Abstract
Agouti-related protein (AgRP) is an orexigenic neuropeptide produced by neurons in the hypothalamic arcuate nucleus (ARC) that is a key component of central neural circuits that control food intake and energy expenditure. Disorders in energy homeostasis, characterized by hypophagia and increased metabolic rate, frequently develop in animals with either acute or chronic diseases. Recently, studies have demonstrated that proopiomelanocortin-expressing neurons in the ARC are activated by the proinflammatory cytokine IL-1β. In the current study, we sought to determine whether inflammatory processes regulate the expression of AgRP mRNA and to characterize the response of AgRP neurons to IL-1β. Here, we show by real-time RT-PCR and in situ hybridization analysis that AgRP mRNA expression in rodents is increased in models of acute and chronic inflammation. AgRP neurons were found to express the type I IL-1 receptor, and the percentage of expression was significantly increased after peripheral administration of lipopolysaccharide. Furthermore, we demonstrate that IL-1β inhibits the release of AgRP from hypothalamic explants. Collectively, these data indicate that proinflammatory signals decrease the secretion of AgRP while increasing the transcription of the AgRP gene. These observations suggest that AgRP neurons may participate with ARC proopiomelanocortin neurons in mediating the anorexic and metabolic responses to acute and chronic disease processes.
DISORDERS IN THE regulation of energy intake and energy expenditure are frequent occurrences during acute and chronic diseases (1,2). Recent studies suggest that the synthesis and release of proinflammatory cytokines, including IL-1β, IL-6, TNF-α, and leukemia inhibitory factor, in response to pathophysiological processes induce anorexia and increased metabolic rate by acting upon centers of the brain that are known to control energy homeostasis (1,3,4,5). One region in the brain that is a candidate target for the actions of inflammatory cytokines is the arcuate nucleus of the hypothalamus (ARC). The ARC is a key site that integrates peripheral signals of energy balance, and regulates energy homeostasis by transducing signals that modulate eating behavior and metabolism (6,7,8). Within the ARC, there are a number of neuropeptide-expressing neurons that play important roles in the regulation of energy homeostasis by providing either anorexigenic or orexigenic signals (8). One key population of neurons in the ARC that provides orexigenic drive is neurons that express the neuropeptide agouti-related protein (AgRP).
AgRP is an endogenous antagonist of the anorexigenic neuropeptide α-MSH [derived from the proopiomelanocortin (POMC) precursor] (9,10). AgRP promotes food intake and positive energy balance via inhibition of α-MSH-stimulated signaling, and inverse agonism at the type 3 and 4 central melanocortin receptors (MC4-R) (9,11,12). In the central nervous system, AgRP is produced exclusively by neurons in the ARC where it is colocalized with neuropeptide Y (NPY) (13) and the inhibitory neurotransmitter γ-aminobutyric acid (14). Central infusion of AgRP (15), or overexpression of AgRP in transgenic mice (16) induces hyperphagia and obesity. AgRP neurons recognize and respond to a number of peripheral signals of energy balance, including insulin and the adipose-derived hormone leptin (17). AgRP neurons express the functional long form of the leptin receptor, a member of the class-I cytokine receptor family (18), demonstrating that this population of neurons is responsive to cytokine signaling.
IL-1β is a proinflammatory cytokine that is rapidly released in response to infection and tissue injury, and can elicit a number of behavioral and metabolic responses, including anorexia, pyrexia, and increased basal metabolic rate (4,19,20,21). In rodents, anorexia can be reliably induced by both peripheral and central administration of recombinant IL-1β (4,22). The effects of IL-1β in the brain are mediated by the type I IL-1 receptor (IL-1RI), and these receptors are expressed by neurons in regions of the brain associated with energy homeostasis, including the ARC (23). In previous work, IL-1β given iv to rats induced Fos protein in NPY mRNA-expressing neurons in the ARC (22). Recently, our laboratory has shown that IL-1β specifically activates a subpopulation of ARC POMC neurons and potently stimulates the release of α-MSH from hypothalamic explants (24). In the current study, we tested the hypothesis that AgRP-expressing neurons in the ARC are targets for IL-1β’s action in the regulation of food intake and metabolism. Specifically, we investigated whether: 1) AgRP gene transcription is regulated by lipopolysaccharide (LPS), or IL-1β given as single injections, or as a result of induced acute and chronic inflammatory states; 2) AgRP peptide secretion from ex vivo hypothalamic explants is regulated by IL-1β and if prostaglandin synthesis is a required step; and 3) AgRP neurons are direct targets for the action of IL-1β by identifying AgRP neurons in the ARC that coexpress IL-1RI mRNA.
Materials and Methods
Animals
Male Sprague Dawley rats (300–350 g; Taconic Farms, Inc., Hudson, NY), F344/NTacfBR rats (200–250 g; Taconic Farms), IL-1RI knockout mice (5–8 wk of age; The Jackson Laboratory, Bar Harbor, ME), and C57BL/6J mice (5–8 wk of age; The Jackson Laboratory) were maintained on a normal 12-h light, 12-h dark cycle with ad libitum access to food (Purina rodent diet 5001; Purina Mills, LLC, St. Louis, MO) and water. Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the Animal Care and Use Committees of Oregon Health & Science University and the Oregon National Primate Research Center.
Peripheral LPS injections in rats and mice for AgRP mRNA expression study
On the day of the experiment at 0900 h, animals received ip injections of LPS [100 μg/kg (Sigma-Aldrich Corp., St. Louis, MO) dissolved in 0.5% BSA (Sigma-Aldrich) in 0.9% saline] or 0.5% BSA in 0.9% saline alone, and were placed in clean cages without food. At 1, 4, or 8 h after treatment, animals were anesthetized with isoflurane and killed by decapitation. The brains were immediately removed from the calvaria, and a hypothalamic block was dissected out, preserved in RNAlater solution (Ambion, Inc., Austin, TX), and stored at −80 C until RNA extraction and RT-PCR analysis.
Implantation of lateral ventricle cannulae
Male Sprague Dawley rats and C57BL/6J mice were anesthetized with a ketamine cocktail and placed in a stereotaxic apparatus (Cartesian Instruments, Sandy, OR). A sterile guide cannula with obturator stylet was stereotaxically implanted into the lateral ventricle. For rats, the coordinates used were: 1.0-mm posterior to bregma, 1.25-mm lateral to midline, and 4.0-mm below the surface of the skull. For mice, the coordinates used were: 1.0-mm posterior to bregma, 0.5-mm lateral to midline, and 2.3-mm below the surface of the skull (25,26). The cannula was then fixed in place with dental cement. The animals were individually housed after surgery for a minimum of 1 wk, and were handled and administered 5 μl (rats) or 1 μl (mice) intracerebroventricular (icv) injections of commercial artificial cerebrospinal fluid (aCSF) (Harvard Apparatus, Holliston, MA) daily.
Central IL-1β injection in rats for AgRP mRNA expression study
On the day of the experiment at 0900 h, rats received icv injections of 10 ng rat IL-1β (R&D Systems, Inc., Minneapolis, MN) dissolved in 5 μl aCSF, or aCSF alone, and were placed in clean cages without food. At 1, 4, or 8 h after treatment, rats were anesthetized with isoflurane and killed by decapitation. The brains were immediately removed from the calvaria, and a hypothalamic block was dissected out, preserved in RNAlater solution, and stored at −80 C until RNA extraction and RT-PCR analysis.
Central IL-1β injection in mice for AgRP mRNA expression study
On the day of the experiment at 0900 h, mice received icv injections of 10 ng murine IL-1β (R&D Systems) dissolved in 1 μl aCSF, or aCSF alone, and were placed in clean cages without food. At 1, 4, or 8 h after treatment, mice were anesthetized with isoflurane and killed by decapitation. The brains were immediately removed from the calvaria, and a hypothalamic block was dissected out, preserved in RNAlater solution, and stored at −80 C until RNA extraction and RT-PCR analysis.
Central IL-1β injection in rats for AgRP mRNA and c-fos mRNA double-label study
On the day of the experiment at 0900 h, rats received icv injections of 10 ng rat IL-1β dissolved in 5 μl aCSF, or aCSF alone, and were placed in clean cages without food. Ninety minutes after treatment, rats were anesthetized with isoflurane and killed by decapitation. The brains were immediately removed from the calvaria and frozen on dry ice. Brains were stored at −80 C until sectioned on a cryostat.
Rat tumor model
Male F344/NTacfBR rats were individually housed, and divided into three and age and weight-matched groups: sham-operated controls; tumor-bearing rats; and sham-operated rats that were pair fed with the tumor-bearing group such that they were given a quantity of food each day equal to the average amount eaten by the tumor-bearing rats from the previous day. On d 0, the animals were anesthetized with a ketamine cocktail, and fresh tumor tissue (0.2–0.3 g) from a rat donor was implanted sc into the flank of the rat as previously described (27). The tumor is a methylcholanthrene-induced sarcoma that does not metastasize. Its characteristic growth curve vs. time is curvilinear and was previously documented (27). On d 13 after tumor implantation, tumor growth had fallen within the predetermined endpoints of the study, according to the Oregon Health & Science University Institutional Animal Care and Use Committee Policy on Tumor Burden, and the animals were killed. The brains were immediately removed from the calvaria, and a hypothalamic block was dissected out, preserved in RNAlater solution, and stored at −80 C until RNA extraction and RT-PCR analysis.
Subtotal nephrectomy procedure
Chronic renal failure was induced in male F344/NTacfBR rats using a two-stage, five-sixth nephrectomy procedure. In the first stage of the procedure, age and weight-matched animals were anesthetized with a ketamine cocktail, and a left flank incision was made to allow visualization of the left kidney. Surgical ablation of two poles of the left kidney resulted in an approximate two-thirds reduction of the total left renal mass. Sham-operated control rats underwent left renal decapsulation during this stage. Animals were allowed 9 d recovery before the second stage of the nephrectomy procedure. In the second stage of the procedure, animals were anesthetized with a ketamine cocktail, and a right flank incision was made to allow visualization and surgical resection of the right kidney. Sham-operated control rats underwent right renal decapsulation during this stage. Two doses of buprenorphine (0.05 mg/kg) were administered before incision and after the operation in each stage. After the second surgery, animals were housed in individual cages, and body weight and food intake were measured daily. Animals were killed 14 d after the second surgery. Blood was collected for blood urea nitrogen and plasma creatinine assays. Brains were collected, and a hypothalamic block was dissected out, preserved in RNAlater solution, and stored at −80 C until RNA extraction and RT-PCR analysis.
Implantation of osmotic minipumps
ALZET micro-osmotic pumps (Model 1007D; DURECT Corp., Cupertino, CA) were filled with ketorolac (K1136; Sigma-Aldrich) dissolved in sterile saline, or sterile saline. Ketorolac-filled pumps were calibrated to deliver a constant infusion of 1.0 mg/kg every 6 h. Male C57BL/6J mice were anesthetized with a ketamine cocktail, and the pumps were implanted sc on the dorsal surface of each mouse. Mice were allowed 2 d recovery before use in studies.
Hypothalamic peptide secretion
Male C57BL/6J mice (n = 30) were anesthetized with isoflurane and killed quickly by decapitation. The brain was removed (with care taken to ensure that there was no contamination of the hypothalamic portion with residual pituitary), and a 2-mm slice was prepared using a vibrating microtome (Leica VS 1000; Leica Microsystems GmbH, Wetzlar, Germany) to include the paraventricular and arcuate nuclei. Individual hypothalami received a 1-h equilibration period with aCSF (aCSF; NaCl 126 mm, Na2HPO 0.09 mm, KCl 6 mm, CaCl2 4 mm, MgSO4 0.09 mm, NaHCO3 20 mm, glucose 8 mm, ascorbic acid 0.18 mg/ml, and 0.6 trypsin inhibitor units aprotinin/ml) at 37 C. Hypothalami were then incubated for 45 min at 37 C in 700 μl aCSF (basal period) before being challenged with a single concentration of murine IL-1β (0.0001–1.0 nm) in 700 μl aCSF for 45 min at 37 C. Tissue viability was verified by a 45-min exposure to 700 μl aCSF containing 56 mm KCl. Treatments were performed in quadruplicate. At the end of each treatment period, supernatants were removed and frozen at −80 C until assayed by RIA. Hypothalamic explants that failed to show peptide release three times above that of basal release in response to aCSF containing 56 mm KCl were excluded from data analysis.
Hypothalamic peptide secretion after ketorolac treatment
Male C57BL/6J mice that had been implanted with ketorolac-filled (n = 6) or saline-filled osmotic pumps (n = 12) were divided into three groups of six animals each. Mice from all groups were anesthetized with isoflurane and killed quickly by decapitation. Brains were removed and processed on a vibrating microtome as described previously. Individual hypothalami received a 1-h equilibration period with aCSF at 37 C. A single group of hypothalami from mice that received saline pumps (n = 6) were incubated for 45 min at 37 C in 700 μl aCSF plus ketorolac (121 μm) (basal period) before being challenged with 0.1 nm murine IL-1β in 700 μl aCSF plus ketorolac (121 μm) for 45 min at 37 C. Hypothalami from the remaining two groups were incubated for 45 min at 37 C in 700 μl aCSF (basal period) before being challenged with 0.1 nm (n = 6 for saline and ketorolac-treated mice) murine IL-1β in 700 μl aCSF for 45 min at 37 C. Tissue viability was verified by a 45-min exposure to 700 μl aCSF containing 56 mm KCl. Treatments were performed in quadruplicate. At the end of each treatment period, supernatants were removed and frozen at −80 C until assayed by RIA. Hypothalamic explants that failed to show peptide release three times above that of basal release in response to aCSF containing 56 mm KCl were excluded from data analysis.
AgRP RIA
AgRP immunoreactivity was measured with a rabbit anti-α-AgRP (Phoenix Pharmaceuticals, Inc., Burlingame, CA). The antibody cross-reacts fully with the mature AgRP. 125I-labeled AgRP was prepared by the iodogen method and purified by high-pressure liquid chromatography (University of Mississippi Peptide Radioiodination Service Center, University, Mississippi). All samples were assayed in duplicate. The assay was performed in a total volume of 350 μl 0.06 m phosphate buffer (pH 7.3), containing 1% BSA. The sample was incubated for 3 d at 4 C before the separation of free and antibody bound label by goat antirabbit IgG serum (Phoenix Pharmaceuticals). There was 100 μl supernatant assayed. The lowest detectable level that could be distinguished from the zero standard was 0.30 fmol/tube. The intraassay coefficient of variation was determined by replicate analysis (n = 10) of two samples at AgRP concentrations of 2 and 10 fmol/tube, and the results were 7.8 and 7.5%, respectively. The interassay coefficients of variation were 10.7 and 12.1% for the range of values measured.
RNA preparation and RT-PCR
Total RNA was extracted from hypothalamic blocks using QIAGEN RNeasy kits (QIAGEN, Inc., Valencia, CA). Hypothalamic blocks were dissected by making coronal cuts at the rostral extent of the optic chiasm and caudal to the mammillary bodies; sagittal cuts were made along the optic tracts. Cortex was then removed at the level of the corpus callosum. DNA was removed from total RNA using ribonuclease (RNase)-free deoxyribonuclease (QIAGEN). Reverse transcriptase (RT) reactions were prepared using a TaqMan Reverse Transcription Kit (Applied Biosystems, Inc., Foster City, CA). For each reaction cDNA synthesis was prepared using 500 ng RNA in a reaction containing 4 μl 10 × RT buffer, 9 μl 25 mm MgCl2, 8 μl 10 mm deoxynucleotide triphosphates, 1.5 μl 50 μm random hexamers, 1 μl RNase inhibitor, 1.5 μl MultiScribe RT, as much as suffices to 40 μl with nuclease-free water. RT reactions were performed on an Eppendorf Mastercycler (Eppendorf AG, Hamburg, Germany) programmed for 25 C for 10 min, 37 C for 1 h, and 95 C for 5 min. Samples were diluted with 40 μl nuclease-free water and stored at 4 C until RT-PCR was performed.
Real-time RT-PCR was performed on an ABI 7300 Real-Time PCR System using rat-specific primer probe sets obtained from Applied Biosystems. Each RT-PCR contained 10 μl TaqMan Universal PCR Master Mix, 1 μl Assays-on-demand Gene Expression Assay Mix, 4 μl nuclease-free water, and 5 μl cDNA. Samples and endogenous controls (eukaryotic 18s rRNA) were run in duplicate to ensure repeatability. Auto comparative threshold values were calculated using 7300 relative quantity (RQ) Study Software version 1.3 and verified.
Double-label in situ hybridization
Simultaneous visualization of AgRP mRNA with either IL-1RI mRNA or c-fos mRNA in the rat brain was performed as previously reported (28), with slight modifications. Coronal brain sections (20 μm) were cut on a cryostat and thaw mounted onto Superfrost Plus slides (VWR Scientific, West Chester, PA). Hypothalamic sections were collected in a 1:6 series from the diagonal band of Broca (bregma 0.50 mm) caudally through the mammillary bodies (bregma −5.00 mm). Hindbrain sections were collected in a 1:6 series from the facial nucleus (bregma −10.00 mm) caudally through the spinal cord (26). Antisense 33P-labeled rat IL-1RI riboprobe (corresponding to bases 207–930 rat IL-1RI; GenBank accession no. M95578) (0.2 pmol/ml), or 33P-labeled rat c-fos riboprobe (corresponding to bases 210–479 rat protooncogene c-fos gene; GenBank accession nos. AY780201 and AY780202) (0.1 pmol/ml) and antisense digoxigenin-labeled rat AgRP riboprobe (concentration determined empirically) were denatured, dissolved in hybridization buffer along with tRNA (1.7 mg/ml), and applied to slides. Slides were covered with glass coverslips, placed in a humid chamber, and incubated overnight at 55 C. The following day, slides were treated with RNase A and washed under conditions of increasing stringency. The sections were incubated in blocking buffer and then in Tris buffer containing antidigoxigenin fragments conjugated to alkaline phosphatase (Roche Molecular Biochemicals, Indianapolis, IN) diluted 1:250 for 3 h at room temperature. AgRP cells were visualized with Vector Red substrate (SK-5100; Vector Laboratories, Burlingame, CA) according to the manufacturer’s protocol. Slides were dipped in 100% ethanol, air-dried, and then dipped in NTB-2 liquid emulsion (Eastman Kodak Co., Rochester, NY) diluted 1:1 with distilled water. Slides were developed 6 d (c-fos), or 11 d (IL-1RI) later and coverslipped. Determination of cells expressing both AgRP mRNA and c-fos mRNA, or IL-1RI mRNA was performed using criteria previously described (28). Briefly, AgRP mRNA-containing cells were identified under fluorescent illumination, and custom-designed software was used to count the silver grains (corresponding to radiolabeled c-fos mRNA or IL-1RI mRNA) over each cell. Signal to background ratios (SBRs) for individual cells were calculated; an individual cell was considered to be double labeled if it had an SBR of 2.5 or more. For each animal the amount of double labeling was calculated as a percentage of the total number of AgRP mRNA-expressing cells and then averaged across animals to produce mean ± sem.
Statistical analysis
Data are expressed as mean ± sem for each group. Statistical analysis was performed using SPSS (version 14.0; SPSS, Inc., Chicago, IL) and Prism (version 3.03; Prism Software Corp., Irvine, CA) software. All data were analyzed with either an unpaired t test, one-way ANOVA followed by a post hoc analysis using a Bonferroni corrected t test, or a two-way ANOVA followed by a one-way ANOVA with post hoc analysis using a Bonferroni multiple comparison test. For all analyses, significance was assigned at the P < 0.05 level.
Results
Acute inflammation increases AgRP mRNA expression
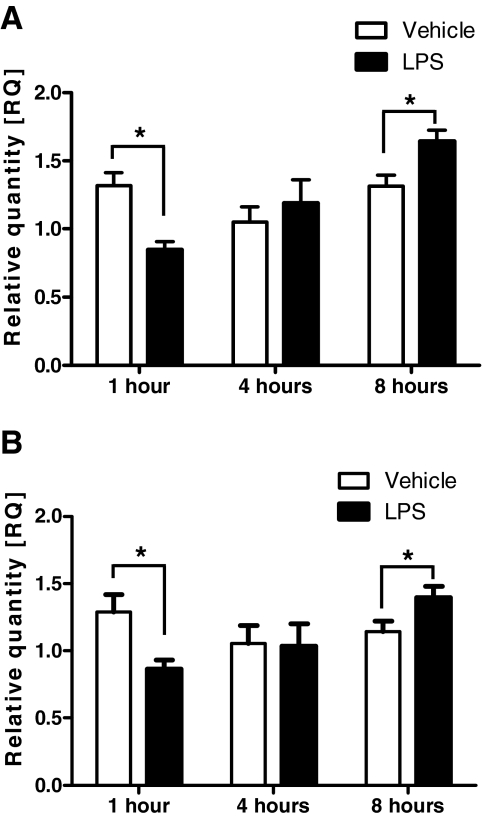
To determine whether AgRP expression is increased during periods of acute inflammation, we measured AgRP mRNA expression in two independent rodent models of acute inflammation. In our first study, we administered ip injections of LPS (100 μg/kg), or vehicle (0.5% BSA in 0.9% saline) to rats and mice at 0900 h, and killed the animals 1, 4, or 8 h later. AgRP mRNA expression was decreased 1 h after LPS treatment in both rats (RQ: LPS 0.846 ± 0.062, n = 7, vs. vehicle 1.317 ± 0.093, n = 7; P < 0.05) (Fig. 1A) and mice (RQ: LPS 0.862 ± 0.070, n = 6, vs. vehicle 1.290 ± 0.131, n = 5; P < 0.05) (Fig. 1B). There was no significant difference in AgRP mRNA expression at 4 h after LPS treatment compared with vehicle in both rats and mice (Fig. 1). At 8 h, AgRP mRNA expression was significantly increased by LPS treatment in both rats (RQ: LPS 1.642 ± 0.082, n = 11, vs. vehicle 1.313 ± 0.084, n = 9; P < 0.05) (Fig. 1A) and mice (RQ: LPS 1.396 ± 0.841, n = 11, vs. vehicle 1.142 ± 0.079, n = 12; P < 0.05) (Fig. 1B).
Figure 1.
AgRP mRNA expression in rats and mice at 1, 4, and 8 h after a single ip injection of LPS (100 μg/kg), or vehicle as determined by real-time RT-PCR. A, AgRP mRNA expression in rats. B, AgRP mRNA expression in mice. *, P < 0.05.
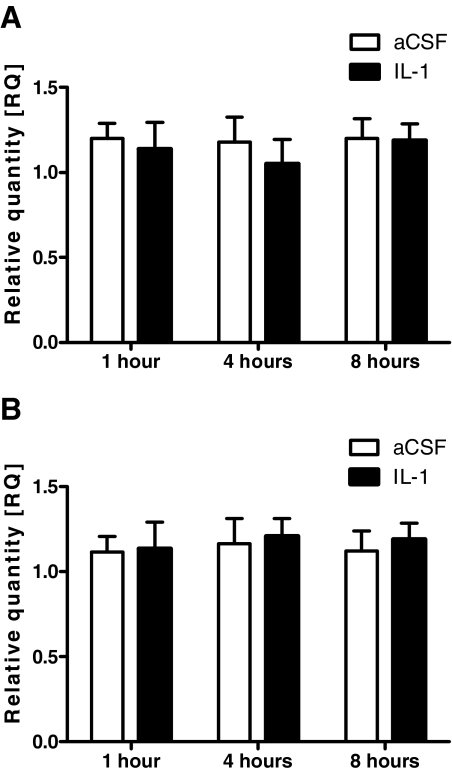
In our second study, we administered icv injections of rat IL-1β (10 ng), or aCSF to rats, and murine IL-1β (10 ng), or aCSF to mice at 0800 h, and killed the animals 1, 4, or 8 h later. Compared with aCSF treatment, IL-1β treatment did not significantly alter AgRP mRNA expression in either rats or mice at any time point measured. (Fig. 2).
Figure 2.
AgRP mRNA expression in rats and mice at 1, 4, and 8 h after a single icv injection of IL-1β (10 ng), or aCSF as determined by real-time RT-PCR. A, AgRP mRNA expression in rats. B, AgRP mRNA expression in mice. P > 0.05 for both groups at all time points studied.
Chronic inflammation increases AgRP mRNA expression
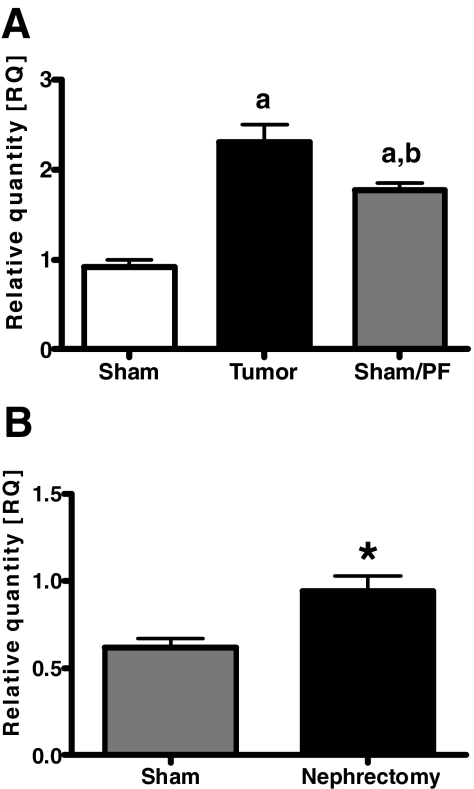
To extend our findings that AgRP mRNA expression is increased in the setting of acute inflammation, we investigated AgRP mRNA expression in two models of chronic inflammation. In our first model of tumor-induced anorexia, sham-operated, ad libitum rats had significantly increased food intake compared with both tumor-bearing rats (sham-operated, ad libitum-fed 223.2 ± 2.68 g, n = 10, vs. tumor-bearing 118.4 ± 4.56 g, n = 10; P < 0.01) and sham-operated, pair-fed rats (120.38 ± 0.24 g, n = 9; P < 0.01). We observed that AgRP mRNA expression was significantly increased in tumor-bearing rats compared with sham-operated, ad libitum fed rats (AgRP to RQ: tumor-bearing 2.31 ± 0.60, n = 10, vs. sham-operated, ad libitum-fed 0.92 ± 0.25, n = 10; P < 0.001) (Fig. 3A). AgRP mRNA expression was also elevated in sham-operated, pair-fed rats (RQ = 1.76 ± 0.26, n = 9) compared with sham-operated, ad libitum fed rats (P < 0.001) but was still significantly lower than tumor-bearing rats (P < 0.05).
Figure 3.
AgRP mRNA expression is increased in rat models of chronic inflammation as determined by real-time RT-PCR. A, Treatment groups: Sham (sham operated, ad libitum fed); Tumor (tumor bearing); and Sham/PF (sham operated, pair fed). a, P < 0.001 vs. sham; b, P < 0.05 vs. tumor. B, Treatment groups: Sham (sham operated) and Nephrectomy (renal failure). Values are presented as the mean ± sem. *, P < 0.05.
In our second model of chronic inflammation, rats that had received a two-stage, five-sixth nephrectomy procedure had significantly elevated blood urea nitrogen and plasma creatinine levels 14 d after the procedure compared with sham-operated rats (data not shown), thus confirming the effectiveness of the procedure in inducing a state of renal failure. At the time the animals were killed, nephrectomy rats were significantly lighter and had consumed less food compared with sham-operated rats (body weight: nephrectomy 164.1 ± 4.51 g, n = 16, vs. sham-operated 176.8 ± 2.65 g, n = 10; P < 0.01) (food consumed: nephrectomy 149.3 ± 5.75 g, n = 16, vs. sham-operated 195.7 ± 8.39 g, n = 10; P < 0.001). AgRP mRNA expression was found to be significantly elevated in rats with renal failure due to nephrectomy compared with sham-operated rats (RQ: nephrectomy 0.94 ± 0.34, n = 16, vs. sham-operated 0.62 ± 0.15, n = 10; P < 0.01) (Fig. 3B).
AgRP neurons express c-fos mRNA after icv IL-1β administration
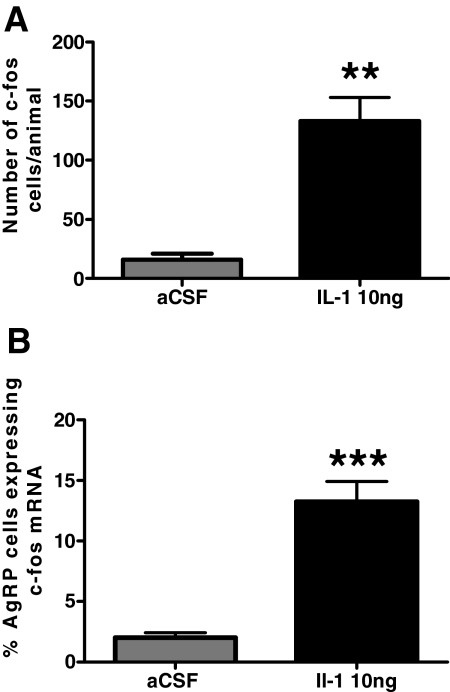
In a previous study, it was reported that an iv injection of IL-1β induced Fos immunoreactivity in ARC neurons and that 23% of these Fos-immunoreactive neurons also expressed NPY mRNA (22). To both confirm and extend upon these findings, we performed double-label in situ hybridization for AgRP mRNA and Fos mRNA on brains from rats that had been given icv injections of IL-1β (10 ng; n = 4) or aCSF (n = 4) and killed 90 min later. IL-1β treatment significantly increased the number of identifiable c-fos mRNA-expressing cells in the ARC (aCSF, 16 ± 5 cells per animal, n = 4; IL-1β, 133 ± 20 cells per animal; P < 0.01) (Fig. 4A). Coexpression of AgRP mRNA and c-fos mRNA was very low in aCSF-treated animals (2 ± 0.5%) and was significantly increased by IL-1β treatment (13.3 ± 1.7%; P < 0.001) (Fig 4B).
Figure 4.
IL-1β activates AgRP mRNA-expressing neurons in the ARC. A, The number of identifiable c-fos mRNA cells per animal after treatment with aCSF or IL-1β (10 ng). B, The percentage of AgRP mRNA-expressing cells that coexpress c-fos mRNA after treatment with aCSF or IL-1β (10 ng). Values are presented as the mean ± sem. **, P < 0.001 vs. aCSF; ***, P < 0.0001 vs. aCSF.
AgRP mRNA coexpression with IL-1RI mRNA
IL-1RI mRNA is densely expressed in the hypothalamus, suggesting that this site is responsive to IL-1β-mediated signaling. Indeed, RT-PCR analysis revealed that in the mouse hypothalamus, the abundance of IL-1RI mRNA (RQ = 2.284 ± 0.186, n = 7) is more than 2-fold higher than leptin receptor isoform Rb mRNA (1.001 ± 0.60, n = 7; P < 0.0001) and MC4-R mRNA (1.016 ± 0.084, n = 7; P < 0.0001). To determine whether AgRP mRNA-expressing cells coexpress IL-1RI mRNA, we performed double-label in situ hybridization on brains from rats that had been administered ip injections of saline (n = 6). In addition, a second group of rats in the same study received ip injections of LPS (100 μg/kg, n = 5) to measure potential changes in coexpression in the setting of acute inflammation. Both group of rats were injected at 0800 h and killed 8 h later. In both groups of rats, a significant number of AgRP mRNA-expressing neurons in the ARC, represented by cell bodies filled with fluorescent red precipitate, had overlying clusters of silver grains, signifying coexpression of IL-1RI. Semiquantitative image analysis revealed that with a SBR of 2.5 set as the threshold for neurons to be considered double labeled, 25.0 ± 2.4% of the digoxigenin-labeled AgRP neurons in saline-treated rats coexpressed IL-1RI mRNA (Fig. 5). LPS treatment caused a significant increase in the number of AgRP mRNA-expressing cells coexpressing IL-1RI mRNA (34.6 ± 2.5%) compared with saline-treated animals (P < 0.05). Coexpression of IL-1RI mRNA by ARC POMC mRNA-expressing cells was not affected by LPS treatment (data not shown).
Figure 5.
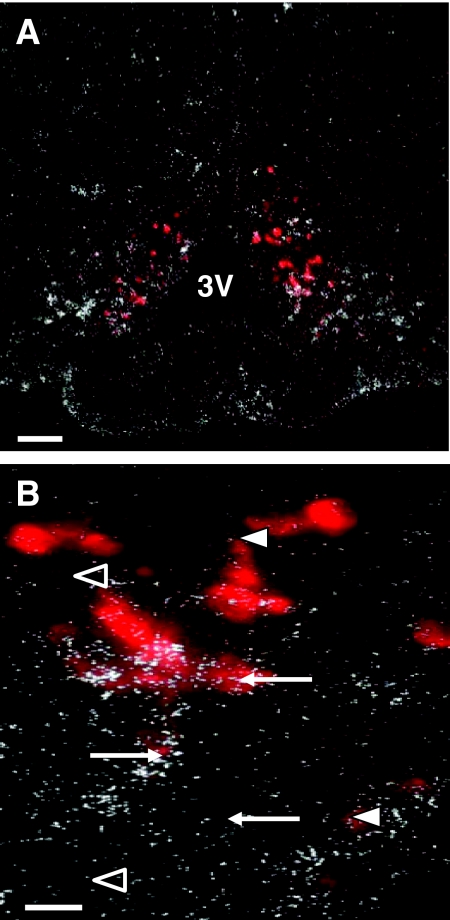
Representative photomicrographs showing coexpression of AgRP mRNA and IL-1RI mRNA in the hypothalamus. A, Photomicrograph showing distribution of IL-1RI mRNA (white-silver grain clusters) in relation to AgRP mRNA (red fluorescent cells) in the ARC of rats. B, Double-label in situ hybridization revealed clusters of silver grains overlying ARC AgRP mRNA-expressing neurons. Arrows point to AgRP neurons that coexpress IL-1RI mRNA. Open arrowheads represent AgRP neurons that do not coexpress IL-1RI mRNA. Arrowheads represent silver grain clusters not overlying AgRP neurons. Scale bars, 100 μm (A) and 25 μm (B). 3V, Third ventricle.
IL-1β decreases AgRP release from murine hypothalamic explants
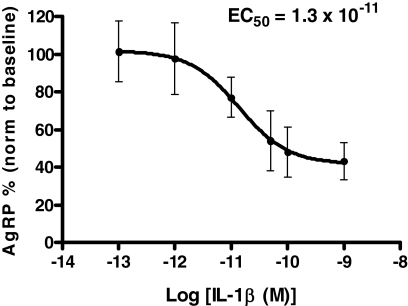
To investigate the effect of IL-1β on AgRP release in vitro, hypothalamic explants harvested from male C57BL/6 mice were incubated with IL-1β (0.01, 0.1, 1.0, 3.0, and 30.0 nm; n = 4 per IL-1β dose). These doses were chosen based on previous work estimating basal IL-1β concentration in the hypothalamus at 0.01–0.02 nm and increasing approximately 10-fold during pathological conditions (29). IL-1β significantly decreased the release of AgRP from hypothalamic explants with a calculated EC50 = 1.3 × 10−11 m (Fig. 6). These results demonstrate that in vivo hypothalamic concentrations of IL-1β that are produced during pathological conditions are able to inhibit potently the in vitro release of AgRP from hypothalamic explants.
Figure 6.
IL-1β decreases the in vitro release of AgRP from murine hypothalamic explants in a dose-dependent manner with a calculated EC50 = 1.3 × 10−11 m. Values are means ± sem (n = 4 for each dose).
Ketorolac treatment does not prevent IL-1β-mediated inhibition of AgRP secretion from murine hypothalamic explants
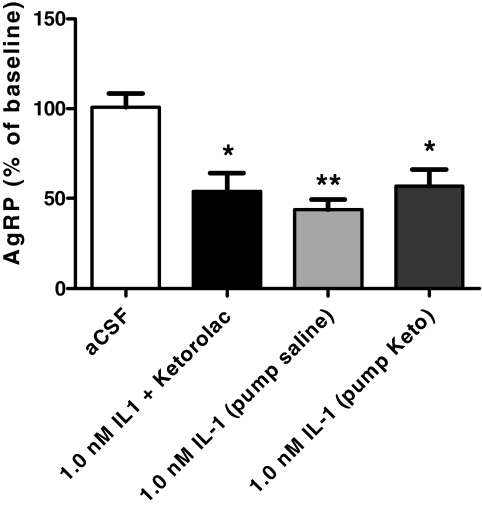
To determine whether inhibition of prostaglandin synthesis would prevent the IL-1β-mediated inhibition of AgRP secretion from hypothalamic explants, we repeated our explant studies in the presence of ketorolac. We observed that acutely blocking prostaglandin synthesis by incubating the explants in aCSF containing ketorolac (121 μm) did not alter IL-1β-mediated inhibition of AgRP release (P < 0.05 vs. aCSF) (Fig. 7). To investigate the effect of chronic blockade of prostaglandin synthesis on IL-1β-mediated inhibition of AgRP release, we implanted mini-osmotic pumps containing either ketorolac or saline 2 d before conducting the explant study. IL-1β (1.0 nm) inhibited AgRP release from hypothalamic explants from mice that had received saline-filled pumps (P < 0.01 vs. aCSF) (Fig. 7). Mice with ketorolac-filled pumps had decreased AgRP release in response to IL-1β (P < 0.05 vs. aCSF) that was not significantly different compared with mice with saline pumps (P > 0.05 vs. saline pump groups). No significant interaction was found between method of infusion and dose of IL-1β by two-way ANOVA.
Figure 7.
Acute and chronic ketorolac treatment does not attenuate IL-1β-mediated decreases in in vitro release of AgRP from murine hypothalamic explants. Data are expressed as mean ± sem (n = 6 for each dose), and were analyzed by one-way ANOVA, followed by a post hoc analysis using a Bonferroni multiple comparison test. *, P < 0.05 vs. aCSF; **, P < 0.01 vs. aCSF.
Discussion
The findings of the present study demonstrate that the transcriptional and secretory activity of AgRP neurons is regulated by inflammatory signals. AgRP mRNA expression was increased in models of both acute and chronic inflammation. AgRP neurons were found to coexpress IL-1RI mRNA, and inflammation after a peripheral dose of LPS increased the number of AgRP neurons that coexpressed IL-1RI mRNA. Finally, the secretion of AgRP from hypothalamic explants was inhibited by IL-1β, and blockade of prostaglandin synthesis failed to attenuate this observed inhibition.
AgRP mRNA expression is increased during periods of fasting. This process is hypothesized to be primarily a leptin-dependent process because leptin replacement can reverse fasting-induced increases in AgRP mRNA expression (13,30,31). In the context of these findings, one could hypothesize that decreased circulating levels of leptin as a result of illness-induced anorexia and weight loss could explain our observation that AgRP mRNA expression was increased in response to acute and chronic inflammatory states. Indeed, circulating leptin levels have been reduced in rats by two different groups investigating tumor-induced anorexia (32,33). However, other groups have demonstrated that in many acute and chronic inflammatory settings, leptin levels are paradoxically elevated despite significant decreases in food intake and body weight (34,35,36,37,38,39). An alternative hypothesis is that cytokines derived from the immune responses may be responsible for overriding classical responses of orexigenic pathways to changes in energy balance. Support for this alternative hypothesis can be found from studies examining the response of the hypothalamic NPY system to inflammation. NPY activity is potently increased in states of noninflammatory negative energy balance including, starvation and dietary restriction (40,41,42). In contrast, this strong NPY activation has not observed in many states of illness-induced anorexia and weight loss, including acute cytokine administration (43,44) and in tumor-bearing rats (45). It should be noted that in some studies, NPY mRNA expression has been increased in the hypothalamus of tumor-bearing rats (46,47). However, in these animals there was still significantly decreased hypothalamic NPY and NPY Y(1) receptor immunostaining, indicating abnormal function of NPY signaling in response to an inflammatory stimulus.
Previously, it had been reported that NPY mRNA-expressing neurons in the hypothalamus express Fos immunoreactivity in response to a peripheral injection of IL-1β (22). Because the majority of ARC NPY neurons coexpress AgRP (13), we hypothesized that IL-1β might play an important role in regulating the activity of AgRP neurons. Using double-label in situ hybridization, we demonstrated that AgRP neurons might be directly targeted by IL-1β because approximately 25% of AgRP neurons in the rat ARC were found to coexpress IL-1RI mRNA. Interestingly, we observed a significant increase in the number of AgRP neurons coexpressing IL-1RI mRNA after a peripheral injection of LPS, suggesting that the sensitivity of this orexigenic system to cytokine signaling may be enhanced during an inflammatory state. AgRP peptide secretion from hypothalamic explants was potently decreased by IL-1β administration, and this effect was not attenuated by the blockade of prostaglandin synthesis. These secretion results suggest that IL-1β has a predominant inhibitory effect on the population of AgRP neurons. This hypothesis is supported by our observation that very few AgRP neurons (13.3 ± 1.7%) coexpress c-fos mRNA in response to IL-1β. We did not observe an increase in AgRP mRNA expression in mice or rats at any time point after they had received a central injection of IL-1β. One explanation for this observation is that the time points we chose to study were either too early or too late to measure changes in AgRP mRNA expression that might have occurred. A second possibility is that central injections of IL-1β may not be sufficient to alter AgRP mRNA expression and that systemic injections of IL-1β may be necessary to see changes in AgRP mRNA expression. Providing support to this possibility is the observation that differential expression of c-fos mRNA is observed in the rat brain in response to either systemic or central injections of IL-1β (48). However, it seems more likely that IL-1β may act principally to regulate the secretory activity of AgRP neurons, and does not have a role in regulating AgRP transcriptional activity. Indeed, we were also unable to detect changes in POMC mRNA expression in mice and rats 8 h after they had received a central injection of IL-1β (data not shown) but have clearly shown in previous experiments that IL-1β activates ARC POMC neurons, leading to the increased release of α-MSH (24).
Currently, there exist conflicting data in the literature regarding the role of the ARC in mediating IL-1β-induced anorexia that results from this study may help to reconcile. In an earlier study, it was shown that destruction of the ARC, by neonatal monosodium glutamate (MSG) treatment, or knife cut disruption of vertical projections from the ARC to the paraventricular nucleus resulted in augmented anorexia in response to IL-1β (22). From these results the authors concluded that the apparent role of the ARC was not to promote but, rather, restrain IL-1β-induced anorexia. However, these data and the authors’ conclusion appear to be at odds with recently published data from our laboratory demonstrating that IL-1β activates ARC POMC neurons and potently stimulates the release of the anorexigenic neuropeptide α-MSH from hypothalamic explants (24). An important caveat to the MSG ablation studies is that IL-1β sensitive POMC-expressing neurons in the lateral hypothalamus were left intact. Previously, in studies comparing the effects of MSG vs. gold thioglucose in mice, it had been noted that the lateral POMC-expressing neurons (largely spared by MSG) may be more important in the control of body weight and feeding than the medial POMC-expressing neurons (49). These observations, when coupled with results from another study showing that AgRP cell body and fiber staining is virtually eliminated after MSG treatment (50), may explain why more pronounced anorexia is observed in MSG-treated animals treated with IL-1β.
In an intact setting, IL-1β-mediated activation of ARC POMC neurons (causing increased α-MSH release) and inhibition of AgRP neurons (causing decreased AgRP release) produce a strong anorexigenic effect via increased MC4-R signaling. Single injections of AgRP have induced hyperphagia for as long as 1 wk in the rat (15). The long duration of the orexigenic effect of AgRP suggests that during periods of illness, the release of AgRP acts as a balancing orexigenic signal that regulates the severity of anorexia and provides a mechanism for a period of rebound hyperphagia when the illness is resolved. Teleologically, an initial period of hypophagia with an acute illness may be beneficial because energy that would otherwise be spent in the acquisition and digestion of food is shunted to systems combating the illness. Hyperphagia after resolution of the illness would allow recovery of energy that had been used during the period of illness. However, lesions of the ARC that ablate both POMC and NPY/AgRP neurons in the ARC, but spare IL-1β-sensitive extra-arcuate POMC neurons in the hypothalamus, disrupt the normal competition that occurs at MC4-Rs between α-MSH and AgRP. Worsened anorexia in response to IL-1β might then occur due to a complete loss of AgRP competition at MC4-Rs that are being activated by α-MSH coming from the remaining IL-1β-sensitive extra-arcuate POMC neurons. Our observations that AgRP secretion is decreased in response to IL-1β, but that the biosynthetic capacity for AgRP neurons to produce AgRP is increased in response to inflammation, support this hypothesized model.
In summary, we have shown that the expression of AgRP mRNA is increased in models of both acute and chronic inflammation, and that the secretion of AgRP from hypothalamic explants is decreased in response to IL-1β. Combined with previous work demonstrating that α-MSH secretion is increased in response to IL-1β, our data support a role for increased hypothalamic melanocortin signaling in mediating cytokine-induced anorexia during acute and chronic inflammatory states.
Acknowledgments
We thank Erin Jobst, Maria Glavas, Pushpa Sinnayah, Aaron Eusterbrock, and Autumn Fletcher for technical assistance, and Dr. Stephanie Krasnow for helpful comments.
Footnotes
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants DK 70333 and NIDDK DK 62207 (to D.L.M), National Institutes of Health Grants RR00163 NIDDK DK62202 (to M.A.C.), American Diabetes Association Grant 1-05-RA-81 (to M.M.M), and American Heart Association Predoctoral Fellowship 0515502Z (to J.M.S.).
Disclosure Summary: J.M.S., X.Z., P.J.E., D.D.B., A.K.B., P.R.L., W.F.G., M.M.M., and D.L.M. have no conflicts of interest to declare. Oregon Health & Science University and M.A.C. have a significant financial interest in Orexigen Therapeutics Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict has been reviewed and managed by the Oregon Health & Science University Conflict of Interest in Research Committee and the Integrity Program Oversight Council.
First Published Online June 26, 2008
Abbreviations: aCSF, Artificial cerebrospinal fluid; AgRP, agouti-related protein; ARC, arcuate nucleus of the hypothalamus; icv, intracerebroventricular; IL-1RI, type I IL-1 receptor; LPS, lipopolysaccharide; MC4-R, type 4 central melanocortin receptor; MSG, monosodium glutamate; NPY, neuropeptide Y; POMC, proopiomelanocortin; RNase, ribonuclease; RQ, relative quantity; RT, reverse transcriptase; SBR, signal to background ratio.
References
- Hart BL 1988 Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–137 [DOI] [PubMed] [Google Scholar]
- Tisdale MJ 1997 Biology of cachexia. J Natl Cancer Inst 89:1763–1773 [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR 1996 Anorexia induced by activators of the signal transducer gp 130. Neuroreport 7:841–844 [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR 1998 Cytokine-induced anorexia. Behavioral, cellular, and molecular mechanisms. Ann NY Acad Sci 856:160–170 [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR 2000 Central nervous system mechanisms contributing to the cachexia-anorexia syndrome. Nutrition 16:1009–1012 [DOI] [PubMed] [Google Scholar]
- Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ 2001 The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord 25(Suppl 5):S63–S67 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ 2001 Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484 [DOI] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS 1999 Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 20:68–100 [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS 1997 Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138 [DOI] [PubMed] [Google Scholar]
- Stutz AM, Morrison CD, Argyropoulos G 2005 The agouti-related protein and its role in energy homeostasis. Peptides 26:1771–1781 [DOI] [PubMed] [Google Scholar]
- Arora S, Anubhuti 2006 Role of neuropeptides in appetite regulation and obesity–a review. Neuropeptides 40:375–401 [DOI] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR 1998 A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of α-melanocyte stimulating hormone in vivo. Endocrinology 139:4428–4431 [DOI] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW 1998 Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1:271–272 [DOI] [PubMed] [Google Scholar]
- Backberg M, Collin M, Ovesjo ML, Meister B 2003 Chemical coding of GABA(B) receptor-immunoreactive neurones in hypothalamic regions regulating body weight. J Neuroendocrinol 15:1–14 [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ 2000 Long-term orexigenic effects of AgRP-(83–132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol 279:R47–R52 [DOI] [PubMed] [Google Scholar]
- Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL 1997 Overexpression of Agrt leads to obesity in transgenic mice. Nat Genet 17:273–274 [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Mobbs CV 1999 Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology 140:814–817 [DOI] [PubMed] [Google Scholar]
- Tartaglia LA 1997 The leptin receptor. J Biol Chem 272:6093–6096 [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE 1994 A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci 14:897–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi G 1994 Pharmacology of interleukin-1 actions in the brain. Adv Pharmacol 25:1–20 [DOI] [PubMed] [Google Scholar]
- Busbridge NJ, Dascombe MJ, Rothwell NJ 1993 Chronic effects of interleukin-1 β on fever, oxygen consumption and food intake in the rat. Horm Metab Res 25:222–227 [DOI] [PubMed] [Google Scholar]
- Reyes TM, Sawchenko PE 2002 Involvement of the arcuate nucleus of the hypothalamus in interleukin-1-induced anorexia. J Neurosci 22:5091–5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Liu C, Hart RP, Sawchenko PE 1995 Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J Comp Neurol 361:681–698 [DOI] [PubMed] [Google Scholar]
- Scarlett JM, Jobst EE, Enriori PJ, Bowe DD, Batra AK, Grant WF, Cowley MA, Marks DL 2007 Regulation of central melanocortin signaling by interleukin-1 β. Endocrinology 148:4217–4225 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ 2001 The mouse brain in stereotaxic coordinates. 2nd ed. San Diego: Academic Press [Google Scholar]
- Paxinos G, Watson C 1998 The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press [Google Scholar]
- Guijarro A, Laviano A, Meguid MM 2006 Hypothalamic integration of immune function and metabolism. Prog Brain Res 153:367–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MJ, Scarlett JM, Steiner RA 2002 Cloning and distribution of galanin-like peptide mRNA in the hypothalamus and pituitary of the macaque. Endocrinology 143:755–763 [DOI] [PubMed] [Google Scholar]
- Cartmell T, Southgate T, Rees GS, Castro MG, Lowenstein PR, Luheshi GN 1999 Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain. J Neurosci 19:1517–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua Jr SC, Leibel RL, Wardlaw SL 2001 Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol 13:959–966 [DOI] [PubMed] [Google Scholar]
- Ziotopoulou M, Erani DM, Hileman SM, Bjorbaek C, Mantzoros CS 2000 Unlike leptin, ciliary neurotrophic factor does not reverse the starvation-induced changes of serum corticosterone and hypothalamic neuropeptide levels but induces expression of hypothalamic inhibitors of leptin signaling. Diabetes 49:1890–1896 [DOI] [PubMed] [Google Scholar]
- Lopez-Soriano J, Carbo N, Tessitore L, Lopez-Soriano FJ, Argiles JM 1999 Leptin and tumor growth in rats. Int J Cancer 81:726–729 [DOI] [PubMed] [Google Scholar]
- Wisse BE, Frayo RS, Schwartz MW, Cummings DE 2001 Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology 142:3292–3301 [DOI] [PubMed] [Google Scholar]
- Cheung W, Yu PX, Little BM, Cone RD, Marks DL, Mak RH 2005 Role of leptin and melanocortin signaling in uremia-associated cachexia. J Clin Invest 115:1659–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C 1998 IL-1 β mediates leptin induction during inflammation. Am J Physiol 274(1 Pt 2):R204–R208 [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR 1996 Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest 97:2152–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva F, Anker SD, Egerer K, Stevenson JC, Kox WJ, Coats AJ 1998 Hyperleptinaemia in chronic heart failure. Relationships with insulin. Eur Heart J 19:1547–1551 [DOI] [PubMed] [Google Scholar]
- Merabet E, Dagogo-Jack S, Coyne DW, Klein S, Santiago JV, Hmiel SP, Landt M 1997 Increased plasma leptin concentration in end-stage renal disease. J Clin Endocrinol Metab 82:847–850 [DOI] [PubMed] [Google Scholar]
- Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet 3rd DJ, Flier JS, Lowell BB, Fraker DL, Alexander HR 1997 Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med 185:171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Kalra PS, Kalra SP 1988 Food deprivation and ingestion induce reciprocal changes in neuropeptide Y concentrations in the paraventricular nucleus. Peptides 9:83–86 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Erickson JC, Baskin DG, Palmiter RD 1998 Effect of fasting and leptin deficiency on hypothalamic neuropeptide Y gene transcription in vivo revealed by expression of a lacZ reporter gene. Endocrinology 139:2629–2635 [DOI] [PubMed] [Google Scholar]
- Widdowson PS, Upton R, Henderson L, Buckingham R, Wilson S, Williams G 1997 Reciprocal regional changes in brain NPY receptor density during dietary restriction and dietary-induced obesity in the rat. Brain Res 774:1–10 [DOI] [PubMed] [Google Scholar]
- McCarthy HD, Dryden S, Williams G 1995 Interleukin-1 β-induced anorexia and pyrexia in rat: relationship to hypothalamic neuropeptide Y. Am J Physiol 269(5 Pt 1):E852–E857 [DOI] [PubMed] [Google Scholar]
- King PJ, Widdowson PS, Doods H, Williams G 2000 Effect of cytokines on hypothalamic neuropeptide Y release in vitro. Peptides 21:143–146 [DOI] [PubMed] [Google Scholar]
- Chance WT, Balasubramaniam A, Dayal R, Brown J, Fischer JE 1994 Hypothalamic concentration and release of neuropeptide Y into microdialysates is reduced in anorectic tumor-bearing rats. Life Sci 54:1869–1874 [DOI] [PubMed] [Google Scholar]
- Chance WT, Sheriff S, Kasckow JW, Regmi A, Balasubramaniam A 1998 NPY messenger RNA is increased in medial hypothalamus of anorectic tumor-bearing rats. Regul Pept 75-76:347–353 [DOI] [PubMed] [Google Scholar]
- Chance WT, Xiao C, Dayal R, Sheriff S 2007 Alteration of NPY and Y1 receptor in dorsomedial and ventromedial areas of hypothalamus in anorectic tumor-bearing rats. Peptides 28:295–301 [DOI] [PubMed] [Google Scholar]
- Day HE, Akil H 1996 Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-β: implications for mechanism of action. Neuroendocrinology 63:207–218 [DOI] [PubMed] [Google Scholar]
- Bergen HT, Mizuno TM, Taylor J, Mobbs CV 1998 Hyperphagia and weight gain after gold-thioglucose: relation to hypothalamic neuropeptide Y and proopiomelanocortin. Endocrinology 139:4483–4488 [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T 1998 The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA 95:15043–15048 [DOI] [PMC free article] [PubMed] [Google Scholar]