Abstract
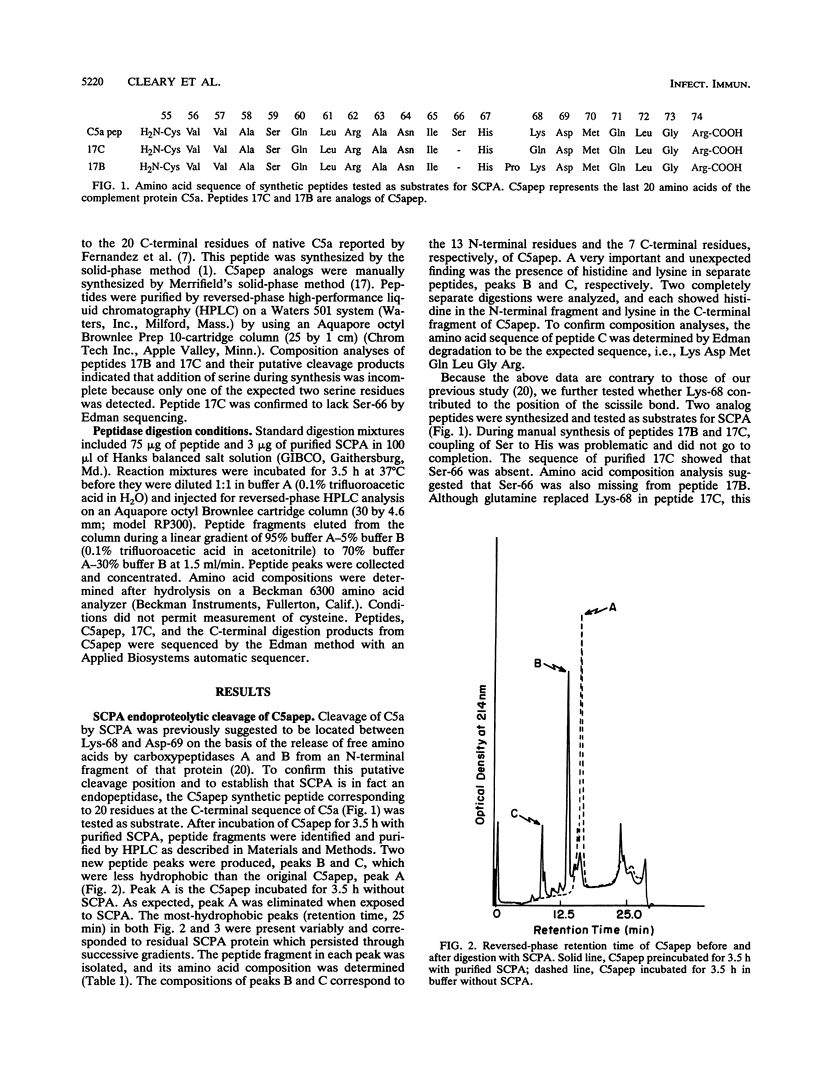
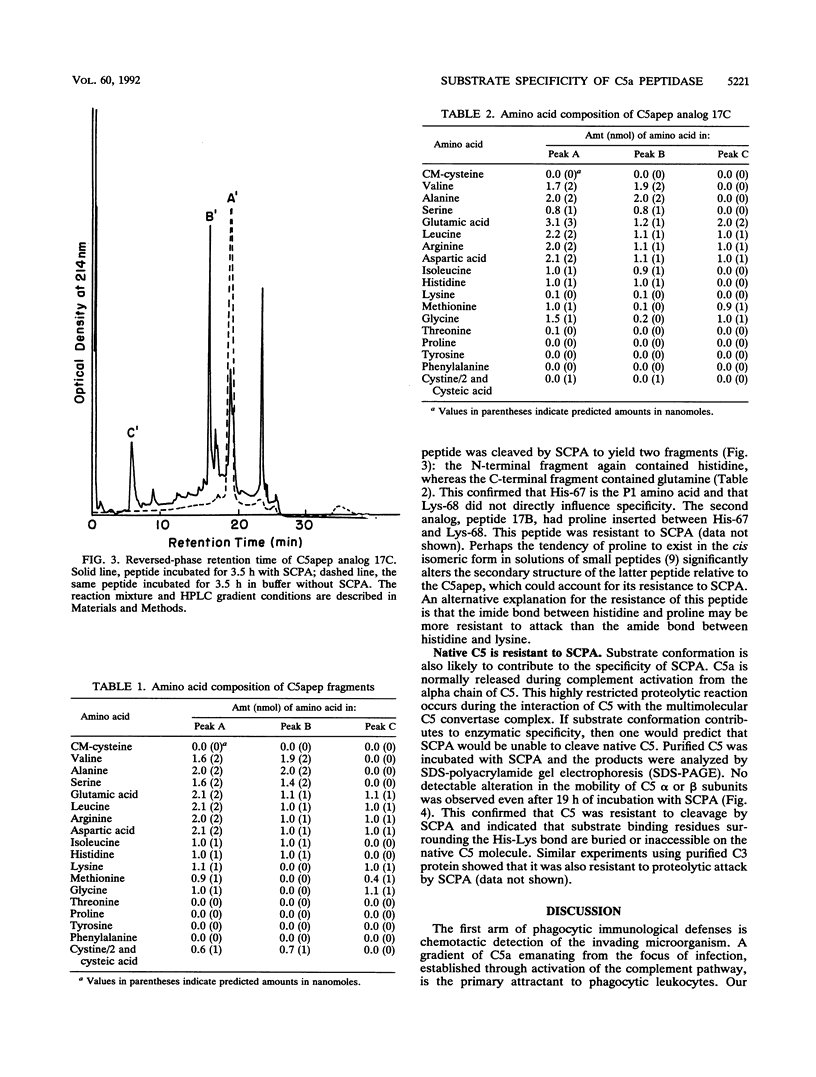
Compositional analysis of streptococcal C5a peptidase (SCPA) cleavage products from a synthetic peptide corresponding to the 20 C-terminal residues of C5a demonstrated that the target cleavage site is His-Lys rather than Lys-Asp, as previously suggested. A C5a peptide analog with Lys replaced by Gln was also subject to cleavage by SCPA. This confirmed that His-Lys rather than Lys-Asp is the scissile bond. Cleavage at histidine is unusual but is the same as that suggested for a peptidase produced by group B streptococci. Native C5 protein was also resistant to SCPA, suggesting that the His-Lys bond is inaccessible prior to proteolytic cleavage by C5 convertase. These experiments showed that the streptococcal C5a peptidase is highly specific for C5a and suggest that its function is not merely to process protein for metabolic consumption but to act primarily to eliminate this chemotactic signal from inflammatory foci.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Tartar A., Seyer J. M., Chedid L. Epitope-specific protective immunogenicity of chemically synthesized 13-, 18-, and 23-residue peptide fragments of streptococcal M protein. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2203–2207. doi: 10.1073/pnas.81.7.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack J. F., Mollison K. W., Buko A. M., Ashworth J. C., Hill H. R. Group B streptococci inactivate complement component C5a by enzymic cleavage at the C-terminus. Biochem J. 1991 Feb 1;273(Pt 3):635–640. doi: 10.1042/bj2730635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Cleary P. P. Complete nucleotide sequence of the streptococcal C5a peptidase gene of Streptococcus pyogenes. J Biol Chem. 1990 Feb 25;265(6):3161–3167. [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Human C5a and C5a analogs as probes of the neutrophil C5a receptor. Mol Immunol. 1980 Feb;17(2):151–161. doi: 10.1016/0161-5890(80)90067-x. [DOI] [PubMed] [Google Scholar]
- Cleary P. P., Peterson J., Chen C., Nelson C. Virulent human strains of group G streptococci express a C5a peptidase enzyme similar to that produced by group A streptococci. Infect Immun. 1991 Jul;59(7):2305–2310. doi: 10.1128/iai.59.7.2305-2310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Primary structural analysis of the polypeptide portion of human C5a anaphylatoxin. Polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J Biol Chem. 1978 Oct 10;253(19):6955–6964. [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grathwohl C., Wüthrich K. Nmr studies of the molecular conformations in the linear oligopeptides H-(L-Ala)n-L-Pro-OH. Biopolymers. 1976 Oct;15(10):2043–2057. doi: 10.1002/bip.1976.360151013. [DOI] [PubMed] [Google Scholar]
- Heath D. G., Cleary P. P. Fc-receptor and M-protein genes of group A streptococci are products of gene duplication. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4741–4745. doi: 10.1073/pnas.86.12.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H. R., Bohnsack J. F., Morris E. Z., Augustine N. H., Parker C. J., Cleary P. P., Wu J. T. Group B streptococci inhibit the chemotactic activity of the fifth component of complement. J Immunol. 1988 Nov 15;141(10):3551–3556. [PubMed] [Google Scholar]
- O'Connor S. P., Cleary P. P. Localization of the streptococcal C5a peptidase to the surface of group A streptococci. Infect Immun. 1986 Aug;53(2):432–434. doi: 10.1128/iai.53.2.432-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Isolation and characterization of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988 Jun;170(6):2618–2624. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., de Vos W. M., Leunissen J. A., Dijkstra B. W. Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteinases. Protein Eng. 1991 Oct;4(7):719–737. doi: 10.1093/protein/4.7.719. [DOI] [PubMed] [Google Scholar]
- Vos P., Boerrigter I. J., Buist G., Haandrikman A. J., Nijhuis M., de Reuver M. B., Siezen R. J., Venema G., de Vos W. M., Kok J. Engineering of the Lactococcus lactis serine proteinase by construction of hybrid enzymes. Protein Eng. 1991 Apr;4(4):479–484. doi: 10.1093/protein/4.4.479. [DOI] [PubMed] [Google Scholar]
- Vos P., Simons G., Siezen R. J., de Vos W. M. Primary structure and organization of the gene for a procaryotic, cell envelope-located serine proteinase. J Biol Chem. 1989 Aug 15;264(23):13579–13585. [PubMed] [Google Scholar]
- Wexler D. E., Chenoweth D. E., Cleary P. P. Mechanism of action of the group A streptococcal C5a inactivator. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8144–8148. doi: 10.1073/pnas.82.23.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler D. E., Cleary P. P. Purification and characteristics of the streptococcal chemotactic factor inactivator. Infect Immun. 1985 Dec;50(3):757–764. doi: 10.1128/iai.50.3.757-764.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler D. E., Nelson R. D., Cleary P. P. Human neutrophil chemotactic response to group A streptococci: bacteria-mediated interference with complement-derived chemotactic factors. Infect Immun. 1983 Jan;39(1):239–246. doi: 10.1128/iai.39.1.239-246.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]