Abstract
Neurons in the ventromedial and arcuate hypothalamic nuclei (VMN and ARC, respectively) mediate many of leptin’s effects on energy homeostasis. Some are also glucosensing, whereby they use glucose as a signaling molecule to regulate their firing rate. We used fura-2 calcium (Ca2+) imaging to determine the interactions between these two important mediators of peripheral metabolism on individual VMN neurons and the mechanisms by which leptin regulates neuronal activity in vitro. Leptin excited 24%, inhibited 20%, and had a biphasic response in 10% of VMN neurons. Excitation occurred with a EC50 of 5.2 fmol/liter and inhibition with a IC50 of 4.2 fmol/liter. These effects were independent of the ambient glucose levels, and both glucosensing and non-glucosensing neurons were affected by leptin. In contrast, the ARC showed a very different distribution of leptin-responsive neurons, with 40% leptin excited, 10% leptin inhibited, and 2% having a biphasic response (χ2 = 60.2; P < 0.0001). Using pharmacological manipulations we found that leptin inhibits VMN neurons via activation of phosphoinositol-3 kinase and activation of the ATP-sensitive K+ channel. In addition, leptin inhibition was antagonized by 5′-AMP-activated protein kinase activation in 39% of neurons but was unaffected by 5′-AMP-activated protein kinase inhibition. No mechanism was delineated for leptin-induced excitation. Thus, within the physiological range of brain glucose levels, leptin has a differential effect on VMN vs. ARC neurons, and acts on both glucosensing and non-glucosensing VMN neurons in a glucose-independent fashion with inhibition primarily dependent upon activation of the ATP-sensitive K+ channel.
LEPTIN SIGNALING in the ventromedial hypothalamus (VMH) plays an important role in regulation of energy homeostasis (1). Early studies showed that “VMH” lesions that included both the arcuate and ventromedial hypothalamic nucleus (ARC and VMN, respectively) produced obesity and hyperphagia (2,3). Subsequent studies cast doubt on a specific role for the VMN in the regulation of energy homeostasis (4). However, additional studies clearly established its importance by showing that highly selective injections of colchicine into the VMN to interrupt all fibers of passage and afferent and efferent projections caused hyperphagia and obesity (5,6).
The importance of the VMN in regulating energy homeostasis was further supported by studies that demonstrated the presence of the signaling form of the leptin receptor (Lepr-b) (7,8,9,10), leptin-induced activation of neurons (11), and downstream signaling pathways in VMN neurons (7,12,13,14). Importantly, selective injections of leptin into the VMN reduce food intake and body weight in lean rats (15). In addition, altered metabolic states such as fasting (16), pregnancy (17), and raising obesity-resistant pups in an obesogenic postnatal environment (18) alter Lepr-b mRNA expression selectively in the VMN. Finally, deletion of Lepr-b selectively in a subpopulation of VMN neurons produces hyperphagia and obesity (19), whereas replacement of Lepr-b selectively in the VMN of Lepr-b-defective Koletsky rats decreases body weight gain and plasma leptin levels, and increases brown adipose tissue uncoupling protein-1 mRNA expression (20). All of these studies point to an important role for VMN leptin signaling in the control of energy homeostasis.
In addition to this role in the regulation of energy homeostasis, the VMN has been studied extensively because it contains a significant population of glucosensing neurons (10,21,22,23,24,25) that appear to play a critical role in regulating the counterregulatory response to hypoglycemia (26,27,28,29). At physiologically relevant glucose concentrations found in the VMH during the diurnal cycle [0.5–2.5 mmol/liter glucose (30,31)], these neurons are either glucose excited (GE) or inhibited (GI) as ambient glucose levels increase (10,22,23,24,28). Of the entire population of VMN neurons, 25–30% of glucosensing and non-glucosensing (NG) neurons also express Lepr-b mRNA, suggesting an important interaction between leptin and glucose sensitivity in these neurons (10). Unfortunately, our understanding of the way in which leptin interacts with VMN neurons is clouded by the fact that existing studies have used a variety of methodologies at widely disparate (and often nonphysiological) glucose concentrations and have focused on different classes of glucosensing neurons when assessing their responsiveness to leptin. Not surprisingly, such studies have provided a variety of conflicting results (19,32,33,34).
Our hypothesis was that glucose and leptin would interact synergistically on glucosensing neurons, to alter their activity when glucose was held within physiologically relevant levels (7). Although the majority of our previous work has focused on VMN neurons, there are many differences between the roles of VMN and ARC neurons in the regulation of energy and glucose homeostasis (28). Given these differences, we predicted that neurons in these two adjacent hypothalamic nuclei would respond differently to leptin. To test these hypotheses, we assessed the effects of glucose and leptin on specific subsets of dissociated VMN and ARC neurons using fura-2 calcium imaging to measure changes in intracellular calcium ([Ca2+]i) oscillations as a surrogate for neuronal activity (10,22,23,28). Finally, because the mechanisms by which leptin acts to alter neuronal activity has never been fully assessed at physiological levels of glucose found in VMN neurons, we also investigated the role of the ATP-sensitive K+ (KATP) channel, phosphoinositol-3 kinase (PI3K), 5′-AMP-activated protein kinase (AMPK), and MAPK in mediating leptin’s effects on VMN neuronal activity.
Materials and Methods
Animals
All work was in compliance with the animal care committee of the East Orange Veterans Affairs Medical Center. Male 3- to 4-wk-old Sprague Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) were housed at 23–24 C on a 12-h light, 12-h dark cycle (lights on at 0700 h). Food (Purina rat chow no. 5001; Purina Mills, LLC, St. Louis, MO) and water were available ad libitum.
Measurement of leptin- and glucose-induced [Ca2+]i responses in dissociated VMN neurons
Single VMN neurons were prepared as described previously (10). The VMN was punched bilaterally, and single cells were papain digested (2 mg/ml, 30 min, 37 C) and mechanically triturated. Cells were plated onto coverslips and allowed to adhere for 60 min before loading with the Ca2+ fluorophore fura-2 acetoxy-methyl ester (Molecular Probes, Inc., Eugene, OR) for 20 min in Hanks’ balanced salt solution buffer containing 2.5 mmol/liter glucose. They were washed twice and transferred to a microscope chamber held at 37 C. Fura-2 fluorescent images were acquired every 5 sec by alternating excitation at 340 and 380 nm, and emissions (420–600 nm) were collected using a cooled, charge-coupled device camera. Neurons were classified as GE, GI, and NG, as previously described (10). The criteria for classification of leptin responsiveness are described below.
Criteria for quantitating changes in [Ca2+]i fluctuations
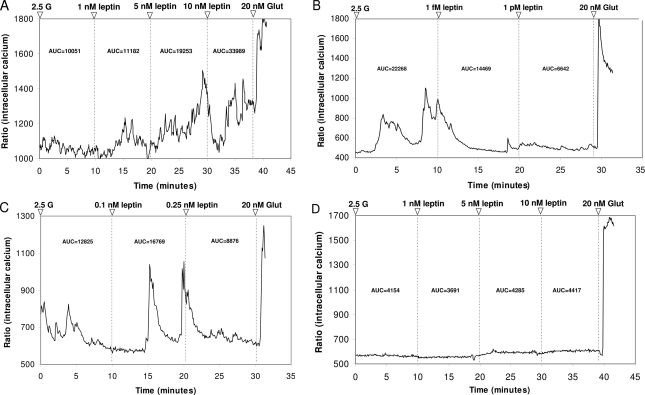
All experiments began with neurons held at 2.5 mmol/liter glucose unless otherwise specified. Changes in glucose and/or addition of drugs were maintained for approximately 10 min after addition. Initially, leptin’s effects were assessed at multiple concentrations between 0.1 fmol/liter and 10 nmol/liter in 512 neurons from nine rats. Significant changes in [Ca2+]i fluctuations were determined by first calculating the integrated area under the curve (AUC) for every 10-min period for a given concentration of leptin or glucose using Origin 7.0 software (OriginLab Corp., Northampton, MA). The neurons were then classified as leptin excited (LepE), leptin inhibited (LepI), biphasic, or leptin unresponsive (Fig. 1). Leptin-excited (Fig. 1A) neurons were defined as those that increased their AUC for [Ca2+]i oscillations by more than 30% of baseline levels at 2.5 mmol/liter glucose. Leptin-inhibited (Fig. 1B) neurons decreased their AUC for [Ca2+]i oscillations after addition of leptin by more than 30% of baseline levels. Biphasic (Fig. 1C) neurons increased their AUC for [Ca2+]i oscillations after addition of leptin by more than 30% and subsequently decreased their AUC after addition of another, higher leptin concentration. Neurons not meeting the minimal AUC criteria (30% increase or decrease) were classified as leptin unresponsive (Fig. 1D).
Figure 1.
Representative changes in [Ca2+]i oscillations after exposure to incremental doses of leptin in freshly dissociated VMN neurons from 3- to 4-wk-old male Sprague Dawley rats. All recordings were performed in 2.5 mmol/liter glucose (2.5 G) followed by two or more doses of leptin. Neurons were tested terminally with 20 nmol/liter glutamate (Glut) to ascertain viability. A, Leptin-excited neuron showing increased [Ca2+]i oscillations at 1, 5, and 10 nmol/liter leptin. B, Leptin-inhibited neuron showing decreased [Ca2+]i oscillations at 1 fmol/liter, which was sustained in 1 pmol/liter leptin. C, Neuron demonstrating a biphasic response (increase at 0.1 nmol/liter, decrease at 0.25 nmol/liter leptin) in [Ca2+]i oscillations to leptin. D, Leptin unresponsive neuron. AUC for each response is given above each tracing after a given manipulation.
Measurement of leptin-induced [Ca2+]i responses in dissociated ARC neurons
Single ARC neurons were obtained from fresh punches of ARC tissue in a similar fashion to VMN neurons. Leptin’s effects were assessed at four concentrations from 1 fmol/liter to 10 nmol/liter in 453 neurons from six rats. All experiments began with neurons held at 2.5 mmol/liter glucose, and ARC neurons were classified as LepE, LepI, or biphasic based on AUC calculations as described previously.
Effects of manipulating PI3K, MAPK, AMPK, and KATP channel activity on leptin-responsive VMN neurons
Dissociated VMN neurons were first classified by their responses to 5 fmol/liter or 1 pmol/liter leptin using the aforementioned criteria. The neurons were then exposed to leptin in 0.005% dimethylsulfoxide (DMSO) (solvent for inhibitors). The 0.005% DMSO concentration was selected based on our preliminary finding that this is the maximal concentration that has no effect on spontaneous [Ca2+]i oscillations in dissociated VMN neurons. After determining the leptin responses, neurons were incubated in the presence of leptin with one of the following inhibitors: PI3K (10 μmol/liter LY 294002); MAPK inhibitor (5 μmol/liter U0126); AMPK (10 μmol/liter Compound C; Calbiochem, San Diego, CA); or KATP channel (200 μmol/liter tolbutamide; Sigma-Aldrich, St. Louis, MO). The effects of activating AMPK on leptin signaling were assessed by comparing AUC changes in [Ca2+]i oscillations produced by 1 pmol/liter leptin to those with leptin plus the AMPK activator 5′-aminoimidazole-4-carboxamide riboside (500 μmol/liter AICAR; Calbiochem) dissolved in 2.5 mmol/liter glucose solution. Finally, to assess any independent effects of the drugs used, tolbutamide, AICAR, and LY294002 were applied to VMN neurons held at 0.5 or 2.5 mm glucose in the absence of leptin.
Statistics
Glucosensing properties, leptin responsiveness, and the effects of pharmacological inhibitors on leptin-responsive neurons were compared using the χ2 test for nonparametric statistics. Comparisons between VMN and ARC neuron responses to leptin were made using Fisher’s exact test. The EC50 of leptin-excited neurons and IC50 of leptin-inhibited neurons were determined by nonlinear regression analysis (sigmoidal dose-response curve fit; GraphPad Prism; GraphPad Software Inc., San Diego, CA).
Results
Leptin-induced changes in [Ca2+]i oscillations of VMN neurons held at 2.5 mmol/liter glucose
We first analyzed the response of 512 freshly dissociated VMN neurons at leptin concentrations of 0.1 fmol/liter-10 nmol/liter. Because the leptin responses were not dependent upon the concentration of glucose between 0.5 and 2.5 mmol/liter (see Table 1), we assessed all neurons at 2.5 mmol/liter glucose. Figure 1 provides examples of neurons that were excited (LepE; Fig. 1A), inhibited (LepI; Fig. 1B), had a biphasic (Fig. 1C), or no response (Fig. 1D) to leptin. All neurons responded to 20 nmol/liter glutamate terminally by increasing [Ca2+]i oscillations as we required to verify viability. In those leptin-excited neurons whose intracellular [Ca2+]i oscillations were increased by leptin, there was a dose-dependent increase with a calculated EC50 of 5.2 fmol/liter (R = 0.96), a threshold of 1 fmol/liter, and maximal response at 0.1 nmol/liter (Fig. 2A). In leptin-inhibited neurons, leptin reduced [Ca2+]i oscillations in a dose-dependent fashion with a calculated IC50 of 4.2 fmol/liter (R = 0.83), a threshold of 1 fmol/liter, and maximum inhibition at 1 pmol/liter (Fig. 2B). Thus, leptin-inhibited neurons had a much narrower dose-response range than did leptin-excited neurons but, within that range, were equally as sensitive to the inhibitory effects of leptin as were leptin-excited neurons to the excitatory effects of leptin. Across the entire range of neurons tested with 1 fmol/liter to 10 nmol/liter leptin, there were approximately equal percentages of leptin-excited (24%) and leptin-inhibited (20%) neurons, whereas half as many (∼10%) demonstrated a biphasic response at one or more leptin concentrations.
Table 1.
Effect of 10 nmol/liter leptin on GE, GI, and glucose nonresponsive (NG) VMN neurons at 2.5 vs. 0.5 mmol/liter glucose
| 2.5 mmol/liter glucose
|
0.5 mmol/liter glucose
|
|||||
|---|---|---|---|---|---|---|
| GE | GI | NG | GE | GI | NG | |
| LepE | 31 (22/71) | 20 (16/80) | 21 (31/146) | 28 (7/25) | 23 (5/22) | 25 (16/64) |
| LepI | 14 (10/71) | 18 (14/80) | 25 (37/146) | 12 (3/25) | 14 (3/22) | 22 (14/64) |
| LepN | 55 (39/71) | 62 (50/80) | 54 (79/146) | 60 (15/25) | 63 (14/22) | 53 (34/64) |
Changes in [Ca2+]i oscillations at varying glucose concentrations from 2.5 to 0.5 to 2.5 or 0.5 to 2.5 to 0.5 mmol/liter glucose, followed by exposure to 10 nmol/liter leptin were assessed in dissociated VMN neurons. Data are mean percentage of total tested, with number of neurons identified over the total number of neurons tested given in parentheses. LepN, Leptin nonresponsive.
Figure 2.
Dose-response curves for leptin-excited (A) and inhibited (B) VMN neurons were calculated for incremental doses of leptin (0.1 fmol/liter-10 nmol/liter) in the presence of 2.5 mmol/liter glucose. Data points (means ± se) were derived by calculating the percent increase [Ca2+]i AUC above baseline fluctuations in the presence of 2.5 mmol/liter glucose. EC50 is the effective concentration of leptin to increase and IC50 the effective concentration of leptin to decrease [Ca2+]i oscillations above and below baseline at 2.5 mmol/liter glucose, respectively.
Characteristics of glucosensing leptin-responsive VMN neurons
To characterize the glucosensing properties of leptin-responsive VMN neurons, they were first classified by their glucose-induced (2.5 to 0.5 to 2.5 mmol/liter) changes in [Ca2+]i oscillations as GE, GI, or NG using previously established criteria (10). Regardless of whether neurons were tested at either 2.5 or 0.5 mmol/liter glucose, 10 nmol/liter leptin increased [Ca2+]i oscillations in 20–31% GE, GI, and NG neurons, and inhibited [Ca2+]i oscillations in 12–25% GE, GI, and NG neurons (Table 1). Overall, there were no statistical differences among the types of neurons (GE, GI, NG) in the leptin-induced proportions of excitation, inhibition, or nonresponsiveness (χ2 = 6.8; P = 0.15). However, for GE neurons there were nearly twice the number of leptin-excited as leptin-inhibited neurons (31 vs. 14%; P = 0.001), and these responses did not vary as a function of the glucose concentrations at which they were tested. Therefore, although twice as many GE neurons were excited than inhibited by leptin, the overall effects of leptin on neuronal activity were independent of the ambient concentrations of glucose within the physiological range, regardless of the glucose sensing properties of the neurons studied.
Leptin-induced changes in [Ca2+]i oscillations of ARC neurons
As a comparison to VMN neuron leptin responses, 453 freshly dissociated ARC neurons were assessed at leptin concentrations of 1 fmol/liter–10 nmol/liter with glucose levels held at 2.5 mmol/liter. In contrast to VMN neurons, leptin excited 40%, leptin inhibited 10%, and had a biphasic response in 2% of ARC neurons (Table 2). There were twice as many leptin-excited, half as many leptin-inhibited, and one fifth as many biphasic neurons in the ARC as in the VMN (χ2 = 60.2; P < 0.0001). Because ARC neurons were assessed at only four concentrations of leptin, it was not possible to calculate EC50 or IC50 responses. However, whereas threshold responses occurred at 1 fmol/liter for leptin-excited and leptin-inhibited neurons in both nuclei, the range of responses to leptin was narrower in ARC than VMN neurons. Maximal leptin excitation occurred at 1000-fold and leptin inhibition at 10-fold lower concentrations in ARC than VMN neurons (Table 2).
Table 2.
Comparison of responses to leptin in VMN vs. ARC neurons
| VMN | ARC | |
|---|---|---|
| % LepE | 24 | 40a |
| % LepI | 20 | 10a |
| % Biphasic | 10 | 2a |
| Leptin-excited threshold concentration | 1 fmol/liter | 1 fmol/liter |
| Leptin-inhibited threshold concentration | 1 fmol/liter | 1 fmol/liter |
| Leptin-excited maximal response concentration | 100 pmol/liter | 100 fmol/liter |
| Leptin-inhibited maximal response concentration | 1 pmol/liter | 10 fmol/liter |
Response of VMN vs. ARC neurons to leptin (0.1 fmol/liter–10 nmol/liter) was characterized by assessing changes in [Ca2+]i oscillations at 2.5 mmol/liter glucose.
P = 0.05 or less by Fischer’s exact test for specific categories of leptin response between VMN and ARC neurons.
Role of KATP channels in mediating leptin’s effects
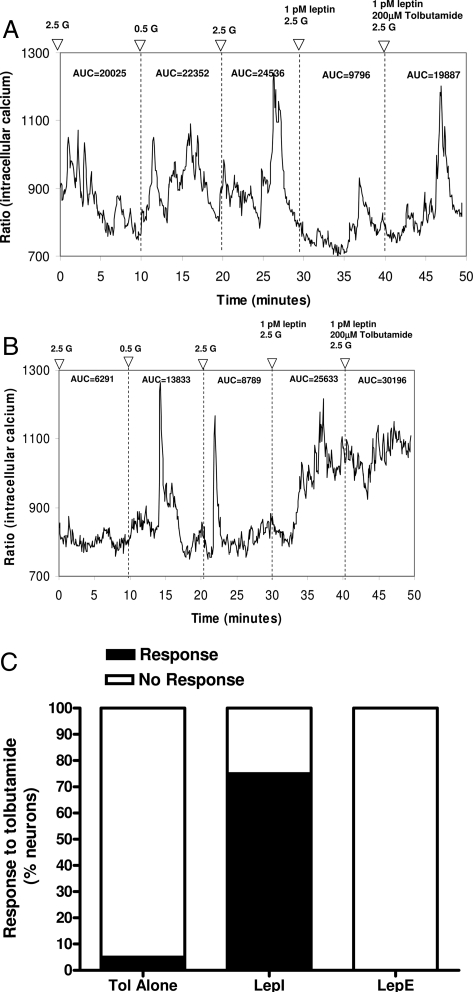
To test the hypothesis that leptin’s effects in the VMN are mediated by the KATP channel, 128 freshly dissociated neurons were first classified by their glucose-induced (2.5 to 0.5 to 2.5 mmol/liter) changes in [Ca2+]i oscillations and then exposed to 1 pmol/liter leptin, followed by 200 μmol/liter tolbutamide in the presence of 1 pmol/liter leptin. Of all the VMN neurons tested at 1 pmol/liter leptin, 20% (25 of 128) were LepE, and 9% (12 of 128) were LepI. Approximately 75% (nine of 12) of leptin-inhibited neurons showed an increase in [Ca2+]i oscillations when the KATP channels were inactivated with tolbutamide (Fig. 3, A and C). Of the nine leptin-inhibited neurons that showed a tolbutamide response, four were GE, and five were NG. These results were unlikely to have been due to an independent effect of tolbutamide alone because none of the leptin-excited neurons showed any change in [Ca2+]i oscillations in the presence of both tolbutamide and leptin (Fig. 3, B and C), and only 5% (four of 77) neurons tested separately in the absence of leptin responded to tolbutamide (Fig. 3C). These results suggest that, in majority of leptin-inhibited neurons, leptin inhibition is dependent upon activation of the KATP channel but is independent of the glucosensing characteristics of the specific neuron. On the other hand, there is no evidence that inactivation of the KATP channel affects leptin excitation of VMN neurons.
Figure 3.
Effect of inactivating the KATP channel on VMN neuron responses to leptin. VMN neurons were first characterized as being GE, GI, or NG by their responses to glucose-induced (2.5 to 0.5 to 2.5 mmol/liter) changes in [Ca2+]i oscillations. Neurons held at 2.5 mmol/liter glucose were then exposed to 1 pmol/liter leptin and then to 200 μmol/liter tolbutamide plus 1 pmol/liter leptin. A, Leptin inhibition of NG neuron is antagonized by tolbutamide. B, Glucose-inhibited neuron excited by leptin is unaffected by addition of tolbutamide. C, Compiled responses of VMN neurons to tolbutamide (Tol) alone and in the presence of leptin. There were significant differences in the tolbutamide responsive vs. nonresponsive leptin-inhibited neurons (P < 0.001), whereas none of the leptin-excited neurons responded to tolbutamide. Tolbutamide alone affected only a small percentage of neurons. 2.5 G, 2.5 mmol/liter glucose.
Role of PI3K in mediating leptin’s effects
To test the hypothesis that PI3K-dependent signaling is required for leptin’s effects on VMN neurons, 109 freshly dissociated VMN neurons were held at 2.5 mmol/liter glucose and treated sequentially with 5 fmol/liter leptin, 5 fmol/liter leptin in 0.005% DMSO, and then the PI3K inhibitor LY 294002 (10 μmol/liter) in 0.005% DMSO in the presence of 5 fmol/liter leptin. Of all the neurons tested at 5 fmol/liter leptin, 8% were LepI. Of these, leptin-induced inhibition was reversed by LY 294002 in 88% (seven of eight; Fig. 4, A and C), and 12% (one of eight) showed no response to LY 294002. In contrast, in leptin-excited neurons (30% of total tested), 90% (27 of 30) demonstrated no effect on leptin’s action in the presence of LY 294002, whereas 3% (one of 30) increased and 7% (two of 30) decreased their [Ca2+]i oscillations (Fig. 4, B and C). As with tolbutamide, there was a small population (five of 116; 4%) of neurons that responded variably to LY 294002 in the absence of leptin (Fig. 4C). These data suggest that PI3K activity is required for leptin’s inhibitory effect in the majority of leptin-inhibited neurons but plays little or no role in mediating leptin’s activating effects in leptin-excited neurons.
Figure 4.
Effect of PI3K inhibition on VMN neuron responses to leptin. Neurons held at 2.5 mmol/liter glucose (2.5 G) were sequentially exposed to leptin (5 fmol/liter), leptin plus vehicle (0.005% DMSO), and leptin plus the PI3K inhibitor LY 294002 (10 μmol/liter) dissolved in 0.005% DMSO. A, Leptin inhibition is unaffected by 0.005% DMSO but is reversed by 10 μmol/liter LY 294002. B, Leptin excitation is unaffected by 0.005% DMSO or 10 μmol/liter LY 294002. C, Compiled responses of VMN neurons to LY 294002 (LY) alone and in the presence of leptin. There were significant differences between the types of leptin-responsive neurons (LepE vs. LepI) in the LY294002-induced proportions of excitation, inhibition, or nonresponsiveness (χ2 = 26.9; P < 0.001). LY294002 alone affected activity in only a small percentage of neurons. Glut, Glutamate.
Role of AMPK in mediating leptin’s effects
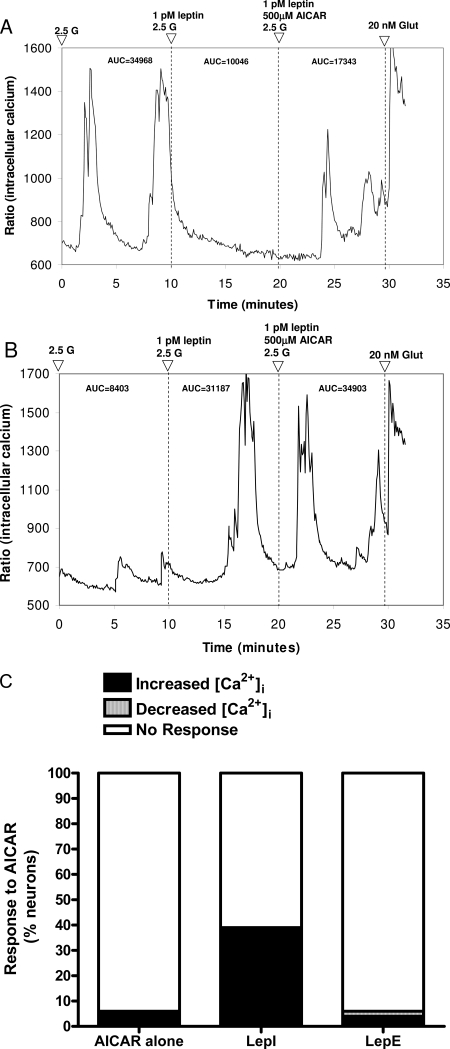
Because previous studies suggested that leptin acts by modulating AMPK activity in the hypothalamus (35), we assessed the effects of pharmacologically increasing and decreasing AMPK activity with AICAR and Compound C, respectively. First, 220 freshly dissociated VMN neurons held at 2.5 mmol/liter glucose were exposed sequentially to 1 pmol/liter leptin and then to AICAR (500 μmol/liter) in the presence of 1 pmol/liter leptin (Table 3 and Fig. 5). Of these, 32% (70 of 220) were LepE, and 10% (23 of 220) were LepI. Of the leptin-inhibited neurons, 39% (nine of 23) responded to AICAR with increased [Ca2+]i oscillations (Fig. 5, A and C). Of the leptin-excited neurons, 94% (66 of 70) were unaffected by AICAR (Fig. 5, B and C), whereas a small population (four of 70) responded variably by increasing or decreasing their [Ca2+]i oscillations in the presence of leptin and AICAR (Fig. 5C). These latter responses were possibly due to the effects of AICAR in the absence of leptin because AICAR affected a similar proportion of neurons (four of 70; 6%) when applied in the absence of leptin (Fig. 5C).
Table 3.
Effects of AICAR and Compound C on leptin-responsive neurons
| AICAR
|
Compound C
|
|||||
|---|---|---|---|---|---|---|
| Total | Increased [Ca2+]i | Decreased [Ca2+]i | Total | Increased [Ca2+]i | Decreased [Ca2+]i | |
| LepE | 32 (70/220) | 4 (3/70) | 1 (1/70) | 35 (56/154) | 0 | 11 (6/56) |
| LepI | 10 (23/220) | 39 (9/23) | 0 | 10 (16/154) | 0 | 0 |
Changes in leptin-induced [Ca2+]i oscillations in dissociated VMN neurons held at 2.5 mmol/liter glucose in the presence of the AMPK activator AICAR (500 μmol/liter) or the AMPK inhibitor Compound C (10 μmol/liter). Data are mean percentage of total neurons tested, with number of neurons identified over the total number of neurons tested given in parentheses. There were significant differences between the types of leptin-responsive neurons (LepE vs. Lep I) in the AICAR-induced proportions of excitation, inhibition, or nonresponsiveness (χ2 = 18.3; P = 0.001).
Figure 5.
Effect of AMPK activation on VMN neuron responses to leptin. A, Leptin inhibition is antagonized by AICAR (500 μmol/liter) dissolved in 2.5 mmol/liter glucose (2.5 G). B, Leptin excitation is unaffected by addition of AICAR. C, Compiled responses of VMN neurons to AICAR alone and in the presence of leptin. AICAR alone affected only a small percentage of neurons. Glut, Glutamate.
To assess the effects of inhibiting AMPK activity, an additional 161 freshly dissociated VMN neurons were held at 2.5 mmol/liter glucose, and then exposed sequentially to 1 pmol/liter leptin, 1 pmol/liter leptin in 0.005% DMSO, and then to Compound C (10 μmol/liter) in 0.005% DMSO in the presence of 1 pmol/liter leptin. Compound C failed to produce any significant effect in the majority (>89%) of either leptin-excited or leptin-inhibited neurons (Table 3). Due to this lack of effect in the presence of leptin, Compound C was not assessed alone. These data suggest that, whereas AMPK activation can antagonize leptin-induced inhibition of [Ca2+]i oscillations in a subpopulation of leptin-inhibited neurons, AMPK inhibition has little effect on leptin signaling in either leptin-inhibited or leptin-excited neurons.
Role of MAPK in mediating leptin’s effects
To test the hypothesis that MAPK-dependent signaling is involved in leptin’s effects in VMN neurons, 154 freshly dissociated neurons were held at 2.5 mmol/liter glucose and exposed sequentially to 5 fmol/liter leptin, 5 fmol/liter leptin in 0.005% DMSO, and then the MAPK inhibitor U0126 (5 μmol/liter) in 0.005% DMSO in the presence of 5 fmol/liter leptin. Of all the VMN neurons tested at 5 fmol/liter leptin, 38% (58 of 154) were LepE, and 9% (14 of 154) were LepI. Of these, U0126 failed to affect leptin-induced alterations in [Ca2+]i oscillations in the majority (>91%) of leptin-responsive neurons. Inhibition of MAPK was not totally without effect because 9% (five of 58) of leptin-excited neurons decreased, whereas 7% (one of 14) of leptin-inhibited neurons increased their [Ca2+]i oscillations in the presence of U0126. However, these data suggest that MAPK-dependent signaling does not participate in leptin’s effects on the vast majority of VMN neurons.
Discussion
These studies represent the first comprehensive, population-based survey of VMN and ARC neuronal responses to leptin performed within the physiological range of glucose levels present in the hypothalamus across the diurnal cycle (30,31). Although at least 50% of VMN and ARC neurons responded to leptin, there were approximately twice as many leptin-excited, half as many leptin-inhibited, and one fifth as many biphasic neurons in the ARC as in the VMN. Importantly, the biphasic response to leptin has not previously been demonstrated and may have important implications for leptin signaling in obesity, in which leptin levels can be many fold higher than in lean individuals. Although VMN neurons were similarly sensitive to the excitatory and inhibitory effects of leptin, leptin-inhibited activity in ARC neurons over a 10-fold narrower range than did leptin excitation. In addition, ARC neurons responded to leptin over a 10- to 1000-fold narrower range than did VMN neurons, suggesting that they were more sensitive to leptin.
Importantly, we also show for the first time here that, at least for VMN neurons, leptin responsiveness is independent of physiologically relevant glucose levels. Thus, we failed to confirm our original hypothesis that there is a synergy between leptin and glucose in the regulation of activity of these neurons (7). On the other hand, in keeping with our previous finding that equal proportions (25–32%) of VMN glucosensing and NG neurons expressed Lepr-b mRNA (10), we found here that approximately equal percentages of GE, GI, and NG VMN neurons responded to leptin. However, whereas equal percentages of GI and NG neurons were excited and inhibited by leptin, twice as many GE neurons were excited as inhibited by leptin. This suggests that GE neurons might possess different leptin signaling pathways than GI or NG neurons.
Our studies using pharmacological inhibitors support those of others (32,36,37,38,39,40) suggesting that leptin’s inhibitory effect on neuronal activity is mediated through activation of PI3K leading to activation of the KATP channel and subsequent membrane hyperpolarization. On the other hand, our studies fail to shed any new light on the mechanism by which leptin excites neurons, although a decrease in potassium conductance was suggested as the ionic mechanism underlying the leptin-induced depolarization in vagal dorsal motor nucleus neurons (40).
Other studies suggest that leptin’s anorectic and neuronal inhibitory effects and AMPK signaling are linked whereby leptin produces inhibition of AMPK activity in the hypothalamus (33,35,41,42). In fact, we did find that AMPK activation with AICAR antagonized leptin-induced inhibition of neuronal [Ca2+]i oscillations in 39% of VMN neurons. However, this finding does not definitively link leptin signaling with AMPK activation because it is possible that maximal activation of AMPK with AICAR might antagonize leptin-induced inhibition of neuronal activity without necessarily being involved directly in the leptin-signaling pathway. In addition, AICAR has other well-described actions besides those on AMPK that might account for its inhibitory effects on leptin’s actions (43,44,45). On the other hand, only a small percentage of leptin-excited VMN neurons were affected by AMPK inhibition with Compound C. In addition, this leaves more than 60% of neurons in which leptin and AMPK signaling do not appear to be linked at all. These findings support those of Claret et al. (46) who found that leptin-induced depolarization and activation still occurred in arcuate proopiomelanocortin neurons in which AMPK had been deleted. Finally, we found little evidence that leptin-induced inhibition or excitation was mediated via the MAPK signaling pathway to any significant degree.
The current studies were undertaken because the existing literature on the interactions among glucose, leptin, and the type of glucosensing neuron studied has produced a wide range of disparate results. Some of these disparities may be due to the use of nonphysiological concentrations of glucose. During the normal diurnal cycle, VMN glucosensing neurons are exposed primarily to brain interstitial glucose levels that range from approximately 0.7 mmol/liter after an overnight fast (∼14% of blood levels) to 1.5–2.5 mmol/liter in fed animals (∼20% of blood levels) (30,31). Glucosensing neurons respond in a concentration-dependent fashion within this range to increments of 0.1–0.2 mmol/liter (10,22,23,24,28). For this reason, it is important to assess the function of glucosensing as well as NG neurons within this physiological range. This is particularly important when assessing functions that are dependent upon the KATP channel, which is highly sensitive to ambient glucose levels, at least in GE neurons (10,22,23,24,28).
The importance of evaluating neurons at physiological levels of glucose is exemplified by comparing our current finding that leptin excited twice as many GE neurons as it inhibited at physiological glucose levels to other studies using nonphysiologically high glucose levels. For example, when neurons were identified as being either GE or NG in VMN slices held at 10 mmol/liter glucose, all of the VMN GE neurons were hyperpolarized and inactivated by 10 nmol/liter leptin via activation of the KATP channel (32), whereas 6% of NG VMN neurons were activated by leptin. In another study, when neuronal responses to high concentrations of leptin (100 nmol/liter) were assessed independently of glucosensing type and at high glucose levels (11.1 mmol/liter) at which all of the KATP channels are likely to be inactivated, 40% of neurons in VMN slices were excited, whereas 19% were inhibited (19). In distinct contrast to those studies, 84% of the dissociated VMN GE neurons held at 1 mmol/liter glucose increased their [Ca2+]i oscillations in the presence of 0.1 nm leptin, suggesting that leptin activated rather than inhibited GE neurons (34). Finally, using a membrane potential sensitive dye to assess responses, leptin inhibited 64% of cultured VMH (ARC plus VMN) GI neurons held at 2.5 mmol/liter glucose (33).
Given this wide range of results, we believe that our current studies significantly clarify the interactions among glucose levels, neuronal glucosensing types, and the responses of VMN neurons to leptin. However, there are several points to consider when interpreting our results. First, we performed Ca2+ imaging in individual freshly dissociated neurons that lack the supportive functions of astrocytes and can potentially have their function altered by dissociation. On the other hand, a distinct advantage of this methodology is that the results reflect only the direct actions of leptin on these neurons that are devoid of interpretive issues raised by the presence of presynaptic inputs (24). An added advantage is that large numbers of neurons can be screened simultaneously under identical physical and temporal conditions. Although, Ca2+ signaling is complex and might raise questions regarding the relationship between changes in [Ca2+]i and action potential frequency, we have shown that [Ca2+]i oscillations are a reasonable surrogate for changes in membrane potential (23). Furthermore, glucose-induced changes in VMN and ARC neurons are qualitatively similar to electrophysiological responses of patch-clamped VMN and ARC glucosensing neurons in slice preparations (24,28,47). Finally, although there are no published data that document the levels of extracellular leptin in the brain, our data demonstrate that individual VMN neurons are sensitive to leptin levels in the femtomolar range in a dose-dependent fashion.
In conclusion, the current studies provide several important new findings with regard to leptin signaling in hypothalamic neurons. First, assessing neurons only within the range of physiological glucose concentrations to which they are exposed in vivo, we show for the first time that the responses to leptin were independent of ambient glucose levels. Second, using population-based simultaneous assessment of large number of neurons, we demonstrate that there are significant numbers of both leptin-excited and leptin-inhibited neurons in both the VMN and ARC that respond at fmol/liter concentrations with a clear dose-related response to incremental increases in leptin concentrations. Third, by using a wide range of leptin concentrations, we show for the first time that there are a significant number of VMN neurons that have a biphasic response to incremental doses of leptin. Fourth, we confirm that the inhibitory effects of leptin are likely due to activation of PI3K with subsequent activation of the KATP channel (32) and that AMPK activation with AICAR may, in a subpopulation of VMN neurons, reverse the inhibitory effects of leptin. Fifth, our data suggest that leptin does not act through the MAPK pathway to alter neuronal activity in the VMN to a significant degree. Sixth, twice as many ARC neurons are excited, half as many are inhibited, and one fifth as many show a biphasic response to leptin as do VMN neurons. Finally, as in other studies, we have identified no potential signaling pathway that mediates the excitatory effects of leptin. In sum, we believe that the current studies add a needed element of clarity to the complex field of neuronal sensing of metabolites such as glucose and hormones, like leptin.
Footnotes
This work was supported by the Research Service of the Department of Veterans Affairs (to B.E.L. and A.D.-M.), the National Institute of Diabetes, Digestive and Kidney Disorders (DK-53181) (to B.E.L.), and by an award from the American Heart Association (to B.G.I.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 12, 2008
Abbreviations: AMPK, 5′-AMP-activated protein kinase; ARC, arcuate hypothalamic nucleus; AUC, area under the curve; DMSO, dimethylsulfoxide; GE, glucose excited; GI, glucose inhibited; [Ca2+]i, intracellular calcium; KATP, ATP-sensitive K+; LepE, leptin excited; LepI, leptin inhibited; NG, non-glucosensing; PI3K, phosphoinositol-3 kinase; VMH, ventromedial hypothalamus; VMN, ventromedial hypothalamic nucleus.
References
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG 2000 Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- Stellar E 1954 The physiology of motivation. Psychol Rev 5:5–22 [DOI] [PubMed] [Google Scholar]
- Hetherington AW 1940 Obesity in the rat following the injection of chronic acid into the hypophysis. Endocrinology 26:264–268 [Google Scholar]
- Gold RM 1973 Hypothalamic obesity: the myth of the ventromedial nucleus. Science 182:488–490 [DOI] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS 2000 Hypothalamic galanin is up-regulated during hyperphagia and increased body weight gain induced by disruption of signaling in the ventromedial nucleus. Peptides 21:519–526 [DOI] [PubMed] [Google Scholar]
- Choi S, Sparks R, Clay M, Dallman MF 1999 Rats with hypothalamic obesity are insensitive to central leptin injections. Endocrinology 140:4426–4433 [DOI] [PubMed] [Google Scholar]
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA 2004 Neuronal glucosensing: what do we know after 50 years? Diabetes 53:2521–2528 [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P 1996 Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett 387:113–116 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte Jr D 1996 Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 2:589–593 [DOI] [PubMed] [Google Scholar]
- Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE 2004 Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53:549–559 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB 1997 Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 138:839–842 [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Banks WA 2004 Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 286:R143–R150 [DOI] [PubMed] [Google Scholar]
- Hubschle T, Thom E, Watson A, Roth J, Klaus S, Meyerhof W 2001 Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci 21:2413–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG 2008 Crossing the border: developmental regulation of leptin transport to the brain. Endocrinology 149:875–876 [DOI] [PubMed] [Google Scholar]
- Jacob RJ, Dziura J, Medwick MB, Leone P, Caprio S, During M, Shulman GI, Sherwin RS 1996 The effect of leptin is enhanced by microinjection into the ventromedial hypothalamus. Diabetes 46:150–152 [DOI] [PubMed] [Google Scholar]
- Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle DS, Erickson JC, Palmiter RD, Schwartz MW 1998 Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting. Diabetes 47:538–543 [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Grattan DR 2005 Suppression of leptin receptor messenger ribonucleic acid and leptin responsiveness in the ventromedial nucleus of the hypothalamus during pregnancy in the rat. Endocrinology 146:3868–3874 [DOI] [PubMed] [Google Scholar]
- Gorski J, Dunn-Meynell AA, Hartman TG, Levin BE 2006 Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol 291:R768–R778 [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua Jr S, Elmquist JK, Lowell BB 2006 Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Kalra SP, Kalra PS 2005 AAV-mediated leptin receptor installation improves energy balance and the reproductive status of obese female Koletsky rats. Peptides 26:2567–2578 [DOI] [PubMed] [Google Scholar]
- Anand BK, Chhina GS, Sharma KN, Dua S, Singh B 1964 Activity of single neurons in the hypothalamus feeding centers: effect of glucose. Am J Physiol 207:1146–1154 [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE 2002 Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 51:2056–2065 [DOI] [PubMed] [Google Scholar]
- Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eikis J, Zhang BB, Levin BE 2006 Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55:412–420 [DOI] [PubMed] [Google Scholar]
- Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH 2001 Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus (VMN). Diabetes 50:2673–2681 [DOI] [PubMed] [Google Scholar]
- Oomura Y, Kimura K, Ooyama H, Maeo T, Iki M, Kuniyoshi N 1964 Reciprocal activities of the ventromedial and lateral hypothalamic area of cats. Science 143:484–485 [DOI] [PubMed] [Google Scholar]
- Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI 1997 Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 99:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI 1995 Local ventromedial hypothalamic glucopenia triggers counterregulatory hormone release. Diabetes 44:180–184 [DOI] [PubMed] [Google Scholar]
- Kang L, Sanders NM, Dunn-Meynell AA, Gaspers LD, Routh VH, Thomas AP, Levin BE 2008 Prior hypoglycemia enhances glucose responsiveness in some ventromedial hypothalamic glucosensing neurons. Am J Physiol Regul Integr Comp Physiol 294:R784–R792 [DOI] [PubMed] [Google Scholar]
- Levin BE, Becker TC, Eiki JI, Zhang BB, Dunn-Meynell AA 2008 Ventromedial hypothalamic glucokinase is an important mediator of the counterregulatory response to insulin-induced hypoglycemia. Diabetes 57:1371–1379 [DOI] [PubMed] [Google Scholar]
- De Vries MG, Arseneau LM, Lawson ME, Beverly JL 2003 Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes 52:2767–2773 [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M 1994 Extracellular glucose concentrations in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci 14:5068–5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML 1997 Leptin inhibits hypothalamic neurons by activation of ATP- sensitive potassium channels. Nature 390:521–525 [DOI] [PubMed] [Google Scholar]
- Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH 2007 Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 292:R1418–R1428 [DOI] [PubMed] [Google Scholar]
- Funahashi H, Yada T, Muroya S, Takigawa M, Ryushi T, Horie S, Nakai Y, Shioda S 1999 The effect of leptin on feeding-regulating neurons in the rat hypothalamus. Neurosci Lett 264:117–120 [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB 2004 AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574 [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS 2005 PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Smith BN 2006 Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: implications for ingestive behaviour. J Physiol 573(Pt 2):395–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Bruening JC 2006 Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest 116:1886–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, Ashford ML 2004 Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci 5:54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TL, Chiou LC, Lin YS, Hsieh JR, Hwang LL 2007 Electrophysiological study on the effects of leptin in rat dorsal motor nucleus of the vagus. Am J Physiol Regul Integr Comp Physiol 292:R2136–R2143 [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ 2004 AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279:12005–12008 [DOI] [PubMed] [Google Scholar]
- Park S, Hong SM, Sung SR, Jung HK 2008 Long-term effects of central leptin and resistin on body weight, insulin resistance, and β-cell function and mass by the modulation of hypothalamic leptin and insulin signaling. Endocrinology 149:445–454 [DOI] [PubMed] [Google Scholar]
- Vincent MF, Marangos PJ, Gruber HE, Van den Berghe G 1991 Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes 40:1259–1266 [DOI] [PubMed] [Google Scholar]
- Jacobs RL, Lingrell S, Dyck JR, Vance DE 2007 Inhibition of hepatic phosphatidylcholine synthesis by 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside is independent of AMP-activated protein kinase activation. J Biol Chem 282:4516–4523 [DOI] [PubMed] [Google Scholar]
- Zang Y, Yu LF, Pang T, Fang LP, Feng X, Wen TQ, Nan FJ, Feng LY, Li J 2008 AICAR induces astroglial differentiation of neural stem cells via activating the JAK/STAT3 pathway independently of AMP-activated protein kinase. J Biol Chem 283:6201–6208 [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ 2007 AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117:2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH 2004 The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes 53:1959–1965 [DOI] [PubMed] [Google Scholar]