα than ERβ (Figs. 4 and 5B). ERα, and to a lesser extent ERβ, are expressed in the hypothalamus (12,13,14,15,16). In the hypothalamus, ERα is necessary for the full expression of rodent female sexual behavior (78,79,80,81,82), whereas ERβ in this region appears to influence anxiety and the stress response (77,83). These different functions of the ER subtypes in brain may be explained in part by the different transcriptional abilities of these receptors. The amino terminus is shorter in ERβ than ERα, which may account for the lower transcriptional activity of ERβ observed in particular cell lines (17). These differences in transcriptional abilities between ERα and ERβ may be attributed to differential recruitment of coactivators, or differences in the ability of the same coactivator to facilitate transcription of the ER subtypes (84). Whereas some studies using recombinant SRC-1 are consistent with our findings that SRC-1 interacts more with ERα than with ERβ (84), other findings suggest that SRC-1 associates equally with each ER subtype (74,85). Whereas these later findings are consistent with our results using SRC-1 from hippocampus, we observed that SRC-1 from hypothalamus interacted more with ERα than ERβ.
These data suggest that ERα is a more efficient transcriptional activator of SRC-1-dependent signaling pathways in the hypothalamus than ERβ. In support, previous findings from our laboratory indicate that SRC-1 function in the hypothalamus is important for maximal expression of ER-mediated female sexual behavior (62), which appears to be ERα dependent (78,79). These differential interactions of SRC-1 from hypothalamus or hippocampus with the ER and PR subtypes suggest that these brain regions have distinct expression patterns of cofactors involved in these important protein-protein interactions. In addition, it is possible that SRC-1 undergoes differential phosphorylation in these two brain regions, leading to distinct patterns of interaction with receptors. Future experiments will need to apply mass spectrometry analysis to determine whether, in a brain region-specific manner, different cofactors are present in the receptor-coactivator complex and/or whether SRC-1 undergoes differential phosphorylation.
These pull-down assays allow us to directly address the differential interactions of SRC-1 with the PR and ER subtypes. In addition, this approach allows the efficient detection of protein-protein interactions and the application of mass spectrometry. However, one must be careful in interpreting the results from these assays, given that nonspecific interactions can occur. In the present studies, little to no interactions were detected between SRC-1 from brain and the fusion protein tags alone (GST or Flag tags) or the resins only (Fig. 1), suggesting there were no significant nonspecific interactions between SRC-1 and fusion tags or resins. Moreover, Western blot analysis and mass spectrometry revealed that SRC-1 interacted with receptor when bound to agonist but not when bound to antagonist or unliganded, suggesting these coactivator-receptor interactions were specific. It should be noted that human ER and PR proteins were used to investigate interactions with SRC-1 protein from rat brain. It is possible that SRC-1 from rat brain may interact differently with human ER and PR, compared with rat receptor. However, the human PR ligand binding domain (LBD), the receptor region most critical for SRC-1 interactions (32,86), has a high degree of protein sequence homology (92%) with the rat PR LBD (BLAST) (18,87). Furthermore, the LBDs of human ERα and ERβ are 89 and 90% identical in protein sequences to the LBDs of rat ERα and ERβ, respectively (88). However, given that discrete differences in protein structure can lead to differences in protein interactions, it will be important to investigate endogenous interactions between SRC-1 and steroid receptors in brain using coimmunoprecipitation assays in future studies. Nevertheless, the high degree of homology between the rat and human LBDs of PR and ER, taken together with the ligand-dependent nature of the interactions in the present studies, suggest that our findings provide important insights into the physical associations of SRC-1 from brain and these receptors.
In conclusion, the present data indicate that SRC-1 from hypothalamus and hippocampus physically associate with ER and PR in a ligand-dependent manner. These findings extend our previous studies showing that SRC-1 is expressed in ER and PR containing cells in brain regions important for reproductive behavior (59). In addition, these protein-protein interaction studies provide further support for work from our laboratory and others that reveal an important role for SRC-1 in ER and PR action in brain (55,60,61,62,63). Moreover, the present studies reveal that SRC-1 from brain interacts differentially with ER and PR subtypes in a brain region-specific manner. Understanding how nuclear receptor coactivators function with various steroid receptors, and their subtypes, is critical to understanding how hormones act in different brain regions to profoundly influence physiology and behavior. Ultimately, investigation of these receptor-coactivator interactions using brain tissue may allow the identification of novel cofactors involved in the steroid receptor complex in brain.
Acknowledgments
We thank Dr. Nancy Forger and members of Dr. Cheryl Sisk’s laboratory for helpful comments on the manuscript.
Footnotes
This work was supported in part by National Institutes of Health Grant RO1 DK61935 (to M.J.T.) and National Institute of Mental Health Training Grant T32MH47538 (to H.A.M.-F.).
Present address for H.A.M.-F.: Neuroscience Program, Michigan State University, East Lansing, Michigan 48824.
Disclosure Statement: The authors have nothing to disclose.
First Published Online June 19, 2008
Abbreviations: AF, Activation function; ER, estrogen receptor; GST, glutathione-S-transferase; LBD, ligand binding domain; NCoA, nuclear receptor coactivator; PR, progestin receptor; SERM, selective estrogen receptor modulator; SPRM, selective PR modulator; SRC, steroid receptor coactivator; TG buffer, Tris-HCl plus glycerol.
References
- Mani SK, O'Malley BW 2002 Mechanism of progesterone receptor action in the brain. In: Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, Pfaff DW, eds. Hormones, brain and behavior. San Diego: Academic Press; 643–682 [Google Scholar]
- Blaustein JD, Erskine MS 2002 Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff DW, ed. Hormones, brain and behavior. New York: Academic Press; 139–214 [Google Scholar]
- Pfaff DW 1980 Estrogens and brain function. New York: Springer-Verlag [Google Scholar]
- Jensen EV, Suzuki T, Kawasima T, Stumpf WE, Jungblut PW, de Sombre ER 1968 A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci USA 59:632–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyamala G, Gorski J 1969 Estrogen receptors in the rat uterus. Studies on the interaction of cytosol and nuclear binding sites. J Biol Chem 244:1097–1103 [PubMed] [Google Scholar]
- Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J 1996 Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KD, Korach KS 2006 Potential biological functions emerging from the different estrogen receptors. Ann NY Acad Sci 1092:361–373 [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J 1997 Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP 1999 The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- Jones PS, Parrott E, White IN 1999 Activation of transcription by estrogen receptor α and β is cell type- and promoter-dependent. J Biol Chem 274:32008–32014 [DOI] [PubMed] [Google Scholar]
- Damdimopoulos AE, Spyrou G, Gustafsson JA 2008 Ligands differentially modify the nuclear mobility of estrogen receptors α and β. Endocrinology 149:339–345 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- Greco B, Allegretto EA, Tetel MJ, Blaustein JD 2001 Coexpression of ER β with ER alpha and progestin receptor proteins in the female rat forebrain: Effects of estradiol treatment. Endocrinology 142:5172–5181 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA 1998 The estrogen receptor β subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol 19:253–286 [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL 1998 Differential distribution and regulation of estrogen receptor-α and -β mRNA within the female rat brain. Brain Res Mol Brain Res 54:175–180 [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE 2003 Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology 144:2055–2067 [DOI] [PubMed] [Google Scholar]
- Delaunay F, Pettersson K, Tujague M, Gustafsson JA 2000 Functional differences between the amino-terminal domains of estrogen receptors α and β. Mol Pharmacol 58:584–590 [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely OM, Maxwell BL, Toft DO, Schrader WT, O'Malley BW 1987 The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun 2:493–501 [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP 1993 Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 7:1244–1255 [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Goldman ME 1994 RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. J Biol Chem 269:11945–11949 [PubMed] [Google Scholar]
- Tung L, Kamel Mohamed M, Hoeffler JP, Takimoto GS, Horwitz KB 1993 Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol 7:1256–1265 [DOI] [PubMed] [Google Scholar]
- Wen DX, Xu YF, Mais DE, Goldman ME, McDonnell DP 1994 The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol 14:8356–8364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangrande PH, Pollio G, McDonnell DP 1997 Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. J Biol Chem 272:32889–32900 [DOI] [PubMed] [Google Scholar]
- Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB 1994 A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol Endocrinol 8:1347–1360 [DOI] [PubMed] [Google Scholar]
- Tung L, Abdel-Hafiz H, Shen T, Harvell DM, Nitao LK, Richer JK, Sartorius CA, Takimoto GS, Horwitz KB 2006 Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Mol Endocrinol 20:2656–2670 [DOI] [PubMed] [Google Scholar]
- Mani SK, Reyna AM, Chen JZ, Mulac-Jericevic B, Conneely OM 2006 Differential response of progesterone receptor isoforms in hormone-dependent and -independent facilitation of female sexual receptivity. Mol Endocrinol 20:1322–1332 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, Demayo FJ, Lydon JP, Conneely OM 2000 Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289:1751–1754 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Conneely OM 2004 Reproductive tissue selective actions of progesterone receptors. Reproduction 128:139–146 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM 2003 Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley BW 2006 Molecular biology. Little molecules with big goals. Science 313:1749–1750 [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK 2006 Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev 20:1405–1428 [DOI] [PubMed] [Google Scholar]
- Oñate SA, Tsai SY, Tsai MJ, O'Malley BW 1995 Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357 [DOI] [PubMed] [Google Scholar]
- Lonard DM, O'Malley BW 2006 The expanding cosmos of nuclear receptor coactivators. Cell 125:411–414 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Oñate SA, Tsai SY, Tsai MJ, O'Malley BW 1997 Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389:194–197 [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T 1996 The CBP co-activator is a histone acetyltransferase. Nature 384:641–643 [DOI] [PubMed] [Google Scholar]
- Voegel JJ, Heine MJS, Zechel C, Chambon P, Gronemeyer H 1996 TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J 15:3667–3675 [PMC free article] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS 1997 AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968 [DOI] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL 1998 The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95:927–937 [DOI] [PubMed] [Google Scholar]
- Margeat E, Poujol N, Boulahtouf A, Chen Y, Muller JD, Gratton E, Cavailles V, Royer CA 2001 The human estrogen receptor α dimer binds a single SRC-1 coactivator molecule with an affinity dictated by agonist structure. J Mol Biol 306:433–442 [DOI] [PubMed] [Google Scholar]
- Tanenbaum DM, Wang Y, Williams SP, Sigler PB 1998 Crystallographic comparison of the estrogen and progesterone receptor’s ligand binding domains. Proc Natl Acad Sci USA 95:5998–6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SP, Sigler PB 1998 Atomic structure of progesterone complexed with its receptor. Nature 393:392–396 [DOI] [PubMed] [Google Scholar]
- Oñate SA, Boonyaratanakornkit V, Spencer TE, Tsai SY, Tsai MJ, Edwards DP, O'Malley BW 1998 The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J Biol Chem 273:12101–12108 [DOI] [PubMed] [Google Scholar]
- McInerney EM, Tsai MJ, O'Malley BW, Katzenellenbogen BS 1996 Analysis of estrogen receptor transcriptional enhancement by a nuclear hormone receptor coactivator. Proc Natl Acad Sci USA 93:10069–10073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre M, Smith CL 2003 Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-α: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol 17:1296–1314 [DOI] [PubMed] [Google Scholar]
- Tremblay A, Tremblay GB, Labrie F, Giguere V 1999 Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol Cell 3:513–519 [DOI] [PubMed] [Google Scholar]
- Lewis-Wambi JS, Jordan VC 2005 Treatment of postmenopausal breast cancer with selective estrogen receptor modulators (SERMs). Breast Dis 24:93–105 [DOI] [PubMed] [Google Scholar]
- Smith CL, Nawaz Z, O'Malley BW 1997 Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol 11:657–666 [DOI] [PubMed] [Google Scholar]
- Han SJ, Tsai SY, Tsai MJ, O'Malley BW 2007 Distinct temporal and spatial activities of RU486 on PR function in reproductive organs of ovariectomized mice. Endocrinology 148:2471–2486 [DOI] [PubMed] [Google Scholar]
- Wardell SE, Edwards DP 2005 Mechanisms controlling agonist and antagonist potential of selective progesterone receptor modulators (SPRMs). Semin Reprod Med 23:9–21 [DOI] [PubMed] [Google Scholar]
- Martinez de Arrieta C, Koibuchi N, Chin WW 2000 Coactivator and corepressor gene expression in rat cerebellum during postnatal development and the effect of altered thyroid status. Endocrinology 141:1693–1698 [DOI] [PubMed] [Google Scholar]
- Meijer OC, Steenbergen PJ, de Kloet ER 2000 Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology 141:2192–2199 [DOI] [PubMed] [Google Scholar]
- Misiti S, Schomburg L, Yen PM, Chin WW 1998 Expression and hormonal regulation of coactivator and corepressor genes. Endocrinology 139:2493–2500 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Nishi M, Kawata M 2001 Localization of nuclear coactivators p300 and steroid receptor coactivator 1 in the rat hippocampus. Brain Res 890:197–202 [DOI] [PubMed] [Google Scholar]
- Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ 2002 Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology 143:436–444 [DOI] [PubMed] [Google Scholar]
- Yousefi B, Jingu H, Ohta M, Umezu M, Koibuchi N 2005 Postnatal changes of steroid receptor coactivator-1 immunoreactivity in rat cerebellar cortex. Thyroid 15:314–319 [DOI] [PubMed] [Google Scholar]
- Charlier TD, Lakaye B, Ball GF, Balthazart J 2002 Steroid receptor coactivator SRC-1 exhibits high expression in steroid-sensitive brain areas regulating reproductive behaviors in the quail brain. Neuroendocrinology 76:297–315 [DOI] [PubMed] [Google Scholar]
- Molenda HA, Kilts C, Allen RL, Tetel MJ 2003 Nuclear receptor coactivator function in reproductive physiology and behavior. Biol Reprod 69:1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetel MJ, Siegal NK, Murphy SD 2007 Cells in behaviourally relevant brain regions coexpress nuclear receptor coactivators and ovarian steroid receptors. J Neuroendocrinol 19:262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolakis EM, Ramamurphy M, Zhou D, Onate S, O'Malley BW 2002 Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol Endocrinol 16:1511–1523 [DOI] [PubMed] [Google Scholar]
- Auger AP, Tetel MJ, McCarthy MM 2000 Steroid receptor coactivator-1 mediates the development of sex specific brain morphology and behavior. Proc Natl Acad Sci USA 97:7551–7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenda-Figueira HA, Williams CA, Griffin AL, Rutledge EM, Blaustein JD, Tetel MJ 2006 Nuclear receptor coactivators function in estrogen receptor- and progestin receptor-dependent aspects of sexual behavior in female rats. Horm Behav 50:383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J 2005 Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J Neurosci 25:906–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Harada N, Ball GF, Balthazart J 2006 Targeting steroid receptor coactivator-1 expression with locked nucleic acids antisense reveals different thresholds for the hormonal regulation of male sexual behavior in relation to aromatase activity and protein expression. Behav Brain Res 172:333–343 [DOI] [PubMed] [Google Scholar]
- Trousson A, Grenier J, Fonte C, Massaad-Massade L, Schumacher M, Massaad C 2007 Recruitment of the p160 coactivators by the glucocorticoid receptor: dependence on the promoter context and cell type but not hypoxic conditions. J Steroid Biochem Mol Biol 104:305–311 [DOI] [PubMed] [Google Scholar]
- Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP 1999 Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol Endocrinol 13:910–924 [DOI] [PubMed] [Google Scholar]
- Melvin VS, Harrell C, Adelman JS, Kraus WL, Churchill M, Edwards DP 2004 The role of the C-terminal extension (CTE) of the estrogen receptor α and β DNA binding domain in DNA binding and interaction with HMGB. J Biol Chem 279:14763–14771 [DOI] [PubMed] [Google Scholar]
- Kraus WL, Kadonaga JT 1998 p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev 12:331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton RG, Haidacher SJ, Lejeune WS, Zhang X, Zhao Y, Kurosky A, Brasier AR, Denner L 2007 Diabetes-induced changes in the renal cortical proteome assessed with two-dimensional gel electrophoresis and mass spectrometry. Proteomics 7:1729–1742 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang W, White MA, Zhao Y 2003 Capillary high-performance liquid chromatography/mass spectrometric analysis of proteins from affinity-purified plasma membrane. Anal Chem 75:3751–3757 [DOI] [PubMed] [Google Scholar]
- Giangrande PH, Kimbrel A, Edwards DP, McDonnell DP 2000 The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol 20:3102–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL 2001 Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci 115:384–393 [PubMed] [Google Scholar]
- Hovland AR, Powell RL, Takimoto GS, Tung L, Horwitz KB 1998 An N-terminal inhibitory function, IF, suppresses transcription by the A-isoform but not the B-isoform of human progesterone receptors. J Biol Chem 273:5455–5460 [DOI] [PubMed] [Google Scholar]
- Cowley SM, Parker MG 1999 A comparison of transcriptional activation by ERα and ERβ. J Steroid Biochem Mol Biol 69:165–175 [DOI] [PubMed] [Google Scholar]
- Yi P, Driscoll MD, Huang J, Bhagat S, Hilf R, Bambara RA, Muyan M 2002 The effects of estrogen-responsive element- and ligand-induced structural changes on the recruitment of cofactors and transcriptional responses by ERα and ERβ. Mol Endocrinol 16:674–693 [DOI] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF 2000 Novel effects of estradiol and estrogen receptor α and beta on cognitive function. Brain Res 883:258–264 [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF 2006 New roles for estrogen receptor β in behavior and neuroendocrinology. Front Neuroendocrinol 27:217–232 [DOI] [PubMed] [Google Scholar]
- Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB 1997 Estrogen receptors are essential for female sexual receptivity. Endocrinology 138:507–510 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW 1998 Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology 139:5070–5081 [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF 2003 Double oestrogen receptor α and β knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol 15:978–983 [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S 2006 RNAi-mediated silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci USA 103:10456–10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW 1999 Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc Natl Acad Sci USA 96:12887–12892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ 2003 Estrogen receptor β in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience 121:837–845 [DOI] [PubMed] [Google Scholar]
- Wong C, Komm B, Cheskis BJ 2001 Structure-Function evaluation of ER α and β interplay with SRC family coactivators. ER selective ligands. Biochemistry 40:6756–6765 [DOI] [PubMed] [Google Scholar]
- Monroe DG, Johnsen SA, Subramaniam M, Getz BJ, Khosla S, Riggs BL, Spelsberg TC 2003 Mutual antagonism of estrogen receptors α and β and their preferred interactions with steroid receptor coactivators in human osteoblastic cell lines. J Endocrinol 176:349–357 [DOI] [PubMed] [Google Scholar]
- Feng W, Ribeiro RCJ, Wagner RL, Nguyen H, Apriletti JW, Fletterick RL, Baxter JD, Kushner PJ, West BL 1998 Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science 280:1747–1750 [DOI] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Mouri N 1993 The ontogeny of gene expression of progestin receptors in the female rat brain. J Steroid Biochem Mol Biol 47:173–182 [DOI] [PubMed] [Google Scholar]
- Harris HA, Bapat AR, Gonder DS, Frail DE 2002 The ligand binding profiles of estrogen receptors α and β are species dependent. Steroids 67:379–384 [DOI] [PubMed] [Google Scholar]