Abstract
GnRH is detectable in the cerebrospinal fluid (CSF), but its source remains unidentified. Previous studies have harvested CSF for GnRH analysis from the median eminence region, but it is unknown whether GnRH in CSF is restricted to this region. If CSF-GnRH plays a physiological role, through volume transmission, to communicate with brain regions that express GnRH receptors but are not evidently innervated by GnRH neurons, then it is essential to establish whether GnRH is more pervasive throughout the cerebroventricular system. Three cannulae were placed in the supraoptic, infundibular, and pineal recesses of the third ventricle. GnRH was undetectable in lateral ventricle CSF. GnRH pulses were detected in all ewes in infundibular recess CSF, but at sites more rostral (supraoptic) and caudal (pineal), GnRH pulse frequency and amplitude significantly (P < 0.05) decreased. A GnRH surge was evident in CSF collected simultaneously from all cannulae, but the amplitude was greatest (P < 0.05) at the infundibular recess. A final study established whether iv administered GnRH enters the CSF. A 250-ng GnRH dose did not affect CSF-GnRH concentrations (1.6 ± 0.3 pg/ml), but 2.5 μg (2.7 ± 0.2 pg/ml; P < 0.001) and 1 mg (38.5 ± 10.6 pg/ml; P < 0.05) significantly increased CSF-GnRH concentrations. The present study shows: 1) the median eminence region is likely to be the major, if not only, source of GnRH entering the cerebroventricular system; and 2) exogenous GnRH crosses the blood-brain barrier, but extremely high doses are required to elevate CSF concentrations to physiological levels. Thus, CSF-GnRH may affect sites that are closer in proximity to the infundibular recess region than previously thought.
WE (1,2,3,4) AND OTHERS (5,6,7) have shown that GnRH is present in mammalian cerebrospinal fluid (CSF). Concentrations of GnRH harvested from CSF in the infundibular region of the third ventricle are notably high and are comparable to those found in the hypophyseal portal blood (2). Like hypophyseal portal-GnRH, this CSF-GnRH displays both a pulsatile and a surge profile (2,5,8). We have previously shown that CSF-GnRH does not contribute to hypophyseal portal-GnRH concentrations (9). Although GnRH is known to be released in the external zone of the median eminence before entering the hypophyseal portal circulation, it remains unclear how and where GnRH enters the CSF.
Considering that the external zone of the median eminence, which is immediately ventral to the infundibular recess, is the established site of GnRH release into the hypophyseal portal vasculature, it is tempting to speculate that this is the source of GnRH in CSF. However, there is compelling evidence that a tight junction barrier limits the penetration of factors from this external zone into the CSF (10,11,12).
GnRH may be secreted directly into the CSF by specific ventricle-contacting neurons (13,14,15,16,17). There is an abundance of GnRH fibers surrounding the infundibular recess of the third ventricle, and some of these axons may terminate in the third ventricle (13,14). Many GnRH fibers are also evident within the organum vasculosum of the lamina terminalis (OVLT), but their function remains unknown. Sparse GnRH neurons and projections have been reported more caudally in the brain (18,19,20). However, studies suggest that only a few GnRH neurons may be required to generate sufficient endogenous release (21,22). To gain further insight into the origins of GnRH in CSF, we placed cannulae in third ventricular regions that were in close proximity to the OVLT, infundibular recess, and pineal recess. We hypothesized that the relative GnRH concentrations in these three locations would indicate which site(s) was the major source of this decapeptide in CSF. Two experiments were performed using ovariectomized ewes during the anestrous season. In the first experiment, GnRH pulsatility was compared with LH pulsatility in peripheral blood. In a second experiment, we investigated the relationship between CSF-GnRH and LH secretion during an estradiol-induced LH surge.
An additional study sought to establish if peripherally administered GnRH was capable of entering the cerebroventricular system. This study sought to clarify why GnRH has been able to induce sexual behavior in some mammalian studies (23,24,25,26) but not in others (27,28).
Materials and Methods
Animals
Sexually mature Ile-de-France ewes were ovariectomized at least 1 month before experimentation, were housed in rooms with natural photoperiod, had free access to water, and were fed daily with hay, straw, and corn. Experiments were performed during anestrus (January to August for this breed in the Northern Hemisphere). Ovariectomy was performed so that the level of steroid exposure, which has a profound effect on the neuroendocrine reproductive axis, was comparable among animals. The steroid-primed LH surge induction model has been used extensively by our laboratory and others (29,30,31), and is not seasonally dependent. The ovariectomized LH pulse model during anestrus was used because large distinct LH pulses are known to occur approximately every hour in the model (32). For experiments, ewes were placed in pens that prevented them from turning around, but they were able to lie down and move forward and backward freely. To prevent the stress of social isolation, ewes were always in contact with other sheep. All procedures were performed in accordance with authorization A37801 (French Ministry of Agriculture).
Surgery
At least 2 wk before a study was performed, CSF guide cannulae (17 gauge, 50 mm, stainless steel luer-lock needle) were introduced stereotaxically into the third ventricle using a modification of a method described in detail previously (1). Briefly, under halothane anesthesia, the sheep head was positioned in a stereotaxic frame, and 1 ml radioopaque liquid (Omnipaque; Nycomed Ingenon, Suresnes, France) was injected into the right lateral ventricle. In experiment 1, three cannulae were positioned in the third ventricle. The first cannula was placed in the optic recess, which is immediately in front of the OVLT, the second in the infundibular recess, which is surrounded by the median eminence, and the third cannula was placed close to the pineal recess (Fig. 1). In experiment 2, in addition to these three cannulae, a fourth cannula (used during the surgical procedure to inject radioopaque material) was retained in the lateral ventricle and used to collect CSF. For experiment 3, a cannula was placed only in the infundibular recess of the third ventricle. Correct placement inside the ventricle was verified by the flow of CSF, and additional x-rays confirmed the precise location within the third ventricle. The cannulae were plugged and fixed in place with dental acrylic cement, and a Teflon cap (DuPont Co., Wilmington, DE) was placed around them for protection.
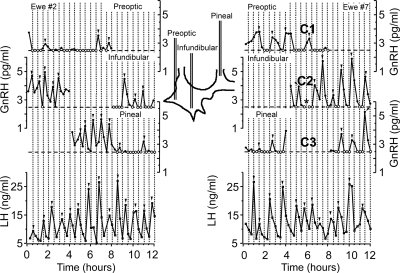
Figure 1.
Surgical placement of four cannulae in the ovine cerebroventricular system. A, Lateral x-ray image showing the placement of cannulae in the supraoptic (C1), infundibular (C2), and pineal (C3) recesses, as well as the lateral ventricle (C4), at the end of the surgery. B, The schematic shows the main landmarks and targets used for placement of the cannulae.
Experiment 1: measurement of GnRH in three different third ventricle locations in relation to LH pulses in ovariectomized ewes
Instantaneous jugular blood and integrated CSF samples were collected every 15 min for 12 h from ovariectomized ewes (n = 8). A polyethylene catheter was inserted through each third ventricular guide cannulae so that the distal end terminated at the tip of the cannula. To avoid the quantity of CSF withdrawal by the pump exceeding CSF production (3), CSF was collected simultaneously only from two of the three cannulae using a Minipulse II peristaltic pump (Gilson, Villiers-le-bel, France) at a flow rate of 250 μl/15 min for 4 h. Every 4 h the paired cannulae from which the CSF was harvested were switched in a random order so that CSF was collected from each cannula twice for two 4-h periods.
Experiment 2: measurement of GnRH in four different ventricle locations in relation to an estradiol-induced LH surge
An LH surge was induced in all ewes (n = 6) using a well-characterized follicular phase model (33). Briefly, a 10-mm SILASTIC brand (Dow Corning, Midland, MI) 17β-estradiol implant was inserted sc, and a progesterone implant (CIDR; InterAg, Hamilton, New Zealand) was also inserted intravaginally. The progesterone implant was then removed after 10 d to simulate luteolysis. Four 30-mm estradiol implants were inserted sc 24 h later to simulate the preovulatory estradiol increase. Instantaneous jugular blood and integrated CSF samples (600 μl/h) were collected hourly for 25 h, starting 12 h after insertion of the four estradiol implants.
Experiment 3: entry of iv GnRH into the infundibular recess of the third ventricle
CSF samples (300 μl/min) were collected every 10 min for 30 min to establish basal CSF-GnRH concentrations. Using an indwelling catheter in the jugular vein, ewes were then injected with 250 ng (n = 7), 2.5 μg (n = 6), or 1 mg (n = 5) GnRH, and 10-min CSF samples were collected for another 30 min. It is known that a 250 ng iv GnRH injection is sufficient to induce an LH pulse comparable to a physiological LH pulse (34).
Assay
GnRH concentrations in CSF were estimated in duplicate using a well-validated RIA (35). GnRH for iodination and standards were purchased from UCB-Bioproducts (Brussels, Belgium). All CSF samples from an individual ewe were measured in the same assay. The intraassay coefficient of variation and assay sensitivity averaged 14% and 2.5 pg/ml, respectively. Concentrations of LH were estimated in duplicate 100-μl jugular plasma samples using an established RIA (2), and all samples from an individual ewe were measured in the same assay. The intraassay coefficient of variation was 7%, and assay sensitivity was 0.13 ng/ml for standard 1051-CY-LH.
Analysis
Data are presented as the mean (±sem). In experiment 1, pulses of plasma LH and GnRH in CSF were detected using the Munro program (Zaristow Software, East Lothian, Scotland) (36). The program identifies secretory peaks by height and duration from a smoothed baseline, using the assay sd as a scale factor. Munro is an adaptation of the Pulsar Program PULSAR algorithm developed by Merriam and Wachter (37). The only essential difference is in the calculation of the baseline; in the Munro program, the baseline is generated by linear interpolation between the nadirs, followed by smoothing, using a moving average. The remaining stages of the Munro algorithm are identical with the Pulsar program. Because the baseline in the Munro program is calculated from the nadirs rather than from the moving average of the data, this program can process data containing pulses with variable widths and amplitudes. The cutoff parameters G1-5 of the Munro program for GnRH and LH were set at 3.98, 2.4, 1.68, 1.24, and 0.93; these values give a 5% false-positive error rate (37). The Baxter parameters (b1-3) were 0.21205, 0.02516, and 0.0004 for GnRH, and 0.09, 0.001, and 0.0001 for LH.
In experiment 2, the onsets of the LH and GnRH surges were defined as the first LH or GnRH sample to exceed the presurge baseline by 2 sd values of this baseline, after which hormone concentrations did not return to baseline levels within 2 h. The presurge baseline and sd were calculated from the samples collected for the first 4 h of the experiment. Because GnRH concentrations were still above the presurge baseline at the end of the experiment for most of the GnRH profiles, surge GnRH secretion for each cannula was estimated by calculation of the area under the curve (AUC) of the concentration vs. time plots above the baseline. AUC was calculated with the trapezoidal rule over the period from the surge onset until the end of the experiment. In experiment 3, the mean concentration of GnRH in CSF was calculated for each ewe for the 30 min before the GnRH injection and the 30 min after injection.
Comparisons between the pulse amplitude and frequency of GnRH and LH pulses were performed using Friedman’s test with repeated measures. Significance was accepted at P < 0.05, and Dunn’s multiple comparison test was used for post hoc repeated measures comparisons. For the CSF-GnRH surge study, the amount of GnRH secreted and the amplitude of the GnRH surge between cannulae were compared using ANOVA with repeated measures. To assess the extent of exogenous GnRH penetration into CSF, both the percent increase and the peak increase in CSF-GnRH concentration above baseline were calculated for each dose and analyzed by ANOVA. Significance was accepted at P < 0.05, and the Bonferroni correction was used for post hoc repeated measures comparisons.
Results
Experiment 1: measurement of GnRH in three different third ventricle locations in relation to LH pulses in ovariectomized ewes
CSF was successfully harvested from each of the three cannula placements in four of the eight animals used in this study. As shown in previous studies (2,3), GnRH secretion is clearly pulsatile in the CSF harvested from the infundibular recess (Fig. 2). GnRH pulses are less apparent in the CSF-GnRH profiles harvested from the supraoptic or pineal recesses. In all ewes, as shown in Fig. 3A, the mean CSF-GnRH concentration (over the two 4 h sampling periods) harvested from the infundibular recess is larger than the CSF-GnRH concentration harvested from the two other locations for all animals. For the four ewes with all three cannulae working, the amplitude of the CSF-GnRH pulses were significantly (P < 0.05) higher in the CSF harvested from the infundibular recess (2.07 ± 0.03 pg/ml) compared with the CSF harvested from either the supraoptic (1.11 ± 0.06 pg/ml) or pineal (1.55 ± 0.15 pg/ml) recesses (Fig. 3C). There was a significant effect (P < 0.05) of cannula placement on GnRH pulse frequency (Fig. 3B). The CSF-GnRH pulse frequency was higher in infundibular recess (3.63 ± 0.11 pulses/4 h) CSF compared with that in the supraoptic recess (1.50 ± 0.40 pulses/4 h; P < 0.05) and pineal recess (2.38 ± 0.27 pulses/4 h; P < 0.01). LH pulse frequency (4.16 ± 0.23 pulses/4 h) in jugular blood was not significantly different from the CSF-GnRH pulse frequency in infundibular recess, but the CSF-GnRH pulse frequencies in the pineal (P < 0.01) and supraoptic (P < 0.05) recesses were significantly lower.
Figure 2.
Results from two ewes showing GnRH concentration profiles (•) in CSF harvested simultaneously from cannulae located in the optic, infundibular, or pineal recess during two periods of 4 h for each cannula. Corresponding LH secretion in the peripheral blood over the 12 h of the sampling period is also shown (▪). The locations of the cannulae in the ventricular system are illustrated in the schematic. The horizontal dashed line indicates the detection limit of the GnRH assay, and values set at this limit are noted by an open circle (○). The vertical dotted lines facilitate visual alignment of the values between the cannulae and jugular blood. Pulses are noted by an arrowhead (▾). The asterisk (*) indicates a missing sample.
Figure 3.
Effect of cannula placement within the third ventricle on CSF-GnRH release during pulses. A, Mean (±sem) concentration of GnRH over the two 4-h periods for each of the eight ewes. It is evident that CSF harvested from the infundibular recess had the greatest GnRH concentration. B, Mean (±sem) GnRH pulse frequency in the CSF for the four animals in which CSF could be collected from all three cannulae. LH pulse frequency in the peripheral blood is also shown (gray bar). C, Mean (±sem) GnRH pulse amplitude in the CSF for the four animals in which CSF could be collected from all three cannulae. The optic recess data are noted by a black bar, infundibular recess data by a dashed bar, and pineal recess by a white bar. Differing letters indicate significant differences P < 0.05.
Experiment 2: measurement of GnRH in four different ventricle locations in relation to an estradiol-induced LH surge
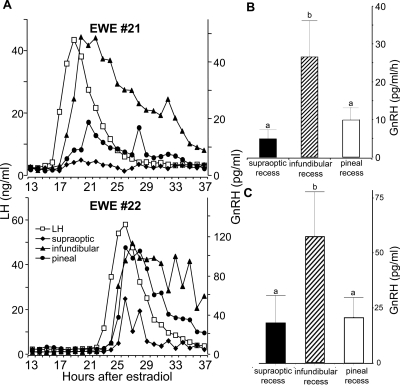
CSF was successfully harvested from the cannula located in the lateral ventricle in three of the animals, but because GnRH levels were below the detection limit for all samples in all these GnRH profiles, the data were not statistically analyzed. CSF was successfully harvested from all three cannulae in the third ventricle in four of the six ewes used. Analysis was only performed on the data from these four animals. It was evident in experiment 1 (Fig. 2, GnRH pulses in pineal recess CSF decline after the infundibular CSF collection commences) that sampling from the infundibular recess could affect CSF-GnRH concentrations at the other loci. Accordingly, these data must be viewed as an underestimate of the physiological concentration at the optic and pineal recesses. A surge of LH that was synchronous with a CSF-GnRH surge was evident in all cannula placements in the third ventricle (Fig. 4A). The amount of GnRH released during the surge at the infundibular recess was significantly (P < 0.05) greater (as estimated by AUC) than the amount detected at the supraoptic or pineal recess areas, which were not significantly different from each other (Fig. 4B). Similarly, the amplitude of the CSF-GnRH surge was significantly greater (P = 0.01) at the infundibular recess compared with either the supraoptic or pineal recesses (Fig. 4C).
Figure 4.
Effect of cannula placement within the third ventricle on CSF-GnRH release during the estradiol-induced surge. A, Representative ewes (nos. 21 and 22) showing the LH concentration profiles (□) and GnRH concentration profiles in CSF harvested simultaneously from cannulae located in the supraoptic (♦), infundibular (▴), and pineal recess (•) during an estradiol-induced GnRH/LH surge. B, Mean (±sem) amount of GnRH (AUC) released into the CSF harvested from cannulae located in the supraoptic, infundibular, and pineal recesses. C, Mean (±sem) amplitude of the GnRH surge in CSF harvested from cannulae located in the supraoptic, infundibular, and pineal recesses. Differing letters indicate significant differences P < 0.05.
Experiment 3: entry of iv GnRH into the infundibular recess of the third ventricle
Basal CSF-GnRH concentrations averaged 1.3 ± 0.2 pg/ml. The 250-ng dose did not cause a significant increase above baseline CSF-GnRH (1.6 ± 0.3 pg/ml). However, both the 2.5 μg (2.7 ± 0.2 pg/ml; P < 0.001) and 1 mg (38.5 ± 10.6 pg/ml; P < 0.05) iv doses of GnRH were clearly capable of entering the CSF of the third ventricle (Fig. 5).
Figure 5.
The percent increase in CSF-GnRH in 30 min above baseline levels after an iv injection of 250 ng, 2.5 μg, and 1 mg. ***, P < 0.001; *, P < 0.05 compared with baseline.
Discussion
Although it is well established that GnRH is detectable in the CSF of the third ventricle (1,2,3,4,5,6,7), how this GnRH gets into the CSF is unknown. The present study provides strong support for the hypothesis that most, if not all, of the GnRH in CSF originates from the GnRH axonal bed in the median eminence that lies in close proximity to, and at times impinges on, the third cerebral ventricle (13,14,15). The present study also confirms that iv administered GnRH is capable of entering the CSF and is not excluded by the blood-brain barrier.
This GnRH may enter the CSF of the infundibular recess in three ways that are not mutually exclusive. The first and most logical way would be for GnRH simply to overflow from the periportal spaces between the GnRH terminals and hypophyseal portal vessels into the extracellular fluid. However, there is compelling evidence that the extracellular fluid occupying this perivascular space is not in free communication with the CSF. Brightman et al. (10) showed that intravascular injections of horseradish peroxidase penetrated the median eminence but not the rest of the brain, whereas intracerebroventricularly (icv) administered horseradish peroxidase was unable to enter the median eminence. Additional electron microscopy studies revealed the presence of tight junctions between the median eminence and the brain (10). Subsequent studies have provided strong support for this tight junction barrier between the median eminence and the rest of the brain (11,12). We have also shown that icv administered GnRH does not enter the hypophyseal portal circulation, providing further support for the integrity of this barrier (9).
Second, a long-standing hypothesis is that hypothalamic factors may be transported to the CSF by tanycytes (38). In this hypothesis, GnRH would be released into the periportal space and then transported back to the third ventricle through tanycytes. Clearly, there is an intimate anatomical relationship between GnRH terminals and tanycytes (39). GnRH administered icv has been taken up by tanycytes (40), but, to our knowledge, studies have not shown that peripherally administered GnRH is taken up by these specialized ependymal cells. Moreover, in contrast to GHRH (41), immunocytochemical studies have not detected GnRH in tanycytes (42,43). Thus, we hypothesize that the role, if any, of infundibular tanycytes in the transport of GnRH from portal vasculature to CSF is likely to be small.
Third, there is compelling evidence that neurons contact the cerebroventricular CSF via their dendrites, axons, or perikarya (for review, see Ref. 15). For the GnRH system, this is conserved through evolution because GnRH-positive CSF-contacting neurons been in both cyclostomes and teleosts (44), as well as mammals (14,16,17). We are unaware of electron-microscopy studies showing specific GnRH terminals in the mammalian cerebroventricular system. Electron-microscopy studies would provide the strongest anatomical support for the release of GnRH by neurons directly into CSF. However, the most compelling evidence comes from a preliminary study by Billings et al. (13), in which cholera toxin was injected into the third ventricle of the ewe. In two sheep, in which it could be ascertained that the cholera toxin did not diffuse beyond the ependymal cell layer, more than half of the GnRH perikarya were labeled with the toxin. Together, these data support the hypothesis that GnRH is directly released into CSF.
Experiment 1 supports the hypothesis that CSF-GnRH pulses are derived predominantly, if not exclusively, from GnRH entering CSF in the infundibular recess region. First, the closer to the median eminence the CSF is collected, the higher the GnRH concentration. Second, when CSF was harvested from the infundibular recess, the CSF-GnRH profiles at the other locations could be perturbed. This is clearly evident in ewe no. 2 (Fig. 2) because GnRH secretion is significantly reduced at the pineal recess as soon as the collection of CSF from the infundibular recess commences. This suggests that most of the GnRH molecules diffusing from the median eminence are being extracted by the infundibular cannula. Consistent with our earlier investigations (2,3), the peak pulse concentration rarely exceeded 4 pg/ml. In contrast, surge CSF-GnRH concentrations were substantially higher at all three locations, and this increased release was evident for several hours and not minutes. From a dynamic perspective, this indicates that more GnRH molecules are diffusing from the median eminence or that the diffusing surface (i.e. the region from which GnRH is originating) is larger during the surge because many more GnRH neurons are recruited at this stage (45).
The concentration profile of GnRH in CSF that we have observed, with highest levels in the immediate vicinity of the infundibular recess and lower levels both more rostral and caudal, may have significant implications for our understanding of the potential physiological role this pool of GnRH plays through volume transmission (46,47). For example, high densities of GnRH receptor-expressing neurons have been consistently reported within the hippocampus of rodents (17,48,49) and recently in humans (50). We have recently confirmed the presence of GnRH receptors in ovine hippocampal neurons (51). Few studies suggest that there is direct GnRH axonal input to the hippocampal area. However, hippocampal pyramidal neurons take up I125-Buserilin, a GnRH receptor agonist, when it is injected icv (48). Importantly, GnRH alters the electrical properties of hippocampal pyramidal cells (52,53) and stimulates increased inositol 1,4,5-triphosphate production within these cells (52). If the CSF-GnRH that we have reported in the present study modulates hippocampal neuronal activity physiologically, it will be imperative to perform such studies in the concentration range that we have detected.
GnRH has affected sexual behavior in numerous mammals (23,24,25,26,54). An elegant series of studies on rats (23,24) provided compelling evidence that GnRH acts within the mesencephalic central gray region to evoke this behavioral response. GnRH binding has been observed in the mesencephalic central gray (48), and we have recently detected GnRH receptor-immunoreactive neurons in the ovine mesencephalic central gray (55). However, there is scant evidence of direct GnRH innervation of the mesencephalic central gray. The pineal recess of the third ventricle is close to the mesencephalic central gray and may provide a possible route through which GnRH may access potential target neurons in this region. It is also of interest that the duration of sexual behavior corresponds quite closely to the duration of the GnRH surge in the CSF.
The present study may also reveal why some, but not all, studies in larger mammals have been successful in inducing sexual behavior with GnRH. Specifically, GnRH stimulates proceptivity in marmosets (54) and, if GnRH is administered icv, can induce sexual receptivity in sheep (25). However, many researchers were unable to elicit sexual behavior in ungulates using GnRH administered via the jugular vein (25,27,28) (Findlay, J. K., C. Fabre-Nys, F. J. Karsch, and K. M. Kendrick, personal communication). It was hypothesized that one possible reason for this failure was that exogenous GnRH did not cross the blood-brain barrier. Although the present study is unable to discriminate between intact and cleaved GnRH [i.e. GnRH (1,2,3,4,5)], compelling evidence that GnRH (1,2,3,4,5) is able to elicit sexual behavior in rodents (56) suggests that GnRH degradation does not underlie the inability of iv GnRH to induce sexual behavior. Moreover, closer examination of rodent studies that were successful at inducing sexual behavior with peripherally administered GnRH suggests that high doses are required for efficacy (57,58). As is evident from the current study, 1 mg GnRH administered iv elevates CSF concentrations into the low surge range, whereas 2.5 μg increases basal CSF-GnRH levels by less than 2 pg/ml. The 250 ng GnRH dose, which can induce an LH pulse, did not elevate CSF-GnRH concentrations significantly. We estimate that if GnRH uptake into the brain is nonsaturable then to increase CSF-GnRH levels in the brain to those detected in the infundibular recess at the peak of the surge, at least 5 mg will be required. Thus, it is likely that in the earlier studies, the central concentrations of GnRH produced by iv GnRH were insufficient to induce a behavioral effect.
The question of “physiologically relevant dose” is important to consider for all potential extra-pituitary sites. If it is assumed that the binding affinity of GnRH receptors at these sites is the same as that of the pituitary GnRH receptors, then it would appear that CSF-GnRH concentrations (∼50 pm) are lower than the reported in vitro ovine pituitary GnRH receptor binding affinity [230 pm (59)]. However, we have previously shown (2) that CSF-GnRH concentrations, at the surge, are comparable to hypophyseal portal-GnRH concentrations, which are clearly sufficient to induce an LH surge. It is well established that a 250-ng GnRH treatment elicits an LH pulse comparable to that produced in vivo (60,61). This 250-ng challenge produces a portal GnRH concentration of 6 pg/min (∼15–20 pg/ml; ∼15 pm), which is indistinguishable from the endogenous release rate of 5.4 pg/min (34). Thus, there appears to be a mismatch between reported GnRH binding affinity at the level of the pituitary and the physiological GnRH concentrations that are capable of eliciting a LH response, and, clearly, more research is required to address this disparity.
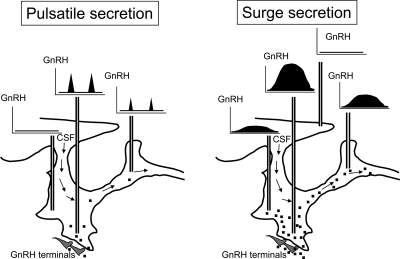
In summary, the present study shows that GnRH is not uniformly distributed throughout the third cerebroventricle but is more concentrated in the infundibular recess region. Accordingly, we hypothesize that the ependyma surrounding the infundibular recess is the source of most, if not all, GnRH in CSF. Thus, we propose that for most of the estrous cycle, when GnRH release is pulsatile, few cerebral regions are exposed to CSF-GnRH (Fig. 6). In contrast, at the preovulatory GnRH surge, potential neuronal targets will receive a stronger, volume-transmitted, signal. Our study also shows unequivocally that iv administered GnRH can penetrate the cerebroventricular system. However, extremely high doses are required to increase the resulting CSF-GnRH concentrations into those found during the estradiol-induced GnRH surge, and this result may explain why several earlier attempts to induce sexual behavior in sheep with exogenous GnRH were unsuccessful.
Figure 6.
Schematic of how we propose that the flow of GnRH in CSF will progress through the cerebroventricular system during tonic release and at the preovulatory GnRH surge. During the surge period, or periods with sustained GnRH release, infundibular GnRH is likely to penetrate more widely in the brain.
Acknowledgments
We thank Alexandre Figwer for his help in experiment 1, which formed part of his candidature for a master of science in Animal Physiology of the University of Tours, and Christine Briant for assistance with the statistical analysis. We are deeply grateful to B. Delaleu, D. Lomet, and A. Duittoz for assisting with the numerous cerebrospinal fluid and blood sampling periods, as well as to the people of “l’hôpital-Abbatoire” for the animal surgeries and the shepherds for taking good care of the animals.
Footnotes
D.C.S. was supported by Grant RR15640 from the National Center for Research Resources, a component of the National Institutes of Health.
Disclosure Statement: The authors have nothing to disclose.
First Published Online June 19, 2008
Abbreviations: AUC, Area under the curve; CSF, cerebrospinal fluid; icv, intracerebroventricularly; OVLT, organum vasculosum of the lamina terminalis.
References
- Skinner DC, Caraty A 2002 Measurement and possible function of GnRH in cerebrospinal fluid in ewes. Reprod Suppl 59:25–39 [PubMed] [Google Scholar]
- Skinner DC, Caraty A, Malpaux B, Evans NP 1997 Simultaneous measurement of gonadotropin-releasing hormone in the third ventricular cerebrospinal fluid and hypophyseal portal blood of the ewe. Endocrinology 138:4699–4704 [DOI] [PubMed] [Google Scholar]
- Skinner DC, Malpaux B, Delaleu B, Caraty A 1995 Luteinizing hormone (LH)-releasing hormone in third ventricular cerebrospinal fluid of the ewe: correlation with LH pulses and the LH surge. Endocrinology 136:3230–3237 [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA 2005 Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazal OS, Leshin LS, Stanko RL, Thomas MG, Keisler DH, Anderson LL, Williams GL 1998 Gonadotropin-releasing hormone secretion into third-ventricle cerebrospinal fluid of cattle: correspondence with the tonic and surge release of luteinizing hormone and its tonic inhibition by suckling and neuropeptide Y. Biol Reprod 59:676–683 [DOI] [PubMed] [Google Scholar]
- van Vugt D, Diefenbach W, Alston E, Ferin M 1985 Gonadotropin-releasing hormone pulses in third ventricular cerebrospinal fluid of ovariectomized rhesus monkeys: correlation with luteinizing hormone pulses. Endocrinology 117:1550–1559 [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Suzuki C, Arai S, Iwamura S, Hirose H 2001 Gonadotropin-releasing hormone in third ventricular cerebrospinal fluid of the heifer during the estrous cycle. Biol Reprod 64:563–570 [DOI] [PubMed] [Google Scholar]
- Xia L, van Vugt D, Alston E, Luckhaus J, Ferin M 1992 A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology 131:2812–2820 [DOI] [PubMed] [Google Scholar]
- Skinner DC, Caraty A, Evans NP 1998 Does gonadotropin-releasing hormone in the cerebrospinal fluid modulate luteinizing hormone release? Neuroendocrinology 67:37–44 [DOI] [PubMed] [Google Scholar]
- Brightman MW, Prescott L, Reese TS 1975 Intercellular junctions of special ependyma. In: Knigge KM, Scott DE, Kobayashi H, Ishii S, eds. Brain-endocrine interaction II; the ventricular system in neuroendocrine mechanisms. Basel: Karger; 146–165 [Google Scholar]
- Petrov T, Howarth AG, Krukoff TL, Stevenson BR 1994 Distribution of the tight junction-associated protein ZO-1 in circumventricular organs of the CNS. Brain Res Mol Brain Res 21:235–246 [DOI] [PubMed] [Google Scholar]
- Ugrumov MV 1992 Development of the median eminence during ontogenesis (morpho-functional aspects). Prog Brain Res 91:349–356 [DOI] [PubMed] [Google Scholar]
- Billings HJ, Lehman ML, Goodman RL, Amstalden M, McManus CJ, Hileman SM GnRH neurons contacting cerebrospinal fluid in estrous ewes. Soc Study Reprod, 38th Annual Meeting, Quebec City, Quebec, 2005 (Abstract T659) [Google Scholar]
- Lehman MN, Robinson JE, Karsch FJ, Silverman A-J 1986 Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J Comp Neurol 244:19–35 [DOI] [PubMed] [Google Scholar]
- Vigh B, Vigh-Teichmann I 1998 Actual problems of the cerebrospinal fluid-contacting neurons. Microsc Res Tech 41:57–83 [DOI] [PubMed] [Google Scholar]
- Jennes L, Stumpf WE 1980 LHRH-neuronal projections to the inner and outer surface of the brain. Neuroendocrinol Lett 2:241–247 [Google Scholar]
- Jennes L, Conn PM 1994 Gonadotropin-releasing hormone and its receptors in rat brain. Front Neuroendocrinol 15:51–77 [DOI] [PubMed] [Google Scholar]
- Jennes L, Stumpf WE 1980 LHRH-systems in the brain of the golden hamster. Cell Tissue Res 209:239–256 [DOI] [PubMed] [Google Scholar]
- Witkin JW, Paden CM, Silverman AJ 1982 The luteinizing hormone-releasing hormone (LHRH) systems in the rat brain. Neuroendocrinology 35:429–438 [DOI] [PubMed] [Google Scholar]
- Caldani M, Batailer M, Thiéry JC, Dubois MP 1988 LHRH-immunoreactive structures in the sheep brain. Histochemistry 89:129–139 [DOI] [PubMed] [Google Scholar]
- Charlton HM 1987 Neural grafts and the restoration of pituitary and gonadal function in hypogonadal (hpg) mice. Ann Endocrinol (Paris) 48:378–384 [PubMed] [Google Scholar]
- Boukhliq R, Goodman RL, Berriman SJ, Adrian B, Lehman MN 1999 A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology 140:5929–5936 [DOI] [PubMed] [Google Scholar]
- Riskind P, Moss RL 1983 Midbrain LHRH infusions enhance lordotic behavior in ovariectomized estrogen-primed rats independently of a hypothalamic responsiveness to LHRH. Brain Res Bull 11:481–485 [DOI] [PubMed] [Google Scholar]
- Riskind P, Moss RL 1979 Midbrain central gray: LHRH infusion enhances lordotic behavior in estrogen-primed ovariectomized rats. Brain Res Bull 4:203–205 [DOI] [PubMed] [Google Scholar]
- Caraty A, Delaleu B, Chesneau D, Fabre-Nys C 2002 Sequential role of e2 and GnRH for the expression of estrous behavior in ewes. Endocrinology 143:139–145 [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Rissman EF 2004 A critical role for the evolutionarily conserved gonadotropin-releasing hormone II: mediation of energy status and female sexual behavior. Endocrinology 145:3639–3646 [DOI] [PubMed] [Google Scholar]
- Cook DL, Winters TA, Horstman LA, Allrich RD 1986 Induction of estrus in ovariectomized cows and heifers: effects of estradiol benzoate and gonadotropin releasing hormone. J Anim Sci 63:546–550 [DOI] [PubMed] [Google Scholar]
- Allrich RD, Cook DL, Horstman LA, Knutson RJ, Winters TA 1989 Influence of dexamethasone, progesterone, gonadotropin-releasing hormone, and testosterone on estrous behavior of estradiol-treated ovariectomized heifers. J Dairy Sci 72:2707–2711 [DOI] [PubMed] [Google Scholar]
- Scanlan N, Skinner DC 2002 Estradiol modulation of growth hormone secretion in the ewe: no growth hormone-releasing hormone neurons and few somatotropes express estradiol receptor α. Biol Reprod 66:1267–1273 [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ 1997 Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology 138:5408–5414 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ 1991 Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129:1175–1182 [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Dahl GE, Evans NP, Manning JM, Mayfield KP, Moenter SM, Foster DL 1993 Seasonal changes in gonadotropin-releasing hormone secretion in the ewe: alteration in response to the negative feedback action of estradiol. Biol Reprod 49:1377–1383 [DOI] [PubMed] [Google Scholar]
- Skinner DC, Harris TG, Evans NP 2000 The duration and amplitude of the luteal phase progesterone increment times the estradiol-induced LH surge in ewes. Biol Reprod 62:1135–1142 [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Cummins JT, Thomas GB, Clarke IJ 1987 Steroid feedback inhibition of pulsatile secretion of gonadotropin-releasing hormone in the ewe. Biol Reprod 36:1207–1218 [DOI] [PubMed] [Google Scholar]
- Caraty A, Locatelli A, Moenter SM, Karsch FJ 1994 Sampling of hypophyseal portal blood of conscious sheep for direct monitoring of hypothalamic neurosecretory substances. In: Levine JE, ed. Methods in neuroscience. San Diego: Academic Press; 163–183 [Google Scholar]
- Taylor PL 1987 Munro. Hormone pulse-profile analysis. Amsterdam: Elsevier [Google Scholar]
- Merriam GR, Wachter KW 1982 Algorithms for the study of episodic hormone secretion. Am J Physiol 243:E310–E318 [DOI] [PubMed] [Google Scholar]
- Akmayev IG, Fidelina OV 1981 Tanycytes and their relation to the hypophyseal gonadotrophic function. Brain Res 210:253–260 [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Blazquez JL, Pastor FE, Pelaez B, Pena P, Peruzzo B, Amat P 2005 Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol 247:89–164 [DOI] [PubMed] [Google Scholar]
- Flament-Durand J, Brion JP 1985 Tanycytes: morphology and functions: a review. Int Rev Cytol 96:121–155 [DOI] [PubMed] [Google Scholar]
- Carretero J, Burks D, Rubio M, Blanco E, Herrero JJ, Bodego P, Juanes JA, Hernandez E, Riesco JM 2002 Immunocytochemical evidence for growth hormone-releasing hormone in the tanycytes of the median eminence of the rat. Folia Morphol (Warsz) 61:209–216 [PubMed] [Google Scholar]
- Gross DS 1976 Distribution of gonadotropin-releasing hormone in the mouse brain as revealed by immunohistochemistry. Endocrinology 98:1408–1417 [DOI] [PubMed] [Google Scholar]
- Gross DS, Baker BL 1977 Immunohistochemical localization of gonadotropin-releasing hormone (GnRH) in the fetal and early postnatal mouse brain. Am J Anat 148:195–215 [DOI] [PubMed] [Google Scholar]
- Vigh-Teichmann I, Vigh B 1983 The system of cerebrospinal fluid-contacting neurons. Arch Histol Jpn 46:427–468 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Karsch FJ, Lehman MN 1993 Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology 133:896–903 [DOI] [PubMed] [Google Scholar]
- Agnati LF, Zoli M, Stromberg I, Fuxe K 1995 Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69:711–726 [DOI] [PubMed] [Google Scholar]
- Zoli M, Torri C, Ferrari R, Jansson A, Zini I, Fuxe K, Agnati LF 1998 The emergence of the volume transmission concept. Brain Res Rev 26:136–147 [DOI] [PubMed] [Google Scholar]
- Jennes L, Dalati B, Conn PM 1988 Distribution of gonadotropin releasing hormone agonist binding sites in the rat central nervous system. Brain Res 452:156–164 [DOI] [PubMed] [Google Scholar]
- Granger A, Ngo-Muller V, Bleux C, Guigon C, Pincas H, Magre S, Daegelen D, Tixier-Vidal A, Counis R, Laverriere JN 2004 The promoter of the rat gonadotropin-releasing hormone receptor gene directs the expression of the human placental alkaline phosphatase reporter gene in gonadotrope cells in the anterior pituitary gland as well as in multiple extrapituitary tissues. Endocrinology 145:983–993 [DOI] [PubMed] [Google Scholar]
- Wilson AC, Salamat MH, RJ, Roche K, Karande A, Meethal SV, Terasawa E, Bowen RL, Atwood CS 2006 Human neurons express type I GnRH receptor and respond to GnRH I by increasing luteinizing hormone expression. J Endocrinol 191:651–663 [DOI] [PubMed] [Google Scholar]
- Albertson AJ, Navratil A, Mignot M, Dufourny L, Cherrington B, Skinner DC 2008 Immunoreactive GnRH type I receptors in the mouse sheep and brain. J Chem Neuroanat 35:326–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennes L, Brame B, Centers A, Janovick JA, Conn PM 1995 Regulation of hippocampal gonadotropin releasing hormone (GnRH) receptor mRNA and GnRH-stimulated inositol phosphate production by gonadal steroid hormones. Brain Res Mol Brain Res 33:104–110 [DOI] [PubMed] [Google Scholar]
- Osada T, Kimura F 1995 LHRH effects on hippocampal-neurons are modulated by estrogen in rats. Endocr J 42:251–257 [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Dixson AF 1985 Luteinizing hormone-releasing hormone enhances proceptivity in a primate. Neuroendocrinology 41:449–453 [DOI] [PubMed] [Google Scholar]
- Albertson AJ, Navratil A, Mignot M, Dufourny L, Cherrington B, Skinner DC 2008 Immunoreactive GnRH type I receptors in the mouse and sheep brain. J Chem Neuroanat 35:326–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TJ, Glucksman MJ, Roberts JL, Mani SK 2006 Facilitation of lordosis in rats by a metabolite of luteinizing hormone releasing hormone. Endocrinology 147:2544–2549 [DOI] [PubMed] [Google Scholar]
- Moss RL, McCann SM 1975 Action of luteinizing hormone-releasing factor (LRF) in the initiation of lordosis behavior in the estrone-primed ovariectomized female rat. Neuroendocrinology 17:309–318 [DOI] [PubMed] [Google Scholar]
- Pfaff DW 1973 Luteinizing hormone-releasing factor potentiates lordosis behavior in hypophysectomized ovariectomized female rats. Science 182:1148–1149 [DOI] [PubMed] [Google Scholar]
- Wagner TO, Adams TE, Nett TM 1979 GnRH interaction with anterior pituitary. I. Determination of the affinity and number of receptors for GnRH in ovine anterior pituitary. Biol Reprod 20:140–149 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT 1984 Direct pituitary effect of estrogen and progesterone on gonadotropin secretion in the ovariectomized ewe. Neuroendocrinology 39:267–274 [DOI] [PubMed] [Google Scholar]
- Breen KM, Davis TL, Doro LC, Nett TM, Oakley AE, Padmanabhan V, Rispoli LA, Wagenmaker ER, Karsch FJ 2008 Insight into the neuroendocrine site and cellular mechanism by which cortisol suppresses pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology 149:767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]