Abstract
One of the major mechanisms by which insulin modulates glucose homeostasis is through regulation of gene expression. Therefore, reduced expression of transcription factors that are required for insulin-regulated gene expression may contribute to insulin resistance. We recently identified insulin response element-binding protein-1 (IRE-BP1) as a transcription factor that binds and transactivates multiple insulin-responsive genes, but the regulation of IRE-BP1 in vivo is largely unknown. In this study, we show that IRE-BP1 interacts with the insulin response sequence of the IGF-I, IGFBP-1, and IGFBP-3 genes using chromatin immunoprecipitation assay. Furthermore, activation by IRE-BP1 is sequence specific and mimics that of the insulin effect on gene transcription. Tissue expression of IRE-BP1 is 50- to 200-fold higher in classical insulin target compared with nontarget tissues in lean animals, with a significantly reduced level of expression in the skeletal muscle and adipose tissue in obese and diabetic animals. In the liver, IRE-BP1 is localized to the nucleus in lean rats but is sequestered to the cytoplasm in obese and diabetic animals. Cytoplasmic sequestration appears to be related to inhibition of insulin-mediated phosphatidylinositol-3 kinase signaling. Therefore, in diabetes and obesity, the mechanisms involved in reducing the transactivation of the insulin response sequence by IRE-BP1 include decreased gene transcription and nuclear exclusion to prevent DNA binding. Our study supports the notion that IRE-BP1 may be relevant to the action of insulin in vivo and may play a role in the development of insulin resistance and diabetes.
INSULIN CONTROLS GENE transcription by modifying the binding and activity of transcription factors on insulin response elements (IREs). Although a conserved cis-acting IRE sequence has been identified in a number of genes known to be inhibited by insulin, no consensus IRE that mediates insulin stimulation of transcription has been identified. Insulin signaling to Forkhead box O1 (FoxO1) has been shown to contribute to insulin suppression of gene expression through the IREs of multiple genes. Consistent with its negative effect on insulin-mediated transcription, FoxO1 functions predominantly as an insulin antagonist and a prodiabetic factor in vivo (1,2,3). On the other hand, sterol response element-binding protein 1 (SREBP-1) and specificity protein-1 (Sp1), among others, have been implicated as mediators in the stimulation of gene transcription by insulin (4,5,6). However, the role of these factors in insulin regulation is not uniform. Transgenic expression of SREBP-1c in adipose tissue produced marked insulin resistance and diabetes (7), whereas Sp1 is a ubiquitous factor that regulates diverse proteins with a variety of functions other than insulin action (4).
We previously identified IRE-binding protein 1 (IRE-BP1) as a candidate factor that interacts with the IRE from multiple genes (8). Our studies suggested that IRE-BP1 may be a target of insulin signaling downstream of the phosphatidylinositol-3 kinase (PI3K)-Akt pathway. Changes in expression level, phosphorylation, and nuclear translocation modulate the transactivation effects of IRE-BP1 on IRE reporter genes (8). A recombinant adenovirus expressing the carboxyl portion of IRE-BP1 in the liver decreased fasting and postprandial hyperglycemia in insulin-resistant diabetic rats, suggesting further that IRE-BP1 may be involved in insulin-regulated metabolism (9). However, the physiological relevance of IRE-BP 1 in vivo is still largely unknown.
The main purpose of this study was to test the hypothesis that IRE-BP1 plays a role in mediating the effect of insulin on gene expression in vivo and to identify the mechanisms by which insulin modulates the function of hepatic IRE-BP1 to mediate gene transcription. We speculated that for IRE-BP1 to be a relevant player in mediating insulin action, it must be expressed in appropriate target tissues and have access to the nucleus, and its expression, translation, posttranslational modification, and protein degradation may be under insulin control (1,4). In this study, we found that IRE-BP1 is regulated physiologically at multiple levels in vivo. Furthermore, the tissue distribution, cellular localization, and pattern of tissue regulation in insulin-resistant animals suggest that IRE-BP1 is regulated at the transcriptional and posttranslational levels by insulin and that IRE-BP1 may be involved in mediating the positive transcriptional effects of insulin in multiple tissues.
Materials and Methods
Chromatin immunoprecipitation (ChIP)
SK-HEP-1 cells were grown in 150-mm dishes and exposed to vehicle, 10−8 m insulin, or 10% fetal calf serum (FCS) for 48 h. Formaldehyde cross-linking, cell lysis, and preparation of nuclei were performed according to the ChIP-IT Express Magnetic Chromatin Immunoprecipitation Kit (Active Motif, Carlsbad, CA). Lysed nuclei were sheared by enzymatic digestion to obtain DNA fragments of 200-1000 bp. After fragmentation of the chromatin, cross-linked DNA/proteins were precipitated by three different antibodies: a control nonimmune antibody (negative control), a rabbit polyclonal antibody against an epitope mapping to amino acids 471–598 of FoxO1 (Santa Cruz Biotechnology, Carlsbad, CA), and a rabbit polyclonal antibody directed against a carboxyl epitope of rat IRE-BP1 (acetylated CTSQNTKSRYIPNGKL, produced by Biosource Inc., Hopkinton, MA) (8). Four micrograms of sheared chromatin DNA was used for each precipitation. Immunoprecipitated complexes were captured by protein G magnetic beads, protein/DNA cross-links were reversed by heating at 65 C for 2.5 h, and the precipitated DNA was used for PCR amplification. An aliquot of chromatin was also treated with ribonuclease A and proteinase K, purified by phenol/chloroform extraction, and used as input DNA.
Primers were designed to amplify the promoter sequences of the human IGF-I gene covering the IRE sequence identified at the +114 to +137 bp downstream of the major transcription initiation site (10) and the IGFBP-1 IRE sequence identified at the −124- to −96-bp region (11). For IGFBP-3, we previously localized the rat IGFBP-3 IRE to the −1150- to −1124-bp region of the promoter region (12). BLAST search using the rat IRE sequence found a 76.7% homologous sequence at the −1203- to −1173-bp region of the human IGFBP-3 gene (13), and the −1248- to −1085-bp region was amplified. Primers were designed from DNA sequence reported in GenBank accession no. J05538 (13), X57025 (14), and J05683 (15), respectively (shown in supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
DNA plasmid constructs and transient transfection
The rat IGFBP-3 promoter region was subcloned into a plasmid that carries the coding region for firefly luciferase (pGL2-basic from Promega, Madison, WI). The −1201/+34 IGFBP-3 construct contained the IRE sequence at −1150 to −1117 bp identified previously (12). Substitution mutations of the IRE region were constructed as previously described (12). Transfection of constructs containing the wild-type −1201/+34-bp plasmid or a series of 5-bp substitution mutations of the IRE (shown in supplemental Table 1), with or without the empty expression vector (pcDNA4-His/Max-TOPO from Invitrogen, Carlsbad, CA) or IRE-BP1 expression vector (containing the carboxyl 50-kDa portion of the protein), was conducted in SK-HEP-1 cells. Lipofectamine LTX and DNA complexes were mixed at a ratio of 8.75 μl/2.5 μg and incubated with the cells overnight. Medium was replaced with serum-free MEM on d 3, with or without the addition of 10−8 m insulin, cells were incubated overnight, and cell extracts were assayed on d 4 for promoter activity using the luciferase assay system. Luciferase activity was normalized to total protein.
Tissue RNA extraction
Ten-week-old male Zucker rats were purchased from Charles River Laboratories (Wilmington, DE) and fed Purina laboratory chow 5008. The use and care of animals were conducted according to the University of Louisville Institutional Animal Care protocol. For tissue cultivation, the rats were anesthesized with a mixture of xylazine (13 mg/kg) and ketamine (87 mg/kg). Tissues were obtained from ad libitum-fed 1) Zucker diabetic fatty (ZDF)-drt rats, 2) Zucker obese rats (fa/fa), and 3) lean Zucker rats (Fa/fa), as described previously (n = 6 rats per group). RNA was extracted using TriReagent kit (Molecular Research Center, Cincinnati, OH).
Quantitative PCR of IRE-BP1
Primers used for amplification of IRE-BP1 mRNA are shown in supplemental Table 1, and the specificity of amplification was confirmed by DNA sequencing (we amplified nucleotides 1026–1223 of XM_001068874). The 5′ end of the 5′ primer was modified with addition of T7 promoter sequence, and oligo(deoxythymidine) sequence was added to the 5′ end of the 3′ primer. Liver cDNA was amplified with the modified primer pair, and the PCR product was used for in vitro transcription with T7 RNA polymerase to produce IRE-BP1 cRNA with poly(A) tail at the 3′ end (16). The cRNA was quantitated by spectrophotometer at 260 nm, then converted to molecule number based on the following formula: N (molecules per microliter) = [C (cRNA in micrograms per microliter)/K (fragment size in base pairs)] × 182.5 × 1013 (16). To establish the standard curve for quantitation, a log dilution series of the cRNA was performed (104 to 1011) and then reverse transcribed into cDNA. The cDNA was amplified in parallel with reverse-transcribed RNA from tissue samples (0.5 μg per sample) and quantitated by the threshold cycle number where SYBR green signals were detected (16,17). The copy number of RNA molecules was determined by plotting the threshold cycle of fluorescence detection in the samples to that of the cycle of detection from the coamplified standard mRNAs. Melting curve analysis for each sample was performed to confirm amplification without formation of primer dimer or nonspecific fragment. An illustration of the methods used to measure adipose tissue mRNA is shown in supplemental Fig. B. Semiquantitative RT-PCR analysis was performed using primers designed to identify GAPDH mRNA to confirm equal loading of RNA.
Small interfering RNA (siRNA) synthesis and transfection
Target selection for silencing of IRE-BP1 was designed using www.ambion.com/techlib/misc/siRNA_design.html, and the siRNA synthesized according to the manufacturer’s protocol (Silencer siRNA Construction kit from Ambion, Austin, TX). Two 29-oligomer DNA oligonucleotides encoding 21-nucleotide sequences corresponding to the sense and antisense strand of IRE-BP1 (shown in supplemental Table 1) were synthesized with an eight-nucleotide leader sequence complementary to the T7 promoter primer sequence (5′-CCTGTCTC-3′) attached at the 3′ ends for both strands. The DNA regions were selected based on lack of homology to coding sequences of other genes. The oligonucleotides were hybridized to T7 promoter primer, extended by Klenow DNA polymerase to create double-stranded templates, and then transcribed by T7 RNA polymerase to produce double-stranded RNA. The T7 primer sequences are removed by digestion with ribonuclease H, and the end product is a double-stranded 21-oligomer siRNA with 3′-terminal uridine dimers that target IRE-BP1 mRNA. The siRNA was transfected into SK-HEP 1 cells using Lipofectamine RNAiMAX (Invitrogen) at a ratio of 25 nmol siRNA to 5 μl Lipofectamine. RNA was isolated, reverse-transcribed into cDNA, and amplified for detection of human IRE-BP1 and IGFBP-1 expression by semiquantitative PCR (primers used are shown in supplemental Table 1).
Cell culture and immunofluorescent microscopy
HepG2 cells were grown on glass slides, maintained in serum-free or 10% FCS-containing MEM, with or without 10 nm insulin. LY294002 and PD98059 were added at 10 μm concentration every 12 h for 3 d. The cells were fixed with 4% paraformaldehyde and then blocked with 5% normal goat serum in 1% (wt/vol) BSA-Tris-buffered saline (TBS) at room temperature for 1 h. This is followed by washing in TBS and then overnight incubation with rabbit anti-IRE-BP1 antibody at 1:150 dilution in TBS-0.5% Triton X-100. Primary antibody incubation was followed by washing and then incubation with Alexa Fluor 488 goat antirabbit IgG (Invitrogen) at 1: 500 dilution. The cells were embedded in mounting medium, and optical sections in the center of the nuclei were obtained with a Zeiss Axiovert 100M confocal microscope.
Statistical methods
Data are expressed as the means ± sem. The significance of differences among groups was determined by ANOVA and Tukey’s test. Differences were considered significant at P < 0.05.
Results
IRE-BP1 interacts with the insulin response sequence of IGFBP-3, IGFBP-1, and IGF-I in the context of chromatin
To strengthen our hypothesis that IRE-BP1 is an endogenous protein that interacts with the IRE sequence, we investigated the binding of IRE-BP1 to the promoter region of IGF-I, IGFBP-1, and IGFBP-3 using ChIP. SK-HEP1 cells, which express IGF-I, IGFBP-1, and IGFBP-3 mRNA and protein (18), were used in this experiment to study the binding of IRE-BP1 to multiple promoter regions. We amplified the IRE region of human IGFBP-1 promoter using FoxO1 antibody-immunoprecipitated DNA and used this experiment as a positive control to validate our ChIP technique. Figure 1 (lane 8 vs. 9) reveals that FoxO1 precipitated IGFBP-1 at a higher level in the absence than in the presence of insulin, consistent with its function as a negative regulator of insulin-mediated IGFBP-1 gene transcription (19,20). On the other hand, amplification of the same IGFBP-1 promoter fragment was increased by insulin when IRE-BP1 antibody-immunoprecipitated DNA was used (lane 7 vs. 6). The DNA samples obtained after immunoprecipitation by the IRE-BP1 antibody were also analyzed for the IGF-I IRE locus. The IGF-I promoter fragment was more abundantly precipitated by IRE-BP1 antibody in insulin-treated compared with vehicle-treated cells (lane 3 vs. 2). By contrast, the nonimmune antibody (rabbit IgG) precipitated a much lower level of DNA (lanes 4 and 5). Amplification of the IRE region of IGFBP-3 after immunoprecipitation with anti-IRE-BP1 antibody also revealed an increase in insulin-treated cells (lane 13 vs. 12) and in cells exposed to serum (lane 15 vs. 14). By contrast, DNA from the same preparation of chromatin that was not subjected to immunoprecipitation showed no such difference (input DNA, lane 10 vs. 11). These results are consistent with increased binding of IRE-BP1 to the promoter region of IGF-I, IGFBP-1, and IGFBP-3 in SK-HEP 1 cells treated with insulin.
Figure 1.
ChIP analysis. SK-HEP-1 cells were exposed to vehicle, 10 nm insulin, or 10% serum. After formaldehyde cross-linking, chromatin was isolated, and cross-linked DNA/protein was precipitated with anti-IRE-BP1, rabbit IgG, or anti-FoxO1 antibodies. Enrichment for IGF-I, IGFBP-1, and IGFBP-3 promoters were tested by amplification of chromatin-associated DNA using primers shown in supplemental Table 1. DNA from the same cells was also amplified without being subjected to immunoprecipitation and labeled as input DNA.
Linker scanning mutations show IRE-BP1 mimics insulin in activating the IGFBP-3 IRE
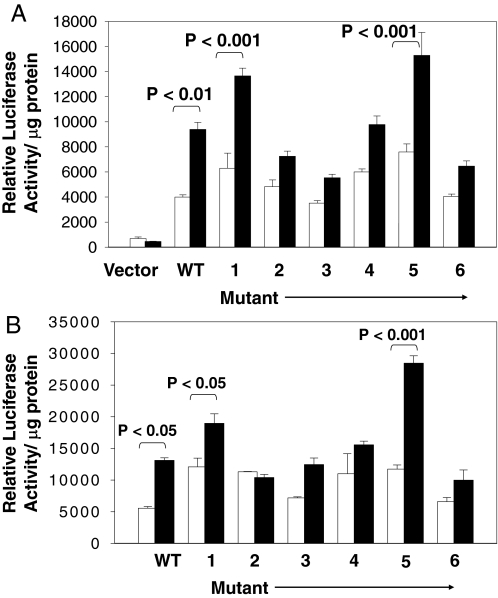
We previously identified the 34-bp IRE localized to the −1150- to −1117-bp region of the IGFBP-3 gene as the binding site for IRE-BP1 (8). To compare the transcriptional activity induced by IRE-BP1 to that induced by insulin on the IRE sequence, a series of 5-bp substitution block mutations scanning the −1150/−1117 region was introduced into the wild-type −1201/+34 promoter fragment of IGFBP-3, and transfected into SK-HEP1 cells. Supplemental Table 1 shows the wild-type IGFBP-3 IRE sequence and the internal substitutions used in these studies, and Fig. 2A shows the luciferase reporter gene activity when cells were transfected with IRE-BP1 and the luciferase reporter linked to the wild-type or mutated IREs. All mutant constructs have basal activities that are not significantly different from the wild-type plasmid (all P > 0.05, wild type vs. mutants). Overexpression of IRE-BP1 increased luciferase activity of the wild-type plasmid by 135 ± 13% (P < 0.01 vs. empty expression vector). Substitution of nucleotides −1148/−1144 (mutant 1), −1143/−1139 (mutant 2), −1138/−1134 (mutant 3), −1133/−1129 (mutant 4), −1128/−1124 (mutant 5), and −1123/−1119 (mutant 6) bases increased response to IRE-BP1 by 117 ± 9 (P < 0.001), 50 ± 8, 57 ± 8, 63 ± 11, 101 ± 24 (P < 0.001), and 60 ± 10%, respectively, compared with vector only. Therefore, mutation of −1148/−1129 bp (mutants 2–4) and −1123/−1119 bp (mutant 6) decreased the ability of IRE-BP1 to stimulate IRE activity. The same DNA constructs were also transfected into SK-HEP1 cells without the IRE-BP1 cDNA because these cells express endogenous IRE-BP1, and then the cells were treated with vehicle or 10 nm insulin for 16 h (Fig. 2B). Luciferase reporter assays show that insulin increased gene transcription of the wild-type plasmid, and that of mutants 1 and 5, significantly (P < 0.05 for wild type and mutant 1, < 0.001 for mutant 5, insulin vs. vehicle), whereas mutants 2–4 and mutant 6 did not respond to insulin stimulation (P > 0.05, vehicle vs. insulin). Therefore, overexpression of IRE-BP1 and insulin provided similar results in stimulating IGFBP-3 IRE in a sequence-specific pattern.
Figure 2.
Transactivation of the IRE by IRE-BP1 and by insulin. A, SK-HEP-1 cells were transfected with pGL2 basic vector alone, wild-type (WT) IGFBP-3 −1200/+34-luciferase reporter or with sequences containing mutations of IGFBP-3 IRE (mutants 1–6, substituted sequences shown in supplemental Table 1) in the absence (white bars) or presence (black bars) of IRE-BP1 expression vector, followed by luciferase reporter assay. Relative luciferase activity was corrected for protein concentration in the lysate. n = 3 each group. This experiment was conducted three times with similar results. B, WT IGFBP-3 −1200/+34 and mutants 1–6 were transfected into SK-HEP-1 cells without IRE-BP1 expression vector. Cells were then treated with vehicle (white bars) or with 10 nm insulin (black bars) overnight and luciferase activity measured as above. n = 3 replicates per condition. The experiment was conducted four times with similar results.
Regulation of IRE-BP1 in diabetes and obesity
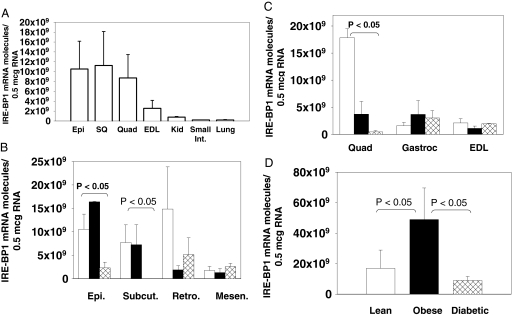
To determine the tissue distribution of IRE-BP1, we obtained RNA from tissues of lean Zucker rats and quantitated IRE-BP1 mRNA. The results show that IRE-BP1 has wide tissue distribution (Fig. 3A), and the expression in tissues that are responsive to insulin (muscle and fat) is higher than in tissues that are considered to be non-insulin-responsive (kidney, small intestine, and lung). To determine whether IRE-BP1 expression is altered in insulin resistance, we compared the level of expression in liver, adipocytes, and skeletal muscle of lean, obese nondiabetic, and obese diabetic Zucker rats (21). As shown in Fig. 3B, there is significantly higher expression of IRE-BP1 in the epididymal, sc, and retroperitoneal tissues than in the mesenteric tissue of lean rats. Compared with lean rats, IRE-BP1 expression was 78 ± 11, 97 ± 3, and 65 ± 24% less in the epididymal (P < 0.05), sc (P < 0.05), and retroperitoneal regions in diabetic rats. In obese rats, IRE-BP1 was also decreased by 25 ± 50 and 87 ± 6% in the sc and the retroperitoneal fat tissue (P = 0.08 vs. lean) and increased by 48 ± 6% in the epididymal fat. With the exception of mesenteric fat tissue, there was a general trend toward decreased expression with insulin resistance, particularly in diabetes.
Figure 3.
Effects of obesity and diabetes on tissue IRE-BP1 mRNA A, Tissues were obtained from epididymal fat (Epi), sc fat (SQ), quadriceps femoris (Quad), EDL muscles, kidney (kid.), small intestine (Int.), and lung of lean Zucker rats. RNA was extracted from the tissues, and the number of molecules of IRE-BP1 mRNA/0.5 μg total RNA was quantitated by quantitative PCR. GAPDH mRNA was used to control for loading and to assess for PCR efficiency. n = 4–6 animals, mean ± sem. B, RNA from white adipose tissues in normal (white bars), obese (black bars), and diabetic (cross-hatched bars) rats were extracted and the relative abundance of IRE-BP1 mRNA analyzed by quantitative PCR. n = 4–6 animals, mean ± sem. C, RNA from the quadriceps femoris, gastrocnemius (Gastroc), and EDL muscles were analyzed for IRE-BP1 mRNA as above, mean ± sem of six animals per group. D, RNA was extracted from the liver of the rats, and the IRE-BP1 mRNA quantitated.
Among the skeletal muscles, the quadriceps expressed IRE-BP1 mRNA at a 6-fold higher level than the gastrocnemius or extensor digitorum longus (EDL) muscles in lean rats (Fig. 3C). Expression was 86 ± 2 and 95 ± 3% less in the quadriceps of obese and diabetic rats, when compared with lean rats (P < 0.05 for lean vs. diabetic). However, there was no significant difference in IRE-BP1 expression in the EDL and gastrocnemius muscles among the various metabolic conditions. The quadriceps is composed predominantly of slow-twitch oxidative fibers, whereas the gastrocnemius and EDL are composed mainly of fast-twitch glycolytic fibers (22,23). Therefore, our results suggest that IRE-BP1 is expressed mainly in the slow-twitch muscle fibers, and the expression in this fiber type is decreased with obesity and diabetes.
Hepatic expression of IRE-BP1 was 2.8 ± 1.6-fold higher in obese than in lean animals (P < 0.05) but was reduced by 31 ± 20% in diabetic animals when compared with normal rats (P > 0.05, diabetic vs. lean, Fig. 3D). In the kidney, small intestine, heart, and lung, we found no significant difference in the expression of IRE-BP1 among the three groups of animals (supplemental Fig. A). Quantitatively, IRE-BP1 mRNA was estimated to be 109-1010 molecules/μg RNA in adipose tissue, muscles, and liver, compared with 107-108 molecules/μg RNA in other tissues. In lean rats, expression of IRE-BP1 in adipose tissue, skeletal muscles, and liver is 50- to 220-fold higher than the expression level in the kidney, small intestine, lung, and heart.
Subcellular localization of IRE-BP1 in hepatocytes
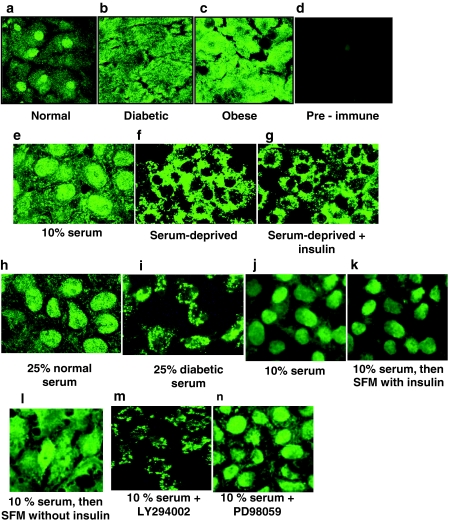
Because hepatic IRE-BP1 mRNA levels varied unexpectedly with the metabolic condition of the animals, we also examined the hypothesis that posttranslational modification may be involved in the regulation of hepatic IRE-BP1 in diabetes and obesity. Using an antibody generated against the carboxy terminus of IRE-BP1, confocal microscopy showed that IRE-BP1 was localized predominantly to the nuclei of hepatocytes in ad libitum-fed normal rats (Fig. 4A). In obese and diabetic rats, by contrast, IRE-BP1 in hepatocytes was diffusely distributed to both the cytoplasm and the nucleus (Fig. 4, B and C). Preimmune serum showed minimal immunostaining of lean rat hepatocytes (Fig. 4D). There was also infiltration of fat cells in the liver of obese rats (supplemental Fig. C), which may account for the increased IRE-BP1 mRNA. Our findings suggest either decreased nuclear translocation or increased nuclear export of IRE-BP1 with diabetes and obesity.
Figure 4.
Subcellular localization of IRE-BP1 in the liver and factors that affect cytoplasmic-nuclear trafficking of IRE-BP1. A–D, Confocal microscope images of liver sections from ad libitum-fed lean (A), diabetic (B), and obese (C) Zucker rats immunostained with IRE-BP1 carboxy antibody or with preimmune serum (D). Magnification, ×100. n = 2 animals per group. E and F, To assess the influence of serum factor on cytoplasmic-nuclear trafficking of IRE-BP1, HepG2 cells were grown in MEM containing 10% FCS (E) or MEM alone (F) for 72 h. G–I, Serum-deprived cells were then stimulated with 10 nm insulin (G), 25% normal mouse serum (H), or 25% diabetic mouse serum (I) for 72 h. Blood was pooled from four normoglycemic Db/db (nondiabetic) and four hyperglycemic db/db (diabetic) mice to provide sera for the studies conducted in H and I. J–L, To determine whether insulin is permissive for IRE-BP1 nuclear localization, cells were grown in MEM containing 10% FCS for 48 h to induce nuclear localization (J), followed by incubation in serum-free MEM containing 10 nm insulin (K) or serum-free MEM without insulin (L) for an additional 96 h. M and N, Cells cultured in 10% FCS-containing MEM for 48 h were treated with 10 μm LY294002 (M) or 10 μm PD 98059 (N) with medium change every 12 h for 3 d. This experiment was conducted three to four times for each condition with similar results.
Because hepatic localization of IRE-BP1 was altered in obese and diabetic rats, we sought to identify the factors that affect its subcellular localization in hepatocytes. In this investigation, we conducted in vitro studies of cultured HepG2 (hepatocellular carcinoma) cells using indirect immunofluorescence and confocal microscopy. Under normal culture conditions (10% FCS in MEM), cells exhibited strong nuclear immunoreaction for IRE-BP1, with a lower cytoplasmic reaction (Fig. 4E, also shown in multiple sections as supplemental Fig. E). After serum deprivation for 72 h, IRE-BP1 was predominantly cytoplasmic (Fig. 4F, also shown in multiple sections as supplemental Fig. F), indicating that serum and/or growth factors in serum are needed for nuclear localization of IRE-BP1. To test whether insulin is the serum factor that enhances nuclear localization, we cultured the cells under serum-free conditions for up to 72 h to induce the nuclear exit of IRE-BP1, followed by treatment with physiological concentration of insulin (10 nm) for 72 h. Under these conditions, treatment with insulin alone did not lead to nuclear translocation of cytoplasmic IRE-BP1 (4 g), whereas exposure of cells to 25% normal mouse serum led to near-complete nuclear entry (4 h). On the other hand, cells exposed to 25% diabetic mouse serum retained their cytoplasmic localization (Fig. 4I), and IRE-BP1 appears to localize mainly to the perinuclear compartment. These results suggest that an as yet unidentified serum factor, which is deficient in diabetes, may induce nuclear import of IRE-BP1.
Because IRE-BP1 contains a consensus nuclear export signal (LPELRL or the LXXLXL pattern of nuclear export signal) (24), we also tested the hypothesis that insulin was permissive for nuclear accumulation of IRE-BP1 by inhibiting nuclear export. In this experiment, we grew cells in 10% FCS-containing medium to induce nuclear enrichment of IRE-BP1, then we changed to serum-free medium in the presence or absence of insulin. Our results show that cells cultured in FCS had strong nuclear immunostaining for IRE-BP1 (Fig. 4J). When shifted to serum-free medium for 4 d, cells cultured in the presence of insulin retained a stronger nuclear immunostain than did cells grown in the absence of insulin (Fig. 4, K vs. L), suggesting that insulin may inhibit nuclear export of IRE-BP1. Furthermore, blockade of the PI3K pathway with LY 294002 led to nuclear exit of IRE-BP1 even when cells were maintained in 10% FCS (Fig. 4, M vs. J), and the protein favored accumulation in the perinuclear compartment, similar in distribution to cells exposed to diabetic serum, whereas blockade of the MAPK pathway with PD98059 did not change the nuclear location of IRE-BP1 when cultured with 10% FCS (Fig. 4N). Therefore, insulin appears to play a role in retaining IRE-BP1 in the nucleus, probably through signals mediated through the PI 3K pathway.
Effect of IRE-BP1 knockdown on IGFBP-1 expression
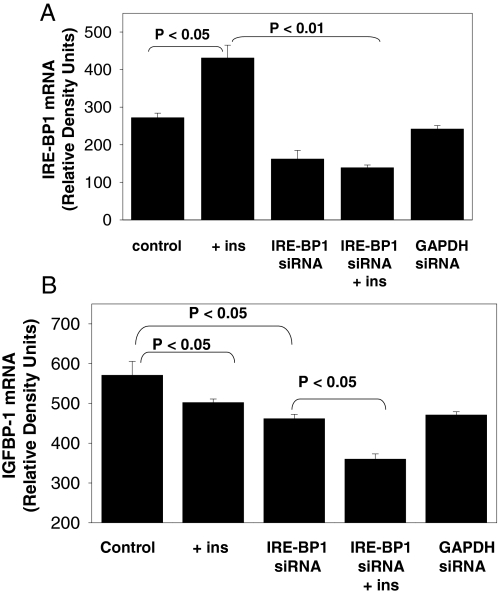
To assess the contribution of IRE-BP1 to insulin regulation of gene expression, IRE-BP1 was knocked down in SK-HEP1 cells. Although three different IRE-BP1-specific siRNAs were synthesized and transfected, only one was effective in decreasing IRE-BP1 expression. As shown in Fig. 5A, insulin stimulated the expression of IRE-BP1 by 58.5 ± 12% (P < 0.05 vs. control). siRNA-mediated knockdown of IRE-BP1 decreased IRE-BP1 expression by 40.4 ± 8% only (P = 0.08 vs. control) and completely abolished insulin stimulation of IRE-BP1. Transfection with GAPDH-specific siRNA had no effect on IRE-BP1 expression. To assess the effect of IRE-BP1 knockdown on insulin control of target genes, we analyzed the expression of IGFBP-1. Figure 5B confirms the negative effect of insulin on IGFBP-1 expression (P < 0.05 vs. control). Knockdown of IRE-BP1 decreased IGFBP-1 expression by 19.1 ± 2% (P < 0.05 vs. control), suggesting that IRE-BP1 stimulates the basal expression of IGFBP-1. Knockdown of IRE-BP1 did not prevent inhibition of IGFBP-1 by insulin (IRE-BP1 siRNA vs. IRE-BP1 siRNA plus insulin, P < 0.05), but rather, inhibition was additive. Therefore, IRE-BP1 alone was not sufficient to induce suppression of IGFBP-1 expression by insulin.
Figure 5.
Effect of IRE-BP1 knockdown on insulin-regulated expression of IGFBP-1. SK-HEP1 cells were transfected with 25 nm IRE-BP1-specific or GAPDH-specific siRNAs in the absence or presence of 10 nm insulin (+ ins) overnight. RNA was extracted and reversed transcribed to cDNA and used to measure IRE-BP1 (A) or IGFBP-1(B) mRNA by semiquantitative PCR. Mean ± sem, n = 3 per group. Experiments were repeated three times with similar results.
Discussion
Using the functional assay of the yeast one-hybrid system, we cloned and identified IRE-BP1 as a hepatic protein that binds to the IRE for the IGFBP-3 gene (8), and further studies indicated that IRE-BP1 may be a common factor that binds to the IREs of other genes. To demonstrate the involvement of IRE-BP1 in the transcriptional action of various genes, we previously showed that gel shift bands formed between rat liver nuclear extracts and insulin response elements (IREs) of the IGFBP-3, IGFBP-1, and IGF-I genes reacted to polyclonal antibodies developed against two separate epitopes of IRE-BP1 (8). In this study, we extend this observation further by showing an enrichment of IRE binding sites for IGFBP-3, IGF-I, and IGFBP-1 in the region of the chromosome bound by IRE-BP1. Enrichment of the IRE promoter fragments was observed when used with chromatin from cells treated with insulin, whereas chromatin from control cells did not produce similar enrichment. Therefore, these data support the idea that IRE-BP1 is a common factor that interacts with multiple insulin-responsive elements and that the function of IRE-BP1 is activated by insulin.
Other investigators have reported that Sp1 and Sp3 also act through the GC-rich region of IGF-I IRE (5,25), whereas FoxO1, in concert with CCAAT enhancer binding protein-βC, peroxisome proliferation-activated receptor-γ coactivator 1α (PGC-1α), and other transcriptional activators, act through IGFBP-1 IRE (26,27). Our findings showed that increased binding of IRE-BP1 occurs in genes that are either positively (IGF-I and IGFBP-3) or negatively (IGFBP-1) regulated by insulin. Therefore, IRE-BP1 may confer a positive effect on gene transcription through the IRE, and protein-protein interactions with other factors may modulate further the gene-specific response to insulin. Such interaction may change with the context of the gene promoter being investigated and whether insulin increases or represses the activity of the gene. Consistent with the role of other interacting proteins, our results showed that IRE-BP1 was not sufficient to inhibit insulin action on IGFBP-1 expression in our knockdown experiments.
We next asked the question of how ubiquitous is the action of IRE-BP1 in mediating the genomic effects of insulin. Although expression of about 400 genes is altered in animal livers that overexpress IRE-BP1 (9), many of which are known to be regulated by insulin, it is not known whether these are direct targets of IRE-BP1 or whether they are activated secondarily to the activation of other genes. To answer this question, we investigated the physiological regulation of IRE-BP1 in two genetic models of insulin resistance. Using quantitative PCR, we compared the tissue-specific expression of IRE-BP1 mRNA in lean, obese, and diabetic Zucker rats and found that IRE-BP1 expression is decreased in multiple tissues in diabetic and obese rats. IRE-BP1 is also expressed at a significantly higher level in insulin target than in nontarget tissues. In adipose tissue, expression is higher in the sc, epididymal, and retroperitoneal fat than in the mesenteric fat region. Because mesenteric fat cells in rodents are less sensitive to the effect of insulin on glucose utilization compared with adipocytes from other regions (28,29,30) and have the highest lipolytic rate (31), IRE-BP1 expression appears to correlate with regional differences in insulin response and/or insulin action rather than with basal glucose metabolism.
In the skeletal muscles, IRE-BP1 appears to be expressed predominantly in the slow-twitch oxidative fibers (22,23) and is physiologically decreased in this muscle fiber type in animals with diabetes. Because slow-twitch oxidative fibers have been reported to take up glucose in an insulin-dependent manner, whereas fast-twitch glycolytic fibers appear to be less insulin sensitive (32,33), our findings suggest that IRE-BP1 may also be associated with insulin response in the skeletal muscles. Therefore, IRE-BP1 appears to be regulated by insulin in the skeletal muscles and in the adipose tissue at the gene expression level in vivo.
By contrast, we found that hepatic IRE-BP1 is regulated mainly at the posttranscriptional level. It is localized predominantly in the nucleus of lean rats, but is diffusely distributed in both the cytoplasm and nucleus of diabetic and obese rat liver. Nuclear exclusion of IRE-BP1 also occurs in hepatocytes of Sprague Dawley rats after prolonged fasting (9). Because hepatic IRE-BP1 inhibits expression of genes involved in gluconeogenesis (9), and both diabetes and fasting are associated with increased gluconeogenesis, it is likely that decreased nuclear IRE-BP1 in both conditions contributes to reduced inhibition of gluconeogenic genes, resulting in hyperglycemia in diabetes and preventing hypoglycemia during fasting. This differential localization of IRE-BP1 in the cytoplasm and nucleus was not observed in the skeletal muscles or adipose tissues of obese and diabetic animals (shown as supplemental Fig. D), suggesting tissue-specific regulation in the liver.
We also searched for a mechanism to link insulin action to IRE-BP1 localization. These experiments imply that a serum factor or a cellular effect induced by serum is involved in inducing nuclear translocation of IRE-BP1. This idea was supported by immunohistochemistry of the liver sections and confirmed by ChIP assay showing enrichment of IRE-BP1-bound chromatin in serum-treated SK-Hep1 cells. Our study also suggests that insulin may limit IRE-BP1 export from the nucleus. This is consistent with our previous findings showing that treatment of hepatocytes with insulin leads to nuclear enrichment of IRE-BP1, as assessed by cell fractionation (8). Blockade of the PI3K pathway also leads to nuclear exit of IRE-BP1, even in the presence of serum. This finding raises the possibility that in diabetes, decreased insulin-mediated PI3K signaling in the liver may contribute to enhanced export of IRE-BP1 from the nucleus, resulting in cytoplasmic accumulation of the protein and reduction of IRE-BP1-mediated gene transcription. It is interesting that cells treated with PI3K inhibitor or cells exposed to diabetic serum exhibited perinuclear distribution of IRE-BP1, raising the possibility that retention of IRE-BP1 in the endoplasmic reticulum occurs under these experimental conditions. Whether shuttling of IRE-BP1 is affected by phosphorylation through Akt signaling, association with cytoplasmic protein, or changes in nuclear transport machinery similar to FoxO1 is presently being investigated in our laboratory.
In conclusion, IRE-BP1 is regulated at the gene transcription and posttranscriptional levels in vivo. In skeletal muscle and adipose tissue, regulation appears to occur predominantly at the transcriptional level, whereas in liver, posttranslational regulation by nucleocytoplasmic shuttling appears to predominate. IRE-BP1 is also expressed at a higher level in classical insulin target tissues than in nontarget tissues and is physically excluded from the site of active transcription in diabetic and obese livers. To our knowledge, none of the previously identified factors thought to mediate insulin effects on target genes exhibited the degree of in vivo correlation that was seen between IRE-BP1 expression and tissue response to insulin. Unanswered questions include whether decreased IRE-BP1 expression is a consequence of insulin resistance or whether decreased IRE-BP1 expression contributes to the development of the diabetic phenotype. However, IRE-BP1 may be functionally related to the transcriptional events that control the positive actions of insulin in multiple tissues, and understanding the regulation of IRE-BP1 may help us understand the pathophysiology of insulin resistance in obesity and type 2 diabetes.
Supplementary Material
Acknowledgments
We thank Dr. Stephen Winters for helpful comments on the manuscript.
Footnotes
This work is supported by grants from the National Institutes of Health (DK067413), the Walter Avis Jacobs Foundation, and the University of Louisville School of Medicine Research Grant (to B.C.V.).
Present address for J.C.: University of Iowa, Department of Internal Medicine, Division of Endocrinology, 200 Hawkins Drive, E401-5GH, Iowa City, Iowa 52242.
Present address for C.-C.C.: China Medical University, No. 2, Yuh-Der Road, Taichung 404, Taiwan.
Disclosure Statement: All authors have nothing to disclose.
First Published Online June 19, 2008
Abbreviations: ChIP, Chromatin immunoprecipitation; EDL, extensor digitorum longus; FCS, fetal calf serum; FoxO1, Forkhead box O1; IRE, insulin response element; IRE-BP1, IRE-binding protein 1; PI3K, phosphatidylinositol-3 kinase; siRNA, small interfering RNA; Sp1, specificity protein-1; TBS, Tris-buffered saline.
References
- Barthel A, Schmoll D, Unterman TG 2005 FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab 16:183–188 [DOI] [PubMed] [Google Scholar]
- Nakae J, Park BC, Accili D 1999 Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a wortmannin-sensitive pathway. J Biol Chem 274:15982–15985 [DOI] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG, Cohen P 1999 Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem 274:17179–17183 [DOI] [PubMed] [Google Scholar]
- Samson SL, Wong NC 2002 Role of Sp1 in insulin regulation of gene expression. J Mol Endocrinol 29:265–279 [DOI] [PubMed] [Google Scholar]
- Zhu JL, Kaytor EN, Pao CI, Meng XP, Phillips LS 2000 Involvement of Sp1 in the transcriptional regulation of the rat insulin-like growth factor-1 gene. Mol Cell Endocrinol 164:205–218 [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR 2005 Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes 54:1314–1323 [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmokov Y, Goldstein JL, Brown MS 1998 Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev 12:3182–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte BC, Phillips LS, Rane MJ, Zhao W 2004 Insulin-response element-binding protein 1, a novel Akt substrate involved in transcriptional action of insulin. J Biol Chem 279:36650–36659 [DOI] [PubMed] [Google Scholar]
- Villafuerte BC, Kaytor EN 2005 An insulin-response element binding protein that ameliorates hyperglycemia in diabetes. J Biol Chem 280:20010–20020 [DOI] [PubMed] [Google Scholar]
- Zhu JL, Pao CI, Hunter EJ, Lin KW, Wu GJ, Phillips LS 1999 Identification of core sequences involved in metabolism-dependent nuclear protein binding to the rat insulin-like growth factor I gene. Endocrinology 140:4761–4771 [DOI] [PubMed] [Google Scholar]
- Durham SK, Suwanichkul A, Scheimann AO, Yee D, Jackson JG, Barr FG, Powell DR 1999 FKHR binds the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology 140:3140–3146 [DOI] [PubMed] [Google Scholar]
- Villafuerte BC, Zhao W, Herington AC, Saffery R, Phillips LS 1997 Identification of an insulin-responsive element in the rat insulin-like growth factor-binding protein-3. J Biol Chem 272:5024–5030 [DOI] [PubMed] [Google Scholar]
- Cubbage ML, Suwanichkul A, Powell DR 1990 Insulin-like growth factor binding protein-3. Organization of the human chromosomal gene and demonstration of promoter activity. J Biol Chem 265:12642–12649 [PubMed] [Google Scholar]
- Steenbergh PH, Koonen Reemsti AM, Cleutjens CB, Sussenbach JS 1991 Complete nucleotide sequence of the high molecular weight human IGF-I mRNA. Biochem Biophys Res Commun 175:507–514 [DOI] [PubMed] [Google Scholar]
- Suwanichkul A, Cubbage ML, Powell DR 1990 The promoter of the human gene for insulin-like growth factor binding protein-1. J Biol Chem 265:21185–21193 [PubMed] [Google Scholar]
- Fronhoffs S, Totzke G, Stier S, Werner N, Rothe M, Brunig T, Koch B, Sachidis A, Vetter H, Ko Y 2002 A method for rapid construction of cRNA standard curves in quantitative real-time reverse transcription polymerase chain reaction. Mol Cell Probes 16:99–110 [DOI] [PubMed] [Google Scholar]
- Totzke G, Sachinidis A, Vetter H 1996 Competitive reverse transcription/polymerase chain reaction for the quantification of p53 and mdm2 mRNA expression. Mol Cell Probes 10:427–433 [DOI] [PubMed] [Google Scholar]
- Gucev ZS, Oh Y, Kelley KM, Labarta JI, Vorwerk P, Rosenfeld RG 1997 Evidence for insulin-like growth factor (IGF)-independent transcriptional regulation of IGF binding protein-3 by growth hormone in SKHEP-1 human hepatocarcinoma cells. Endocrinology 138:1464–1470 [DOI] [PubMed] [Google Scholar]
- Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matilka R, Xiao X, Franks R, Heidenreich KA, Sajan MP, Farese RV, Stolz DB, Tso P, Koo SH, Montiminy M, Unterman TG 2006 FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem 281:10105–10117 [DOI] [PubMed] [Google Scholar]
- Zhang X, Gan L, Guo S, He X, Olson ST, Mesecar A, Adam S, Unterman TG 2002 Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem 277:45276–45284 [DOI] [PubMed] [Google Scholar]
- Peterson RG, Shaw WN, Neel MA, Little LA, Eichberg J 1990 Zucker diabetic fatty rat as a model for non-insulin-dependent diabetes mellitus. ILAR News 32:16–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey M, Weidner M, Gavigan K, Zheng D, Tyndall G, Houmard J 1995 The insulin action-fiber type relationship in humans is muscle group specific. Am J Physiol 269:E150–E154 [DOI] [PubMed] [Google Scholar]
- Song X, Ryder J, Kawano Y, Chibalin A, Krook A, Zierath J 1999 Muscle fiber type specificity in insulin signal transduction. Am J Physiol 277:R1690–R1696 [DOI] [PubMed] [Google Scholar]
- Moede T, Leibiger B, Pour HG, Berggren PO, Leibiger IB 1999 Identification of a nuclear localization signal, RRMKWKK, in the homeodomain transcription factor PDX-1. FEBS Lett 461:229–234 [DOI] [PubMed] [Google Scholar]
- Kaytor EN, Zhu JL, Pao CI, Phillips LS 2001 Physiological concentrations of insulin promote binding of nuclear proteins to the insulin-like growth factor I gene. Endocrinology 142:1041–1049 [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Accili D, Spiegelman BM 2003 Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC1α interaction. Nature 423:550–555 [DOI] [PubMed] [Google Scholar]
- Suwanichkul A, Allander SV, Morris SL, Powell DR 1994 Glucocorticoids and insulin regulate expression of the human gene for insulin-like growth factor-binding protein-1 through proximal promoter elements. J Biol Chem 269:30835–30841 [PubMed] [Google Scholar]
- Newby FD, Sykes MN, DiGirolamo M 1988 Regional Differences in adipocyte lactate production from glucose. Am J Physiol 255:E716–E722 [DOI] [PubMed] [Google Scholar]
- King JL, DigGirolamo M 1998 Lactate production from glucose and response to insulin in perifused adipocytes from mesenteric and epididymal regions of lean and obese rats. Obes Res 6:69–75 [DOI] [PubMed] [Google Scholar]
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ 2001 The biology of white adipocyte proliferation. Obes Rev 2:239–254 [DOI] [PubMed] [Google Scholar]
- Wajchenberg B 2000 Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21:697–738 [DOI] [PubMed] [Google Scholar]
- Halseth A, Bracy D, Wasserman D 2001 Functional limitations to glucose uptake in muscles comprised of different fiber types. Am J Physiol Endocrinol Metab 280:E994–E999 [DOI] [PubMed] [Google Scholar]
- Petersen H, Fueger P, Bracy D, Wasserman D, Halseth A 2003 Fiber type-specific determinants of Vmax for insulin-stimulated muscle glucose uptake in vivo. Am J Physiol Endocrinol Metab 284:E541–E548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.