Abstract
Chronic early-life stress (ES) exerts profound acute and long-lasting effects on the hypothalamic-pituitary-adrenal system, with relevance to cognitive function and affective disorders. Our ability to determine the molecular mechanisms underlying these effects should benefit greatly from appropriate mouse models because these would enable use of powerful transgenic methods. Therefore, we have characterized a mouse model of chronic ES, which was provoked in mouse pups by abnormal, fragmented interactions with the dam. Dam-pup interaction was disrupted by limiting the nesting and bedding material in the cages, a manipulation that affected this parameter in a dose-dependent manner. At the end of their week-long rearing in the limited-nesting cages, mouse pups were stressed, as apparent from elevated basal plasma corticosterone levels. In addition, steady-state mRNA levels of CRH in the hypothalamic paraventricular nucleus of ES-experiencing pups were reduced, without significant change in mRNA levels of arginine vasopressin. Rearing mouse pups in this stress-provoking cage environment resulted in enduring effects: basal plasma corticosterone levels were still increased, and CRH mRNA levels in paraventricular nucleus remained reduced in adult ES mice, compared with those of controls. In addition, hippocampus-dependent learning and memory functions were impaired in 4- to 8-month-old ES mice. In summary, this novel, robust model of chronic early life stress in the mouse results in acute and enduring neuroendocrine and cognitive abnormalities. This model should facilitate the examination of the specific genes and molecules involved in the generation of this stress as well as in its consequences.
CHRONIC EARLY-LIFE stress (ES) in children is associated with later life morbidity, including depression and anxiety (1,2). These are accompanied by dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis, a system that is central in orchestrating the stress response (2,3). Therefore, understanding how ES influences the developing brain both short- and long-term and specifically how chronic ES affects the HPA axis and stress-sensitive brain functions including learning and memory has significant clinical importance. Animal models enable studies that dissect out the direct effects of ES from potential genetic and other confounders. Therefore, rodent and primate models of acute and repeated ES have been set up and investigated over the past half a century (3,4,5,6,7,8,9,10,11,12).
In several models of recurrent or chronic ES using immature rats, changes were evident at every level of the HPA axis. In these models, basal plasma corticosterone (CORT) and ACTH levels were significantly elevated, as were relative adrenal weights, indicative of chronic stress. In contrast, expression levels of the stress-regulating neuropeptide CRH were reduced in the hypothalamic paraventricular nucleus (PVN) (6,13). In addition to these alterations of basal levels of the components of the HPA axis, plasma CORT and ACTH responses to subsequent stress were higher in rats that had experienced ES and remained elevated longer than their control counterparts (14,15). This may indicate decreased negative feedback to the HPA axis after chronic ES (13,14,15).
The consequences of chronic ES on basal HPA axis levels and on its tone, i.e. the activation of this system in response to stress, were enduring. Thus, in response to an acute stressor, CORT responses of adult rats that had experienced chronic ES might be higher (16,17,18). In addition, chronic ES was found to lead to anatomical derangements in the hippocampus, a structure that plays a role in dampening the stress response (19,20,21,22,23,24) and is critical for spatial learning and memory (18,21,25). Following chronic ES, dendritic length and branching complexity were reduced in hippocampal pyramidal neurons (18), and this was associated with both cellular and cognitive/functional deficits. Specifically, long-term potentiation was severely impaired in hippocampal CA3 and CA1 synapses, and performance in tests examining spatial learning and memory was deficient in adult male rats that had experienced chronic ES (3,18,25,26).
Whereas rat models have contributed significantly to our understanding of the profound consequences of chronic ES on the integrity and function of the hippocampal-HPA axis both acutely and long term, the molecular mechanisms involved are only partially resolved. Genetic engineering in mice provides a powerful tool to probe the role of individual genes and molecules, even within specific anatomical locations, in these mechanisms (27,28,29,30,31,32). Therefore, we set out to characterize a chronic ES model in the mouse and define the molecular basis of the HPA axis changes involved.
Separation from the mother, a powerful stressor for the developing rodent, is useful as an acute or recurrent stressor but is impractical chronically because of the resulting inanition and hypothermia of the pups. In addition, the mother may be present in human chronic ES, but her interaction with the infant may be unpredictable and erratic (33,34,35). Therefore, in the model presented here, pup stress was evoked via fragmented maternal care, generated by reducing the amount of nesting material available to the dam. We built on data from the rat (13,15,18,36) and tested the hypothesis that modifying the nesting and bedding material provided to C57BL mouse dams would influence maternal behavior and that this altered behavior, in turn, would result in ES in the pups. We examined the effects of this ES model on HPA axis components including basal plasma corticosterone levels, hypothalamic CRH expression levels as well as arginine vasopressin (AVP) expression. Finally, we evaluated the longevity of the effects of this ES on HPA axis parameters and, in addition, analyzed selective behavioral measures in adult ES-experiencing mice compared with controls.
Materials and Methods
Animals
A total of 28 C57BL/6J primiparous, timed pregnant mice (Jackson Laboratory, Bar Harbor, ME) and their offspring of both genders (n = 132) were initially used to adapt an existing rat model of chronic early life stress to the mouse. Dams assigned to either the control or the ES group were shipped concurrently during embryonic d 11–13 to eliminate potential variation in prenatal shipment stress. Nine additional dams and their male progeny (n = 23) were used to study the long-term effects of chronic ES.
All animals were housed in temperature-controlled, quiet, uncrowded conditions on a 12-h light, 12-h dark schedule (lights on at 0600 h, lights off at 1800 h) with free access to food and water. Experiments were approved by the Institutional Animal Care Committee and carried out according to National Institutes of Health guidelines for experimental animals.
Chronic early-life stress paradigm
Dams were housed singly and cages were monitored every 12 h for the birth of pups. The day of birth was termed postnatal day (P) 0. On the morning of P2, litters were adjusted to five pups if needed and included both genders.
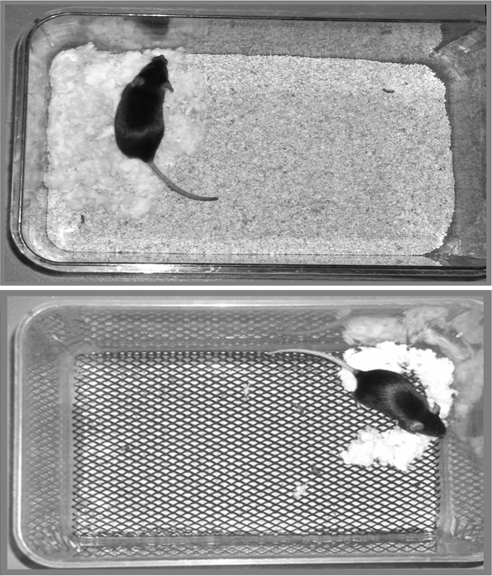
The ES paradigm was initiated on P2. Control dams (n = 7) were placed in cages with standard amounts of corn husk bedding (∼650 ml) and nesting material, i.e. one square piece of felt-like material measuring 5 × 5 cm. This material was shredded by the dam to create a nest area. In contrast, reduced amount of nesting material was available to ES dams (n = 16). For the initial characterization of the model, experimental dams with their pups were placed in cages containing varying amounts of nesting material, consisting of one third, one half, or two thirds of one felt square. In addition, in the ES cages, this nesting material was placed on a fine-gauge aluminum mesh platform (mesh dimensions 0.4 × 0.9 cm, catalog no. 57398; McNichols Co., Tampa, FL), layered approximately 2.5 cm above the cage floor. The cage floor was covered with a small amount of corn husk bedding (∼60 ml). This setup permitted mouse droppings to fall below the platform without trapping the pups. In addition, all cages were housed in a room with robust ventilation, avoiding the accumulation of ammonia. Figure 1 shows examples of the stress-provoking and standard cage setups. Both groups were completely undisturbed from P2 to P9.
Figure 1.
Photographs demonstrating the setup of a control cage in comparison with cages designed to provoke ES in immature mice. Top, A control dam in a cage with the standard amount (∼650 ml) of bedding, and one square of felt-like nesting material. Bottom, A dam in the limited-nesting cage, with minimal bedding and limited nesting material (one half square). Note the elevated mesh platform that permits dropping elimination. In both cages, the dam has shredded the nesting material to create a confined nest area.
For short-term studies, animals were killed on P9 between 0700 and 0830 h (1–2.5 h after lights on). Pups were rapidly removed from their cages, weighed, and killed by decapitation. Trunk blood was collected for plasma CORT levels. Attempts at systematic removal of both adrenal glands were unsuccessful because of their size. Brains were rapidly removed onto powdered dry ice and stored at −80 C for analysis of CRH and AVP mRNA.
To examine whether the consequences of chronic ES were persistent, 9-d-old graduates of the control (n = 4 dams) and ES treatment (n = 5 dams, two thirds square nesting material) were permitted to mature. They were transferred to standard control cages as define above on P9 and weaned at P21. Males (n = 10 control,13 ES) were housed by group and age (two to five per cage). These mice were tested for hippocampus-dependent learning and memory functions as well as in the open field test at 4–8 months. To examine plasma CORT and PVN-CRH expression, these adult mice were rapidly killed by decapitation between 0800 and 0900 h (2–3 h after lights on), trunk blood was collected and brains were dissected onto powdered dry ice and stored at −80 C for in situ hybridization for CRH mRNA. In addition, adrenals were removed and weighed.
Assessment and analysis of maternal behaviors
The duration of dam presence within the nest area, where she was in contact with the pups, was evaluated on day of life 2–8 of the pups. Control (n = 6 litters) and ES dams (n = 12) were observed three times a day in both the light (0900 and 1500 h) and dark (2000 h) phases. Each maternal observation session consisted of 30 min, during which dam-pup interaction was scored every other minute (resulting in 15 1 min epochs). Within each epoch, the duration of dam-pup contact was recorded as well as the number of times the dam left the nest (sorties). To optimize visibility and minimize interference with normal cage activities, the observer used mirrors placed above the cages and employed a transparent screen. Still, observation of licking and grooming of pups was not possible in control cages because the nesting material often formed a dome covering the nest, obstructing the observer’s view. Therefore, these parameters were not included for either control or ES groups. However, nest size in both groups was such that the dam was in contact with the pups constantly during her presence in the nest area. Therefore, the duration of interaction of dams with pups could be ascertained and was recorded, together with the frequency of sorties of the dam from the nest. These were analyzed for each observation session and correlated to both treatment group and each postpartum day, (for lights-on or -off periods) using two-way ANOVA.
Assessment of learning and memory function and anxiety-like behavior in adult mice
Three functional tests, spaced a minimum of 4 wk apart, were carried out on adult ES graduates and control cohorts. These tests were conducted in a quiet room between 0800 and 1400 h. Before learning and memory tests, animals were habituated by handling for 5 consecutive days.
Morris water-maze test.
To assess hippocampus-mediated learning and memory, the hidden platform variation of the test was administered to 4-month-old male mice (18,37,38,39). Briefly, spatial cues were placed on four walls surrounding the circular pool (120 cm diameter). Water was opacified with powdered milk and adjusted to 23 ± 1 C. Before the first training day, mice were placed in the water, led to the platform, and trained to sit on the platform for 15 sec. For the acquisition portion of the task, mice were then trained for 8 consecutive days, and given two trials per day with a 5-min intertrial interval. Between the two trials, mice were placed in individual cages lined with paper towels and covered with lids (to avoid hypothermia). The starting quadrant differed randomly for each trial, and latency to reach the platform was measured. If the platform was not reached within 60 sec, the mouse was placed on it (for 15 sec). On the ninth day, a probe trial was conducted (on five mice per group). The platform was removed from the pool, and mice were placed in the quadrant opposite from the platform quadrant and allowed to swim for 60 sec. The amount of time spent in the platform quadrant was measured.
Learning/memory analysis was carried out using two-way ANOVA. Short-term memory was assessed in the second daily trial because the mouse simply had to remember the platform location from 5 min earlier (the first daily test). Long-term memory was assessed from the first daily trial because the mouse had to remember the platform location from the previous day. For this analysis, we commenced on the second day because the first trial of the first day involved no memory.
Object recognition test.
Seven 8-month-old mice were tested for their ability to remember an object seen on the previous day (18,38,40). This test uses two objects that are presented on the first day. On the second day, one of the objects is replaced with a novel one. The test is based on the observation that mice remember an object explored 24 h earlier and typically spend more time with the novel object. The object recognition test was conducted in a white melamine box measuring 25 × 31 × 25 cm with some bedding. The box was lit indirectly with one 60-W bulb at a distance of approximately 8 feet. Before testing, mice were habituated to the box for 3 d (10 min/d). Mice were allowed to explore objects for 15 min on both the training and testing day, and exploration was defined as facing the object at a distance of 1 cm or less. For both test days, objects were wiped with soapy water after every test. Time spent exploring each object was recorded and object preference during the testing (second) day was expressed as a ratio of time spent with the novel compared with the familiar object. Total exploration time was also compared among groups.
Open field.
This test for anxiety-like behavior (36,41) was conducted using a custom white plexiglas box measuring 43 × 43 × 30 cm (MED Associates Inc., St. Albans, VT). The box floor was marked with a grid of 36 squares (6 × 6). The center 16 (4 × 4) were defined as the center, and the perimeter consisted of the squares adjacent to the box’s walls. The box was uniformly lit under bright lighting conditions. Mice aged 5–7 months were placed in the center of the box and allowed to explore for 5 min. The time spent in the periphery of the box was compared with time in the center to generate a measure of anxiety-like behavior.
RIA
Plasma CORT levels were measured in pups or adults killed within 5 min of their disturbance. The RIA for plasma CORT levels was performed using a commercial kit (MP Biomedicals, Solon, OH) as previously described (13,18). Assay sensitivity was 0.5 μg/dl.
Semiquantitative in situ hybridization (ISH)
Expression levels of CRH and of AVP mRNA were determined in the PVN using methods described previously (39,42,43). Briefly, 20-μm coronal sections were collected on gelatin-coated slides and stored at −80 C. Sections were thawed, air dried, and postfixed in 4% paraformaldehyde in phosphate buffer for 20 min. Sections were dehydrated and rehydrated through graded ethanols (3 min each) and exposed to 0.25% acetic anhydride in 0.1 m triethanolamine (pH 8) for 8 min. After graded dehydration (1 min each), sections were incubated with prehybridization/hybridization buffer containing 50% formamide, 5× SET (3 m NaCl, 0.05 m EDTA, 0.6 m Tris buffer) 0.2% sodium dodecyl sulfate, 5 × Denhardt’s solution, 0.5 mg/ml salmon sperm sheared DNA, 250 μg/μl yeast tRNA, 100 mm dithiothreitol, and 10% dextran sulfate in a humidified chamber for 1 h at 42 C.
Expression levels of CRH and AVP mRNA were investigated using 35S-labeled deoxyoligonucleotide probes complementary to the 39-bp coding to the COOH-most 13 amino acids of CRH (GATAATCTCCATCAGTTTCCTGTTGCTGTGAGCTTGCTG) and 48 bp for AVP mRNA (GTAGACCCGGGGCTTGGCAGAATCCACGGACTCTTGTGTCCCAGCCAG). Concentrations applied per section were 0.15 × 106 and 0.13 × 106 cpm for CRH and AVP probes, respectively. Briefly, sections were hybridized at 42 C overnight in 0.03 ml hybridization buffer and washed with 2× saline sodium citrate (SSC) for 1 h at 42 C, followed by 30 min each in 1× SSC and 0.3× SSC at room temperature. All sections were then dehydrated in graded ethanols containing 0.3 m ammonium acetate, followed by 95 and 100% ethanol, and apposed to film (BioMax MR film, MR-1; Eastman Kodak, Rochester, NY) for 9 h (AVP) or 4–7 d (CRH).
Data analysis and statistical considerations
All analyses were performed without knowledge of treatment group (blindly). mRNA signal was quantified by measuring OD of incorporated radioactivity over the PVN of the hypothalamus using Image Tool (University of Texas Health Science Center, San Antonio, TX; version 1.27). Linearity of hybridization signal was ascertained using 14C standards (American Radiolabeled Chemicals, St. Louis, MO). Background signal was determined over the thalamus and subtracted from hybridization signal measured over the PVN. Statistical analyses for maternal behaviors are described above. For learning and memory tests, plasma CORT levels, and ISH analyses included t test, one-factor ANOVA followed by Bonferroni’s post hoc test, or two-factor ANOVA, as appropriate. Significance level was set at 0.05, and data are presented as means with se. Analyses were performed using GraphPad Prism software (GraphPad, San Diego, CA).
Results
Reduced bedding and nesting material in the cage influences maternal interaction with her offspring
Reduction of nesting and bedding material (ES cage, shown in Fig. 1) influenced the interactions of mouse dams with their pups. Because nest size was small, dams had contact with offspring for the duration of their presence in the nest area. This duration of dam presence in the nest in contact with the pups (dam-pup interaction) was not affected by the altered cage environment (Fig. 2B). However, the frequency of leaving the nest area (dam sorties) increased significantly in dams housed in ES cages (n = 12), compared with those housed in standard cages (controls; n = 6). Figure 2A demonstrates the increased number of sorties from the nest by ES dams on P2–8, the duration of the modified cage environment [F(1,6) = 12.61, P < 0.001, effect of nesting material, two-way ANOVA].
Figure 2.
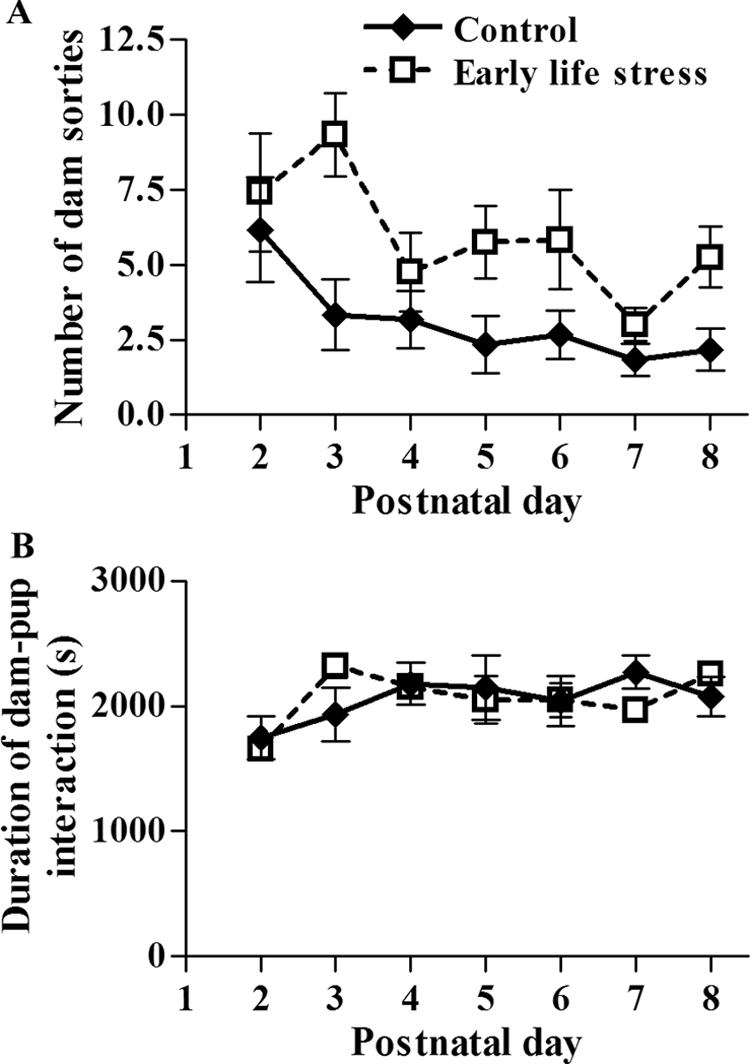
Duration of dam-pup interaction is similar for limited-nesting (ES) and control dams. In contrast, the experimental dams leave the nest area more frequently than control dams. A, Graphic depiction of the average number of sorties from the nest for control (n = 6) and ES (n = 12) dams throughout P2–P8, the period of their stay in the modified cages. The number of sorties from the nest was significantly higher for ES dams than controls [F(1,6) = 12.61; P < 0.001, effect of nesting material, two-way ANOVA]. B, Graphic depiction of the duration of dam-pup interaction (measured as time spent by each dam in contact with pups within the nest area) did not differ significantly between the two groups [F(1,6) = 0.01; P > 0.05, two-way ANOVA].
The increased number of sorties from the nest area without change in the overall duration of the dam’s interaction with the pups [Fig. 2B; F(1,6) = 0.01, P = 0.91, two-way ANOVA] resulted in fragmentation of the dam’s nurturing interactions with the pups (Fig. 3A). In other words, whereas the duration or quantity of maternal interaction with the pups was not affected by the limited nesting material, the quality of maternal care, as measured by its continuity and consistency, was disrupted (Fig. 3A). The causal relationship between reduced nesting material and altered dam-pup interaction was supported by the fact that progressive decline in the nesting material was associated with progressive increase in the frequency of dams’ sorties from the nest area [Fig. 3B; F(3,11) = 7.89, P < 0.005, one-way ANOVA].
Figure 3.
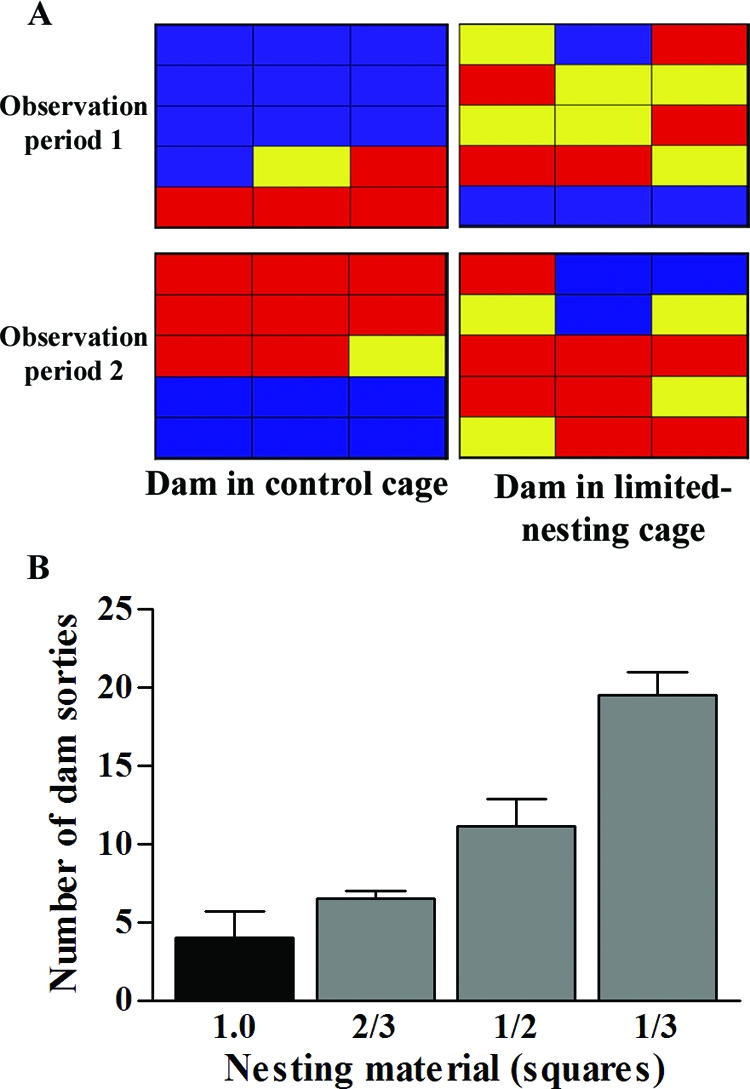
A, Representative activity grids of control and ES dams, during two matched observation periods. Each grid depicts one dam’s activity during 15 1-min epochs, and individual panes are color coded to represent the dam’s location/activity during that epoch. Blue, Dam in nest in contact with pups for the total epoch; red, outside nest area; yellow, a mixed epoch. The consistency of control dam behavior is contrasted with the fragmented pattern in the ES dam. B, Correlation of the number of sorties from the nest on P3 and the amount of nesting material provided to the dam. The number of dam sorties from the nest progressively increased as the amount of nesting material (number of felt squares) was reduced [F(3,11) = 7.89, P < 0.005, one-way ANOVA].
Fragmentation of dam-pup interaction leads to chronic stress in the pups
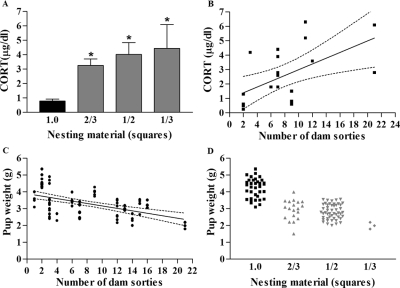
Both fragmentation of maternal interaction with her offspring, as well as the provoking reduction of nesting material, correlated with the pups’ plasma CORT levels on the last day of this chronic ES experience: on the morning of P9, plasma CORT levels of ES mice (n = 15) were significantly higher than those of controls [n = 10; Fig. 4A; F(3,22) = 12.52, P < 0.0001, one-way ANOVA]. In addition, plasma CORT levels correlated with the degree of fragmentation of dam-pup interaction, measured by the frequency of dam sorties from the nest (Fig. 4B; r2 = 0.33, linear regression). Whereas adrenal weight in relation to body weight might provide an optimal measure of chronic stress in neonatal rodents (18), we were unable to obtain these organs systematically and consistently from immature mice. Body weight itself, typically reduced in chronic stress, was influenced by this chronic stress paradigm. As shown in Fig. 4C, average pup weight was inversely proportional to the fragmentation of dam-pup interaction (r2 = 0.26, linear regression). Because this quality of the dams’ behavior was a function of the nesting material, pup weight also correlated with the amount of nesting material (Fig. 4D).
Figure 4.
Influence of reduced nesting material and fragmented dam-pup interaction on pups’ plasma CORT levels and weight on P9. A, Plasma CORT levels were 3- to 4-fold higher in ES pups (n = 15), compared with controls (n = 11), and increased significantly as nesting material amounts were progressively reduced [F(3,22) = 12.52; *, P < 0.0001, one-way ANOVA]. B, Basal plasma CORT levels correlated significantly with the number of dam sorties from the nest (measured on P3; r2 = 0.33, linear regression, 95% confidence intervals are depicted between dotted lines). C, Pup weight decreased as a function of the number of dam sorties from the nest (r2 = 0.26, linear regression, 95% confidence intervals depicted between dotted lines). D, Pup weight was positively correlated with the amount of nesting material [F(3,103) = 62.33, P < 0.0001, one-way ANOVA].
No significant differences in morning plasma CORT levels, obtained 1–2 h after lights on, were found among groups raised in cages provided with one third, one half, or two thirds felt squares, consistent with a ceiling effect, i.e. maximal stimulation of the adrenals provoked already by the more modest reduction of nesting material (Fig. 4A). In addition, a correlation was found between the number of dam sorties from the nest (fragmentation of dam-pup interaction) and plasma CORT levels of the pups (Fig. 4B). The degree of this fragmentation also correlated with the pups’ weights (Fig. 4C). Together, these data suggest that body weight and plasma CORT were independently regulated by dam-pup interaction and that plasma CORT elevations were not simply a function of reduced body weight.
The maximal reduction in nesting material used during the design and testing of this stress paradigm (one third of a felt square) led to a significant reduction in body weight and excess mortality. In contrast, weight of ES mice raised in cages with two thirds or one half felt squares was only moderately lower (30 and 35%) than that of controls (Fig. 4D, 4.2 ± 0.1 vs. 2.9 ± 0.1 and 2.8 ± 0.1 g), although larger than the reduction of weight in rat pups subjected to a similar alteration of cage environment and of dam-pup interactions (13,18). These data led to the exclusion of mice raised in cages with one third square of nesting material from further study. In addition, these data indicate that chronic ES can be provoked in mouse pups without excessive morbidity by providing the cages with one half to two thirds squares of nesting material.
CRH mRNA expression is drastically lower in chronically stressed immature mice
Release of adrenal glucocorticoids in response to stress during the first week of life in the rodent is regulated by hypothalamic CRH (44,45). In addition, during repetitive or chronic stress, AVP expression may be detectable in CRH-expressing parvocellular neurons in the PVN, in addition to the expression of this peptide in the magnocellular PVN (46,47). Therefore, we used semiquantitative ISH to measure the steady-state expression levels of these two stress hormones in control and ES P9 mice.
Levels of CRH mRNA in the PVN of ES pups were reduced by approximately 75%, compared with those of controls (Fig. 5, P < 0.0001, t test). In addition, CRH mRNA levels in P9 mice correlated inversely with the number of dam sorties on P3 (r = −0.57, P = 0.03, Spearman correlation). Expression levels of AVP mRNA did not distinguish ES from control mice (P = 0.50, t test; Fig. 6).
Figure 5.
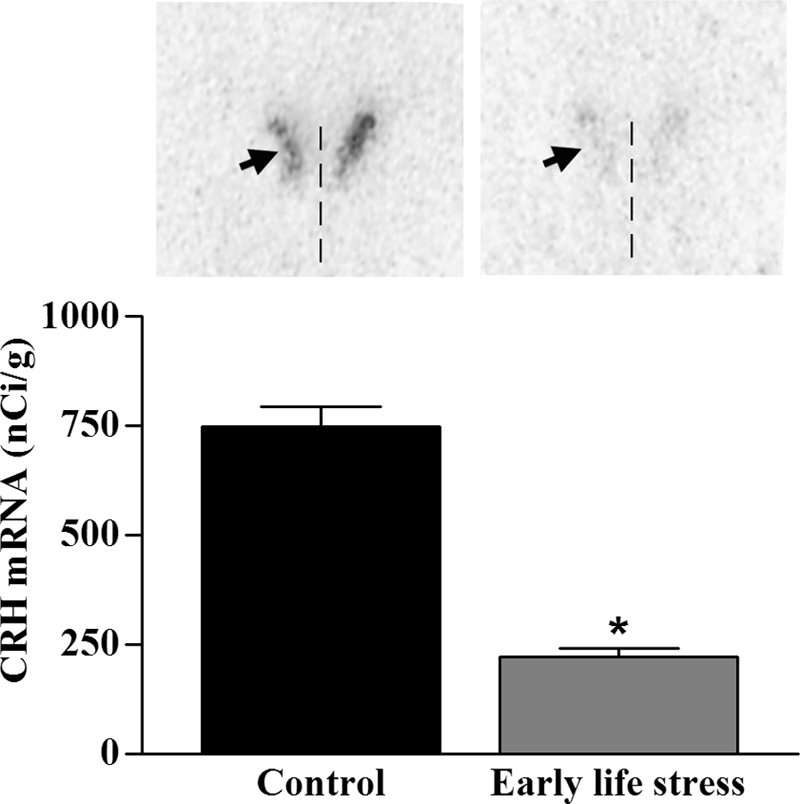
CRH mRNA expression levels in hypothalamic PVN of pups raised in control (n = 6) vs. ES (n = 8; one half square nesting material) cages. Semiquantitative analysis of the OD over PVN revealed a significant decrease in CRH mRNA in ES pups, compared with controls (*, P < 0.0001, t test). Representative sections are shown. Arrowhead, PVN; vertical line, third ventricle.
Figure 6.
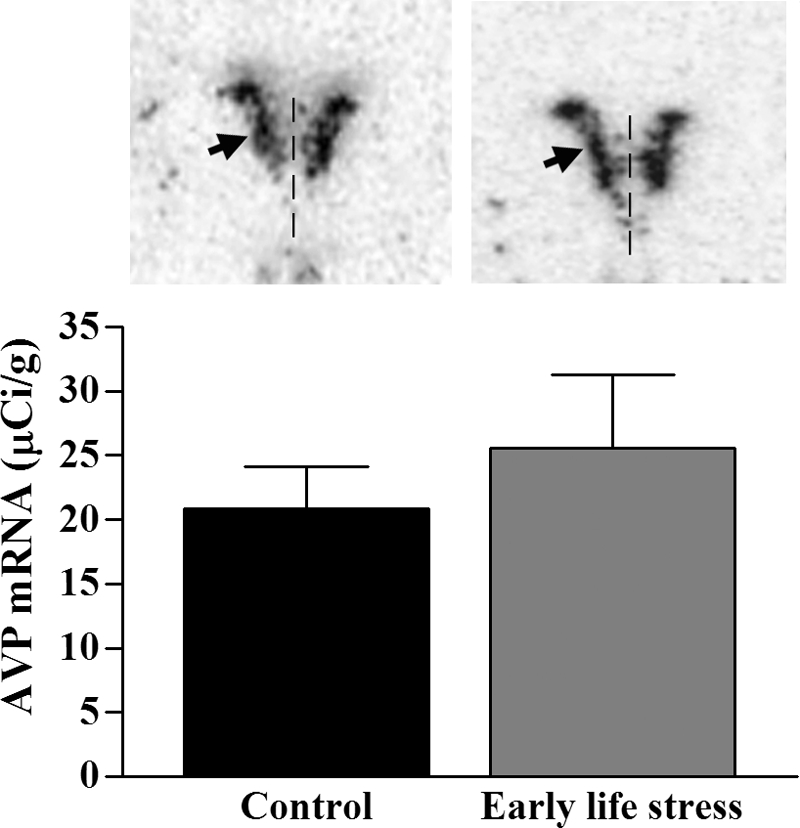
mRNA expression levels of AVP were not significantly altered in PVN of pups raised in ES (n = 4; one half and two thirds squares nesting material) vs. control (n = 4) cages. Analysis of the OD over PVN after semiquantitative ISH histochemistry revealed comparable expression levels over the PVN (P = 0.5, t test). Representative sections are shown. Arrowhead, PVN; vertical line, third ventricle.
Neuroendocrine consequences of ES persist to adulthood
The alterations in HPA axis parameters by chronic ES, found on P9 (reduced CRH mRNA expression and increased morning plasma CORT), persisted to adulthood. Basal plasma CORT levels, measured 2–3 h after lights on, remained elevated in 4- to 7-month-old ES mice, compared with those of controls (5.5 ± 1.1 vs. 2.1 ± 0.4 μg/dl; P < 0.05, t test; Fig. 7A). In addition, steady-state levels of hypothalamic CRH mRNA remained about 50% lower in adult ES mice, compared with those of controls (P < 0.05 t test; Fig. 7B). However, the reduction in weight found in ES pups normalized by adulthood (P = 0.11, t test, Fig. 7C). In addition, adrenal weight relative to body weight was not significantly different between control (n = 3) and ES (n = 4) mice (2.0 ± 0.4 × 10−4 vs. 1.7 ± 0.2 × 10−4, respectively; P = 0.46, t test) similar to findings in the rat (18).
Figure 7.
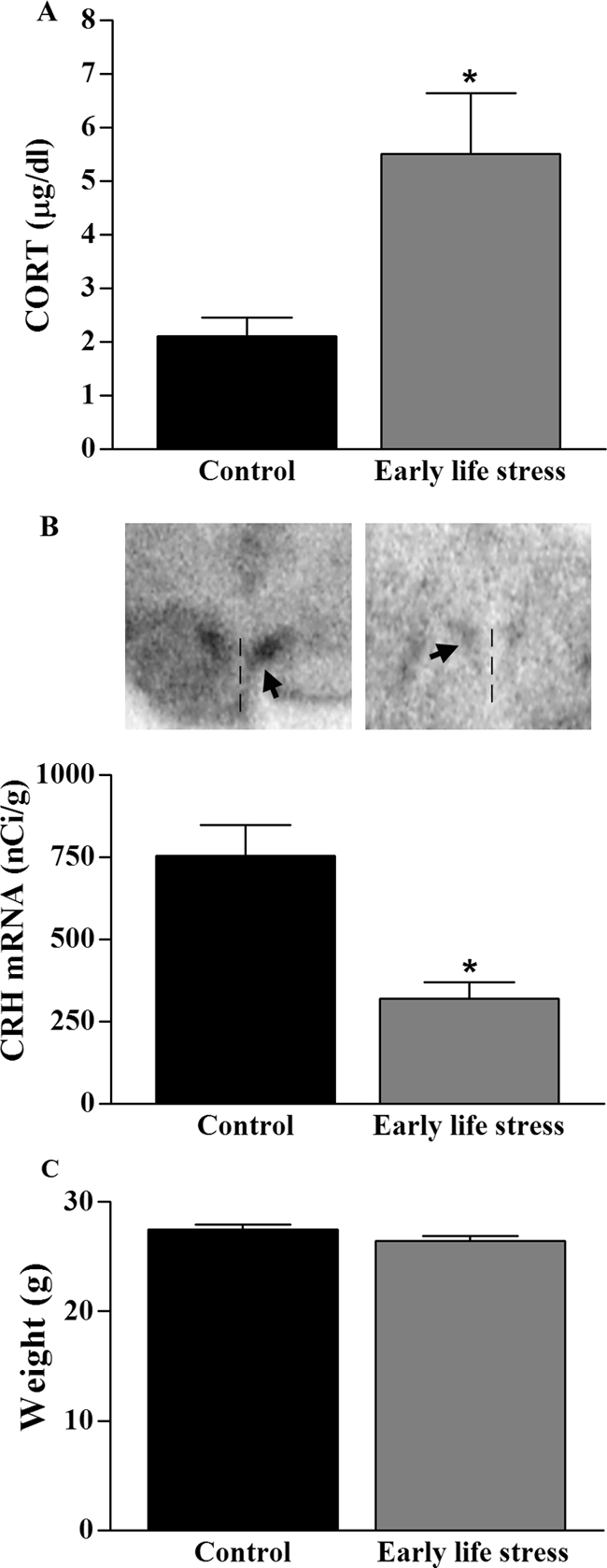
Neuroendocrine changes after ES persist to adulthood. A, ES led to elevated basal plasma CORT levels (n = 3/group; *, P < 0.05, t test). B, CRH mRNA in the PVN was significantly reduced in ES mice (n = 3), compared with controls (n = 4; *, P < 0.05, t test). Representative sections are shown. Arrowhead, PVN; vertical line, third ventricle. C. Weights of 3- to 4-month-old ES (n = 11) and control (n = 10) mice were not significantly different (P > 0.05, t test).
Hippocampal learning and memory deficits occur in 4- to 8-month-old mouse graduates of ES without overt anxiety-like behavior
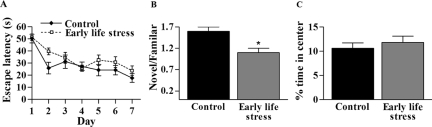
Spatial learning and memory were tested in adult mice using the Morris water maze test. Performance on this test is known to require an intact hippocampus and has been found to be impaired in early-life stressed rats. In this test, ES mice took significantly longer to reach the platform [Fig. 8A; F(1,6) = 6.13, P = 0.01, two-way ANOVA]. Further dissection of the nature of the learning and memory deficits demonstrated that both long-term memory (apparent from performance of the first test each day) and short-term memory (apparent from performance on the second daily test) were impaired. Thus, testing memory for the previous day (starting on the second trial day) revealed a significant increase in time required to reach the platform for ES mice vs. controls [F(1,5) = 5.31, P = 0.02, two-way ANOVA]. Similarly, short-term memory, assessed by the second daily trial, was also impaired in ES mice [F(1,6) = 4.43, P = 0.04, two-way ANOVA]. Performance in the probe test, a long-term spatial memory test, did not differ between groups when these were tested after 8 d of training (probably because of the ES mice eventually learning the spatial cues).
Figure 8.
Hippocampus-dependent learning and memory impairments were evident in adult ES mice. A, Acquisition rate of Morris water maze learning (average of two trials/day) over 7 d. ES mice (n = 13) took significantly longer to find the hidden platform, compared with controls (n = 10; P < 0.05, two-way ANOVA). B, The ratio of time spent with a novel object vs. a familiar one, a measure of memory, was significantly lower in adult ES mice (n = 8), compared with controls (n = 6; *, P = 0.03, t test). C, Anxiety-like behavior was not more pronounced in adult ES (n = 13) vs. control (n = 10) mice. In the open field test, percent time spent in the center of the chamber was similar between groups (P > 0.05).
Because the Morris water maze includes forced swimming, which might be stressful, the test might be confounded when comparing naive and ES groups. Therefore, this test was complemented by the object recognition paradigm, which is free from adverse situations. This test has been shown to also require an intact dorsal hippocampus (48). Seven- to 8-month-old control and ES mice subjected to the object recognition paradigm readily explored both objects on both the first (training) and second (testing) day of this memory test, and total exploration time did not differ between the groups (212 ± 14 vs. 190 ± 6 sec; P = 0.14, t test). However, on the second testing day, the controls explored the novel object for significantly longer periods than the familiar object, whereas ES mice spent almost equal time exploring the novel object and a replicate of the object from the previous day (Fig. 8B). This is consistent with the notion that the ES mice did not remember the familiar object explored the day before (18). Taken together, the results of these two learning and memory tests indicate that hippocampus-dependent cognitive function was impaired in ES mice as long as 7 months after the provoking early stress period. In addition, poor performance on the stress-free object recognition test indicates that these deficits are not simply a result of inability of the ES group to cope with the test conditions.
Indeed, in a test designed to examine for anxiety-like behavior, the open field paradigm, there were no significant differences in percent time spent in the center of the open field by ES vs. control mice (11 ± 1 vs. 12 ± 1%; P = 0.51, t test; Fig. 8C).
Discussion
The major findings of these experiments are: 1) chronic ES can be provoked in the mouse by manipulating cage environment during the first postnatal week; 2) the mechanism by which pup stress is generated involves abnormal dam-pup interaction, induced by limiting nesting material; 3) limited nesting material results in frequent sorties of the dam from the nest which, in turn, fragments dam-pup interaction, provoking stress in the pups; 4) elevated plasma CORT levels in pups are associated with depletion of CRH mRNA stores in the PVN, without changes in AVP expression; and 5) finally, the ES provoked in this novel mouse model results in profound and enduring changes in CRH expression, morning CORT levels, and hippocampus-dependent learning and memory functions. Taken together, these data provide a molecular, neuroendocrine, and cognitive characterization of a reliable model of chronic ES in the mouse.
Disruption of dam-pup interaction results in chronic ES
The crucial role of maternal presence and the role of dam-derived sensory input in governing the stress response of neonatal rodents has been amply documented (6,7,25,36,43,49,50). In addition, disruption of the maternal behavior by limiting the nesting material provided to the dams has been shown to create chronic ES in rat pups (13,36). In the current studies, we found that the total duration of dam-pup interaction did not decline in nesting-material depleted cages. Rather, dams tended to leave the nest area more frequently so that each episode of interaction with pups in the nest was shorter. In other words, in the presence of similar duration (quantity) of dam-pup interactions, poor quality of this interaction, defined as lack of consistency and frequent interruption, promoted elevated plasma CORT levels in ES pups. Indeed, the frequency of leaving the nest area (sorties) by each mouse dam was the best predictor of plasma CORT and body weight in her pups.
Note that we cannot comment on the precise nature of dam-pup interactions (e.g. nursing, sensory input from licking/grooming) in the nest and the relative disruption of each of these behaviors in the current chronic ES model. This fact is a result of inability to directly observe nurturing behavior in control cages because the abundant bedding and nesting materials obstructed the domed nest. Therefore, the duration of time the dam spent in contact with pups within the nest area was used as a surrogate marker for these activities, and whether subtle disruption of the type or sequence of specific nurturing activities accompanied the frequent sorties from the nest remains unclear. Still, using fragmentation of dam-pup interaction as an indicator of the quality of this interaction strongly suggests that quality rather than the overall quantity of maternal interaction governed the generation of ES in the pups. This distinction is consistent with reports in the rat on the lack of correlation between total duration of maternal care and HPA axis tone in offspring (51) and on the influence of qualitative measures of dams’ nurturing behaviors on HPA-related gene expression in pups (36,43).
Comparison of this to other models of ES
Existing studies of ES in the mouse have relied on separating the pups from the mother (52,53,54,55). This was done repeatedly, for 3 h per day (52,54,55) or once, for a protracted time (53). Single prolonged separation from the dam led to activation of the HPA axis, which involved metabolic signals such as hypoglycemia. Indeed, it was not possible to separate physiological and emotional stress in neonatal mice separated from the dam, and because of the overwhelming metabolic consequences, the duration of the ES generated by maternal separation is limited (6,53,56). In the chronic ES model described here, plasma CORT was highly elevated when nesting material was reduced, and pups experiencing chronic ES weighed less than unstressed controls. Interestingly, plasma CORT levels did not correlate with pup weight (r2 = 0.17), suggesting that the abnormal interaction with the dam affected stress hormone levels and body weight independently and that plasma CORT elevations were not simply a function of reduced body weight. Because the overall duration of interaction of the dam with the pups was similar in the control and ES groups it is unlikely that prolonged activation of metabolic signals such as hypoglycemia were the dominant means for generation of the chronic ES in the pups. Rather, the fragmented nature of dam-pup interaction might be at play, as suggested from studies manipulating this parameter in the neonatal rat (43,57).
Recurrent stress has recently been used in neonatal mice to explore the long-lasting effects of ES, but this approach resulted in minimal behavioral effects during adulthood (52,55). In a second study (54), recurrent maternal separation failed to provoke the tested outcome, anxiety/depression-like behaviors, in adult graduates from eight different mouse strains. In contrast, mice subjected to the chronic ES model described here had enduring changes in both molecular components of the HPA axis, as well as cognitive function, at 4–8 months of age. Similar to the recurrent ES studies, anxiety-like behaviors in conventional tests were not affected (Fig. 8C). However, defective cognitive function, accompanying severe depression in humans (58), and reported after early-life human stress (59,60) was observed. These derangements, together with enduring plasma CORT elevation and aberrant CRH mRNA expression, render the current model a useful tool to study the long-lasting consequences of ES and their neurobiological underpinnings.
The current mouse model of ES shares similarities with the rat model, but there are also differences. Similar to the rat, normalization of body weight together with enduring deficits of hippocampus-dependent learning and memory have been found in the current model (11,18). In contrast to the rat model, plasma CORT levels remain elevated in adult ES graduates and are associated with reduced CRH mRNA expression.
Selective and enduring disruption of stress-activated neuropeptide expression in the hypothalamus
Elevation of plasma CORT levels in the ES pups led to our examination of the mechanism(s) that might drive the secretion of this stress hormone. Previous work from our group has demonstrated that in the rat and throughout the first 2 postnatal weeks, CRH release was required for stress-induced elevation of plasma CORT (44). In contrast, others found that hypoglycemia-induced elevation of CORT required AVP but not CRH (61). To better understand the potential roles of CRH and AVP in the chronic ES model, in mouse, we examined the hypothalamic expression levels of these two stress-activated regulators of ACTH and CORT secretion. We found a robust reduction of steady-state CRH-mRNA levels and little change in AVP expression. These data are in line with previous findings in the chronic ES rat model (13), in which, in addition, reduced number and activity of CRH receptors was found in the pituitary (13). The lack of change in the AVP expression is distinct from the increased expression of this peptide in adult recurrent stress (47,62,63) and does not support (but also does not exclude) the possibility that AVP might assume the role of driving the HPA axis in chronic ES (64).
The reduction of CRH mRNA levels persisted in 5- to 7-month-old ES mice. This finding indicates that the cascade of events set in motion during the neonatal ES reprogrammed the set point of the HPA axis, leading to persistently altered CRH mRNA expression even long after the ES was removed. The nature of the molecular mechanisms responsible for this reprogramming is unclear. Elevated plasma CORT may be partially responsible, exerting negative feedback on CRH expression in PVN (65). More intriguing is the question of what drives high plasma CORT levels in immature and adult ES rats in which PVN CRH expression is low. A role for AVP has not been excluded; alternative hypotheses include a dissociation of CRH mRNA expression and peptide levels and release or ES-induced changes in the sensitivity of the pituitary or adrenal to signals promoting release of stress hormones.
Conclusions
In summary, a model of chronic ES in neonatal mouse is described, which likely involves both psychological and physiological components. The chronic ES derives from abnormal quality (fragmentation) of dam-pup interaction. Importantly, the ES generated in this model results in enduring derangements of the HPA axis and in neuronal dysfunction in the hippocampus.
This model will be a useful tool to study chronic ES. In a practical sense, we recommend the use of one half to two thirds felt squares as nesting material in cages with restricted bedding, to provoke reliable chronic ES with minimal cannibalism. Importantly, we hope that this mouse model will prove a widely usable and powerful tool for using genetic engineering methodologies to investigate the roles of specific genes in the mechanisms of chronic stress early in life as well as the consequences of this stress on the structure and function of HPA axis and cognitive function.
Footnotes
This work was supported by National Institutes of Health National Institute of Neurological Disorders and Stroke Grant NS28912 and National Institute of Mental Health Grant MH73136.
Disclosure Statement: The authors have nothing to disclose.
First Published Online June 19, 2008
Abbreviations: AVP, Arginine vasopressin; CORT, corticosterone; ES, early-life stress; HPA, hypothalamic-pituitary-adrenal; ISH, in situ hybridization; P, postnatal day; PVN, paraventricular nucleus; SSC, saline sodium citrate.
References
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS 1998 Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14:245–258 [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB 2004 Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 29:641–648 [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM 2001 Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol 13:419–449 [DOI] [PubMed] [Google Scholar]
- Spencer-Booth Y, Hinde RA 1971 The effects of 13 days maternal separation on infant rhesus monkeys compared with those of shorter and repeated separations. Anim Behav 19:595–605 [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Paully GS 1984 The effects of varying environmental demands on maternal and infant behavior. Child Dev 54:773–780 [PubMed] [Google Scholar]
- Levine S, Huchton DM, Wiener SG, Rosenfeld P 1991 Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev Psychobiol 24:547–558 [DOI] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Cascio CS, Dallman MF 1991 The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology 128:1385–1395 [DOI] [PubMed] [Google Scholar]
- Clarke AS 1993 Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Dev Psychobiol 26:433–446 [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ 1993 Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 18:195–200 [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Oitzl MS, Levine S, de Kloet ER 2002 The HPA system during the postnatal development of CD1 mice and the effects of maternal deprivation. Brain Res Dev Brain Res 139:39–49 [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Baram TZ 2006 Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Front Neuroendocrinol 27:180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Baram TZ 2008 The central corticotropin releasing factor system during development and adulthood. Eur J Pharmacol 583:204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ 2001 Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol 13:799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S 1993 Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology 57:204–212 [DOI] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, Baram TZ 1996 Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol 15:114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S 1988 Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav Neurosci 102:692–700 [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG 2002 Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav 73:131–140 [DOI] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ 2005 Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci 25:9328–9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM 1985 Glucocorticoid toxicity in the hippocampus: temporal aspects of neuronal vulnerability. Brain Res 359:300–305 [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R 1991 The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 12:118–134 [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS 1995 Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience 69:83–88 [DOI] [PubMed] [Google Scholar]
- Herman JP, Dolgas CM, Carlson SL 1998 Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience 86:449–459 [DOI] [PubMed] [Google Scholar]
- Dallman MF 2005 Fast glucocorticoid actions on brain: back to the future. Front Neuroendocrinol 26:103–108 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Karst H, Joels M 2008 Corticosteroid hormones in the central stress response: quick-and-slow. Front Neuroendocrinol 29:268–272 [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ 2002 Stressed-out, or in (utero)? Trends Neurosci 25:518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK 2002 Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res 950:52–63 [DOI] [PubMed] [Google Scholar]
- Karolyi IJ, Burrows HL, Ramesh TM, Nakajima M, Lesh JS, Seong E, Camper SA, Seasholtz AF 1999 Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice. Proc Natl Acad Sci USA 96:11595–11600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP 2000 Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 24:403–409 [DOI] [PubMed] [Google Scholar]
- Müller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kühn R, Reul JM, Holsboer F, Wurst W 2003 Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci 6:1100–1107 [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW 2004 CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557 [DOI] [PubMed] [Google Scholar]
- Rissman RA, Lee KF, Vale W, Sawchenko PE 2007 Corticotropin-releasing factor receptors differentially regulate stress-induced τ phosphorylation. J Neurosci 27:6552–6562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Hebda-Bauer EK, Pletsch A, Luo J, Hoversten MT, Osetek AJ, Evans SJ, Watson SJ, Seasholtz AF, Akil H 2007 Overexpressing the glucocorticoid receptor in forebrain causes an aging-like neuroendocrine phenotype and mild cognitive dysfunction. J Neurosci 27:8836–8844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin JM, Polansky NA, Kilpatrick AC, Shilton P 1996 Family functioning in neglectful families. Child Abuse Negl 20:363–377 [DOI] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Caspi A, Taylor A, Purcell S 2003 Domestic violence is associated with environmental suppression of IQ in young children. Dev Psychopathol 15:297–311 [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett KA 2007 Violence against women and the perinatal period: the impact of lifetime violence and abuse on pregnancy, postpartum, and breastfeeding. Trauma Violence Abuse 8:344–353 [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ 2008 Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 154:1132–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R 1984 Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60 [DOI] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ 2001 Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA 98:8856–8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ 2005 Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology 146:4090–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, West AN, Zola SM, Squire LR 2001 Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus 11:176–186 [DOI] [PubMed] [Google Scholar]
- Rozeboom AM, Akil H, Seasholtz AF 2007 Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci USA 104:4688–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ 2001 Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology 142:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ 2006 Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J Neurosci 26:2434–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SJ, Baram TZ 1994 Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology 135:2364–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, Oitzl MS, Muller MB, Ohl F, Wurst W, Holsboer F, Levine S, de Kloet ER 2003 Regulation of the developing hypothalamic-pituitary-adrenal axis in corticotropin releasing hormone receptor 1-deficient mice. Neuroscience 119:589–595 [DOI] [PubMed] [Google Scholar]
- Whitnall MH, Mezey E, Gainer H 1985 Co-localization of corticotropin-releasing factor and vasopressin in median eminence neurosecretory vesicles. Nature 317:248–250 [DOI] [PubMed] [Google Scholar]
- de Goeij DC, Kvetnansky R, Whitnall MH, Jezova D, Berkenbosch F, Tilders FJ 1991 Repeated stress-induced activation of corticotropin-releasing factor neurons enhances vasopressin stores and colocalization with corticotropin-releasing factor in the median eminence of rats. Neuroendocrinology 53:150–159 [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE 2007 Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci 8:872–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA, Brunelli SA, Masmela J, Shair HN 1996 Maternal interactions prior to separation potentiate isolation-induced calling in rat pups. Behav Neurosci 110:1158–1167 [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ 1997 Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277:1659–1662 [DOI] [PubMed] [Google Scholar]
- Macrí S, Mason GJ, Würbel H 2004 Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. Eur J Neurosci 20:1017–1024 [DOI] [PubMed] [Google Scholar]
- Parfitt DB, Levin JK, Saltstein KP, Klayman AS, Greer LM, Helmreich DL 2004 Differential early rearing environments can accentuate or attenuate the responses to stress in male C57BL/6 mice. Brain Res 1016:111–118 [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Levine S, Alam S, Harbich D, Sterlemann V, Ganea K, de Kloet ER, Holsboer F, Muller MB 2006 Metabolic signals modulate hypothalamic-pituitary-adrenal axis activation during maternal separation of the neonatal mouse. J Neuroendocrinol 18:865–874 [DOI] [PubMed] [Google Scholar]
- Millstein RA, Holmes A 2007 Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev 31:3–17 [DOI] [PubMed] [Google Scholar]
- Parfitt DB, Walton JR, Corriveau EA, Helmreich DL 2007 Early life stress effects on adult stress-induced corticosterone secretion and anxiety-like behavior in the C57BL/6 mouse are not as robust as initially thought. Horm Behav 52:417–426 [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Newth CJ, Baram TZ 1995 Effects of maternal and sibling deprivation on basal and stress induced hypothalamic-pituitary-adrenal components in the infant rat. Neurosci Lett 192:49–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ 1999 Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. J Neurosci 19:3982–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S 2002 Preclinical models: status of basic research in depression. Biol Psychiatry 52:503–528 [DOI] [PubMed] [Google Scholar]
- Kaplan Z, Iancu I, Bodner E 2001 A review of psychological debriefing after extreme stress. Psychiatr Serv 52:824–827 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA 2007 Chronic distress and incidence of mild cognitive impairment. Neurology 68:2085–2092 [DOI] [PubMed] [Google Scholar]
- Muret L, Priou A, Oliver C, Grino M 1992 Stimulation of adrenocorticotropin secretion by insulin-induced hypoglycemia in the developing rat involves arginine vasopressin but not corticotropin-releasing factor. Endocrinology 130:2725–2732 [DOI] [PubMed] [Google Scholar]
- Ma XM, Levy A, Lightman SL 1997 Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: a study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology 138:4351–4357 [DOI] [PubMed] [Google Scholar]
- Volpi S, Rabadan-Diehl C, Aguilera G 2004 Regulation of vasopressin V1b receptors and stress adaptation. Ann NY Acad Sci 1018:293–301 [DOI] [PubMed] [Google Scholar]
- Grino M, Paulmyer-Lacroix O, Faudon M, Renard M, Anglade G 1994 Blockade of α2-adrenoceptors stimulates basal and stress-induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocrinology 135:2549–2557 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM 1989 Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J Comp Neurol 285:413–435 [DOI] [PubMed] [Google Scholar]