Abstract
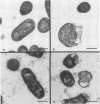
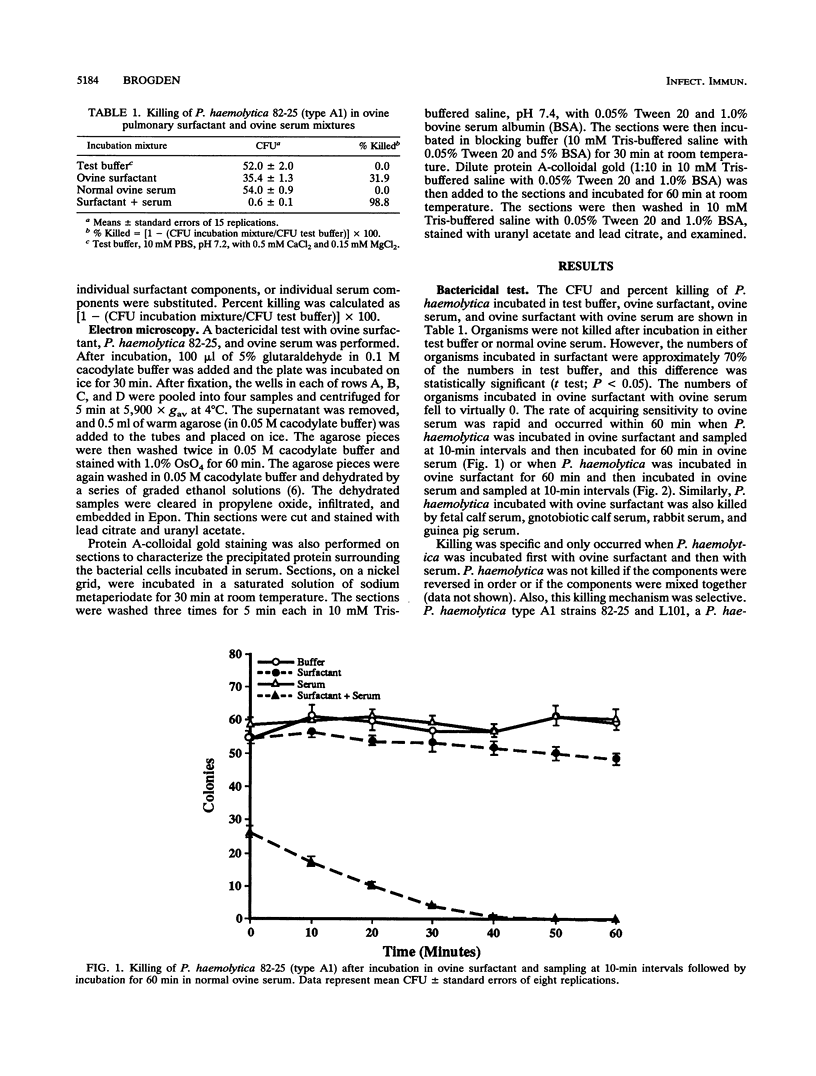
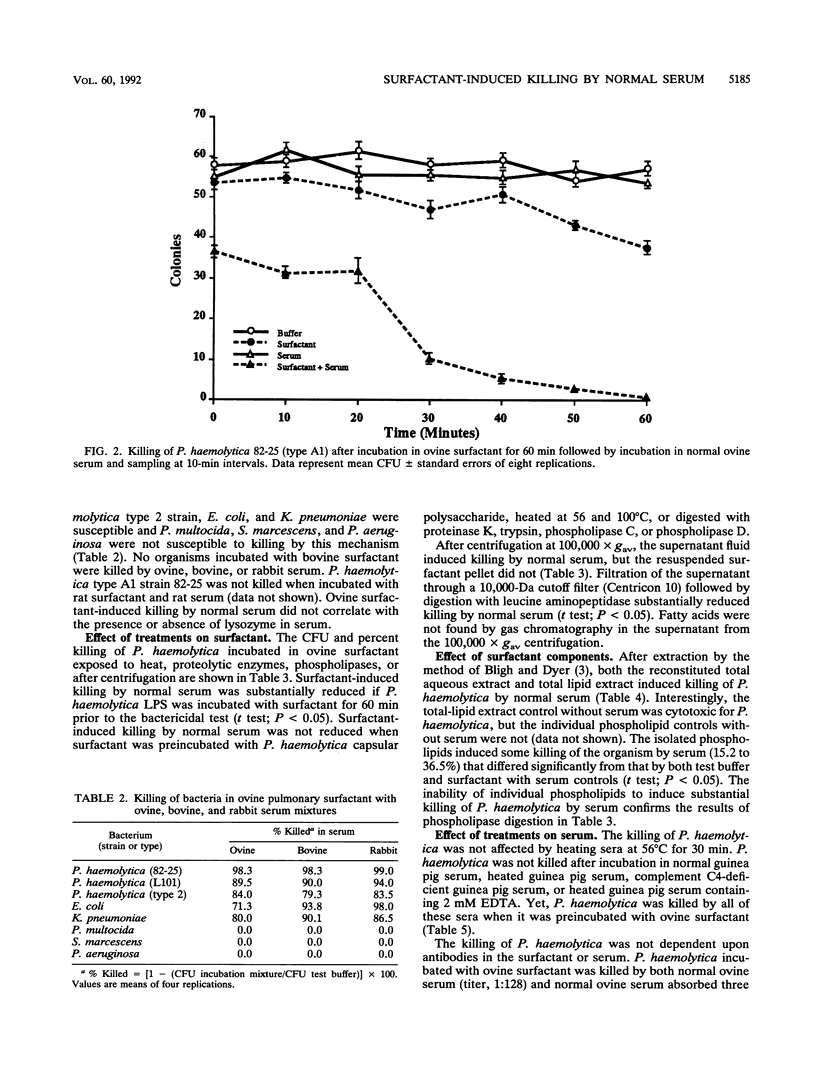
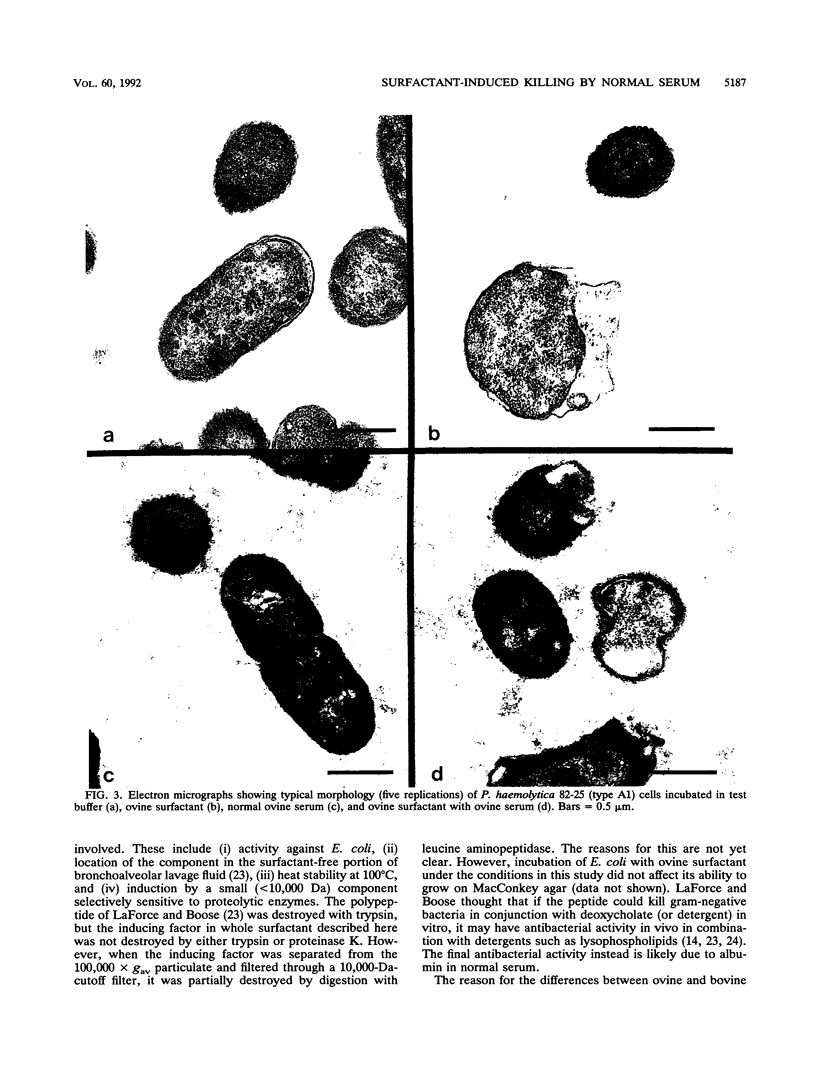
Pulmonary surfactant has been shown to play an increasingly important role in bacterial clearance at the alveolar surface in the lung. This study describes a bactericidal mechanism in which ovine pulmonary surfactant induces killing of Pasteurella haemolytica by normal serum. To demonstrate killing, six bacterial species were incubated first with pulmonary surfactant for 60 min at 37 degrees C and then with serum for an additional 60 min at 37 degrees C. P. haemolytica type A1 strains 82-25 and L101, a P. haemolytica type 2 strain, Escherichia coli, and Klebsiella pneumoniae were susceptible and Pasteurella multocida, Serratia marcescens, and Pseudomonas aeruginosa were not susceptible to killing by ovine pulmonary surfactant and normal serum. No bacteria incubated with bovine pulmonary surfactant were killed by normal serum. Although the species origin of pulmonary surfactant was selective, the species origin of serum was not. P. haemolytica incubated with ovine pulmonary surfactant was killed by fetal calf serum, gnotobiotic calf serum, pooled normal sheep serum, pooled normal rabbit serum, and pooled guinea pig serum. Ultrastructurally, killed P. haemolytica suspensions contained dead cells and cells distorted with vacuoles between the cytoplasmic membrane and the cytoplasm. The mechanism of killing did not correlate with concentrations of complement or lysozyme or titers of residual antibody in either the pulmonary surfactant or the serum, and killing was reduced by preincubation of surfactant with P. haemolytica lipopolysaccharide. Preliminary characterization of both surfactant and serum implicate a low-molecular-weight proteinaceous component in the surfactant and serum albumin in the serum. This mechanism may help clear certain gram-negative bacteria from the lungs of sheep as a part of the pulmonary innate defense system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlam C., Knights J. M., Mugridge A., Lindon J. C., Baker P. R., Beesley J. E., Spacey B., Craig G. R., Nagy L. K. Purification, characterization and immunological properties of the serotype-specific capsular polysaccharide of Pasteurella haemolytica (serotype A1) organisms. J Gen Microbiol. 1984 Sep;130(9):2415–2426. doi: 10.1099/00221287-130-9-2415. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BORGMAN R. F., WILSON C. E. Pasteurellosis and enzootic pneumonia in goats. J Am Vet Med Assoc. 1955 Mar;126(936):198–204. [PubMed] [Google Scholar]
- Blau K. A., Ward A. C., Prieur D. J., Corbeil L. B. Serum susceptibility of bovine pasteurellas. Can J Vet Res. 1987 Apr;51(2):157–161. [PMC free article] [PubMed] [Google Scholar]
- Brogden K. A., Adlam C., Lehmkuhl H. D., Cutlip R. C., Knights J. M., Engen R. L. Effect of Pasteurella haemolytica (A1) capsular polysaccharide on sheep lung in vivo and on pulmonary surfactant in vitro. Am J Vet Res. 1989 Apr;50(4):555–559. [PubMed] [Google Scholar]
- Brogden K. A., Cutlip R. C., Lehmkuhl H. D. Complexing of bacterial lipopolysaccharide with lung surfactant. Infect Immun. 1986 Jun;52(3):644–649. doi: 10.1128/iai.52.3.644-649.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden K. A., Rimler R. B., Cutlip R. C., Lehmkuhl H. D. Incubation of Pasteurella haemolytica and Pasteurella multocida lipopolysaccharide with sheep lung surfactant. Am J Vet Res. 1986 Apr;47(4):727–729. [PubMed] [Google Scholar]
- Cham B. E., Knowles B. R. A solvent system for delipidation of plasma or serum without protein precipitation. J Lipid Res. 1976 Mar;17(2):176–181. [PubMed] [Google Scholar]
- Coonrod J. D., Lester R. L., Hsu L. C. Characterization of the extracellular bactericidal factors of rat alveolar lining material. J Clin Invest. 1984 Oct;74(4):1269–1279. doi: 10.1172/JCI111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Yoneda K. Detection and partial characterization of antibacterial factor(s) in alveolar lining material of rats. J Clin Invest. 1983 Jan;71(1):129–141. doi: 10.1172/JCI110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G., Zasloff M., Eck H., Brasseur M., Maloy W. L., Bevins C. L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L. G. Pulmonary surfactant. Annu Rev Med. 1989;40:431–446. doi: 10.1146/annurev.me.40.020189.002243. [DOI] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Boose D., LaForce F. M. Isolation of an antibacterial peptide from human lung lavage fluid. J Infect Dis. 1985 Jun;151(6):1123–1129. doi: 10.1093/infdis/151.6.1123. [DOI] [PubMed] [Google Scholar]
- Gilmour N. J. Pasteurella haemolytica infections in sheep. Tijdschr Diergeneeskd. 1980 Oct 15;105(20):191–198. [PubMed] [Google Scholar]
- Haagsman H. P., Hawgood S., Sargeant T., Buckley D., White R. T., Drickamer K., Benson B. J. The major lung surfactant protein, SP 28-36, is a calcium-dependent, carbohydrate-binding protein. J Biol Chem. 1987 Oct 15;262(29):13877–13880. [PubMed] [Google Scholar]
- Harwood J. L., Desai R., Hext P., Tetley T., Richards R. Characterization of pulmonary surfactant from ox, rabbit, rat and sheep. Biochem J. 1975 Dec;151(3):707–714. doi: 10.1042/bj1510707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S., Musher D. M., Goree A., Lawrence E. C. Human alveolar lining material and antibacterial defenses. Am Rev Respir Dis. 1986 Jan;133(1):136–140. doi: 10.1164/arrd.1986.133.1.136. [DOI] [PubMed] [Google Scholar]
- LaForce F. M., Boose D. S. Effect of zinc and phosphate on an antibacterial peptide isolated from lung lavage. Infect Immun. 1984 Sep;45(3):692–696. doi: 10.1128/iai.45.3.692-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforce F. M., Boose D. S. Sublethal damage of Escherichia coli by lung lavage. Am Rev Respir Dis. 1981 Dec;124(6):733–737. doi: 10.1164/arrd.1981.124.6.733. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Selsted M. E., Szklarek D., Fleischmann J. Antibacterial activity of microbicidal cationic proteins 1 and 2, natural peptide antibiotics of rabbit lung macrophages. Infect Immun. 1983 Oct;42(1):10–14. doi: 10.1128/iai.42.1.10-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. T., Maheswaran S. K., Opuda-Asibo J., Townsend E. L., Thies E. S. Susceptibility of Pasteurella haemolytica to the bactericidal effects of serum, nasal secretions and bronchoalveolar washings from cattle. Vet Microbiol. 1983 Nov;8(6):585–599. doi: 10.1016/0378-1135(83)90007-x. [DOI] [PubMed] [Google Scholar]
- Markham R. J., Wilkie B. N. Interaction between Pasteurella haemolytica and bovine alveolar macrophages: cytotoxic effect on macrophages and impaired phagocytosis. Am J Vet Res. 1980 Jan;41(1):18–22. [PubMed] [Google Scholar]
- Moore V. L., Tobolski S. L. A modified macro-method for the quantitation of the hemolytic activity of rabbit complement. J Immunol Methods. 1974 May;5(1):71–76. doi: 10.1016/0022-1759(74)90047-7. [DOI] [PubMed] [Google Scholar]
- O'Neill S. J., Lesperance E., Klass D. J. Human lung lavage surfactant enhances staphylococcal phagocytosis by alveolar macrophages. Am Rev Respir Dis. 1984 Dec;130(6):1177–1179. doi: 10.1164/arrd.1984.130.6.1177. [DOI] [PubMed] [Google Scholar]
- Phillips M., Rimler R. B., Rebers P. A. Failure of ribosomes from nonencapsulated Pasteurella multocida to protect CF-1 mice. Am J Vet Res. 1981 Oct;42(10):1769–1774. [PubMed] [Google Scholar]
- Possmayer F. A proposed nomenclature for pulmonary surfactant-associated proteins. Am Rev Respir Dis. 1988 Oct;138(4):990–998. doi: 10.1164/ajrccm/138.4.990. [DOI] [PubMed] [Google Scholar]
- Purdy C. W., Richards A. B., Foster G. S. Blood bactericidal assay (Pasteurella haemolytica) comparison of morbidity in marketed feeder calves. Am J Vet Res. 1989 Feb;50(2):221–225. [PubMed] [Google Scholar]
- Reynolds H. Y. Lung inflammation: role of endogenous chemotactic factors in attracting polymorphonuclear granulocytes. Am Rev Respir Dis. 1983 Feb;127(2):S16–S25. doi: 10.1164/arrd.1983.127.2P2.S16. [DOI] [PubMed] [Google Scholar]
- Rimler R. B., Brown W. E. Pasteurella multocida: plasma lysozyme in bacteremic and lipopolysaccharide-exposed turkeys. J Infect Dis. 1980 Oct;142(4):614–617. doi: 10.1093/infdis/142.4.614. [DOI] [PubMed] [Google Scholar]
- Shelley S. A., Paciga J. E., Balis J. U. Lung surfactant phospholipids in different animal species. Lipids. 1984 Nov;19(11):857–862. doi: 10.1007/BF02534515. [DOI] [PubMed] [Google Scholar]
- Shewen P. E., Wilkie B. N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982 Jan;35(1):91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston J. R., Cook W., Driftmier K., Richard J. L., Sacks J. M. Decreased complement and bacteriostatic activities in the sera of cattle given single or multiple doses of aflatoxin. Am J Vet Res. 1986 Apr;47(4):846–849. [PubMed] [Google Scholar]
- Van Golde L. M., Batenburg J. J., Robertson B. The pulmonary surfactant system: biochemical aspects and functional significance. Physiol Rev. 1988 Apr;68(2):374–455. doi: 10.1152/physrev.1988.68.2.374. [DOI] [PubMed] [Google Scholar]