Abstract
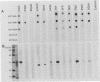
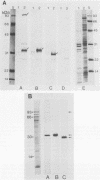
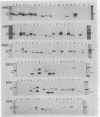
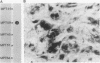
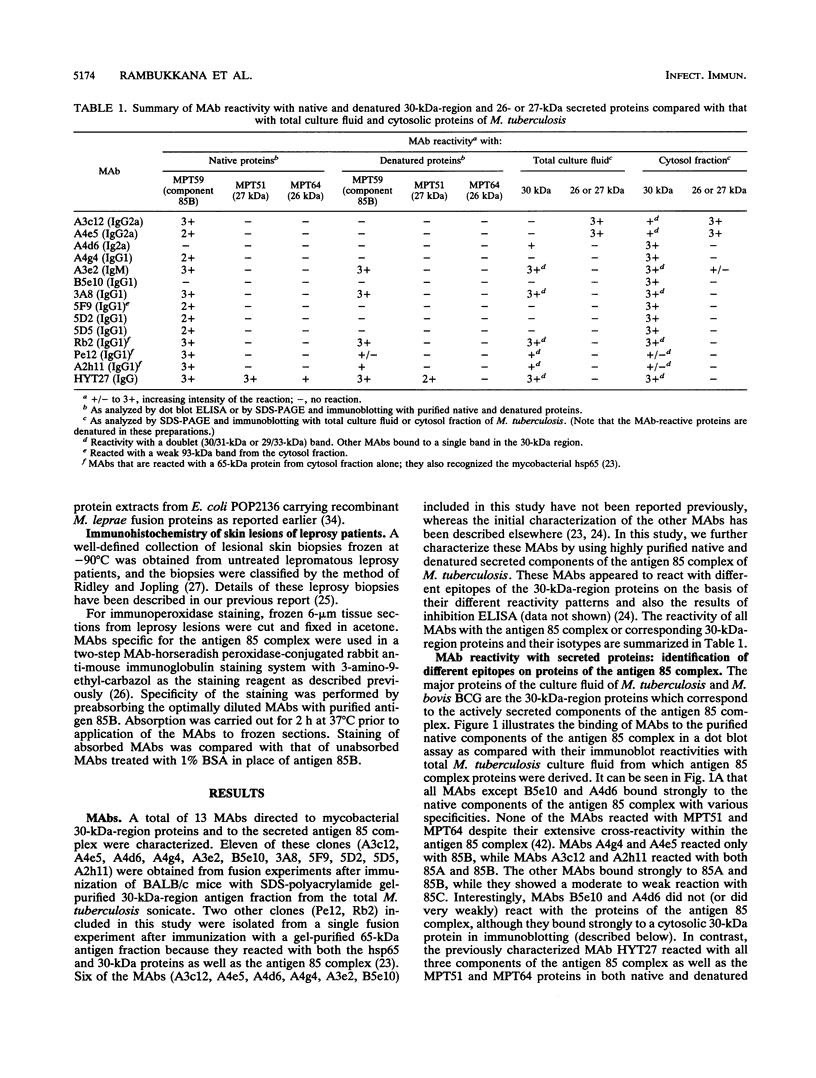
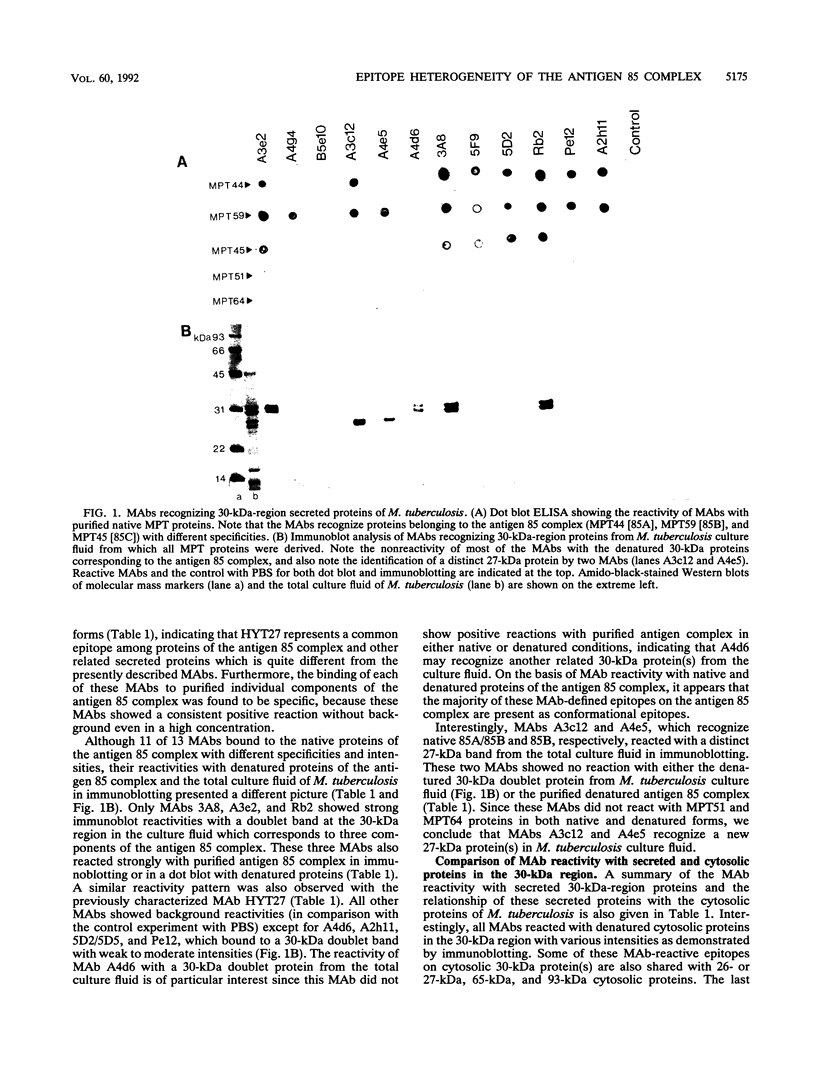
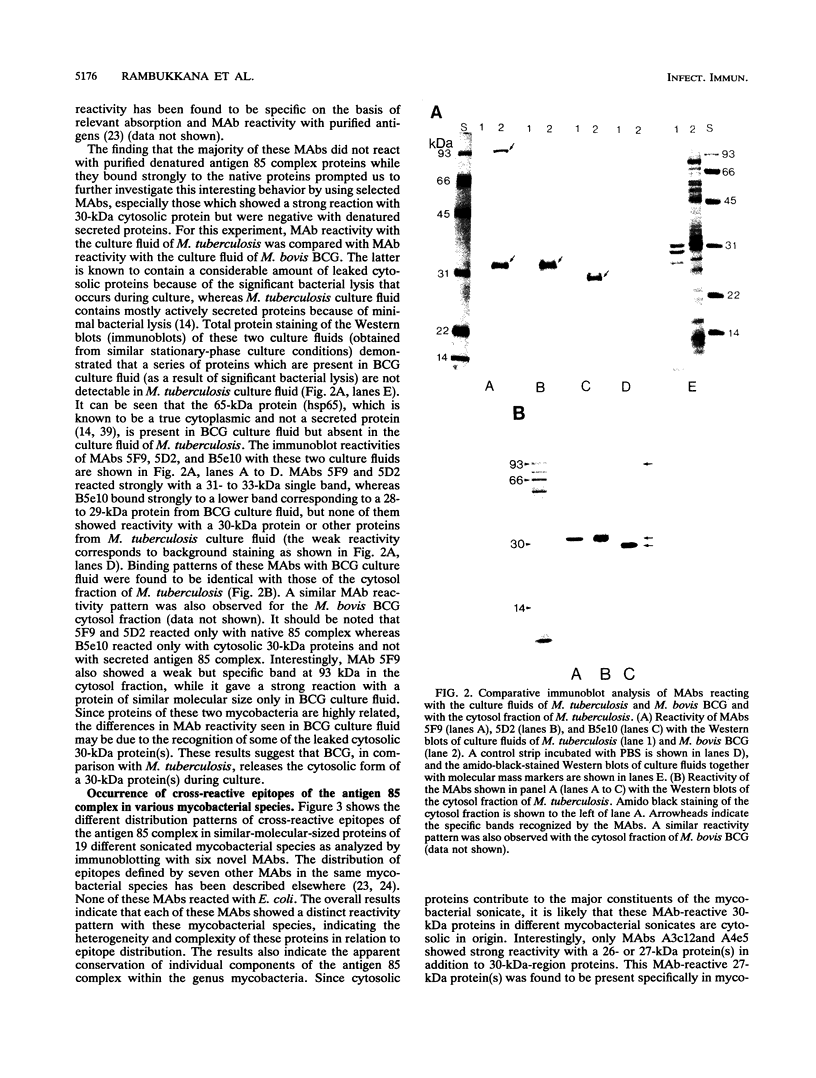
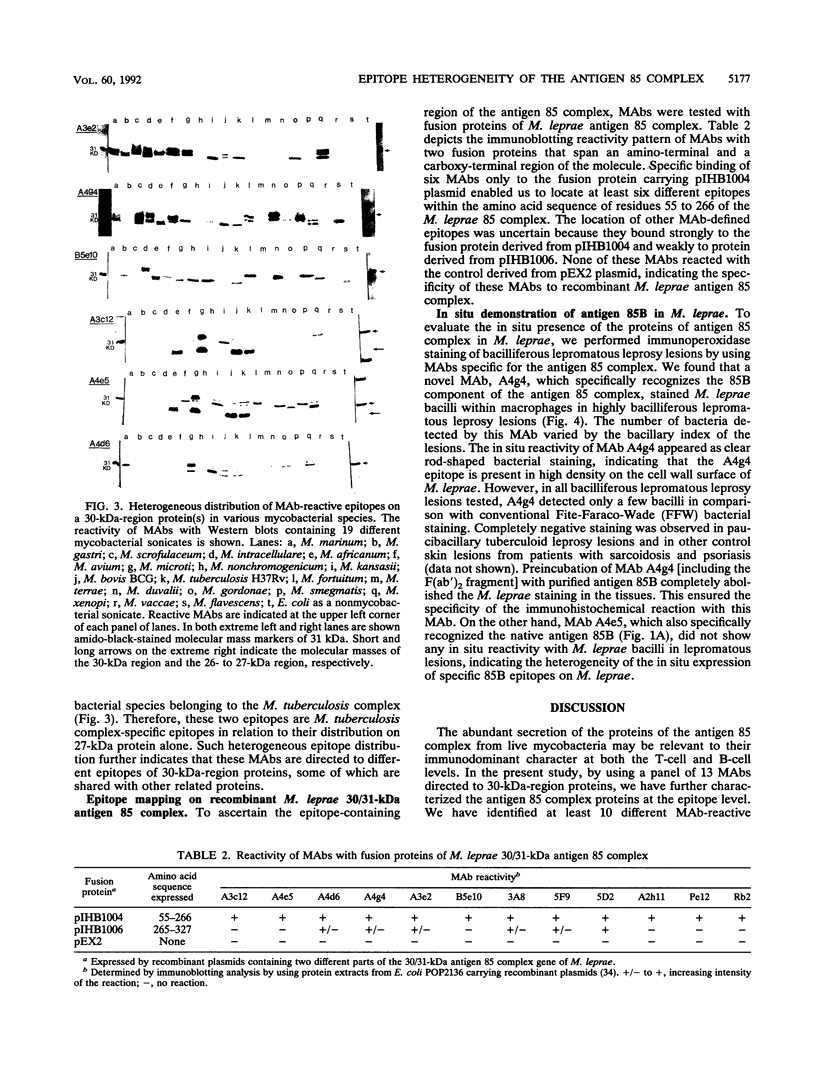
Proteins of the antigen 85 complex in the 30-kDa region secreted by live mycobacteria are important in the immune response against mycobacterial infections and may play an important biological role in the host-parasite interaction. In the present study, we have characterized epitopes of the 30-kDa-region proteins and the antigen 85 complex by using a panel of 13 monoclonal antibodies (MAbs) reacting with these antigens, 6 of which have not been described before. By using five previously characterized related secreted proteins of Mycobacterium tuberculosis, MPT44 (85A), MPT59 (85B), MPT45 (85C), MPT51 (27 kDa), and MPT64 (26 kDa), we have identified at least 10 different MAb-reactive epitopes on the proteins of the antigen 85 complex. A heterogeneous distribution of epitopes was observed within the components of the antigen 85 complex. Two distinct epitopes specific for antigen 85B and two other epitopes restricted to the 85A and 85B components were recognized. Two of them were shared with a previously unidentified 27-kDa protein present in M. tuberculosis culture fluid from which all MPT proteins were derived. The rest of the MAb-reactive epitopes were found to be present mostly in antigens 85A and 85B and to a lesser extent in antigen 85C. None of these MAbs recognized component 85C alone nor did they bind to the related MPT51 and MPT64 proteins. Interestingly, most of the MAbs reacted with purified native proteins of the antigen 85 complex but not to them in their denatured forms. In contrast, reactivity of the MAbs with the cytosol fraction of M. tuberculosis in immunoblotting revealed that they bound to a closely related cytosolic 30-kDa protein(s) even when they were denatured. Heterogeneity of these MAb-reactive epitopes of the antigen 85 complex was further evident as they were found to be distributed in various patterns among 19 different mycobacterial species. By using fusion proteins of the Mycobacterium leprae 30/31-kDa antigen 85 complex, we have localized at least six different epitopes within amino acid residues 55 to 266 of the M. leprae antigen 85 complex. Finally, by immunohistochemical analysis, we have demonstrated the in situ expression of one of the novel MAb-reactive epitopes specific for antigen 85B on the cell wall surface of M. leprae within macrophages in lepromatous leprosy lesions and thus provide direct evidence for the presence of the B component of the antigen 85 complex on the surface of intact M. leprae.
Full text
PDF

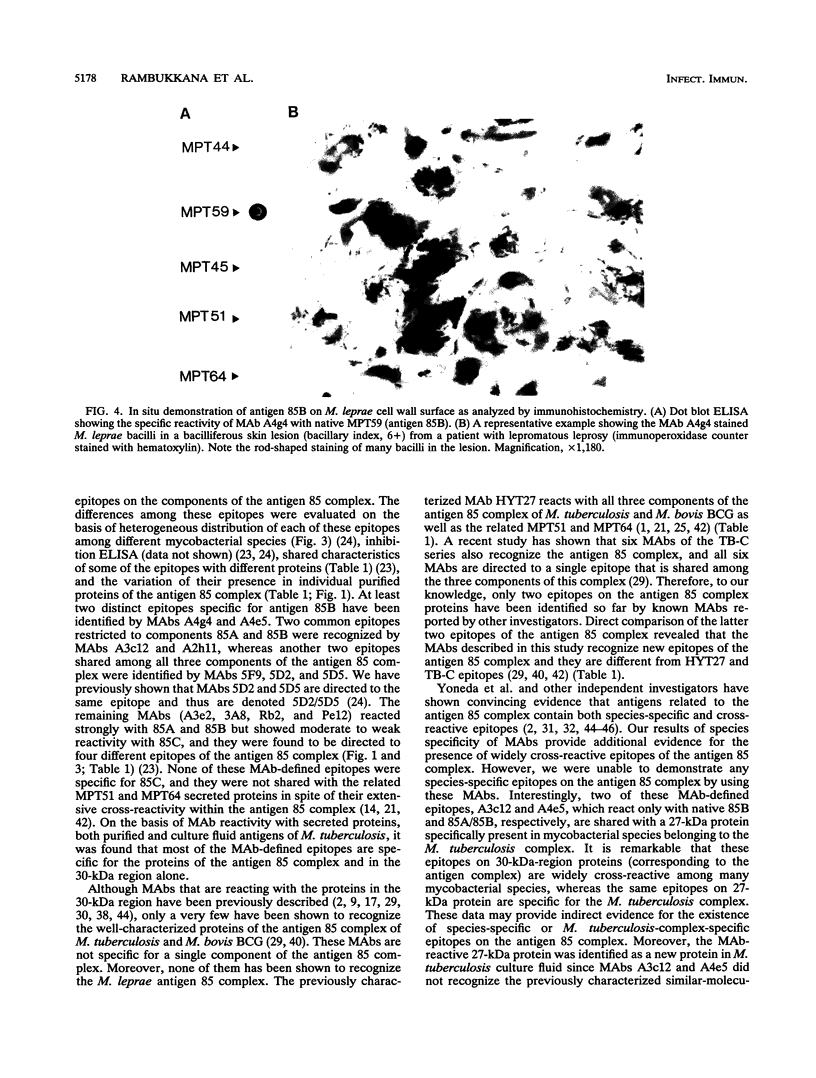



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A. B., Yuan Z. L., Hasløv K., Vergmann B., Bennedsen J. Interspecies reactivity of five monoclonal antibodies to Mycobacterium tuberculosis as examined by immunoblotting and enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Mar;23(3):446–451. doi: 10.1128/jcm.23.3.446-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bentzon M. W., Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991 Apr;59(4):1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Mehra V., Hirschfield G. R., Fong S. J., Abou-Zeid C., Rook G. A., Hunter S. W., Brennan P. J., Modlin R. L. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989 Oct 15;143(8):2656–2662. [PubMed] [Google Scholar]
- Borremans M., de Wit L., Volckaert G., Ooms J., de Bruyn J., Huygen K., van Vooren J. P., Stelandre M., Verhofstadt R., Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989 Oct;57(10):3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Garsia R. J., Basten A. Antigens of Mycobacterium leprae identified by immunoprecipitation with sera from leprosy and tuberculosis patients. Clin Exp Immunol. 1988 Mar;71(3):394–398. [PMC free article] [PubMed] [Google Scholar]
- Content J., de la Cuvellerie A., De Wit L., Vincent-Levy-Frébault V., Ooms J., De Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect Immun. 1991 Sep;59(9):3205–3212. doi: 10.1128/iai.59.9.3205-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani G., Biano A., Beltrame A., Vismara D., Mezzopreti M. F., Colizzi V., Young D. B., Bloom B. R. Generation and characterization of monoclonal antibodies to 28-, 35-, and 65-kilodalton proteins of Mycobacterium tuberculosis. Infect Immun. 1988 May;56(5):1281–1287. doi: 10.1128/iai.56.5.1281-1287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. M., Ferguson L. E. Purification and Characterization of Two Proteins from Culture Filtrates of Mycobacterium tuberculosis H(37)Ra Strain. Infect Immun. 1970 Feb;1(2):164–168. doi: 10.1128/iai.1.2.164-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P. K., Rambukkana A., Baas J. G., Groothuis D. G., Halperin M. Enzyme-linked immunosorbent assay for distinguishing serological responses of lepromatous and tuberculoid leprosies to the 29/33-kilodalton doublet and 64-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Feb;28(2):379–382. doi: 10.1128/jcm.28.2.379-382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espitia C., Sciutto E., Bottasso O., González-Amaro R., Hernández-Pando R., Mancilla R. High antibody levels to the mycobacterial fibronectin-binding antigen of 30-31 kD in tuberculosis and lepromatous leprosy. Clin Exp Immunol. 1992 Mar;87(3):362–367. doi: 10.1111/j.1365-2249.1992.tb03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Wiker H. G., Duncan J. R., Garcia M. M., Dukes T. W., Brooks B. W., Turcotte C., Nagai S. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J Clin Microbiol. 1990 May;28(5):913–921. doi: 10.1128/jcm.28.5.913-921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Wiker H. G., Nagai S. Protein antigens of mycobacteria studied by quantitative immunologic techniques. Clin Infect Dis. 1992 Jan;14(1):313–319. doi: 10.1093/clinids/14.1.313. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., McNeil M., Modlin R. L., Mehra V., Bloom B. R., Brennan P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J Immunol. 1989 Apr 15;142(8):2864–2872. [PubMed] [Google Scholar]
- Janicki B. W., Chaparas S. D., Daniel T. M., Kubica G. P., Wright G. L., Yee G. S. A reference system for antigens of Mycobacterium tuberculosis. Am Rev Respir Dis. 1971 Oct;104(4):602–604. doi: 10.1164/arrd.1971.104.4.602. [DOI] [PubMed] [Google Scholar]
- Kolk A. H., Ho M. L., Klatser P. R., Eggelte T. A., Kuijper S., de Jonge S., van Leeuwen J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol. 1984 Dec;58(3):511–521. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Terasaka K., Yamada T. Cloning and expression of the gene for the cross-reactive alpha antigen of Mycobacterium kansasii. Infect Immun. 1990 Feb;58(2):550–556. doi: 10.1128/iai.58.2.550-556.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988 Sep;170(9):3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Wiker H. G., Harboe M., Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991 Jan;59(1):372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessolani M. C., Rumjanek F. D., Marques M. A., de Melo F. S., Sarno E. N. Serological response of patients with leprosy to a 28- to 30-kilodalton protein doublet from early cultures of Mycobacterium bovis BCG. J Clin Microbiol. 1989 Oct;27(10):2184–2189. doi: 10.1128/jcm.27.10.2184-2189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Burggraaf J. D., Faber W. R., Teeling P., Krieg S., Thole J. E., Harboe M. Identification and characterization of epitopes shared between the mycobacterial 65-kilodalton heat shock protein and the actively secreted antigen 85 complex: their in situ expression on the cell wall surface of Mycobacterium leprae. Infect Immun. 1992 Nov;60(11):4517–4527. doi: 10.1128/iai.60.11.4517-4527.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Chand A., Baas J. G., Groothuis D. G., Kolk A. H. Subcellular distribution of monoclonal antibody defined epitopes on immunodominant Mycobacterium tuberculosis proteins in the 30-kDa region: identification and localization of 29/33-kDa doublet proteins on mycobacterial cell wall. Scand J Immunol. 1991 Jun;33(6):763–775. doi: 10.1111/j.1365-3083.1991.tb02551.x. [DOI] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Krieg S., Faber W. R. Association of the mycobacterial 30-kDa region proteins with the cutaneous infiltrates of leprosy lesions. Evidence for the involvement of the major mycobacterial secreted proteins in the local immune response of leprosy. Scand J Immunol. 1992 Jul;36(1):35–48. doi: 10.1111/j.1365-3083.1992.tb02938.x. [DOI] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Krieg S., Young S., Le Poole I. C., Bos J. D. Mycobacterial 65,000 MW heat-shock protein shares a carboxy-terminal epitope with human epidermal cytokeratin 1/2. Immunology. 1992 Oct;77(2):267–276. [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Rumschlag H. S., Shinnick T. M., Cohen M. L. Serological responses of patients with lepromatous and tuberculoid leprosy to 30-, 31-, and 32-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol. 1988 Oct;26(10):2200–2202. doi: 10.1128/jcm.26.10.2200-2202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata R. A., Sanson A. J., Malhotra I. J., Wiker H. G., Harboe M., Phillips N. B., Daniel T. M. Purification and characterization of the 30,000 dalton native antigen of Mycobacterium tuberculosis and characterization of six monoclonal antibodies reactive with a major epitope of this antigen. J Lab Clin Med. 1991 Dec;118(6):589–598. [PubMed] [Google Scholar]
- Schou C., Yuan Z. L., Andersen A. B., Bennedsen J. Production and partial characterization of monoclonal hybridoma antibodies to Mycobacterium tuberculosis. Acta Pathol Microbiol Immunol Scand C. 1985 Dec;93(6):265–272. doi: 10.1111/j.1699-0463.1985.tb02955.x. [DOI] [PubMed] [Google Scholar]
- Tasaka H., Matsuo Y. Specificity and distribution of alpha antigens of Mycobacterium kansasii and Mycobacterium marinum. Am Rev Respir Dis. 1984 Oct;130(4):647–649. doi: 10.1164/arrd.1984.130.4.647. [DOI] [PubMed] [Google Scholar]
- Tasaka H., Nomura T., Matsuo Y. Specificity and distribution of alpha antigens of Mycobacterium avium-intracellulare, Mycobacterium scrofulaceum, and related species of mycobacteria. Am Rev Respir Dis. 1985 Jul;132(1):173–174. doi: 10.1164/arrd.1985.132.1.173. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Schöningh R., Janson A. A., Garbe T., Cornelisse Y. E., Clark-Curtiss J. E., Kolk A. H., Ottenhoff T. H., De Vries R. R., Abou-Zeid C. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol Microbiol. 1992 Jan;6(2):153–163. doi: 10.1111/j.1365-2958.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turneer M., Van Vooren J. P., De Bruyn J., Serruys E., Dierckx P., Yernault J. C. Humoral immune response in human tuberculosis: immunoglobulins G, A, and M directed against the purified P32 protein antigen of Mycobacterium bovis bacillus Calmette-Guérin. J Clin Microbiol. 1988 Sep;26(9):1714–1719. doi: 10.1128/jcm.26.9.1714-1719.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vooren J. P., Drowart A., De Bruyn J., Launois P., Millan J., Delaporte E., Develoux M., Yernault J. C., Huygen K. Humoral responses against the 85A and 85B antigens of Mycobacterium bovis BCG in patients with leprosy and tuberculosis. J Clin Microbiol. 1992 Jun;30(6):1608–1610. doi: 10.1128/jcm.30.6.1608-1610.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbon A., Kuijper S., Jansen H. M., Speelman P., Kolk A. H. Antigens in culture supernatant of Mycobacterium tuberculosis: epitopes defined by monoclonal and human antibodies. J Gen Microbiol. 1990 May;136(5):955–964. doi: 10.1099/00221287-136-5-955. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S. A localization index for distinction between extracellular and intracellular antigens of Mycobacterium tuberculosis. J Gen Microbiol. 1991 Apr;137(4):875–884. doi: 10.1099/00221287-137-4-875. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Bennedsen J. Quantitative and qualitative studies on the major extracellular antigen of Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):830–838. doi: 10.1164/ajrccm/141.4_Pt_1.830. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Patarroyo M. E., Ramirez C., Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Nagai S., Harboe M., Ljungqvist L. A family of cross-reacting proteins secreted by Mycobacterium tuberculosis. Scand J Immunol. 1992 Aug;36(2):307–319. doi: 10.1111/j.1365-3083.1992.tb03104.x. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Sletten K., Nagai S., Harboe M. Evidence for three separate genes encoding the proteins of the mycobacterial antigen 85 complex. Infect Immun. 1990 Jan;58(1):272–274. doi: 10.1128/iai.58.1.272-274.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsaae A., Ljungqvist L., Heron I. Monoclonal antibodies produced in BALB.B10 mice define new antigenic determinants in culture filtrate preparations of Mycobacterium tuberculosis. J Clin Microbiol. 1988 Dec;26(12):2608–2614. doi: 10.1128/jcm.26.12.2608-2614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda M., Fukui Y. Isolation, purification, and characterization of extracellular antigens of Mycobacterium tuberculosis. Am Rev Respir Dis. 1965 Dec;92(6):9–18. doi: 10.1164/arrd.1965.92.6P2.9. [DOI] [PubMed] [Google Scholar]
- Yoneda M., Fukui Y., Yamanouchi T. Extracellular proteins of tubercle bacilli. V. Distribution of alpha and beta antigens in various mycobacteria. Biken J. 1965 Dec;8(4):201–223. [PubMed] [Google Scholar]
- Young D. B. Structure of mycobacterial antigens. Br Med Bull. 1988 Jul;44(3):562–583. doi: 10.1093/oxfordjournals.bmb.a072268. [DOI] [PubMed] [Google Scholar]