Abstract
Herein we describe generation of the hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd mouse line, which carries human functional CYP1A1 and CYP1A2 genes in the absence of mouse Cyp1a1 and Cyp1a2 genes, in a (>99.8%) background of the C57BL/6J genome and harboring the poor-affinity aryl hydrocarbon receptor (AHR) from the DBA/2J mouse. We have characterized this line by comparing it to our previously created hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 line––which carries the same but has the high-affinity AHR of the C57BL/6J mouse. By quantifying CYP1A1 and CYP1A2 mRNA in liver, lung and kidney of dioxin-treated mice, we show that dose-response curves in hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd mice are shifted to the right of those in hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 mice––similar to, but not as robust as, dose-response curves in DBA/2J versus C57BL/6J mice. This new mouse line is perhaps more relevant than the former to human risk assessment vis-à-vis human CYP1A1 and CYP1A2 substrates, because poor-affinity rather than high-affinity AHR occurs in the vast majority of the human population.
Keywords: Cytochrome P450 1 (CYP1) genes, humanized mouse line, human risk assessment, CYP1A1 and CYP1A2 substrates, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD;dioxin) as a P450 inducer, aryl hydrocarbon receptor (AHR)
INTRODUCTION
Since the 1960s, cytochrome P450 (CYP) enzymes were known to be heme-thiolate proteins, localized principally in liver and metabolizing drugs and other foreign chemicals. It is now realized, however, that CYP enzymes are involved in innumerable endogenous functions such as: the metabolism of eicosanoids [1]; biosynthesis of cholesterol and bile acids; steroid synthesis and metabolism; synthesis and degradation of biogenic amines; vitamin D3 synthesis and metabolism; and hydroxylation of retinoic acid and presumably other morphogens. There are still a few CYP enzymes having unknown functions [2–5].
The human and mouse genomes carry 57 and 102 functional CYP genes, respectively, with almost all of the additional mouse genes occurring in the Cyp2, Cyp3 and Cyp4 families; of the 18 mammalian families, CYP1 has three members in both human and mouse––CYP1A1, CYP1A2 and CYP1B1 [3–6]. Ancestors of the CYP1A and CYP1B subfamily diverged from one another probably more than 500 million years ago, whereas CYP1A2 likely arose as a gene duplication event from CYP1A1 about 450 million years ago. Thus, land animals (including fowl) carry both CYP1A1 and CYP1A2; sea animals do not have the CYP1A2 gene [2]. The CYP1A1 and CYP1A2 genes are located at human chromosome 15q24.1, in head-to-head orientation, 23,306 bases from one transcription start-site to the other [7]. Among three mammalian genomes studied, estimates are that about 10% of gene duplication pairs share bidirectional promoters [8].
Human/rodent CYP1A2 orthologs are well known to exhibit species-specific differences in the rates by which various substrates are metabolized [9]. For example, human and mouse CYP1A2 differ by 3- to 7-fold in ethoxyresorufin O-deethylation [10] and uroporphyrinogen oxidation [11]. Drug or carcinogen metabolism can differ in the rodent, and extrapolation of rodent data to human populations is thus prone to error; therefore, one of the long-term goals of this laboratory has been to insert human metabolism gene(s) in place of mouse orthologous gene(s).
Cyp1a1(−/−) [12] and Cyp1a2(−/−) [13] single-knockout and the Cyp1a1/1a2(−/−) double-knockout [14] mouse lines have been created. Insertion of a bacterial artificial chromosome (BAC) containing the human CYP1A1_CYP1A2 locus––including 56 kb 3′-ward of CYP1A1 and 86 kb 3′-ward of CYP1A2 [7; 15; 16]––resulted in the successful generation of “humanized” hCYP1A1_1A2_Cyp1a1(−/−) and hCYP1A1_1A2_Cyp1a2(−/−) lines, which contain both human CYP1A1 and CYP1A2 genes in the absence of either the mouse Cyp1a1 or Cyp1a2 ortholog, respectively. These lines have been used for theophylline [15] and the food mutagen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) [16; 17]. In the first two instances [15; 16], human hepatic CYP1A2 was demonstrated to be responsible for the “human metabolite profile” in the absence of mouse CYP1A2; in the third case [17], human lung CYP1A1 was shown to be accountable for the human PhIP metabolite profile when the mouse Cyp1a1 gene was lacking.
Studying the hCYP1A1_1A2_Cyp1a1(−/−) and hCYP1A1_1A2_Cyp1a2(−/−) lines separately, however, is cumbersome. For human risk assessment of CYP1A1 or CYP1A2 substrates, it would be preferable to have a mouse line carrying both human CYP1A1 and CYP1A2 genes in the absence of both mouse orthologs. We therefore generated the hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 mouse line [14]; this line has functional human CYP1A1 and CYP1A2 genes, in the absence of both mouse Cyp1a1 and Cyp1a2 genes, and is on a theoretically >99.8% C57BL/6J (B6) genetic background [14]. Because this line expresses the B6 high-affinity AHR encoded by the Ahrb1 allele, however, there is still some concern––because the vast majority of humans (i.e. >90–95%) carries the poor-affinity receptor, which is closer in function to that of the DBA/2J (D2) mouse harboring the Ahrd allele [6]. Hence, we have now developed a humanized line containing the homozygous Ahrd/d genotype. Characterization of this new line is the focus of the present report.
MATERIALS AND METHODS
Mice
C57BL/6J (B6) and DBA/2J (D2) inbred mouse strains and the B6.D2-Ahrd congenic line were purchased from The Jackson Laboratory (Bar Harbor, ME). Characterization of the humanized hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 mouse line, which has >99.8% C57BL/6J genetic background harboring the Ahrb1 allele, has been reported; this line has a single copy of the BAC containing the human CYP1A1_CYP1A2 locus, inserted randomly into the genome [14]. In the present study––the new humanized hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd mouse line is also on a >99.8% C57BL/6J genetic background, except it is harboring the Ahrd allele. This line was developed by breeding hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 with B6.D2-Ahrd mice. All experiments involving these mice were conducted in accordance with the National Institutes of Health (NIH) standards for the care and use of experimental animals and the University Cincinnati Institutional Animal Care and Use Committee.
Genotyping mice by PCR
Crude genomic DNA for genotyping was prepared from a 4-mm tail biopsy using the DirectPCR lysis reagent according to the manufacturer’s protocol (Viagen Biotech Inc.; Los Angeles, CA). PCR primers used to detect individual alleles are summarized in Table 1. The Ahrb1 and Ahrd alleles were detected using the same primer pair––with a 300-bp product representing the Ahrb1 allele and a 260-bp product representing the Ahrd allele.
Table 1.
Primer pairs used in genotyping and in qRT-PCR
| Genotyping
| ||
|---|---|---|
| Allele | Forward | Reverse |
| hCYP1A1(+) | 5′-GCAGCCCTGTTTGTTCCTG-3′ | 5′-AGGCTGGCCTATGTGGTCTA-3′ |
| hCYP1A2(+) | 5′-AGGATTGGCATTGTTGAAGG-3′ | 5′-GGGCACTGGCCATAGTATTC-3′ |
| Cyp1a1/1a2(−/−) | 5′-GTCAAAGTAACCAGACACATCCTGC-3′ | 5′-GACATAGGAGCTACCTACAC-3′ |
| Cyp1a1(+) | 5′-CTGTCTCTGAATCTTACTGCAGCC-3′ | 5′-GGGCATAGAGCAGGACAGAGCTT-3′ |
| Cyp1a1(−) | 5′-CTGTCTCTGAATCTTACTGCAGCC-3′ | 5′-GTCAAAGTAACCAGACACATCCTG C-3′ |
| Ahr(b1) or Ahr(d) | 5′-CAGTGGGAATAAGGCAAGAGTGA-3′ | 5′-AGGGAGATGAAGTATGTGTATGTA-3′ |
|
qRT-PCR
| ||
| MRNA | Forward | Reverse |
|
| ||
| Human CYP1A1 | 5′-CACTTCCGCTTGCCCATG-3′ | 5′-ACAACAAGAGACACAAGTTTG-3′ |
| Human CYP1A2 | 5′-CACAACAAGGGACACAACG-3′ | 5′-CTTGCCCATGCCAAACAGC-3′ |
| Mouse CYP1A1 | 5′-CAGACCTCAGCTGCCCTATC-3′ | 5′-CTTGCCCAAACCAAAGAGAG-3′ |
| Mouse CYP1A2 | 5′-AAGACAATGGCGGTCTCATC-3′ | 5′-GACGGTCAGAAAGCCGTGGT-3′ |
| Mouse β-actin | 5′-CATCCGTAAAGACCTCTATGCC-3′ | 5′-ACGCAGCTCAGTAACAGTCC-3′ |
Treatment of the mice
For all four genotype groups, female mice (age 2–3 months) were treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) intraperitoneally at a single dose of 0.1, 1.0, 10 or 100 μg/kg, respectively; the vehicle only (corn oil) was used for the untreated groups (0 μg/kg). Mice were sacrificed 48 h later.
Biohazard precaution
TCDD is highly toxic and a presumed human carcinogen. All personnel were instructed in safe handling procedures. Lab coats, gloves and masks were worn at all times, and contaminated materials were collected separately for disposal by the Hazardous Waste Unit or by independent contractors. TCDD-treated mice were housed separately, and their carcasses regarded as contaminated biological materials.
Reverse transcription and quantitative real-time polymerase chain reaction (qRT-PCR)
Mouse tissues were harvested and frozen in liquid nitrogen, and stored at ™80°C until use. Total RNA was isolated using Tri Reagent (Molecular Research Center, Inc.; Cincinnati, OH). First-strand cDNA was synthesized from 1 μg of total RNA with Verso™ cDNA kit (Thermal Fisher Scientific Inc.; Waltham, MA). Reaction mixtures of 20 μl containing 125 nM gene-specific primer sets, 1 μl of cDNA template, and 10 μl of iQ™SYBR Green Supermix (Bio-Rad) was used for qRT-PCR in a DNA Engine Opticon-2 Real Time PCR Detection System (MJ Research; Waltham, MA), and results were analyzed using the software provided by the manufacturer. Primers used in qRT-PCR are summarized in Table 1. For each examined tissue, individual TCDD-induced mRNA levels are reported as the fold increase over that by β-actin (ACTB) mRNA. Thus, one can compare the relative mRNA levels within a tissue, but not between tissues. The cycle numbers for the detection of ACTB mRNA did not differ significantly between untreated and treated groups.
Statistical analyses
Statistics were performed using SigmaStat Statistical Analysis software (SPSS Inc.; Chicago, IL). Group means of the cycle difference (ΔCt) after normalization of ACTB mRNA were compared by one-way ANOVA, followed by the Tukey Post-hoc test for pairwise comparison-of-means. All data were normally distributed and reported as the means ± S.E. P-values of <0.05 are considered as statistically significant.
RESULTS AND DISCUSSION
Generation of the hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd mouse line
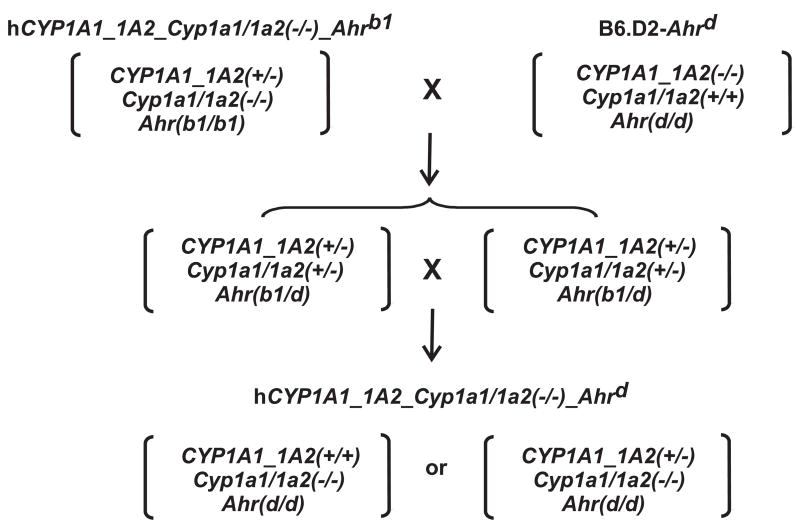
Given the fact that human poor-affinity AHR resembles more closely poor-affinity AHR of D2 mice, we developed a humanized line containing the Ahrd allele. This was achieved by mating hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 mice with B6.D2-Ahrd congenic mice (Fig. 1). Historically, congenic lines were developed by George Snell in the 1940s, with the idea to place the major histocompatibility complex (H2) of one inbred mouse into the genome of a second type of mouse; graft-versus-host diseases could thus be studied––as related to the H2 locus and in the absence of modifying genes located in trans. To develop a congenic line that contains a >99.8% homogeneous genetic background, a selected genotype (or phenotype) from one inbred strain must be backcrossed 20 generations into another inbred strain.
FIG. 1.
Breeding scheme to generate the hCYP1A1_CYP1A2_Cyp1a1/1a2(−/−)-Ahrd (Ahrd) mouse line. The hCYP1A1_CYP1A2_Cyp1a1/1a2(−/−)-Ahrb1 mice were crossed with B6.D2-Ahrd congenic mice to produce heterozygotes, following which homozygous humanized mice harboring two Ahrd alleles on a >99.8% C57BL/6J genetic background were created. Homozygotes for the hCYP1A1_1A2 insertion can be generated one-fourth of the time by mating two heterozygotes, but we cannot distinguish heterozygotes from homozygotes by our current PCR genotyping, because the region of chromosomal insertion is unknown. The only way to distinguish between these two would be to measure the copy number of the hCYP1A1_1A2 genes. Consequently, the data from both hCYP1A1_1A2 lines represent a mixture of heterozygotes and homozygotes at the hCYP1A1_CYP1A2 locus.
The B6.D2-Ahrd congenic line carries the Ahrd locus (and unknown amounts of adjacent DNA from the D2 mouse on chromosome 12), on a theoretically >99.8% B6 genetic background [18]. Intercrossing of hCYP1A1_1A2(+/−)_Cyp1a1/1a2(+/−)_Ahr(b1/d) heterozygotes (Fig. 1) thus gave rise to the hCYP1A1_1A2(+/+)_Cyp1a1/1a2(−/−)_Ahr(d/d) homozygous line, which basically differs from our previous humanized line only at the Ahr locus.
Homozygous hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd offspring are healthy, fertile and exhibit a normal Mendelian frequency––indistinguishable from the hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 line (not shown). Both of these humanized mouse lines carry human functional CYP1A1 and CYP1A2 genes replacing the mouse orthologs. The two human genes are regulated by the endogenous mouse AHR: high-affinity AHR in the hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 line [14], versus poor-affinity AHR in the hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd line. To initially characterize this newly created humanized mouse line, we compared the induction profiles of human versus mouse CYP1A1 and CYP1A2 mRNA in selected tissues, using a wide range of TCDD doses. This experimental approach had originally been carried out, examining the induction of aryl hydrocarbon hydroxylase (CYP1A1) activity in liver of TCDD-treated B6 and D2 mice; from the 10- to 15-fold shift-to-the-right in the dose-response curves between B6 and D2, a receptor was hypothesized to explain these findings [19].
Induction of human CYP1A1
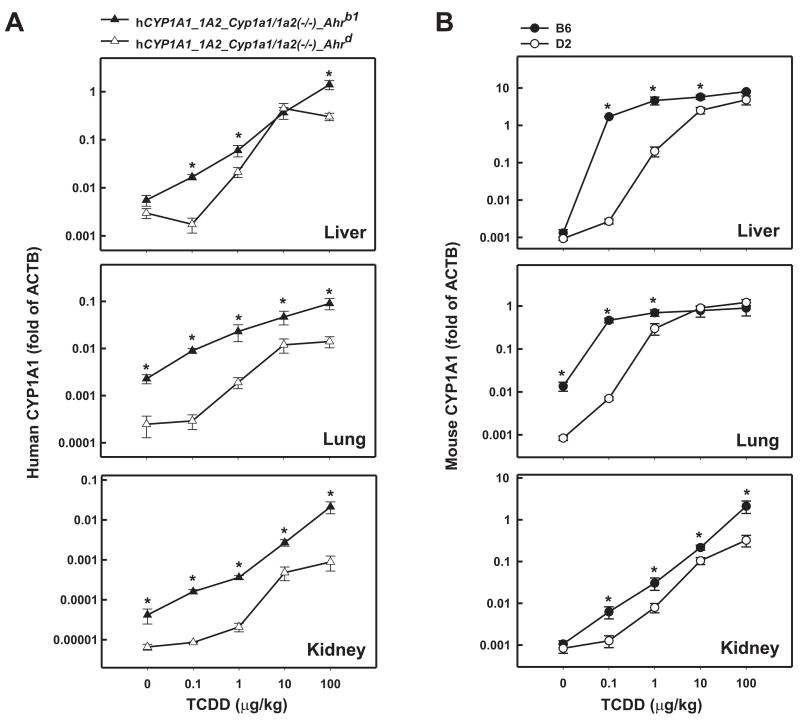
In hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 mice containing the high-affinity AHR (Fig. 2A), human CYP1A1 mRNA levels became elevated already at the 0.1 μg/kg dose of TCDD and accumulated in a dose-dependent manner reaching ~250-, ~40- and ~500-fold induction in liver, lung and kidney, respectively, at the highest TCDD dose. In these same tissues of the hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd mouse carrying the poor-affinity AHR, the dose-response curve was shifted-to-the-right ~3- to 4-fold: human CYP1A1 mRNA induction was not significantly detectable until TCDD doses of 1.0 μg/kg or higher and the highest accumulated induction levels were ~150-, ~56- and ~130-fold in liver, lung and kidney, respectively. Whereas human CYP1A1 mRNA amounts in both lines were not significantly different in liver at the 10 μg/kg dose of TCDD, the mRNA levels were significantly greater at the other doses in liver and at all doses in lung and kidney of hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1, compared with those of hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd mice. In either humanized line, as expected, mouse CYP1A1 mRNA was undetectable (not shown).
FIG. 2.
Log-log dose-response plots of CYP1A1 mRNA as a function of administered TCDD in liver, lung and kidney. Mice received either vehicle only or one of four doses of intraperitoneal TCDD 48 h before killing. Human (A) and mouse (B) CYP1A1 mRNA levels were compared by normalizing their concentrations to that of β-actin (ACTB) mRNA; PCR reactions for CYP1A1 mRNA and ACTB mRNA were performed on the same plate. Based on the plate reader, the efficiency for each single well was >90%. Note the striking differences in values on the ordinates. Data are reported as means ± S.E.M. (N=3–4 mice per group). *P <0.05, when comparing mRNA levels for that gene, at the same dose of TCDD, between the two mouse lines (A) or between the two inbred strains (B).
Induction of mouse CYP1A1
Using the same induction regimen, we also studied the mouse CYP1A1 mRNA in B6 (high-affinity-AHR) and D2 (poor-affinity-AHR) mice (Fig. 2B). In all three tissues, the B6 Cyp1a1 gene responded more robustly to TCDD, when compared with the human CYP1A1 gene under the control of the same mouse AHR. Mouse CYP1A1 mRNA also showed dose-dependent responses at all four TCDD doses.
In B6 animals (Fig. 2B), mouse CYP1A1 mRNA levels were strikingly elevated already at the 0.1 μg/kg dose of TCDD and accumulated in a dose-dependent manner reaching ~6100-, ~66- and ~2000-fold induction in liver, lung and kidney, respectively, at the highest TCDD dose. In these same tissues of the D2 mouse, the dose-response curve was shifted ~10- to 12-fold to the right; the highest accumulated induction levels of mouse CYP1A1 mRNA were ~5200-, ~1400- and ~390-fold in liver, lung and kidney, respectively. Note that B6 lung basal CYP1A1 mRNA amounts are ~16-fold greater than that in D2.
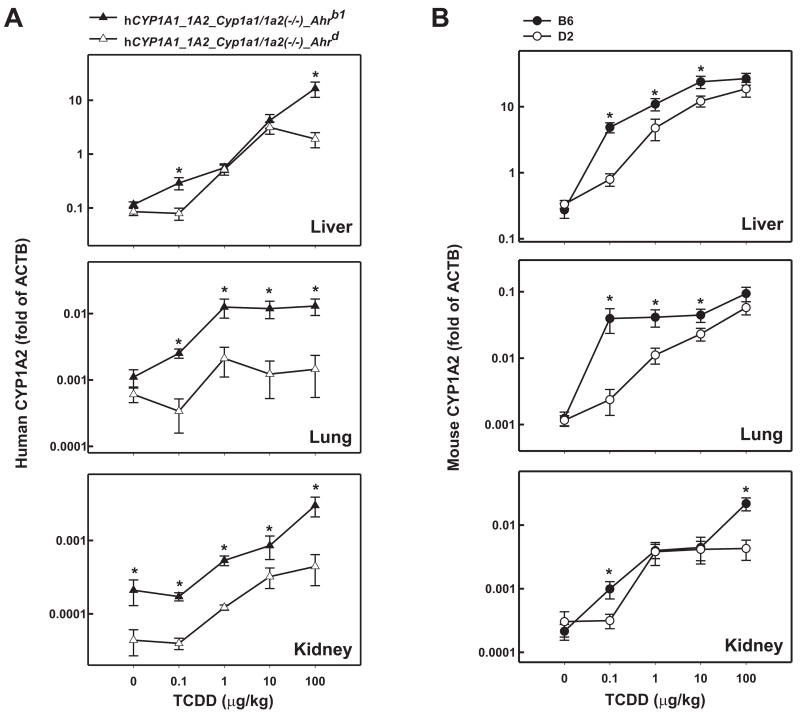
Induction of human CYP1A2
In TCDD-treated hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 mice (Fig. 3A), human CYP1A2 mRNA levels were increased in a dose-dependent manner (at all four TCDD doses) in liver, reaching ~140-fold maximal induction over basal levels; in lung, maximal induction of ~12-fold was accomplished at the 1.0 μg/kg TCDD dose; in kidney, accumulation of human CYP1A2 mRNA occurred at doses of only 1.0 μg/kg and higher, with maximal induction of ~14-fold over basal levels. In TCDD-treated hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd mice, human CYP1A2 mRNA in liver was maximally induced ~37-fold at the 10 μg/kg TCDD dose; in lung maximal increases of ~3.5-fold occurred at the 1 μg/kg dose; in kidney maximal induction of ~10-fold were seen at the 100 μg/kg dose. While evidence of a shift-to-the-right in the dose-response curves was seen in lung and kidney, in liver any differences in accumulated human CYP1A2 mRNA between the two humanized lines were found only at the 0.1 and 100 μg/kg doses. Although extremely low, human CYP1A2 basal levels in the kidney were ~5 times greater in hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 mice than in hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd mice. In either humanized line, as expected, mouse CYP1A2 mRNA was undetectable (not shown).
FIG. 3.
Log-log dose-response plots of CYP1A2 mRNA as a function of administered TCDD in liver, lung and kidney from the same mice as shown in Fig. 2. Human (A) and mouse (B) CYP1A2 mRNA levels were measured identically to that described in the Fig. 2 legend. *P <0.05, when comparing mRNA levels for that gene, at the same dose of TCDD, between the two mouse lines (A) or between the two inbred strains (B).
Induction of mouse CYP1A2
We also examined mouse Cyp1a2 gene expression in these three tissues (Fig. 3B). Mouse CYP1A2 mRNA from B6 liver revealed a dose-dependent response at doses from 0.1 to 10 μg/kg of TCDD, with maximal increases of ~100-fold. In lung and kidney dose-dependent responses were also found––with maximal induction of ~75- and 100-fold, respectively, reached at the 100 μg/kg TCDD dose. In B6 lung, mouse CYP1A2 mRNA amounts were no different at 0.1, 1.0 and 10 μg/kg doses of TCDD.
In liver and lung of the D2 mouse, the dose-response curve was shifted-to-the-right ~10- to 15-fold (Fig. 3B), whereas B6-D2 differences in kidney were apparent only at the 0.1 and 100 μg/kg doses of TCDD; the highest accumulated induction levels of mouse CYP1A2 mRNA were ~56-, ~50- and ~14-fold in liver, lung and kidney, respectively.
Absolute CYP1A1 and CYP1A2 mRNA levels in humanized lines versus B6 mouse
It is known that mammalian CYP1A1 basal mRNA is negligible, resulting in no detectable CYP1A1 protein in virtually any tissue, whereas basal levels of CYP1A2 mRNA and protein are relatively high in liver; induction by TCDD increases these levels, but––due to negligible basal levels––”fold-induction” is generally not a useful parameter [6; 20]. In the present study, we found that mouse CYP1A1 maximally induced mRNA concentrations were roughly 10 times higher than human CYP1A1 in liver and lung and 100-fold greater in kidney (Fig. 2). In contrast, mouse CYP1A2 maximally induced mRNA levels were <2-fold higher than human CYP1A2 in liver, but ~7-fold greater in lung, and ~9-fold higher in kidney (Fig. 3).
Human maximally induced CYP1A2 in liver was ~12 times higher than human maximally induced CYP1A1 mRNA, whereas mouse maximally induced CYP1A2 in liver was ~3-fold greater than mouse maximally induced CYP1A1 mRNA. Human maximally induced CYP1A1 levels in lung and kidney were both ~7-fold greater than human maximally induced CYP1A2 mRNA, whereas mouse maximally induced CYP1A1 in lung and kidney was ~13- and ~96-fold higher than mouse maximally induced CYP1A2 mRNA.
How representative are these humanized CYP1A mouse lines to individuals in a human population? Clearly, it is possible that the individual from whom the BAC library was derived [7] might represent an “outlier” as far as CYP1A1 and CYP1A2 expression.
In both lung and kidney, maximally induced expression of human CYP1A2 mRNA seen in the humanized lines and mouse CYP1A2 mRNA seen in the B6 mouse is quite low (~0.01-fold of ACTB mRNA); therefore, these levels are negligible––and no CYP1A2 protein is usually detected on Western immunoblots of these tissues. Maximally induced expression of human CYP1A1 mRNA in kidney shows much lower levels (~1%) than maximally induced expression of mouse CYP1A1 mRNA seen in B6 kidney. These findings call into question how important human CYP1A-dependent metabolism might be in both lung and kidney of these two humanized mouse lines. Alternatively, it is possible that the 180-kb BAC containing the human CYP1A1 and CYP1A2 genes [7] does not include all of the cis and/or trans regulatory sites needed for “normal” expression of these two transgenes in mouse lung and kidney. In addition, lung and kidney (more so than liver) are organs comprised of numerous heterogeneous cell types; qRT-PCR is so sensitive that it might detect CYP1A1 or CYP1A2 mRNA at quite high levels in one cell type that represents a minute amount of the entire organ. One might be able to resolve these questions by determining precise copy numbers of CYP1A1 and CYP1A2 mRNA in the various humanized CYP1A mouse lines, as well as in established tissue culture lines that are derived from human versus mouse specific cell types.
Conclusions
By breeding hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 mice with B6.D2-Ahrd congenic mice, we generated the humanized hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd line, which carries the human CYP1A1 and CYP1A2 genes in the absence of the mouse Cyp1a1 and Cyp1a2 orthologs. Human CYP1A1 and CYP1A2 are controlled by the endogenous mouse high-affinity AHR in the hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 line, and by the endogenous mouse poor-affinity AHR in the hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd line. We have characterized both humanized lines by quantifying human CYP1A mRNA levels in liver, lung and kidney––as a function of four doses of the potent inducer, dioxin. We have compared dose-response curves of those human mRNA levels with those of mouse CYP1A mRNA levels in B6 (high-affinity) versus D2 (poor-affinity) mice. Although subtle differences exist, the shift-to-the-right in dose-response curves can be visualized in all three organs between the hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 and hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd lines, and more robustly in the three organs between the B6 and D2 inbred strains.
We believe this newly-developed hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrd line will complement the previously described hCYP1A1_1A2_Cyp1a1/1a2(−/−)_Ahrb1 line, in carrying out pharmacokinetic and human risk assessment studies––involving any drug or environmental toxicant that is an effective substrate for CYP1A1 or CYP1A2. Researchers should now be able to compare the poor-affinity-AHR line with the high-affinity-AHR line, with regard to varying doses of environmental toxicants and/or drugs. Upon publication of this report, this line will be made commercially available by The Jackson Laboratories (Bar Harbor, Maine).
Acknowledgments
We thank our colleagues for many fruitful discussions and careful readings of this manuscript. We appreciate the success by Drs. Shige Uno and Tim Dalton in generating the original Cyp1a1/1a2(−/−) double-knockout genotype, from which the present humanized line was subsequently possible. Supported, in part, by NIH Grants R01 ES014403 (D.W.N.) and P30 ES06096 (D.W.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nebert DW, Karp CL. Endogenous functions of the aryl hydrocarbon receptor: intersection of cytochrome P450 (CYP)1-metabolized eicosanoids and AHR biology. J Biol Chem. 2008;283 doi: 10.1074/jbc.R800053200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW, Gunsalus IC, Nebert DW. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 5.Stark K, Guengerich FP. Characterization of orphan human cytochromes P450. Drug Metab Rev. 2007;39:627–637. doi: 10.1080/03602530701467708. [DOI] [PubMed] [Google Scholar]
- 6.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Z, Dalton TP, Jin L, Wang B, Tsuneoka Y, Shertzer HG, Deka R, Nebert DW. Toward the evaluation of function in genetic variability: characterizing human SNP frequencies and establishing BAC-transgenic mice carrying the human CYP1A1_CYP1A2 locus. Hum Mutat. 2005;25:196–206. doi: 10.1002/humu.20134. [DOI] [PubMed] [Google Scholar]
- 8.Li YY, Yu H, Guo ZM, Guo TQ, Tu K, Li YX. Systematic analysis of head-to-head gene organization: evolutionary conservation and potential biological relevance. PLoS Comput Biol. 2006;2:e74. doi: 10.1371/journal.pcbi.0020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turesky RJ. Interspecies metabolism of heterocyclic aromatic amines and the uncertainties in extrapolation of animal toxicity data for human risk assessment. Mol Nutr Food Res. 2005;49:101–117. doi: 10.1002/mnfr.200400076. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama T, Gonzalez FJ, Gelboin HV. Human cDNA-expressed cytochrome P450 IA2: mutagen activation and substrate specificity. Mol Carcinog. 1989;2:192–198. doi: 10.1002/mc.2940020405. [DOI] [PubMed] [Google Scholar]
- 11.Nichols RC, Cooper S, Trask HW, Gorman N, Dalton TP, Nebert DW, Sinclair JF, Sinclair PR. Uroporphyrin accumulation in hepatoma cells expressing human or mouse CYP1A2: relation to the role of CYP1A2 in human porphyria cutanea tarda. Biochem Pharmacol. 2003;65:545–550. doi: 10.1016/s0006-2952(02)01550-2. [DOI] [PubMed] [Google Scholar]
- 12.Dalton TP, Dieter MZ, Matlib RS, Childs NL, Shertzer HG, Genter MB, Nebert DW. Targeted knockout of Cyp1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem Biophys Res Commun. 2000;267:184–189. doi: 10.1006/bbrc.1999.1913. [DOI] [PubMed] [Google Scholar]
- 13.Liang HC, Li H, McKinnon RA, Duffy JJ, Potter SS, Puga A, Nebert DW. Cyp1a2(−/−) null mutant mice develop normally but show deficient drug metabolism. Proc Natl Acad Sci USA. 1996;93:1671–1676. doi: 10.1073/pnas.93.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragin N, Uno S, Wang B, Dalton TP, Nebert DW. Generation of a ‘humanized’ hCYP1A1_1A2_Cyp1a1/1a2(−/−) mouse line. Biochem Biophys Res Commun. 2007;359:635–642. doi: 10.1016/j.bbrc.2007.05.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derkenne S, Curran CP, Shertzer HG, Dalton TP, Dragin N, Nebert DW. Theophylline pharmacokinetics: comparison of Cyp1a1(−/−) and Cyp1a2(−/−) knockout mice, humanized hCYP1A1_1A2 knock-in mice lacking either the mouse Cyp1a1 or Cyp1a2 gene, and Cyp1(+/+) wild-type mice. Pharmacogenet Genomics. 2005;15:503–511. doi: 10.1097/01.fpc.0000167326.00411.50. [DOI] [PubMed] [Google Scholar]
- 16.Cheung C, Ma X, Krausz KW, Kimura S, Feigenbaum L, Dalton TP, Nebert DW, Idle JR, Gonzalez FJ. Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in mice humanized for CYP1A1 and CYP1A2. Chem Res Toxicol. 2005;18:1471–1478. doi: 10.1021/tx050136g. [DOI] [PubMed] [Google Scholar]
- 17.Ma X, Idle JR, Malfatti MA, Krausz KW, Nebert DW, Chen CS, Felton JS, Waxman DJ, Gonzalez FJ. Mouse lung CYP1A1 catalyzes the metabolic activation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Carcinogenesis. 2007;28:732–737. doi: 10.1093/carcin/bgl184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nebert DW. Pharmacogenetics: An approach to understanding chemical and biologic aspects of cancer. J Natl Cancer Inst. 1980;64:1279–1290. doi: 10.1093/jnci/64.6.1279. [DOI] [PubMed] [Google Scholar]
- 19.Poland AP, Glover E, Robinson JR, Nebert DW. Genetic expression of aryl hydrocarbon hydroxylase activity: induction of monooxygenase activities and cytochrome P1-450 formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice genetically “nonresponsive” to other aromatic hydrocarbons. J Biol Chem. 1974;249:5599–5606. [PubMed] [Google Scholar]
- 20.Eaton DL, Gallagher EP, Bammler TK, Kunze KL. Role of cytochrome P450 1A2 in chemical carcinogenesis: implications for human variability in expression and enzyme activity. Pharmacogenetics. 1995;5:259–274. doi: 10.1097/00008571-199510000-00001. [DOI] [PubMed] [Google Scholar]