Abstract
Diabetic neuropathy (DN) is a common complication of diabetes. Currently, there is no drug treatment to prevent or slow the development of DN. Rosiglitazone (Rosi) is a potent insulin sensitizer and may also slow the development of DN by a mechanism independent of its effect on hyperglycemia. A two by two design was used to test the effect of Rosi treatment on the development of DN. Streptozotocin-induced diabetic DBA/2J mice were treated with Rosi. DN and oxidative stress were quantified, and gene expression was profiled using the Affymetrix Mouse Genome 430 2.0 microarray platform. An informatics approach identified key regulatory elements activated by Rosi. Diabetic DBA/2J mice developed severe hyperglycemia, DN, and elevated oxidative stress. Rosi treatment did not affect hyperglycemia but did reduce oxidative stress and prevented the development of thermal hypoalgesia. Two novel transcription factor binding modules were identified that may control genes correlated to changes in DN after Rosi treatment: SP1F_ZBPF and EGRF_EGRF. These targets may be useful in designing drugs with the same efficacy as Rosi in treating DN but with fewer undesirable effects.
DIABETIC NEUROPATHY (DN) is a common complication of type 1 and 2 diabetes, and affects approximately 20% of adult diabetic patients (1). Patients with DN experience a progressive loss of sensation following a “stocking and glove” pattern, resulting from length-dependent axonal loss (2). Clinical trials have failed to demonstrate the effectiveness of any drug treatment in stabilizing or improving nerve function (1). In the absence of promising results from the currently available drugs and drug targets, new classes of drugs are being evaluated for the treatment of DN.
Thiazolidinediones (TZDs) are potent insulin sensitizers used to treat type 2 diabetes and insulin-resistant type 1 diabetes (3). TZDs were first identified as insulin-sensitizing agents by Chang (4,5) and Fujita et al. (6), who evaluated their antidiabetic effects in mouse models of type 2 diabetes and a rat model of type 1 diabetes. The mechanism of this antidiabetic activity was determined to be activation of peroxisome proliferator-activated receptor (PPAR)-γ, a transcription factor (TF) in the nuclear receptor family (7). Although other PPAR TFs are ubiquitously expressed, PPAR-γ is specific to adipose tissue, kidney, liver, and Schwann cells (8,9). Microarray studies of gene regulation by TZDs found that they alter steady-state mRNA levels of several hundred genes, primarily those involved in glucose and lipid metabolism (10).
Recent evidence suggests that TZDs reduce the development of diabetic complications independent of insulin sensitization. TZD treatment reduces the formation of atherosclerotic plaques (11), a macrovascular complication, and also reduces oxidative stress and the inflammatory response (12), two mechanisms of microvascular complications. TZD effects on oxidative stress are attributable to a number of potential causes, including altered expression of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and improvement of mitochondrial health (13,14). Two studies were performed demonstrating that TZDs slow or prevent the development of neuropathy in models of type 1 diabetes. In streptozotocin (STZ)-treated rats, troglitazone protects against nerve conduction velocity (NCV) slowing, and maintains normal myelinated fiber architecture and number (15). Pioglitazone also has neuroprotective effects on NCV and reduces macrophage infiltration in the sciatic nerve (8). These improvements in the DN profile of the diabetic rats cannot be explained by insulin sensitization and are due to some other effect of TZDs. Troglitazone is an innate antioxidant as well as glitazones (16), and may reduce oxidative stress independent of TF activation. No such alternative mechanism is known for pioglitazone, and changes in protein expression suggest that a gene regulatory mechanism was activated (9). A systems biology approach may elucidate this gene regulatory mechanism, but a rat model of diabetes was used by both research groups, which is nonideal for gene expression profiling.
Although TZDs have significant promise in the treatment of DN, their side effects may limit their usefulness (17,18,19). Due to the heterogeneous complications of members of the TZD family, we hypothesize that the beneficial effects of TZDs on diabetic complications are independent of the mechanisms that cause adverse effects. Screening the gene expression patterns induced in the nerve by PPAR-γ agonist activity may allow us to separate these positive and negative effects, and identify regulatory mechanisms that affect the development of DN. We examined changes in gene expression in sciatic nerves of mice with STZ-induced diabetes and TZD treatment to define downstream transcriptional responses. Using systems biology, we inferred likely regulatory mechanisms based on those changes. Such regulatory mechanisms constitute starting points for more specific drug target development with the potential to separate the beneficial and harmful effects of TZDs. A TZD with no known innate antioxidant capacity, rosiglitazone (Rosi), was chosen as the treatment to minimize the potential confounding effects on oxidative stress. Gene expression changes were measured using Affymetrix microarrays (Affymetrix Inc., Santa Clara, CA), revealing that novel transcriptional control sequences are present in genes strongly correlated with DN.
Materials and Methods
Mice
DBA/2J mice (stock no. 000671) were purchased from The Jackson Laboratory (Bar Harbor, ME). Breeding colonies established at the University of Michigan provided the animals used in this study. Mice were housed in a pathogen-free environment, and cared for following the University of Michigan Committee on the Care and Use of Animals guidelines. Mice were fed powdered mouse chow (Purina 5001; Purina Mills, LLC, St. Louis, MO).
Induction of diabetes and Rosi treatment
Four treatment groups were defined: control (n = 17), diabetic (n = 15), control plus Rosi (n = 18), and diabetic plus Rosi (n = 13). Diabetes was induced by low-dose STZ treatment (40 mg/kg for 5 d) when the animals reached a weight of 22 g (∼10 wk old). Two weeks after STZ treatment, or the equivalent time point for control mice, Rosi (3 mg/kg orally·d) was given in Nutra-Cal (Vetoquinol USA, Buena, NJ) to half of the diabetic and control animals. To increase survival, diabetic and diabetic plus Rosi mice were implanted with a sustained release insulin implant (Linbit for mice 0.1 U insulin/d/implant; LinShin Canada, Inc., Scarborough, Toronto, Ontario, Canada) 10 wk after STZ treatment. The second and third implants were inserted after 15 and 20 wk diabetes.
Measurement of blood glucose and glycosylated hemoglobin (GHb)
Fasting blood glucose levels were measured every 4 wk to document the persistence of diabetes. After a 6-h fast, one drop of tail blood was analyzed using a standard Glucometer (OneTouch; LifeScan Inc., Milpitas, CA). At 24 wk, GHb was measured using the Helena Laboratories Test Kit, Glyco-Tek Affinity Column Method (Helena Laboratories Corp., Beaumont, TX).
Thermal latency
Mice were placed in a plantar test apparatus (model 336TG; Life Sciences, Woodland Hills, CA) on a warm (32 C) glass plate and allowed to habituate for 10 min. An adjustable red light emitter (range 60–170 C) was maneuvered under the hind paw, and the time of activation of the beam to the time of paw withdrawal was recorded. The light source was set at 25 C and the temperature increased to 70 C over the course of 10 sec.
Nerve conduction studies
NCVs were recorded as previously described (20,21). For the sciatic nerve, the recording electrodes were placed in the dorsum of the foot, and the stimulating electrodes at the knee and sciatic notch. For the sural nerve, the anode was placed on the third toe of the foot, and the cathode was placed on the heel of the foot. The cathode and anode were placed 5-mm apart. The frequency band was inclusive of two, 10 Hz muscle potential recordings (orthodromic, motor) and 10, 2-Hz potential recordings (antidromic, sensory).
Tissue harvest
Twenty-four weeks after induction of diabetes, mice were euthanized by sodium pentobarbital overdose and tissues harvested as previously described (20,21). A blood sample (50 μl) was collected for measurement of GHb [see Measurement of blood glucose and glycosylated hemoglobin (GHb)]. The left sciatic nerve was dissected and stored in 30 μl RNA later (Ambion, Inc., An Applied Biosystems Business, Austin, TX) for gene expression analysis. The right sciatic nerve was dissected and immediately submerged in ice-cold antioxidant buffer, rapidly frozen by immersion in liquid nitrogen, and stored at −70 C for quantification of end products of oxidative damage.
Oxidative stress measures
Five samples from each experimental group were analyzed as previously described (22,23,24). Tissue was homogenized at 4 C in buffer [100 μm diethylenetriaminepentaacetic acid, 50 μm butylated hydroxytoluene, 1% (vol/vol) ethanol, 10 mm 3-amino-1,2,4-triazole, and 50 mm sodium phosphate buffer (pH 7.4)], frozen, and thawed. Protein was precipitated with ice-cold trichloroacetic acid (10% vol/vol), collected by centrifugation, washed with 10% trichloroacetic acid, and delipidated twice with water/methanol/water-washed diethyl ether (1:3:7 vol/vol). Isotopically labeled internal standards were added, and samples were hydrolyzed in 4 n methane sulfonic acid at 110 C for 24 h under argon as described previously (24). Hydroxyoctadecadienoic acids (HODEs) were quantified by reverse-phase C-18 HPLC analysis of triphenylphosphine-reduced lipid extracts after base hydrolysis. The protein content of tissue pellets was determined by a modified Lowry protein assay using BSA as a standard (23).
Amino acids were isolated from the acid hydrolysate using a solid-phase C-18 column (Supelclean SPE; Supelco Inc., Bellefonte, PA). Analyses of the dabsylated derivatives of tyrosine and O,O′-dityrosine were performed by reverse-phase HPLC (JASCO HPLC; JASCO, Essex, UK; Column; and Beckman ODS Ultrasphere C-18 column; Beckman Coulter, Inc., Fullerton, CA) as described previously (22). The identity of the compounds was confirmed by identical retention times of authentic O,O′-dityrosine and tyrosine. Quantification was performed by constructing standard curves using authentic O,O′-dityrosine and tyrosine.
RNA preparation
Total RNA was isolated using a commercially available silica gel-based isolation protocol (RNeasy Mini Kit; QIAGEN, Inc., Valencia, CA), including an on-column deoxyribonuclease digestion following the manufacturer’s protocol. RNA quantity and quality were measured by microfluid electrophoresis using the RNA 6000 Pico LabChip on a 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA).
Affymetrix microarrays
Five samples from each experimental group were analyzed for gene expression. Of total RNA, 75 ng was amplified and biotin labeled using the Ovation Biotin-RNA Amplification and Labeling System (NuGEN Technologies, Inc., San Carlos, CA) according to the manufacturer’s protocol. Amplification and hybridization were performed by the University of Michigan Comprehensive Cancer Center Affymetrix and Microarray Core Facility (University of Michigan, Ann Arbor, MI) using the GeneChip Mouse Genome 430 2.0 Array (Affymetrix). Intensities of target hybridization to the respective probe features on the chip were detected by laser scan of the array. Image files were generated by Affymetrix GeneChip software (MAS5). Microarray data are available via the Gene Expression Omnibus, accession no. GSE11343.
Real-time RT-PCR
The expression of eight regulated genes from the microarray was confirmed by real-time RT-PCR performed on five independent samples from each experimental group. The amount of mRNA isolated from each sciatic nerve was first normalized to an endogenous reference [TATA box binding protein; Δ cycle threshold (CT)] and then relative to a control group (ΔΔCT), and was expressed as 2−ΔΔCT.
Data analysis
The Affymetrix CEL files were analyzed using the National Center for Integrative Biomedical Informatics (http://portal.ncibi.org/gateway/) GenePattern server. The samples were Robust Multichip Average normalized using the BrainArray Custom CDF Mm430A2_Mm_ENTREZG version 9 (http://brainarray.mbni.med.umich.edu/brainarray/default. asp) (25) and log2 transformed. A Student’s t test, CyberT False Discovery Rate (FDR) (26), and fold change were calculated. Standard libraries from the bioconductor project (www.bioconductor.org), the R project (www.r-project.org), and GenePattern (www.broad.mit.edu/cancer/software/genepattern/) were used. The Database for Annotation, Visualization and Integrated Discovery (DAVID) 2007 (27) functional annotation clustering tool was used to attribute functional annotation to the lists of significantly regulated genes.
Data analysis for phenotypical and oxidative stress data was performed using GraphPad Prism 3.0 (GraphPad Software Inc., San Diego, CA). When performing comparisons across more than two groups, a one-way ANOVA test was performed, and Bonferroni corrected P values were used. If the multiple group comparison did not reach significance, the directed hypothesis that diabetic mice develop complications was tested using a one-way t test.
TF binding sites
The Genomatix Transcription Factor binding motif database was used to search for TFs common to regulated genes. Only sequences with an empirically verified transcript were searched. Five hundred base pairs upstream of the first transcription start site (TSS) and 100 bp downstream of the last TSS were designated as the promoter region. FrameWorker was used to identify new transcription minding motifs, with a threshold of 40% genes containing the motif and an intersite variability of 20 bp or less. ModelInspector was used with the default settings to search for previously characterized TF binding modules, with queries limited to the top strand.
The National Center for Integrative Biomedical Informatics GenePattern server was used to run the Broad Institute Gene Set Enrichment Analysis (GSEA) module (version 1) for GenePattern 3.0 (28). Default settings were used aside from increasing the permutation count to 1000 and disabling the probe set collapsing option.
Results
Fasting blood glucose, GHb
Fasting blood glucose was significantly elevated in diabetic mice 6, 12, 16, 20, and 24 wk after STZ (P < 0.001). GHb was also significantly increased after 24 wk, indicative of prolonged hyperglycemia (P < 0.001). Diabetic mice lost a significant amount of weight compared with control mice (P < 0.001). Rosi treatment had no significant effect on blood glucose, GHb, or weight loss in the DBA/2J mice (Table 1). There is no evidence that the levels of insulin provided to the mice resolved their diabetes or that Rosi treatment enhanced the efficacy of the insulin supplement.
Table 1.
Metabolic parameters of the DBA/2J mice
| Glycemic status | Treatment | Final weight | 20-wk blood glucose | Final GHb |
|---|---|---|---|---|
| Nondiabetic | None | 29.7 ± 3.9 (n = 17) | 122.1 ± 18.5 (n = 17) | 5.8 ± 0.9 (n = 15) |
| Diabetic | STZ | 23.5 ± 1.7 (n = 18) | 455.2 ± 163.6 (n = 18) | 14.2 ± 1.7 (n = 18) |
| Nondiabetic | Rosi | 29.2 ± 3.2 (n = 15) | 122.8 ± 16.3 (n = 15) | 6.0 ± 0.7 (n = 15) |
| Diabetic | STZ and Rosi | 23.7 ± 1.5 (n = 13) | 509 ± 153.5 (n = 13) | 13.6 ± 2.0 (n = 13) |
Final weight was reduced in diabetic mice (P < 0.001); 20-wk blood glucose and final GHb were increased (P < 0.001).
Neuropathy
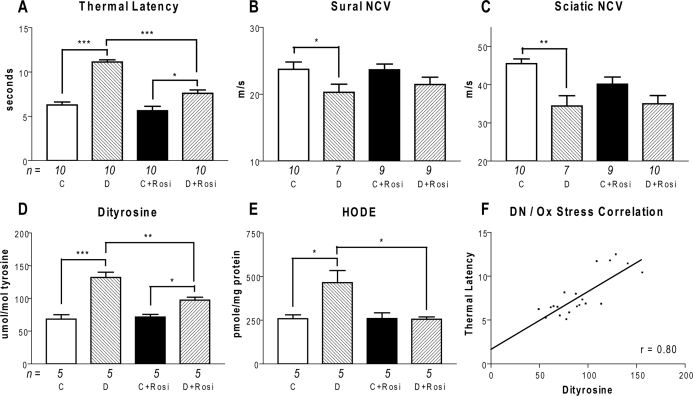
DN in the DBA/2J mice was assessed by thermal latency in the hind paw, and NCV in the sciatic and sural nerves. Thermal latency was significantly increased after 24 wk diabetes (control = 6.3 ± 1.0 sec, diabetic = 11.1 ± 0.8 sec; P < 0.001; Fig. 1). Despite maintaining the same mean blood glucose as diabetic mice (Table 1), the diabetic plus Rosi mice had significantly lower thermal latency (diabetic plus Rosi = 7.6 ± 1.2; P < 0.001). Sensory and motor NCVs were measured in the sural and sciatic nerves. Sural NCV was decreased by 14% in diabetic mice (control = 23.7 ± 3.5 m/sec, diabetic = 20.3 ± 3.2 m/sec; P < 0.05), whereas sciatic NCV was decreased by 24% in diabetic mice (control = 45.5 ± 3.9 m/sec, diabetic = 34.4 ± 7.1 m/sec; P < 0.01). Although there was a trend toward reduced impairment of NCV after Rosi treatment, it did not reach significance.
Figure 1.
DN and oxidative (Ox) stress measures. Panel A, Hind paw withdrawal latency was increased in diabetic (D) mice and reduced by Rosi treatment (D+Rosi). Panel, B, Sural sensory NCV was reduced in diabetic mice. Panel, C, Sciatic motor NCV was reduced in diabetic mice. Hind paw latency, sural sensory NCV, and sciatic motor NCV were not affected by Rosi treatment in control (C) mice (C, C plus Rosi). Panel D, The dityrosine to tyrosine ratio was increased in diabetic mice, and reduced by Rosi treatment. Dityrosine was still increased in diabetic mice treated with Rosi compared with control mice treated with Rosi. E, HODE was increased in diabetic mice and decreased by Rosi treatment. There was no difference between control and diabetic mice treated with Rosi. F, There is a significant correlation between thermal latency and dityrosine ratio. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Oxidative stress measures
Oxidative stress is a major mechanism of hyperglycemia-induced DN in humans, particularly through the oxidation of proteins and lipids. To determine whether oxidative stress was elevated in the DBA/2J-STZ mice, we quantified levels of protein-bound O,O′-dityrosine and HODE in the nerve. Dityrosine was significantly increased by diabetes (control = 68.4 ± 15.0 μmol/mol tyrosine, diabetic = 131.9 ± 17.9 μmol/mol; P < 0.001). This effect was reduced significantly by treatment with Rosi (diabetic plus Rosi = 97.3 ± 10.1 μmol/mol; P < 0.01). HODE in the nerve was also significantly increased by diabetes (control = 259.2 ± 47.4 pmol/mg protein, diabetic = 465.4 ± 154.7 pmol/mg; P < 0.05), and the effect was attenuated by Rosi (diabetic plus Rosi = 255.2 ± 31.1 pmol/mg; P < 0.05). Rosi treatment did not affect dityrosine or HODE in nondiabetic DBA/2J mice.
The correlation between thermal latency and dityrosine was found to be highly significant (r = 0.80; P < 0.001), as was the correlation between thermal latency and HODE (r = 0.71; P < 0.001). We hypothesized that Rosi activates cellular mechanisms of free radical management. We tested this hypothesis by analyzing gene expression patterns in DBA/2J mice.
Gene expression changes in diabetes
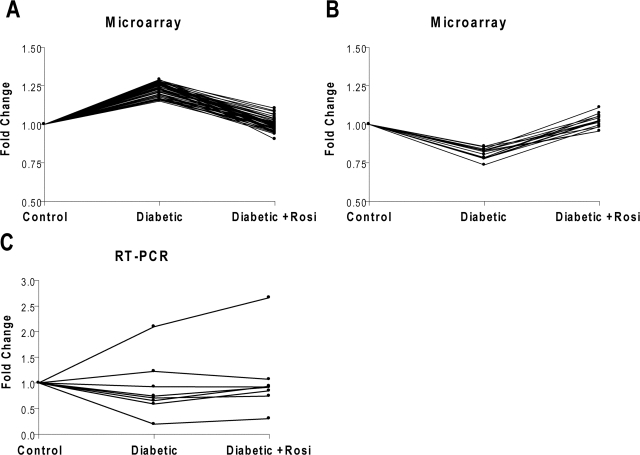
Affymetrix Mouse Genome 430 2.0 microarrays were used to measure the expression of 12,150 genes in the sciatic nerve (n = 5 mice per group). One array of a control animal did not pass quality control and was excluded from further analysis (data not shown). Changes in expression between groups were tested by a t test and the CyberT FDR (26). Genes with both a P value and FDR less than 0.05 were considered differentially expressed genes (DEGs). A total of 526 DEGs was found between control and diabetic, 318 between diabetic and diabetic plus Rosi, and 345 between control and control plus Rosi. The DEGs between control and diabetic mice were clustered into functional categories using the DAVID functional annotation tool (Table 2). Some diabetic DEGs were also significantly regulated by Rosi treatment. There were 15 genes down-regulated by diabetes and up-regulated by Rosi, and 68 up-regulated by diabetes and down-regulated by Rosi (Fig. 2, A and B). Eight genes regulated by diabetes and Rosi were validated by quantitative real-time RT-PCR (Table 3). Although changes in expression did not consistently reach significance, the trend of down-regulation in diabetes and partial normalization with Rosi treatment was confirmed in four of eight genes (Fig. 2C).
Table 2.
DAVID enrichment categories
| Overall function | Median enrichment P value |
|---|---|
| Metabolism | 3.75 × 10−5 |
| Metal ion binding | 9.57 × 10−4 |
| mRNA processing | 3.60 × 10−4 |
| Cellular regulation | 7.75 × 10−3 |
| Mitochondria and energy production | 1.25 × 10−2 |
Genes significantly regulated in diabetes relative to control were analyzed with the DAVID Functional Annotation Clustering. The table lists the top five nonredundant functional clusters and the median P value of each member of the cluster. The enrichment P value is the probability of finding as many regulated genes in a functional category as were observed, calculated using Fisher’s exact test. Because DAVID clusters annotation from over 40 different sources, including GO, KEGG, and Swiss-prot, the median P value from each cluster was used to rank its relative significance.
Figure 2.
Patterns of gene regulation. Each line is the fold change vs. control of one gene across the three different conditions. Genes regulated by both diabetes and Rosi were found to either increase with diabetes and decrease with Rosi treatment (A), or decrease in expression in diabetes and increase with Rosi treatment (B). C, The gene expression pattern of eight genes down-regulated by diabetes and up-regulated by Rosi was confirmed by real-time RT-PCR.
Table 3.
Genes tested by RT-PCR
| Gene ID | Gene name | Correlation |
|---|---|---|
| 99543 | Olfactomedin-like 3 | 0.960348 |
| 228357 | Low-density lipoprotein receptor-related protein 4 | 0.864936 |
| 94249 | Solute carrier family 24 (sodium/potassium/calcium exchanger), member 3 | 0.706296 |
| 17183 | Matrilin 4 | 0.683156 |
| 11865 | Aryl hydrocarbon receptor nuclear translocator-like | 0.374882 |
| 14194 | Fumarate hydratase 1 | 0.353724 |
| 192650 | Calcium binding protein 7 | −0.40936 |
| 56758 | Muscleblind-like 1 (Drosophila) | −0.92576 |
Gene identifier (ID) is the EntrezGene accession number that uniquely identifies the gene. Correlation is between the expression level of each gene as measured by the Affymetrix chip and RT-PCR.
Promoter module enrichment
Groups of functionally related genes regulated in the same direction by diabetes may be under the control of conserved TF binding modules. TFs bind to specific DNA sequences called motifs. Two or more motifs arranged in a consistent order with a consistent spacing in the promoter sequence are designated a TF binding module. Such modules are core features of translation initiation sites. The genes regulated by diabetes identified as either metabolic or mitochondrial by the DAVID functional annotation tool (Table 4) were separated into up-regulated and down-regulated genes. The promoter regions of the DEGs were compared against the Genomatix database of experimentally validated TF modules (www.genomatix.de). Some TF modules were found to be significantly overrepresented or underrepresented in the regulated genes (Table 5). Results were sorted by the fold change in the frequency of each module in the promoter region of these regulated genes compared with the frequency in all promoter regions in the mouse genome. Fold change greater than one is indicative of overrepresentation and a fold change less than one underrepresentation. Modules that are overrepresented in up-regulated genes are likely to have been up-regulated, and vice versa for modules overrepresented in down-regulated genes.
Table 4.
Significantly regulated genes related to mitochondrial function and metabolism
| Gene ID | Gene name | Fold change |
|---|---|---|
| 18746 | Pyruvate kinase, muscle | 1.64 |
| 18618 | Phosphatidylethanolamine n-methyltransferase | 1.43 |
| 67681 | Mitochondrial ribosomal protein L18 | 1.43 |
| 67582 | Solute carrier family 25 (mitochondrial carrier, phosphate carrier), member 26 | 1.42 |
| 12856 | Cytochrome c oxidase, subunit XVII assembly protein homolog (yeast) | 1.36 |
| 20655 | Superoxide dismutase 1, soluble | 1.35 |
| 14467 | Glioblastoma amplified sequence | 1.34 |
| 22262 | Urate oxidase | 1.33 |
| 28030 | G elongation factor, mitochondrial 1 | 1.32 |
| 68375 | NADH dehydrogenase (ubiquinone) 1 α-subcomplex, 8 | 1.29 |
| 66988 | Leucine aminopeptidase 3 | 1.29 |
| 13171 | Dihydrolipoamide branched chain transacylase E2 | 1.28 |
| 50776 | Polymerase (DNA directed), γ-2, accessory subunit | 1.26 |
| 20916 | Succinate-coenzyme A ligase, ADP-forming, β subunit | 1.25 |
| 15494 | Hydroxy-δ-5-steroid dehydrogenase, 3 β- and steroid δ-isomerase 3 | 1.25 |
| 15512 | Heat shock protein 2 | 1.25 |
| 12039 | Branched chain ketoacid dehydrogenase E1, α polypeptide | 1.24 |
| 104910 | Expressed sequence AI132487 | 1.24 |
| 57423 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit f, isoform 2 | 1.23 |
| 18975 | Polymerase (DNA directed), γ | 1.20 |
| 50529 | Mitochondrial ribosomal protein S7 | 1.20 |
| 69955 | Phenylalanine-tRNA synthetase 2 (mitochondrial) | 1.18 |
| 53895 | Caseinolytic peptidase, ATP-dependent, proteolytic subunit homolog (Escherichia coli) | 1.16 |
| 269951 | Isocitrate dehydrogenase 2 (NADP+), mitochondrial | 1.15 |
| 15488 | Hydroxysteroid (17-β) dehydrogenase 4 | 0.87 |
| 29876 | Chloride intracellular channel 4 (mitochondrial) | 0.85 |
| 71701 | Polyribonucleotide nucleotidyltransferase 1 | 0.84 |
| 30057 | Translocase of inner mitochondrial membrane 8 homolog b (yeast) | 0.83 |
| 28295 | DNA segment, Chr 10, Johns Hopkins University 81 expressed | 0.83 |
| 14194 | Fumarate hydratase 1 | 0.83 |
| 66841 | Electron transferring flavoprotein, dehydrogenase | 0.81 |
| 68463 | Mitochondrial ribosomal protein L14 | 0.81 |
| 16973 | Low-density lipoprotein receptor-related protein 5 | 0.80 |
| 53333 | Translocase of outer mitochondrial membrane 40 homolog (yeast) | 0.80 |
| 67270 | DNA segment, Chr 10, ERATO Doi 322, expressed | 0.53 |
Gene identifier (ID) is the EntrezGene accession number that uniquely identifies the gene. Fold change is the ratio of the mean expression of the indicated gene in diabetic mice to control mice. Chr, Chromosome; NADH, reduced nicotinamide adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate.
Table 5.
Promoter modules significantly altered in regulated metabolic and mitochondrial genes
| Promoter module | Fold change in frequency | P value |
|---|---|---|
| Up-regulated genes | ||
| NF1F_NR2F_01 | 3.88 | 0.001 |
| SP1F_MYOD_01 | 2.44 | 0.03 |
| STAT_ETSF_03 | 2.44 | <0.001 |
| ETSF_ETSF_01 | 2.34 | 0.02 |
| ETSF_SP1F_04 | 2.18 | 0.006 |
| SORY_ETSF_01 | 1.70 | <0.001 |
| ETSF_IRFF_01 | 1.18 | <0.001 |
| IRFF_ETSF_01 | 1.13 | 0.001 |
| NFKB_NKXH_01 | 0.82 | <0.001 |
| Down-regulated genes | ||
| NFKB_AP1F_01 | 10.01 | 0.003 |
| SP1F_EBOX_SP1F_01 | 7.16 | 0.002 |
| EREF_SF1F_01 | 4.32 | 0.03 |
| SP1F_SP1F_01 | 2.75 | 0.03 |
| ETSF_SP1F_04 | 2.18 | 0.05 |
| SORY_ETSF_01 | 2.12 | 0.002 |
| NFKB_NKXH_01 | 1.53 | <0.001 |
| ETSF_IRFF_01 | 0.94 | 0.03 |
Promoter modules are the experimentally characterized combination of TF binding sites found in the promoter region of the regulated genes. Fold change in frequency is the fold change in the percentage of genes with this promoter module in the regulated genes compared with all mouse promoters. The P value is the probability of finding this degree of change in frequency by chance, calculated using Fisher’s exact test.
Identifying common TF modules
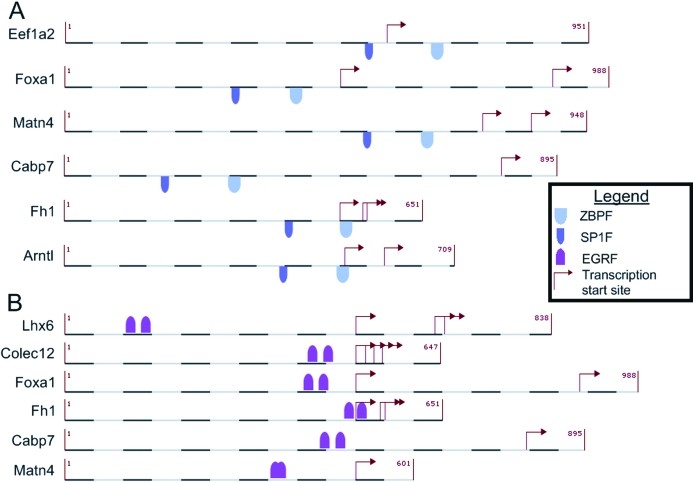
Common TFs control the regulation of the genes regulated by both diabetes and Rosi. The promoter regions of the two gene groups were searched for conserved TF binding modules using the Genomatix FrameWorker tool. No modules were significantly enriched in the promoter region of genes up-regulated by diabetes and down-regulated by Rosi. A total of 29 novel modules was enriched in the genes down-regulated by diabetes and up-regulated by Rosi (P < 0.05) (Table 6). Genes containing these modules were identified by screening the promoter region of all mouse genes. The GSEA was used to test the hypothesis that these genes are up-regulated by Rosi by comparing gene expression levels between control and control plus Rosi mice. The genes containing the SP1F_ZBPF or EGRF_EGRF modules were found to be significantly up-regulated by Rosi treatment (P < 0.05) (Fig. 3).
Table 6.
Top five significantly enriched and regulated TF binding motifs
| TF module | FrameWorker P value | GSEA P value |
|---|---|---|
| SP1F_ZBPF | 9.94E-04 | 1.50E-02 |
| EGRF_EGRF | 2.27E-03 | 3.07E-02 |
| ZBPF_EGRF | 2.16E-03 | 5.77E-02 |
| ETSF_ETSF | 7.77E-06 | 1.22E-01 |
| ZBPF_SP1F_SP1F | 7.81E-06 | 1.27E-01 |
FrameWorker P values are the probability that the binding motif could be found in the promoter region of a set of genes by chance. The module search is performed repeatedly on randomly selected promoter regions to approximate the frequency of matching a promoter sequence by chance. This distribution is the basis for a Z test to determine how likely it is that the genes were found by chance. GSEA P values are the probability that a random gene set could show the same degree of directional regulation as was found in the query gene set.
Figure 3.
Significant promoter modules. The conserved binding motifs found to be significant by FrameWorker and GSEA. A, The SP1F_ZBPF motif. B, The EGRF_EGRF motif.
Discussion
DN is a common complication of type 1 and 2 diabetes, resulting from hyperglycemia-induced oxidative stress (29). The current study evaluated DBA/2J-STZ mice and the effect of Rosi treatment on the development of DN. We found that: 1) the DBA/2J-STZ mice developed DN; 2) Rosi treatment significantly improved thermal latency and reduced oxidative stress in the sciatic nerve; and 3) two novel promoter modules were regulated by both diabetes and Rosi, and are likely relevant to the development of DN.
The DBA/2J-STZ mouse is an established model of type 1 diabetes that develops a profound nephropathy (30). Using a low-dose STZ protocol, we confirmed diabetes induction and animal survival for 24 wk. The mice were administered insulin to improve long-term survival. The dose was insufficient to restore euglycemia, even when combined with the insulin-sensitizing effects of Rosi. The DBA/2J-STZ mice developed increased fasting blood glucose, GHb, and lost weight relative to control mice, consistent with previous reports (30). No change in blood glucose, GHb, or weight was observed after Rosi treatment.
DBA/2J-STZ mice developed persistent DN after 24 wk diabetes as measured by both increased thermal latency and decreased NCV. Thermal latency was significantly reduced by Rosi treatment, and NCV studies demonstrated a corresponding trend toward improvement. The discrepancy between these measures may be due to the small effect size of DN on NCV in DBA/2J mice because TZDs have attenuated NCV impairment in STZ-treated Wistar rats (15). Little is known about the effect of TZDs on peripheral nerve independent of its effect on hyperglycemia in DN. In a model of spinal injury, Rosi significantly reduces damage to both axons and the myelin sheath by a mechanism independent of blood glucose concentration (31). One factor in this protective effect is likely the reduction of inflammation near the injury site, and correspondingly lower levels of activated microglia and macrophages.
Rosi treatment partially corrected DN but not hyperglycemia, therefore, alternative mechanisms were investigated. To determine whether the amelioration of DN observed is due to a reduction of diabetes-induced oxidative stress, biomarkers of oxidative damage were assayed in the sciatic nerve. Both dityrosine and HODE were significantly elevated in the DBA/2J-STZ mice, consistent with previous work showing that diabetes increases oxidized lipids (32,33) and protein (34). Our findings are consistent with the oxidative stress model of DN (29) because thermal latency is highly correlated with increased levels of dityrosine and HODE. Treatment with Rosi reduced both biomarkers significantly, to levels near that of control animals. Because Rosi did not correct hyperglycemia but did reduce oxidative stress, we hypothesize that Rosi promotes antioxidant activity. Several drugs with antioxidant function are effective in treating animal models of DN, including the antioxidant response element activator resveratrol (33) and innate antioxidant edaravone (32). One TZD, troglitazone, is an innate antioxidant, and reduces oxidative stress in a non-PPAR-γ dependent fashion (16,35). The antioxidant capacity is derived from an active chromanol ring, similar to that present in vitamin E (16). Rosi does not have this functional group, and there are no published reports documenting direct antioxidant activity. There is also no evidence that Rosi binds the antioxidant response element. Therefore, we examined the effects of Rosi on gene expression related to its PPAR-γ agonist activity.
Microarray analyses of the sciatic nerves of the experimental animals found significant and coordinated changes in gene expression due to both diabetes and Rosi treatment. Very little is known about how the hyperglycemic, oxidative diabetes microenvironment alters gene expression in the peripheral nerve, or whether diabetes therapies ameliorate any changes in gene expression. To date, the only microarray experiment performed using diabetic peripheral nerve tissue was performed by Price et al. (36) on the dorsal root ganglia of STZ-treated male Wistar rats. They found that the Gene Ontology categories glucose metabolism, oxidoreductase activity, and manganese ion binding were significantly enriched for regulated genes. The results of the current study are consistent with these findings. Price et al. (36) also reported other enriched categories that we did not reproduce. This may either reflect inherent differences in gene expression between mice and rats and between dorsal root ganglia and sciatic nerve, or it may be due to the more stringent significance criteria in the current study. Gene expression changes that overlap between these two studies are consistent with a model in which hyperglycemia induces neural oxidative stress, and in turn stimulates the expression of compensatory genes in the peripheral nerve.
Common promoter elements among these functionally related, diabetes-altered genes could explain their regulation. The search for metabolic genes regulated by diabetes led to the identification of several well-characterized regulatory elements involved in hyperglycemia-induced changes in gene expression. The most highly up-regulated module, NF1F_NR2F_01, was first identified in the promoter region of pyruvate kinase L (37). The promoter is activated by increased dietary glucose in healthy mice and insulin in diabetic mice, resulting in an expression change of pyruvate kinase L in the liver (38). High glucose levels in diabetic mice may result in high baseline activity for this module and lead to the observed increase in gene expression of its targets. Therefore, the activation of this module is likely an effect of diabetes, however, its status as a therapeutic target for DN has yet to be defined.
Surprisingly, we find that the NFKB_AP1F_01 module is significantly down-regulated in the peripheral nerve of diabetic mice. This differs from previous reports in diabetic nephropathy. Both nuclear factor-κB (NFKB) alone (39,40) as well as NFKB acting in tandem with activator protein-1 (41,42) are up-regulated in diabetic kidney. However, analysis of NFKB in a peripheral nerve model of DN found no change in NFKB expression (43). This difference in gene regulation between DN and nephropathy may be one factor that contributes to the different complication profiles of the two tissues. Further study of the tissue-specific function of this module is necessary to understand the effect of its suppression.
Because of the large number of genes regulated between diabetes and control, we only had the statistical power to search for previously characterized promoter modules. By focusing on the smaller set of genes significantly regulated by both diabetes and Rosi, we identified novel promoter modules. Modules with significant sequence homology across the regulated genes were then tested for functional activity by measuring trends in gene expression in the control and control plus Rosi data. These separate lines of evidence identified two promoter modules significantly involved in gene regulation by diabetes and Rosi.
The SP1F_ZBPF binding site is a module that contains binding domains for a stimulating protein 1 (Sp1) family TF and a Krüppel-like zinc finger TF. The apparent regulation of this domain is consistent with studies showing that Sp1 is activated by insulin-stimulated glucose metabolism (44), whereas Krüppel-like factors are associated with apoptosis, survival, and neurite outgrowth (45). Although no PPAR-γ binding motif was found associated with this module, PPAR-γ stimulates a similar promoter module of Sp1, sterol response element and double E box in mouse adipocytes (46), independent of a specific DNA binding motif. Sp1 is an intermediate step in the PPAR-γ induction of resistin (47) and hormone-sensitive lipase (48), whereas PPAR-γ suppresses Sp1 expression in a lung carcinoma cell line (49). Similarly, both TZD (50) and non-TZD (51) activation of PPAR-γ induces Krüppel-like-factor 4 in colon cancer cells. Both of the TFs identified in this module have functional associations with diabetes, and both respond to PPAR-γ activation. These separate lines of evidence support our contention that the SP1F_ZBPF module may constitute a novel therapeutic target in the treatment of DN.
The EGRF_EGRF motif is a double binding site for early growth factors (Egrs) 1 and 2, and similar C2H2 zinc finger factors. A similar artificial motif was used to detect Egr-1 activity (52), lending plausibility to the functionality of this module. In the developing nervous system, Egr-2 mutations are associated with childhood demyelinating diseases (53), and Egr-1 plays a continuing role in the maintenance of the myelin sheath in the adult peripheral nervous system (54). In the present study, genes with this promoter module are down-regulated by diabetes and up-regulated by Rosi treatment. A non-TZD class of PPAR-γ agonists, methylene-substituted diindolylmethanes, indirectly activates Egr-1 through a phosphatidylinositol 3-kinase dependent mechanism in prostate and colon cancer cell lines (55). Conversely, PPAR-γ inhibits Egr-1 in carbon monoxide-treated macrophages (56), lung tissue after ischemia reperfusion (57), and in response to spinal cord injury (31). Although the direction of regulation varies by tissue type and insult, PPAR-γ clearly affects the expression and activity of Egr-1, consistent with our experimental findings. Our experimental findings and known regulatory association indicate that the EGRF_EGRF module may also constitute a novel therapeutic target in the treatment of DN.
The Genomatix promoter search was limited to cis-acting transcriptional elements to reduce the scope of the searches performed. However, it is possible that regulatory elements more than 500-bp upstream (58) or located in an intron more than 100-bp downstream (59) of the TSS play a significant role in the transcription of these genes. If these promoter modules prove to be insufficiently specific in further experiments, it would be necessary to assess the activity of such trans-acting elements more carefully.
In conclusion, this study demonstrates that the DBA/2J-STZ mouse develops and maintains DN. DN is highly correlated with the oxidative stress present in the sciatic nerve, and both are reduced by Rosi treatment. Using a systems biology approach, we found significant changes in gene expression induced by diabetes and Rosi, and that two TF binding modules, SP1F_ZBPF and EGRF_EGRF, are likely involved. These modules constitute novel drug targets and merit future study.
Acknowledgments
We thank Ms. Julie Erwin for expert manuscript preparation and Dr. Gilbert Omenn for expert editorial advice. We also acknowledge the expertise of Ms. Hongyu Zhang, Ms. Marylee Schin, Ms. Jharna Saha, Mr. John Hayes, Dr. Sang Su Oh, and Ms. Carey Backus with regard to animal models of diabetes and neuropathy phenotyping methods, and Ms. Yu Hong and Ms. Anna Henger with regard to mRNA quantification.
Footnotes
This work was supported by the National Institutes of Health (NIH) (U54-DA021519, NS38849, and DK60994), the Juvenile Diabetes Research Foundation Center for the Study of Complications in Diabetes, the Office of Research Development (Medical Research Service), and the Program for Neurology Research and Discovery (http://www.med. umich.edu/pnrd/). This work was supported in part by a Michigan Institute for Clinical and Health Research-Clinical and Translational Science Awards multidisciplinary pilot grant (to M.K.). S.P. is supported by a Career Development Award from Juvenile Diabetes Research Foundation (2-2003-149). F.C.B. is supported by the NIH (U01DK076139).
Disclosure Statement: T.D.W., M.K., K.A.S., F.C.B., and E.L.F have nothing to declare. S.P. received lecture fees from Merck/Schering Plough.
First Published Online June 26, 2008
Abbreviations: CT, Cycle threshold; DAVID, Database for Annotation, Visualization and Integrated Discovery; DEG, differentially expressed gene; DN, diabetic neuropathy; Egr, early growth factor; FDR, False Discovery Rate; GHb, glycosylated hemoglobin; GSEA, Gene Set Enrichment Analysis; HODE, hydroxyoctadecadienoic acid; NCV, nerve conduction velocity; NFKB, nuclear factor-κB; PPAR, peroxisome proliferator-activated receptor; Rosi, rosiglitazone; Sp1, stimulating protein 1; STZ, streptozotocin; TF, transcription factor; TSS, transcription start site; TZD, thiazolidinediones.
References
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D, American Diabetes Association 2005 Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 28:956–962 [DOI] [PubMed] [Google Scholar]
- Said G, Goulon-Goeau C, Slama G, Tchobroutsky G 1992 Severe early-onset polyneuropathy in insulin-dependent diabetes mellitus—a clinical and pathological study. N Engl J Med 326:1257–1263 [DOI] [PubMed] [Google Scholar]
- Bhatia V, Viswanathan P 2006 Insulin resistance and PPAR insulin sensitizers. Curr Opin Investig Drugs 7:891–897 [PubMed] [Google Scholar]
- Chang AY, Wyse BM, Gilchrist BJ, Peterson T, Diani AR 1983 Ciglitazone, a new hypoglycemic agent. I. Studies in ob/ob and db/db mice, diabetic Chinese hamsters, and normal and streptozotocin-diabetic rats. Diabetes 32:830–838 [DOI] [PubMed] [Google Scholar]
- Chang AY, Wyse BM, Gilchrist BJ 1983 Ciglitazone, a new hypoglycemic agent. II. Effect on glucose and lipid metabolisms and insulin binding in the adipose tissue of C57BL/6J-ob/ob and −+/? mice. Diabetes 32:839–845 [DOI] [PubMed] [Google Scholar]
- Fujita T, Sugiyama Y, Taketomi S, Sohda T, Kawamatsu Y, Iwatsuka H, Suzuoki Z 1983 Reduction of insulin resistance in obese and/or diabetic animals by 5-[4-(1-methylcyclohexylmethoxy)benzyl]-thiazolidine-2,4-dione (ADD-3878, U-63,287, ciglitazone), a new antidiabetic agent. Diabetes 32:804–810 [DOI] [PubMed] [Google Scholar]
- Vasudevan AR, Balasubramanyam A 2004 Thiazolidinediones: a review of their mechanisms of insulin sensitization, therapeutic potential, clinical efficacy, and tolerability. Diabetes Technol Ther 6:850–863 [DOI] [PubMed] [Google Scholar]
- Yamagishi SI, Ogasawara S, Mizukami H, Yajima N, Wada RI, Sugawara A, Yagihashi S 2008 Correction of protein kinase C activity and macrophage migration in peripheral nerve by pioglitazone, peroxisome proliferator activated-γ-ligand, in insulin-deficient diabetic rats. J Neurochem 104:491–499 [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W 1999 Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 20:649–688 [DOI] [PubMed] [Google Scholar]
- Sears DD, Hsiao A, Ofrecio JM, Chapman J, He W, Olefsky JM 2007 Selective modulation of promoter recruitment and transcriptional activity of PPARγ. Biochem Biophys Res Commun 364:515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumasia R, Eagle KA, Kline-Rogers E, May N, Cho L, Mukherjee D 2005 Role of PPAR-γ agonist thiazolidinediones in treatment of pre-diabetic and diabetic individuals: a cardiovascular perspective. Curr Drug Targets 5:377–386 [DOI] [PubMed] [Google Scholar]
- Giannini S, Serio M, Galli A 2004 Pleiotropic effects of thiazolidinediones: taking a look beyond antidiabetic activity. J Endocrinol Invest 27:982–991 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Patel N, Rahn D, McAllister J, Sadeghi S, Horwitz G, Berry D, Wang KX, Swerdlow RH 2007 The thiazolidinedione pioglitazone alters mitochondrial function in human neuron-like cells. Mol Pharmacol 71:1695–1702 [DOI] [PubMed] [Google Scholar]
- Hwang J, Kleinhenz DJ, Rupnow HL, Campbell AG, Thule PM, Sutliff RL, Hart CM 2007 The PPARγ ligand, rosiglitazone, reduces vascular oxidative stress and NADPH oxidase expression in diabetic mice. Vascul Pharmacol 46:456–462 [DOI] [PubMed] [Google Scholar]
- Qiang X, Satoh J, Sagara M, Fukuzawa M, Masuda T, Sakata Y, Muto G, Muto Y, Takahashi K, Toyota T 1998 Inhibitory effect of troglitazone on diabetic neuropathy in streptozotocin-induced diabetic rats. Diabetologia 41:1321–1326 [DOI] [PubMed] [Google Scholar]
- Nagasaka Y, Kaku K, Nakamura K, Kaneko T 1995 The new oral hypoglycemic agent, CS-045, inhibits the lipid peroxidation of human plasma low density lipoprotein in vitro. Biochem Pharmacol 50:1109–1111 [DOI] [PubMed] [Google Scholar]
- Deeg MA, Buse JB, Goldberg RB, Kendall DM, Zagar AJ, Jacober SJ, Khan MA, Perez AT, Tan MH 2007 Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 30:2458–2464 [DOI] [PubMed] [Google Scholar]
- Singh S, Loke YK, Furberg CD 2007 Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 298:1189–1195 [DOI] [PubMed] [Google Scholar]
- Faich GA, Moseley RH 2001 Troglitazone (Rezulin) and hepatic injury. Pharmacoepidemiol Drug Saf 10:537–547 [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius 3rd F, Feldman EL 2007 Mouse models of diabetic neuropathy. Neurobiol Dis 28:276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Russell JW, Sullivan KA, Backus C, Hayes JM, McLean LL, Feldman EL 2007 SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp Neurol 208:216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malencik DA, Sprouse JF, Swanson CA, Anderson SR 1996 Dityrosine: preparation, isolation, and analysis. Anal Biochem 242:202–213 [DOI] [PubMed] [Google Scholar]
- Pennathur S, Ido Y, Heller JI, Byun J, Danda R, Pergola P, Williamson JR, Heinecke JW 2005 Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem 280:22706–22714 [DOI] [PubMed] [Google Scholar]
- Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, O'Brien K, Geary RL, Heinecke JW 2004 Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem 279:42977–42983 [DOI] [PubMed] [Google Scholar]
- Dai M, Wang P, Boyd AD, Kostov G, Athey B, Jones EG, Bunney WE, Myers RM, Speed TP, Akil H, Watson SJ, Meng F 2005 Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res 33:e175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi P, Long AD 2001 A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17:509–519 [DOI] [PubMed] [Google Scholar]
- Dennis Jr G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA 2003 DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4:P3 [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP 2005 Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102:15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Russell JW, Low P, Feldman EL 2004 Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 25:612–628 [DOI] [PubMed] [Google Scholar]
- Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD 2005 Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54:2628–2637 [DOI] [PubMed] [Google Scholar]
- Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R 2007 Thiazolidinedione class of peroxisome proliferator-activated receptor γ agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther 320:1002–1012 [DOI] [PubMed] [Google Scholar]
- Saini AK, Kumar HSA, Sharma SS 2007 Preventive and curative effect of edaravone on nerve functions and oxidative stress in experimental diabetic neuropathy. Eur J Pharmacol 568:164–172 [DOI] [PubMed] [Google Scholar]
- Kumar A, Kaundal RK, Iyer S, Sharma SS 2007 Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci 80:1236–1244 [DOI] [PubMed] [Google Scholar]
- Ueno Y, Horio F, Uchida K, Naito M, Nomura H, Kato Y, Tsuda T, Toyokuni S, Osawa T 2002 Increase in oxidative stress in kidneys of diabetic Akita mice. Biosci Biotechnol Biochem 66:869–872 [DOI] [PubMed] [Google Scholar]
- May JM, Qu ZC 2000 Troglitazone protects human erythrocytes from oxidant damage. Antioxid Redox Signal 2:243–250 [DOI] [PubMed] [Google Scholar]
- Price SA, Zeef LA, Wardleworth L, Hayes A, Tomlinson DR 2006 Identification of changes in gene expression in dorsal root ganglia in diabetic neuropathy: correlation with functional deficits. J Neuropathol Exp Neurol 65:722–732 [DOI] [PubMed] [Google Scholar]
- Tremp GL, Boquet D, Ripoche MA, Cognet M, Lone YC, Jami J, Kahn A, Daegelen D 1989 Expression of the rat L-type pyruvate kinase gene from its dual erythroid- and liver-specific promoter in transgenic mice. J Biol Chem 264:19904–19910 [PubMed] [Google Scholar]
- Yamada K, Noguchi T, Miyazaki J, Matsuda T, Takenaka M, Yamamura K, Tanaka T 1990 Tissue-specific expression of rat pyruvate kinase L/chloramphenicol acetyltransferase fusion gene in transgenic mice and its regulation by diet and insulin. Biochem Biophys Res Commun 171:243–249 [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Kloting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Haring HU, Schleicher E, Nawroth PP 2001 Diabetes-associated sustained activation of the transcription factor nuclear factor-κB. Diabetes 50:2792–2808 [DOI] [PubMed] [Google Scholar]
- Schmid H, Boucherot A, Yasuda Y, Henger A, Brunner B, Eichinger F, Nitsche A, Kiss E, Bleich M, Grone HJ, Nelson PJ, Schlondorff D, Cohen CD, Kretzler M 2006 Modular activation of nuclear factor-κB transcriptional programs in human diabetic nephropathy. Diabetes 55:2993–3003 [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Lorenzo O, Egido J 2000 Angiotensin III increases MCP-1 and activates NF-κB and AP-1 in cultured mesangial and mononuclear cells. Kidney Int 57:2285–2298 [DOI] [PubMed] [Google Scholar]
- Mezzano SA, Barria M, Droguett MA, Burgos ME, Ardiles LG, Flores C, Egido J 2001 Tubular NF-κB and AP-1 activation in human proteinuric renal disease. Kidney Int 60:1366–1377 [DOI] [PubMed] [Google Scholar]
- Burnand RC, Price SA, McElhaney M, Barker D, Tomlinson DR 2004 Expression of axotomy-inducible and apoptosis-related genes in sensory nerves of rats with experimental diabetes. Brain Res Mol Brain Res 132:235–240 [DOI] [PubMed] [Google Scholar]
- Moreno-Aliaga MJ, Swarbrick MM, Lorente-Cebrian S, Stanhope KL, Havel PJ, Martinez JA 2007 Sp1-mediated transcription is involved in the induction of leptin by insulin-stimulated glucose metabolism. J Mol Endocrinol 38:537–546 [DOI] [PubMed] [Google Scholar]
- Kaczynski J, Cook T, Urrutia R 2003 Sp1- and Kruppel-like transcription factors. Genome Biol 4:206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AV, Snedden SK, Raimbault S, Ricquier D, Collins S 2001 Transcriptional regulation of the mouse uncoupling protein-2 gene. Double E-box motif is required for peroxisome proliferator-activated receptor-γ-dependent activation. J Biol Chem 276:10817–10823 [DOI] [PubMed] [Google Scholar]
- Chung SS, Choi HH, Cho YM, Lee HK, Park KS 2006 Sp1 mediates repression of the resistin gene by PPARγ agonists in 3T3-L1 adipocytes. Biochem Biophys Res Commun 348:253–258 [DOI] [PubMed] [Google Scholar]
- Deng T, Shan S, Li PP, Shen ZF, Lu XP, Cheng J, Ning ZQ 2006 Peroxisome proliferator-activated receptor-γ transcriptionally up-regulates hormone-sensitive lipase via the involvement of specificity protein-1. Endocrinology 147:875–884 [DOI] [PubMed] [Google Scholar]
- Han S, Ritzenthaler JD, Rivera HN, Roman J 2005 Peroxisome proliferator-activated receptor-γ ligands suppress fibronectin gene expression in human lung carcinoma cells: involvement of both CRE and Sp1. Am J Physiol Lung Cell Mol Physiol 289:L419–L428 [DOI] [PubMed] [Google Scholar]
- Drori S, Girnun GD, Tou L, Szwaya JD, Mueller E, Xia K, Shivdasani RA, Spiegelman BM 2005 Hic-5 regulates an epithelial program mediated by PPARγ. Genes Dev [Erratum (2005) 19:875] 19:362–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Jutooru I, McAlees A, Safe S 2007 Structure-dependent activity of glycyrrhetinic acid derivatives as peroxisome proliferator-activated receptor γ agonists in colon cancer cells. Mol Cancer Ther 6:1588–1598 [DOI] [PubMed] [Google Scholar]
- Cibelli G, Policastro V, Rossler OG, Thiel G 2002 Nitric oxide-induced programmed cell death in human neuroblastoma cells is accompanied by the synthesis of Egr-1, a zinc finger transcription factor. J Neurosci Res 67:450–460 [DOI] [PubMed] [Google Scholar]
- Berger P, Niemann A, Suter U 2006 Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease). Glia 54:243–257 [DOI] [PubMed] [Google Scholar]
- Decker L, Desmarquet-Trin-Dinh C, Taillebourg E, Ghislain J, Vallat JM, Charnay P 2006 Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. J Neurosci 26:9771–9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Baek SJ, Liu S, Safe S 2005 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome proliferator-activated receptor γ agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and nonsteroidal anti-inflammatory drug-activated gene-1. Mol Pharmacol 68:1782–1792 [DOI] [PubMed] [Google Scholar]
- Bilban M, Bach FH, Otterbein SL, Ifedigbo E, de Costa d'Avila JC, Esterbauer H, Chin BY, Usheva A, Robson SC, Wagner O, Otterbein LE 2006 Carbon monoxide orchestrates a protective response through PPARγ. Immunity 24:601–610 [DOI] [PubMed] [Google Scholar]
- Okada M, Yan SF, Pinsky DJ 2002 Peroxisome proliferator-activated receptor-γ (PPAR-γ) activation suppresses ischemic induction of Egr-1 and its inflammatory gene targets. FASEB J 16:1861–1868 [DOI] [PubMed] [Google Scholar]
- Thellmann M, Hatzold J, Conradt B 2003 The Snail-like CES-1 protein of C. elegans can block the expression of the BH3-only cell-death activator gene egl-1 by antagonizing the function of bHLH proteins. Development 130:4057–4071 [DOI] [PubMed] [Google Scholar]
- Nam S, Jin YH, Li QL, Lee KY, Jeong GB, Ito Y, Lee J, Bae SC 2002 Expression pattern, regulation, and biological role of runt domain transcription factor, run, in Caenorhabditis elegans. Mol Cell Biol 22:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]