Abstract
Pituitary FSH promotes pubertal timing and normal gametogenesis by binding its receptor (FSHR) located on Sertoli and granulosa cells of the testis and ovary, respectively. Studies on Fshr transcription provide substantial evidence that upstream stimulatory factor (USF) 1 and USF2, basic helix-loop-helix leucine zipper proteins, regulate Fshr through an E-box within its promoter. However, despite the strong in vitro support for USF1 and USF2 in Fshr regulation, there is currently no in vivo corroborating evidence. In the present study, chromatin immunoprecipitation demonstrated specific binding of USF1 and USF2 to the Fshr promoter in both Sertoli and granulosa cells, in vivo. Control cells lacking Fshr expression showed no USF-Fshr promoter binding, thus correlating USF-promoter binding to gene activity. Evaluation of Fshr expression in Usf1 and Usf2 null mice further explored USF’s role in Fshr transcription. Loss of either gene significantly reduced ovarian Fshr levels, whereas testis levels were unaltered. Chromatin immunoprecipitation analysis of USF-Fshr promoter binding in Usf-null mice indicated differences in the composition of promoter-bound USF dimers in granulosa and Sertoli cells. Promoter-bound USF dimer levels declined in granulosa cells from both null mice, despite increased USF2 levels in Usf1-null ovaries. However, compensatory increases in promoter-bound USF homodimers were evident in Usf-null Sertoli cells. In summary, this study provides the first in vivo evidence that USF1 and USF2 bind the Fshr promoter and revealed differences between Sertoli and granulosa cells in compensatory responses to USF loss and the USF dimeric composition required for Fshr transcription.
FSH AND THE related LH are key components of the hypothalamic pituitary gonadal axis that regulate gonad function, gamete development, and fertility (1,2,3,4). These hormones are produced in the pituitary in response to the hypothalamic decapeptide GnRH and, once released into circulation, elicit activity by binding their specific cell-surface receptors located on target cells. In the case of FSH, its receptor (FSHR) is produced only by Sertoli cells of the testis, granulosa cells of the ovary, and osteoclasts of the bone (5,6,7,8). This highly restricted expression of FSHR and its role in endocrine signaling instigated numerous studies to identify the transcriptional mechanisms required for its unique cell specificity and changes in receptor levels.
Transcriptional studies of Fshr have focused predominantly on its 5′ flanking or promoter region, and identified several DNA elements and proteins important for promoter function. Transient transfection analysis and promoter mutagenesis showed that basal promoter activity was largely retained within 100 bp of 5′ flanking sequence and driven predominantly by an E-box element (9,10,11,12,13,14). The E-box (5′-CACRTG-3′) has been the target of several in vitro studies that indicate it plays a key role in Fshr transcription (9,10,11,15). Furthermore, in vivo genomic footprinting, which demonstrated E-box occupancy in primary Sertoli cells, and studies correlating E-box methylation status to Fshr transcriptional activity provide in vivo evidence for E-box control of Fshr transcription (11,16,17). Analysis of proteins binding to the Fshr E-box, via in vitro binding studies, identified upstream stimulatory factor (USF) 1 and USF2, two ubiquitous members of the basic-helix-loop-helix leucine zipper protein family (9,10,11,12). Formation of either heterodimers or homodimers of USF is required for binding to its DNA element, the E-box, and thus transcriptional regulation (18,19). Transient transfection experiments, demonstrating Fshr promoter activation with USF overexpression, further supported a role for the USF proteins (11). In addition to controlling Fshr basal transcription, the E-box and USF were required to convey transcriptional signals that activate Fshr by the orphan nuclear receptor NR5A1, better known as steroidogenic factor 1 (SF-1) (13,15,21). Thus, the E-box, together with USF1 and USF2, likely coordinates Fshr transcription by integrating signals from other transcription factors bound elsewhere on the promoter or to more distal regulatory elements (14).
Despite the strong support for USF1 and USF2 involvement, there is currently no in vivo evidence that they regulate Fshr transcription within a normal cellular or physiological environment. Yet, confirmation that these proteins regulate Fshr, in vivo, is essential to the accurate understanding of Fshr transcription and the direction of future studies. The current study used chromatin immunoprecipitation (ChIP) and Fshr expression analysis in USF-deficient mice to demonstrate, in vivo, that USF1 and USF2 bind the Fshr E-box and regulate gene transcription. The study also showed that Fshr expression responded differently in Sertoli and granulosa cells to the loss of one USF partner, implying that the USF proteins modulate Fshr activity differently in males and females.
Materials and Methods
Experimental animals
All experiments using animals were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center (assurance no. A3237-01), and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animal husbandry and genotyping
Usf1+/− and Usf2+/− breeder pairs were used to generate wild-type, Usf1+/−, Usf1−/−, Usf2+/−, and Usf2−/− mice (22). Tail biopsy DNA was prepared and used for genotyping by Southern blot as described (22,23).
Cell preparation and culture
Mouse Sertoli cells were isolated from 21-d-old wild-type animals essentially as described, except cells were resuspended in PBS prewarmed to 37 C (11). Granulosa cells were prepared from ovaries of 21-d-old mice essentially as described (24). Briefly, ovaries were dissected in prewarmed collection media [DMEM, 10 mm HEPES (pH 7.2), 1% penicillin-streptomycin, and 0.3% BSA], and granulosa cells were subsequently released from ovarian follicles by repeated puncture with 27-gauge needles in prewarmed PBS. Primary Sertoli cells and peritubular myoid cells were prepared from 15-d-old Sprague Dawley rats and cultured as described (25). Rats were used for studies involving peritubular myoid cells to minimize the number of animals used to obtain the necessary cell yields. This allowed comparison of USF binding in Fshr expressing (Sertoli) and nonexpressing cells (myoid) using cells that were comparable with respect to the animal, tissue, and experimental manipulation.
ChIP
ChIP was performed as described (25,26). Cross-linked chromatin was prepared from rat Sertoli cells, rat myoid cells, mouse Sertoli cells, and mouse granulosa cells as described (25). Mouse Sertoli and granulosa cells were pooled by genotype from multiple animals. Antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and 5 μg each was used for immunoprecipitation: rabbit anti-USF1 IgG (sc-229), rabbit anti-USF2 IgG (sc-862), mouse monoclonal anti-MYC IgG1 (sc-40), and normal rabbit IgG (sc-2027). PCR was performed using 5 μl immunoprecipitated DNA or 1 μl input material, and primers were directed to sequences within the mouse and rat Fshr loci. For both mouse and rat Fshr, one primer set spanned the promoter region, and another primer set spanned a negative control region 5 or 6 kb upstream of the Fshr transcriptional start sites, respectively (Table 1). Products were resolved by agarose gel electrophoresis. Band intensities were quantified from multiple ethidium bromide-stained agarose gels by densitometry using a Kodak EDAS 290 gel documentation system (Carestream Molecular Imaging, New Haven, CT) and ImageQuant TL software (GE Healthcare Bio-Sciences Corp., Piscataway, NJ).
Table 1.
Oligodeoxynucleotides used as primers for Fshr RPA probe generation, ChIP PCR, EMSA probe, and competitors
| Target | Use | Oligodeoxynucleotide sequence(s) (5′–3′) |
|---|---|---|
| Fshr exons 9–10 | RNase protection probe | CCCCAAGCTTATAGATGAGGTTTCTGTCT |
| CCCCGTCGACCTCCTAGTTCTGTTCTACCC | ||
| Mouse Fshr promoter | ChIP | CTTGAAGGATAAGACAGGTGC |
| CTGCTTTCTGCCTGCTCC | ||
| Mouse Fshr −5 kb | ChIP | CCACACGACTACACAGAAAGAAG |
| CTGCTTGGGGTAGCGTTG | ||
| Rat Fshr promoter | ChIP | GCAGAAGATTATTGACACACATTAGTC |
| ACAGATTCCCCAGGCTCCTC | ||
| Rat Fshr −6 kb | ChIP | GAAATAACGGGGTTGGGT |
| GATACTTAGACTTCTTCCTTTACG | ||
| E-box | EMSA | TCTTGGTGGGTCACGTGACTTTGCCCGT |
| NS | EMSA | CTAGAGTCGACCTGCAGGCATGCAAGCTTGGCATTC |
Underlined bases indicate the core E-box element.
RNA sample preparation and ribonuclease protection assay (RPA)
Total RNA was isolated from gonads of 21-d-old mice using TRIzol reagent (Invitrogen Corp., Carlsbad, CA) according to manufacturer recommendations. RPAs were preformed as described elsewhere using 5 × 105 cpm Fshr or Nr5a1 probes and 2 × 104 cpm βActin probe per assay sample (23,27). A plasmid containing a portion of the mouse Fshr mRNA sequence, mFshr9/10-GEM, which was used as template for synthesizing the Fshr cRNA probe, was generated as follows. cDNA was synthesized from mouse testis RNA in the presence of reverse transcriptase as described (11). Exons 9–10 of mouse Fshr were amplified by PCR from mouse testis cDNA using the primers noted in Table 1. The PCR product was digested with the restriction endonucleases SalI and HindIII, and cloned directionally into SalI and HindIII restriction sites of pGEM4Z (Promega Corp., Madison, WI). The in vitro transcription template was generated by linearizing mFshr9/10-GEM with EcoR I and purifying the linear plasmid DNA with an Ultra-clean PCR cleanup kit (MO BIO Laboratories, Inc., Solana Beach, CA). In vitro transcription was performed using linearized mFshr9/10-GEM template and T7 RNA polymerase (Promega), producing a 200 nucleotide Fshr cRNA probe that protected 154 nucleotides of mouse Fshr mRNA. The 192-nucleotide βActin cRNA probe was generated as described previously and protects 126 nucleotides of rat βActin [nucleotides 899–774 of accession no. NM031144 (28)]. Sequence mismatches between the rat βActin probe and mouse βActin mRNA (accession no. NM_007393) caused partial internal RNase A cleavage, producing two protected fragments of approximately 119 and 95 nucleotides, respectively. The Nr5a1 cRNA probe was synthesized as described (23). For the combined detection of Fshr and βActin mRNAs, cRNA probes were hybridized with either 5 μg (ovary) or 60 μg (testis) total RNA, whereas for the combined detection of Nr5a1 and βActin mRNAs, probes were hybridized with 10 μg (ovary) or 5 μg (testis) total RNA. After hybridization, samples were digested with RNase T1 and RNase A (Roche Diagnostics Corp., Indianapolis, IN), resolved by denaturing PAGE, and visualized by autoradiography. Band intensities from multiple experiments were quantified by phosphorimagery using a Phosphoimager SI (GE Healthcare Bio-Sciences) and ImageQuant software.
Nuclear extract preparation and EMSAs
Nuclear proteins were prepared from pooled whole testes and ovaries of 21-d-old mice, as described (29). Studies used three independent preparations of nuclear proteins from wild-type mice and two from null mice. Each nuclear protein preparation was analyzed at least twice. EMSAs were performed as previously described using a radiolabeled double-stranded E-box oligodeoxynucleotide (Table 1) using either 10 or 3.5 μg nuclear protein from testes or ovaries, respectively (30). EMSA results with 10 μg nuclear proteins from wild-type ovaries (n = 1; see Fig. 3A) were similar to those performed with 3.5 μg. Where indicated, a 100-fold m excess of unlabeled competitor oligodeoxynucleotide (Table 1) or 2 μg specific antibody was added to the reaction before the addition of probe. Antibodies were as described for ChIP. Band intensities of the USF complexes were quantified from three EMSAs of wild-type ovaries and six EMSAs of wild-type testes using phosphorimagery (Storm 860 PhosphorImager; GE Healthcare Bio-Sciences) and ImageQuant TL software. A value for each USF complex was calculated from the signal intensity determined for the specific complex minus that from an area of equal size and migration distance within the sample lane containing both USF1 and USF2 antibodies. The relative abundance of USF1 and USF2 homodimers was determined by dividing their intensity values (unshifted complex when opposing antibody was present) by that of the total USF complex (no competitor or antibody).
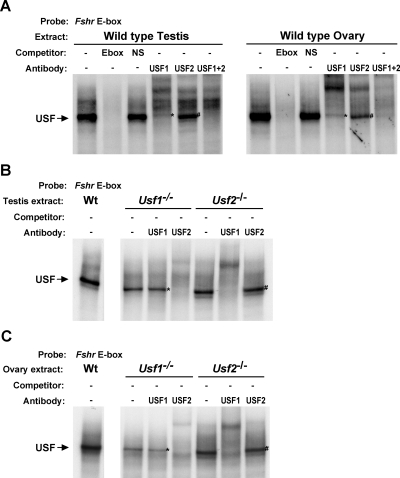
Figure 3.
In vitro binding to the Fshr E-box in wild-type (Wt) and Usf-null testes and ovaries. EMSAs evaluated transcription factor binding to the E-box using nuclear protein extracts isolated from testis and ovaries of 21-d-old wild-type mice (A) or testes (B) and ovaries (C) of Usf1−/− and Usf2−/− mice. Mouse genotypes from which nuclear protein extracts were prepared, the inclusion of competitor oligodeoxynucleotides at 100-fold m excess, and 2 μg antibodies against USF1 and/or USF2 are noted above the autoradiograms. Labeled arrows to the left of the autoradiograms indicate the position of the USF binding complex. Asterisks note USF2 homodimers, and the number sign (#) notes USF1 homodimers. Densitometric quantification of wild-type EMSAs is shown in Table 2. NS, Nonspecific.
Western blot
Western blot analysis was performed as described (11). Briefly, nuclear extracts from 21-d-old mice (20 μg ovary or 40 μg testis) were resolved by SDS-PAGE, transferred to polyvinylidene fluoride (Millipore Corp., Billerica, MA), blocked, and probed overnight at 4 C with USF1 primary antibody (1:10,000 dilution). After incubation with secondary antibody, protein complexes were visualized by chemiluminescence using the SuperSignal West Femto Maximum Sensitivity kit (Pierce, Rockford, IL). Primary antibodies were rabbit anti-USF1 (Santa Cruz Biotechnology), rabbit anti-USF2 (AnaSpec, San Jose, CA), and mouse anti-RNA polymerase II (Covance Research Products, Denver, PA). Horseradish peroxidase-conjugated secondary antibodies were donkey antirabbit IgG or donkey antimouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Blots were stripped [100 mm 2-mercaptoethanol, 2% sodium dodecyl sulfate, and 62.5 mm Tris-HCl (pH 6.7)] for 30 min at 55 C according to manufacturer’s recommendations (Millipore), and reprobed sequentially for USF2 (1:500 dilution) and RNA polymerase II (1:1000 dilution). USF1 and USF2 protein levels were measured by densitometry using ImageQuant TL software, and resulting values were made relative to levels of RNA polymerase II.
Results
USF binding to the Fshr promoter correlates with gene activity
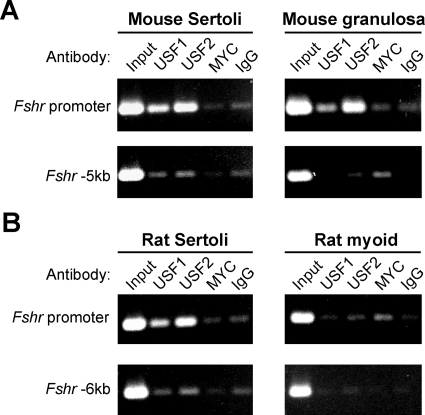
To determine whether the USF proteins bound the Fshr E-box, in vivo, their interaction with the endogenous promoter was evaluated using ChIP and primary mouse Sertoli and granulosa cells as sources of transcriptionally active Fshr. In both Sertoli and granulosa cells, immunoprecipitations with either USF1 or USF2 antibodies significantly enriched for DNA containing the Fshr promoter, compared with immunoprecipitations with control antibodies (MYC and normal IgG) (Fig. 1A). Importantly, USF immunoprecipitations did not enrich DNA containing a region located 5-kb upstream of the promoter that does not contain USF binding sites, indicating that the USF-promoter interactions were specific. The apparent modest enrichment of upstream region DNA in USF immunoprecipitations likely results from slight differences in chromatin shearing or nonspecific immunoprecipitation (Fig. 1A). USF-Fshr promoter binding was also compared between rat Sertoli cells and a nonexpressing cell type, peritubular myoid cells, isolated concurrently from the same animals (Fig. 1B). Although USF1 and USF2 immunoprecipitation enriched Fshr promoter DNA in Sertoli cells, no such enhancement occurred with myoid cells. These data provide the first direct evidence that USF1 and USF2 bind the endogenous Fshr promoter, and indicate that the interactions depend on transcriptionally active Fshr.
Figure 1.
The USF proteins bind the endogenous Fshr promoter in expressing cells. Interactions between the USF proteins and Fshr promoter, in vivo, were evaluated using ChIP with cross-linked chromatin from primary Sertoli cells and granulosa cells isolated from wild-type immature (21 d old) mice (A) or 15-d-old primary rat Sertoli and peritubular myoid cells (B). In DNA isolated from each immunoprecipitated chromatin or input sample, the promoter region (upper) and a negative control region (lower) located 5000 bp (mouse) or 6000 bp (rat) upstream of exon 1 were amplified by PCR. Immunoprecipitations for USF1 or USF2 were compared with immunoprecipitations with a control antibody against MYC, which does not bind the Fshr E-box, in vitro, and with normal rabbit IgG. Chromatin samples and antibodies used for immunoprecipitation are noted above each ethidium bromide-stained agarose gel.
USF deficiency impairs Fshr expression in the ovary
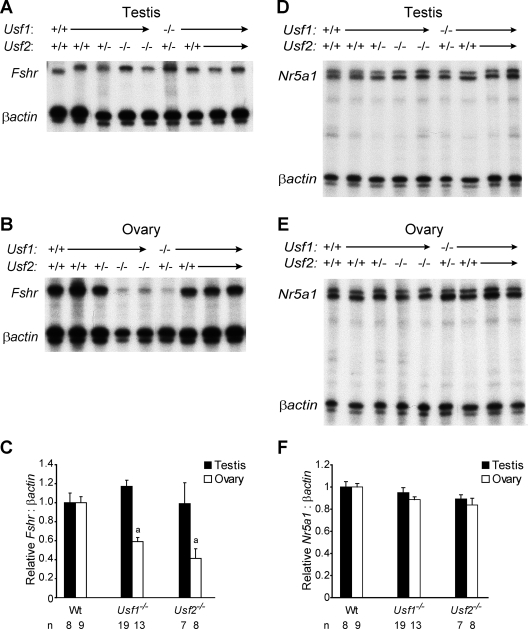
To examine further the role of USF-E-box interactions in Fshr expression, Fshr mRNA levels were quantified in the testes and ovaries of wild-type and USF-deficient mice (Usf1−/− and Usf2−/−), using RNase protection and phosphorimagery (Fig. 2). Evaluation of Fshr mRNA in the testis (normalized to βActin mRNA) showed comparable levels in wild-type, Usf1−/−, and Usf2−/− mice (Fig. 2, A and C). On the contrary, ovarian Fshr mRNA levels in Usf1−/− and Usf2−/− mice were reduced to 60 and 40% of wild type, respectively (Fig. 2, B and C). When considered together with the results of ChIP analysis, the finding suggests that reduced Fshr expression results from the direct loss of USF at the Fshr promoter. However, previous studies identify another potential mechanism involving the transcription factor SF-1 (or NR5A1), which was identified as both a target of USF and an activator of Fshr (15,31,32,33). Because Sertoli cells and granulosa cells express both SF-1 and FSHR, a reduction in SF-1, due to USF loss, could be responsible for the reduction in Fshr mRNA (34,35). To determine the potential involvement of SF-1, its transcript levels were compared in wild-type and Usf-deficient gonads. Notably, no significant difference in Nr5a1 mRNA levels was detected between wild-type, Usf1−/−, and Usf2−/− mice in either the testis or ovary (Fig. 2, D–F). Thus, transcriptional changes in SF-1 are not responsible for the diminished expression of Fshr in the ovary. Unexpectedly, Fshr mRNA levels in Sertoli cells were similar between wild-type and USF-deficient animals. This indicated that testes and ovaries respond differently to USF loss and suggested that a single USF protein is sufficient for testis expression. Although such a mechanism could be revealed by determining Fshr levels in the absence of both USF proteins, testis mRNA could not be measured in Usf1−/− Usf2−/− double mutant mice due to their embryonic lethality (22).
Figure 2.
USF deficiency compromises ovarian Fshr expression. RPA measured levels of Fshr mRNA in testes (A) and ovaries (B) of 21-d-old mice of the genotype indicated above each autoradiogram. In all RPAs, βActin mRNA levels controlled for RNA loading. C, Densitometric quantification of the RPAs presents the Fshr to βActin ratio of each genotype group relative to the Fshr to βActin ratio of wild-type (Wt) gonads. RPAs also quantified levels of Nr5a1 (SF-1) mRNA in testes (D), ovaries (E) from the same 21-d-old mice. F, Densitometric quantification of the RPAs presents the Nr5a1 to βActin ratio relative to wild type. Numbers below each bar on the graphs note the number of animals included in each group. Error bars reflect the sems, and “a” notations above bars reflect expression levels that are significantly different from wild type (P ≤ 0.0005) as determined by the Student’s t test.
USF promoter binding differs between granulosa and Sertoli cells in Usf-null animals
The differential response of Fshr to USF loss in Sertoli and granulosa cells offered several potential explanations, including differences in: 1) USF dimer requirements, 2) compensatory responses to USF loss, or 3) participating transcription factors. To help distinguish these possibilities, the composition of Fshr E-box binding complexes were evaluated in vitro and in vivo using EMSA and ChIP, respectively. In vitro binding complexes were characterized using nuclear proteins isolated from the testes and ovaries of wild-type, Usf1−/−, and Usf2−/− mice, and with each, one major complex bound the E-box (Fig. 3A). Notably, the complex was abolished by addition of homologous unlabeled E-box competitor, but not nonspecific competitor, demonstrating its specificity for the E-box sequence. As expected, in both testis and ovary, antibodies to USF1 and USF2 cross-reacted with the major binding complex (Fig. 3A).
Because dimer formation (either homodimer or heterodimer) is required for USF binding to DNA (i.e. E-box), the composition of bound complexes can be assessed by their cross-reactivity with each antibody. Thus, homodimers were identified as USF complexes bound to the DNA in the presence of one antibody but lost with addition of both antibodies; in Fig. 3A the asterisk (*) marks USF2 homodimers, and the number sign (#) marks USF1 homodimers. In both the ovary and testis of wild-type mice, heterodimers were more prevalent than either USF homodimers (Fig. 3A and Table 2). However, the relative contributions of dimer complexes differed between the testis and ovary, with fewer homodimers and more heterodimers present in the ovary than the testis. Evaluation of E-box binding using extracts from Usf-null animals showed that, in both testis and ovary, USF was retained as the major E-box binding complex, albeit as homodimers of the remaining protein (Fig. 3, B and C). Notably, there was no evidence that USF loss causes induction of other binding proteins as a method of compensation.
Table 2.
Relative abundance of USF dimers bound to the Fshr E-box in testis and ovary
| Dimer | Relative dimer abundance (%total USF binding)a
|
|
|---|---|---|
| Ovary | Testis | |
| USF1/USF2 heterodimersb | 74.3 ± 6.5 | 58.9 ± 3.7 |
| USF1 homodimersc | 23.0 ± 6.6 | 34.5 ± 4.1 |
| USF2 homodimersc | 2.7 ± 0.9 | 6.6 ± 3.1 |
Relative dimer abundance was determined by dividing the signal intensity from the specific dimer complex by that of the total USF complex (see Materials and Methods).
Heterodimer values represent the total USF signal minus that of both USF homodimers.
Homodimer values represent the signal intensities for complexes not lost in the presence of opposing antibody.
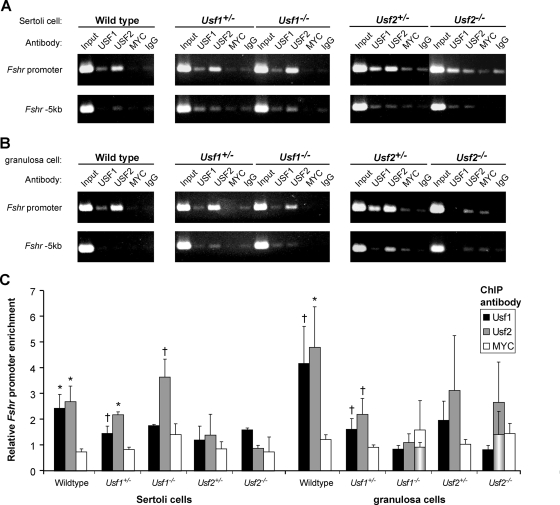
To assess whether alterations to in vivo homodimer binding were responsible for the discrepant changes in Fshr mRNA levels in USF-deficient testes and ovaries, proteins bound to the Fshr E-box in gonads from wild-type and Usf-null mice were evaluated by ChIP. Examination of USF binding in Sertoli cells indicated that, in the absence of one USF protein (i.e. Usf1−/− or Usf2−/−), promoter binding by homodimers of the remaining partner (i.e. USF2 or USF1, respectively) was enhanced or maintained (Fig. 4, A and C). In contrast, USF binding to the E-box was lost in granulosa cells lacking one binding partner (Fig. 4, B and C). Normalized data were assessed across multiple experiments and represented graphically as the relative promoter to −5-kb signals for each specific antibody, relative to that of the control antibody (IgG) (Fig. 4C). In this analysis, comparison of USF signals to that of a second control antibody, MYC, revealed that similar levels of USF1 and USF2 bound to the promoter in Sertoli and granulosa cells of wild-type mice. In contrast, signals for promoter bound USF1 and USF2, when compared with signals for antibodies against MYC (or antibodies to the deleted USF), were markedly different between Sertoli and granulosa cells of Usf-null mice. Thus, in both Usf1−/− and Usf2−/− Sertoli cells, specific promoter binding by the remaining USF protein was approximately 2-fold above that for MYC (Fig. 4C). However, in granulosa cells, there was no difference between specific promoter binding by the remaining USF protein and MYC. Thus, in Sertoli cells the absence of one USF protein seemed to favor promoter occupancy by homodimers of the remaining partner, whereas in granulosa cells, USF homodimers were not observed bound to the promoter when one partner was missing. Thus, upon the loss of one dimeric partner, homodimers of the companion protein maintained promoter occupancy in Sertoli cells, but not in granulosa cells.
Figure 4.
USF dimer binding to the endogenous Fshr promoter differs between ovaries and testes of Usf-null mice. Binding to the endogenous Fshr promoter was evaluated using ChIP in cross-linked chromatin from Sertoli cells (A) or granulosa cells (B) isolated from wild-type, Usf1+/−, Usf1−/−, Usf2+/−, and Usf2−/− mice. The promoter region and a negative control region located 5-kb upstream of the first transcriptional start site were amplified by PCR from each sample of DNA from immunoprecipitated chromatin or input. Immunoprecipitations for USF1 or USF2 were compared with immunoprecipitations with a control antibody against MYC, which does not bind the Fshr E-box, in vitro, and with normal rabbit IgG. Source cell type and genotype for chromatin samples and antibodies used for immunoprecipitation are noted above each ethidium bromide-stained agarose gel. C, Amplified DNA signals of ethidium bromide-stained agarose gels were quantified by densitometric analysis, and normalized data from multiple experiments combined and graphed. The signal for each specific antibody relative to that of control antibody (IgG) was determined for both the promoter and −5-kb regions and the normalized data, representing the ratio of the promoter to −5-kb values, were graphed as the relative promoter to −5-kb signals for each specific antibody relative to that of IgG. Bars represent mean values for USF1 (black), USF2 (gray), and MYC (white), and error bars are sems. The quantified data represent results of at least two independent PCRs for each immunoprecipitation. Granulosa cell data represented by two bars (USF2 immunoprecipitations in Usf2−/− mice and MYC immunoprecipitations in Usf1−/− mice) show data derived from all evaluated samples (rear bar) as well as that derived only from samples considered experimentally valid (front cylindrical bars). A single data point was omitted from the USF2/Usf2−/− immunoprecipitations because its relative signal (3 fold above background) contradicted the absence of this protein in the sample, indicating it was invalid. Two data points from the MYC/Usf1−/− immunoprecipitations were omitted, due to their significant deviation from the mean of all MYC samples (>2 sd values). Values for USF immunoprecipitations that were significantly higher than the corresponding MYC immunoprecipitation from the same chromatin are noted by asterisks (P ≤ 0.05) or crosses (P ≤ 0.2) located immediately above the bars. Statistical significance was determined by a Student’s t test.
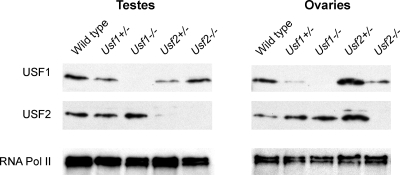
Western blot analysis was used to determine whether differences in promoter binding reflected gonadal changes in protein expression that occurred in response to the Usf deletions. Comparison of USF1 and USF2 levels in wild-type, Usf1+/−, Usf1−/−, Usf2+/−, and Usf2−/− gonads revealed significant differences in the relative changes of USF1 and USF2 across testes and ovaries with various genotypes (Fig. 5 and Table 3). Most notable was the expression difference between wild-type and Usf2+/− mice. In Usf2+/− testes, levels of both USF proteins decreased, whereas in the ovary they increased (Fig. 5). Interestingly, USF expression changes in the testis correlated well to those observed for promoter binding via ChIP, whereas protein expression in the ovary correlated poorly to promoter binding (compare Fig. 5 and Table 3 with Fig. 4C). These findings suggest that USF protein levels account for E-box occupancy in the testis, but not the ovary.
Figure 5.
Tissue-specific changes in USF protein levels accompany the loss of one USF partner. Protein levels of USF1 (top) and USF2 (middle) were measured by Western blot using nuclear extracts from testes and ovaries of wild-type, Usf1+/−, Usf1−/−, Usf2+/−, and Usf2−/− animals. Levels of RNA polymerase II (bottom) were also measured to control for protein loading. Densitometric quantification of these Western blots is shown in Table 3.
Table 3.
USF Western blot quantification
| Relative levelsa | Usf1+/− | Usf1−/− | Usf2+/− | Usf2−/− |
|---|---|---|---|---|
| Testis | ||||
| USF1 | 0.53 | 0 | 0.34 | 1.05 |
| USF2 | 0.98 | 1.29 | 0.29 | 0 |
| Ovary | ||||
| USF1 | 0.11 | 0 | 1.96 | 0.48 |
| USF2 | 2.77 | 3.37 | 4.06 | 0 |
Relative USF levels were determined by normalizing the relative USF-to-RNA polymerase II values of the indicated genotype to the relative USF-to-RNA polymerase II value of wild-type animals.
Discussion
Numerous in vitro studies substantiate the importance of a proximal E-box element and its binding factors, USF1 and USF2, in Fshr promoter function and as primary determinants of Fshr transcription (reviewed in Refs. 13 and 14). Promoter mutagenesis and transient transfection analyses demonstrated that the E-box is the principal element responsible for basal Fshr promoter activity, and mutations in the E-box that abolished USF binding, in vitro, correlated with a loss of promoter activity (10). A functional role for USF in Fshr transcription was further substantiated by experiments demonstrating Fshr promoter activation in transfected cells that overexpressed USF and promoter inhibition with dominant-negative USF proteins (lacking transactivation domains) (11). However, despite significant in vitro evidence for the E-box and USF, their in vivo function in Fshr regulation has remained poorly documented. Thus, before the present investigation, we were aware of only one study that directly supported in vivo relevance of the E-box and no studies that verified the in vivo significance of USF1 and USF2 in Fshr regulation. That is, previous in vivo genomic footprinting demonstrated E-box occupancy in Sertoli cells but did not reveal the responsible binding protein(s) (11). Thus, the current study provides the first direct in vivo evidence that USF-E-box interactions occur in the context of the endogenous Fshr promoter (Fig. 6). Although the resolution of ChIP (several hundred base pairs) precluded precise location of USF binding within the promoter region, these data combined with the previous genomic footprinting studies make a strong case for functional interaction between the USF proteins and Fshr E-box, in vivo.
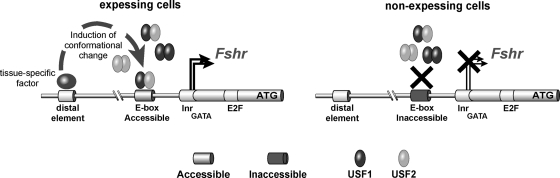
Figure 6.
A model for USF binding to the Fshr promoter and transcriptional activity of the gene. The results of this study, together with previous in vivo data, support a mechanism of Fshr regulation whereby cell-specific transcription factors bind distal regulatory elements and initiate conformational changes in the Fshr locus that are unique to Sertoli and granulosa cells and permissive to transcription. One component of these chromatin changes is an alteration to the promoter that makes it accessible to other transcription factors (e.g. USF1 and USF2) that are required for Fshr transcription. Thus, in Sertoli and granulosa cells (left), USF dimers can bind the E-box within the promoter, leading to Fshr transcription. However, in nonexpressing cells (right), the chromatin conformation is nonpermissive, leaving the E-box inaccessible. Thus, whereas the USF proteins are ubiquitous, they fail to bind the Fshr promoter in nonexpressing cells. ATG, Translational initiation codon; E2F, E2F transcription factor binding site; GATA, GATA binding protein binding site; Inr, initiator region.
The finding that USF/promoter interactions were only detected in cells expressing Fshr is another significant finding of the current study, which is also consistent with the previous in vivo footprinting data, which showed cell-specific E-box occupancy (11). Restriction of USF-Fshr E-box interactions to granulosa and Sertoli cells, despite broad USF expression, implies that a key component of Fshr gene activation, and thus its cell specificity, is the formation of a permissive state to allow USF occupancy of the E-box (36). This further suggests that other factors, which function in a cell-specific manner, facilitate USF binding to the E-box. Thus, in Sertoli and granulosa cells, it appears local chromatin changes, via modifications to histones and/or DNA, serve to reduce or eliminate constraints that normally prevent protein binding in nontranscribing cells (Fig. 6). In support of this hypothesis, previous studies showed that CpG methylation of the E-box prevented USF binding and abrogated Fshr transcription, whereas promoter demethylation was associated with transcriptionally active Fshr (17). Thus, the promoter’s methylation state appears to be one mechanism that restricts Fshr expression, by preventing USF binding in nonexpressing cell types, via methylation, or by permitting USF binding in expressing cells, via demethylation.
Currently, the factors that manage cell-specific promoter accessibility and their respective binding sites within the Fshr locus are unknown, but mounting evidence implicates a prominent role for distal regulatory elements in control of Fshr transcription. Studies in transgenic mice suggest that such cis-regulatory elements reside at significant distances from the Fshr promoter (11,37,38). Four separate reports have used DNA constructs containing different amounts of Fshr promoter sequence to test the promoter’s ability to direct cell-specific expression in transgenic mice (11,20,37,38). Notably, two of the studies showed that the promoter lacked regulatory sequences required to direct proper cell specificity (11,37). In contrast, one transgenic study demonstrated gonad-specific expression with the Fshr promoter, but the question of cell specificity was inconclusive because the identity of expressing cells was not explored (39). Recent transgenic analysis of a 413-kb yeast artificial chromosome corroborated the promoter deficiency and added additional support for the importance of distal regulatory regions in Fshr transcription (38). Despite containing a large genomic region with the entire Fshr coding sequence (from 97 kb upstream of exon 1 to 57 kb downstream of exon 10), this transgene failed to express in either Sertoli or granulosa cells (14). Furthermore, like the two previous promoter studies that investigated cellular expression, YAC transgene expression was observed in the testis but only ectopically in germ cells. These results led to the conclusion that regulatory elements required for cell-specific expression of Fshr are located at great distances from the coding sequence. In all, the data support a mechanism whereby a unique combination of transcription factors bind distally located regulatory elements, and induce a conformational change in the Fshr locus that is unique to Sertoli and granulosa cells. This change in chromatin environment includes alterations in the promoter that make it accessible to other transcription factors (e.g. USF1 and USF2) that initiate and maintain Fshr transcription (Fig. 6).
Also unique to the current study were the findings that deletion of one USF protein caused changes in USF-Fshr promoter binding and Fshr expression that suggested differences between Sertoli and granulosa cells in promoter regulation or adaptive changes in response to USF loss. Although results of Fshr expression analysis support an ovarian role for USF in Fshr transcription, neither knockout mouse indicated a role for USF in testicular Fshr expression. However, in considering the extensive evidence supporting USF control of Fshr transcription in Sertoli cells, other interpretations may be more suitable than one concluding that USF has no functional role. Alternative explanations include variations in the complement of USF dimers capable of promoter activation and/or mechanistic differences in how granulosa and Sertoli cells respond to (or compensate for) the loss of one USF protein.
Both the in vitro and in vivo binding data suggested that homodimers are less prevalent and/or less active in the ovary than in the testis, and consequently, indicated a greater dependency on heterodimers to direct Fshr transcription in the ovary. In vitro, EMSA results suggested that homodimers, under normal conditions (i.e. wild type), do not occupy the promoter in the ovary to the same extent as they do in the testis. Furthermore, comparison of in vivo promoter binding and protein levels in mice with different USF genotypes revealed discrepancies that suggested that homodimer binding and activity are less potent in the ovary than in the testis. In particular, diminished USF2 binding in Usf1−/− ovaries, despite enhanced USF2 protein levels, identified a bias against USF2 dimer interaction at the promoter that was not evident in the testis. Furthermore, comparison of USF1 ChIP results and protein levels from Usf1+/− and Usf2−/− ovaries implicated a preference for USF heterodimers because it suggested that the greater USF1 binding in Usf1+/− mice was due to the presence of USF2 and, thus, bound heterodimers. However, because protein levels were assessed using whole tissue, one must recognize that the identified changes in USF1 and USF2 might not accurately reflect changes within Sertoli and granulosa cells, the site of Fshr promoter binding. Thus, the implicated relationship between protein levels and E-box occupancy, which suggested cellular differences in promoter regulation, requires further validation of expression profiles within these specific cell populations.
In contrast to the ovary, evidence from Usf-null mice demonstrated that both USF1 and USF2 homodimers bound the Fshr promoter in the testis and presumably sustained transcriptional activity to the same level as that supported by an environment capable of forming heterodimers (i.e. wild type, Usf1+/−, and Usf2+/−). Although the role of USF1 and USF2 in differential regulation of Fshr in granulosa and Sertoli cells is still uncertain, it is tempting to speculate that the differential changes in Fshr mRNA and promoter binding, in response to different USF genetic backgrounds, represent distinct requirements for USF in ovarian and testicular Fshr regulation.
In summary, the current study confirms the importance of USF1 and USF2 in Fshr regulation by demonstrating their interaction with its promoter, in vivo. Moreover, promoter binding by these ubiquitous proteins only occurred in Fshr-expressing cells, implicating promoter accessibility as a key factor controlling cell-specific expression. Undoubtedly, additional cis-regulatory elements within the Fshr gene and their cognate binding factors contribute to promoter accessibility, and their identification is certain to shed light on the mechanisms controlling cell-specific Fshr transcription. Surprisingly, our results also revealed differences between Sertoli and granulosa cells in Fshr expression and promoter binding in response to USF loss, which suggests differences in promoter regulation and cellular responses between Sertoli and granulosa cells. This subtle difference in transcriptional regulation between two Fshr-expressing cell types could be the foundation for discrete mechanisms controlling the ability to produce receptor protein and respond to the FSH signal in the ovary and testis.
Acknowledgments
We thank Dr. Ning Lei for technical assistance and thoughtful discussions, Dr. Lane Christenson for assistance with chromatin immunoprecipitation, and Dr. David Albertini for assistance with granulosa cell isolation.
Footnotes
This work was supported by the National Institutes of Child Health & Human Development (HD35217) (to L.L.H.), a University of Kansas Medical Center Biomedical Training fellowship (to B.P.H.), and the University of Kansas Medical Center’s Center for Reproductive Sciences (National Institute of Child Health and Human Development Specialized Cooperative Centers Program in Reproduction Research; U54-HD33994).
Present address for B.P.H.: University of Pittsburgh School of Medicine, Magee-Womens Research Institute and Department of Obstetrics, Gynecology, and Reproductive Sciences, 204 Craft Avenue, Pittsburgh, Pennsylvania 15213.
Disclosure Statement: The authors have nothing to disclose.
First Published Online June 19, 2008
Abbreviations: ChIP, Chromatin immunoprecipitation; FSHR, FSH receptor; RNase, ribonuclease; RPA, ribonuclease protection assay; SF-1, steroidogenic factor 1; USF, upstream stimulatory factor.
References
- Simoni M, Gromoll J, Hoppner W, Kamischke A, Krafft T, Stahle D, Nieschlag E 1999 Mutational analysis of the follicle-stimulating hormone (FSH) receptor in normal and infertile men: identification and characterization of two discrete FSH receptor isoforms. J Clin Endocrinol Metab 84:751–755 [DOI] [PubMed] [Google Scholar]
- Sriraman V, Sairam MR, Jagannadha RA 2004 Evaluation of relative role of LH and FSH in restoration of spermatogenesis using ethanedimethylsulphonate-treated adult rats. Reprod Biomed Online 8:167–174 [DOI] [PubMed] [Google Scholar]
- Gromoll J, Simoni M, Nordhoff V, Behre HM, De Geyter C, Nieschlag E 1996 Functional and clinical consequences of mutations in the FSH receptor. Mol Cell Endocrinol 125:177–182 [DOI] [PubMed] [Google Scholar]
- Themmen APN, Huhtaniemi IT 2000 Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev 21:551–583 [DOI] [PubMed] [Google Scholar]
- Heckert LL, Griswold MD 1991 Expression of follicle-stimulating hormone receptor mRNA in rat testes and Sertoli cells. Mol Endocrinol 5:670–677 [DOI] [PubMed] [Google Scholar]
- Dankbar B, Brinkworth MH, Schlatt S, Weinbauer GF, Nieschlag E, Gromoll J 1995 Ubiquitous expression of the androgen receptor and testis-specific expression of the FSH receptor in the cynomolgus monkey (Macaca fascicularis) revealed by a ribonuclease protection assay. J Steroid Biochem Mol Biol 55:35–41 [DOI] [PubMed] [Google Scholar]
- Skinner MK, Griswold MD 2005 Sertoli cell biology. San Diego: Elsevier Academic Press [Google Scholar]
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M 2006 FSH directly regulates bone mass. Cell 125:247–260 [DOI] [PubMed] [Google Scholar]
- Goetz TL, Lloyd TL, Griswold MD 1996 Role of E-box and initiatory region in the expression of the rat follicle-stimulating hormone receptor. J Biol Chem 271:33317–33324 [DOI] [PubMed] [Google Scholar]
- Heckert LL, Daggett MA, Chen J 1998 Multiple promoter elements contribute to activity of the follicle-stimulating hormone receptor (FSHR) gene in testicular Sertoli cells. Mol Endocrinol 12:1499–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckert LL, Sawadogo M, Daggett MA, Chen J 2000 The USF proteins regulate transcription of the follicle-stimulating hormone receptor but are insufficient for cell-specific expression. Mol Endocrinol 14:1836–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing W, Sairam MR 2001 Characterization of regulatory elements of ovine follicle-stimulating hormone (FSH) receptor gene: the role of E-box in the regulation of ovine FSHreceptor expression. Biol Reprod 64:579–589 [DOI] [PubMed] [Google Scholar]
- Heckert LL 2005 Structure and regulation of the FSH receptor gene. In: Skinner MK, Griswold MD, eds. Sertoli cell biology. San Diego: Elsevier Academic Press; 281–302 [Google Scholar]
- Hermann BP, Heckert LL 2007 Transcriptional regulation of the FSH receptor: new perspectives. Mol Cell Endocrinol 260–262:100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckert LL 2001 Activation of the rat follicle-stimulating hormone receptor promoter by steroidogenic factor 1 is blocked by protein kinase a and requires upstream stimulatory factor binding to a proximal E-box element. Mol Endocrinol 15:704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness MP, Linder CC, Morales CR, Heckert LL, Pikus P, Griswold MD 1994 Relationship of a mouse Sertoli cell line (MSC-1) to normal Sertoli cells. Biol Reprod 51:116–124 [DOI] [PubMed] [Google Scholar]
- Griswold MD, Kim JS 2001 Site-specific methylation of the promoter alters deoxyribonucleic acid-protein interactions and prevents follicle-stimulating hormone receptor gene transcription. Biol Reprod 64:602–610 [DOI] [PubMed] [Google Scholar]
- Sirito M, Walker S, Lin Q, Kozlowski MT, Klein WH, Sawadogo M 1992 Members of the USF family of helix-loop-helix proteins bind DNA as homo- as well as heterodimers. Gene Expr 2:231–240 [PMC free article] [PubMed] [Google Scholar]
- Corre S, Galibert MD 2005 Upstream stimulating factors: highly versatile stress-responsive transcription factors. Pigment Cell Res 18:337–348 [DOI] [PubMed] [Google Scholar]
- Linder CC, Heckert LL, Goetz TL, Griswold MD 1994 Follicle-stimulating hormone receptor gene promoter activity. Endocrine 2:957–966 [Google Scholar]
- Levallet J, Koskimies P, Rahman N, Huhtaniemi I 2001 The promoter of murine follicle-stimulating hormone receptor: functional characterization and regulation by transcription factor steroidogenic factor 1. Mol Endocrinol 15:80–92 [DOI] [PubMed] [Google Scholar]
- Sirito M, Lin Q, Deng JM, Behringer RR, Sawadogo M 1998 Overlapping roles and asymmetrical cross-regulation of the USF proteins in mice. Proc Natl Acad Sci USA 95:3758–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova T, Presley J, Manimaran RR, Scherrer SP, Tejada L, Peterson KR, Heckert LL 2005 A FTZ-F1-containing yeast artificial chromosome recapitulates expression of steroidogenic factor 1 in vivo. Mol Endocrinol 19:2549–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao IM, Mills TM, Anderson E, Mahesh VB 1991 Heterogeneity in granulosa cells of developing rat follicles. Anat Rec 229:177–185 [DOI] [PubMed] [Google Scholar]
- Hermann BP, Heckert LL 2005 Silencing of Fshr occurs through a conserved, hypersensitive site in the first intron. Mol Endocrinol 19:2112–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi H, Christenson LK, Strauss JF 2004 Regulation of transcription of the steroidogenic acute regulatory protein (StAR) gene: temporal and spatial changes in transcription factor binding and histone modification. Mol Cell Endocrinol 215:119–126 [DOI] [PubMed] [Google Scholar]
- Harju S, Peterson KR 2001 Sensitive ribonuclease protection assay employing glycogen as a carrier and a single inactivation/precipitation step. Biotechniques 30:1198–1200 [DOI] [PubMed] [Google Scholar]
- Chen JK, Heckert LL 2001 Dmrt1 expression is regulated by follicle-stimulating hormone and phorbol esters in postnatal Sertoli cells. Endocrinology 142:1167–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuchle O, Wellauer PK 1992 A rapid method for the isolation of DNA-binding proteins from purified nuclei of tissues and cells in culture. Nucleic Acids Res 20:3555–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei N, Heckert LL 2002 Sp1 and Egr1 regulate transcription of the Dmrt1 gene in Sertoli cells. Biol Reprod 66:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daggett MA, Rice DA, Heckert LL 2000 Expression of steroidogenic factor 1 in the testis requires an E-box and CCAAT box in its promoter proximal region. Biol Reprod 62:670–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer SP, Rice DA, Heckert LL 2002 Expression of steroidogenic factor 1 in the testis requires an interactive array of elements within its proximal promoter. Biol Reprod 67:1509–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya H, Cheng YH, Lin Z, Reierstad S, Yin P, Attar E, Xue Q, Imir G, Thung S, Trukhacheva E, Suzuki T, Sasano H, Kim JJ, Yaegashi N, Bulun SE 2007 Upstream stimulatory factor-2 regulates steroidogenic factor-1 expression in endometriosis. Mol Endocrinol 22:904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K, Iida H, Nomura M, Hatano O, Honda S, Tsukiyama T, Niwa O, Hara T, Takakusu A, Shibata Y, Omura T 1994 Functional difference between Ad4BP and ELP, and their distributions in steroidogenic tissues. Mol Endocrinol 8:643–653 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Shen WH, Ingraham HA, Parker KL 1994 Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol 8:654–662 [DOI] [PubMed] [Google Scholar]
- Sirito M, Lin Q, Maity T, Sawadogo M 1994 Ubiquitous expression of the 43- and 44- kDa forms of transcription factor USF in mammalian cells. Nucleic Acids Res 22:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordhoff V, Gromoll J, Foppiani L, Luetjens CM, Schlatt S, Kostova E, Huhtaniemi I, Nieschlag E, Simoni M 2003 Targeted expression of human FSH receptor Asp567Gly mutant mRNA in testis of transgenic mice: role of human FSH receptor promoter. Asian J Androl 5:267–275 [PubMed] [Google Scholar]
- Hermann BP, Hornbaker KI, Maran RR, Heckert LL 2007 Distal regulatory elements are required for Fshr expression, in vivo. Mol Cell Endocrinol 260- 262:49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]