Abstract
Carboxyl ester lipase (bile salt-stimulated lipase) is a pancreatic enzyme capable of hydrolyzing esters of cholesterol and fat-soluble vitamins. It also efficiently digests triglycerides (TG) into free fatty acids and glycerol and is abundant in the milk of humans and several other species. We used the mouse as a model to test the hypothesis that milk-derived carboxyl ester lipase (CEL) digests milk TG and that without its activity milk lipids and their digestion intermediates can disrupt the intestinal epithelium of neonates. CEL protein and enzymatic activity were shown to be abundant in mouse milk. After 24-h administration of the CEL-specific inhibitor, WAY-121,751-5, the small intestines of treated and control neonates were analyzed histologically for signs of fat malabsorption and injury to their villus epithelium. In vehicle-fed controls, TG were digested and absorbed in the duodenum and jejunum, whereas, in inhibitor-fed littermates, large intracellular neutral lipid droplets accumulated in enterocytes of the ileum, resulting in damage to the villus epithelium. Similar results were observed in neonates nursed by CEL knockout females compared with heterozygous controls. The results suggest that lack of CEL activity causes incomplete digestion of milk fat and lipid accumulation by enterocytes in the ileum of neonatal mice.
Keywords: infant nutrition disorders, intestinal absorption, dietary fats, small intestine, enterocolitis, bile salt-stimulated lipase
AS NEONATAL CARE HAS BECOME more sophisticated, the survival of premature and low birth weight infants has increased dramatically. As a result, there has been an increase in the incidence of intestinal disorders, especially ileal perforation and necrotizing enterocolitis (NEC) (17, 31, 49). NEC is characterized by mucosal and transmural necrosis and inflammation, usually involving the terminal ileum or colon. The inflammation and loss of mucosal integrity is often accompanied by rupture of the intestinal wall and sepsis. Despite advances in the diagnosis and treatment of this disease, NEC remains a major cause of morbidity and mortality in nurseries caring for premature and low birth weight infants (17, 25, 53).
The etiology of NEC is not clear and is likely to derive from several causes. The only consistent predisposing factors remain prematurity and enteral feeding, suggesting that the immature intestinal mucosae of these infants are unable to withstand the stress associated with processing a complex diet (31). Early observations indicated that there was a reduced incidence of NEC in babies fed breast milk as opposed to formula (2, 6, 38). Subsequent work has investigated various components of breast milk that might be responsible for its protective properties. The role of immunoglobulins in protecting against infection (6, 16, 23), the roles of cytokines and growth factors in promoting maturation of the intestinal epithelium (9), and the importance of antiinflammatory factors (24) have been investigated with mixed results. Acetyl hydrolase activity against platelet-activating factor (PAF) has been found in milk, and its role in protecting against excessive inflammatory responses to PAF has been shown in rat models of ischemia-induced NEC (21, 22, 43-45). However, recent reports suggest that other factors in milk also protect against PAF- and ischemia-induced injury (5, 40).
Dietary fat has been shown to influence both the morphology and the function of the intestinal epithelium. Several investigators have found that the type and amount of lipid in formulas and milk significantly affect the transport properties and permeability of the neonatal intestinal epithelium and the rate at which it proliferates and matures (46, 54, 61). In a neonatal pig model of NEC, Crissinger and co-workers (12, 56, 57) showed that the degree of permeability and intestinal damage directly relates to the presence and type of fat in various neonatal formulas. Luminal lipids were also found to exacerbate intestinal injury in a rat model of NEC (4).
Carboxyl ester lipase (CEL), also called bile salt-stimulated lipase (BSSL), is an enzyme present at high levels in the milk of humans and several other species (15, 20, 47). This lipase has a broad range of potential substrates, including mono-, di-, and triacylglycerols, cholesteryl esters, vitamin esters, and lysophospholipids (reviewed in Ref. 50). CEL constitutes 1-2% of milk protein (15) and has been proposed to play an important role in neonatal nutrition, specifically in the digestion and absorption of milk fat (1, 10, 27, 30, 47). Evidence to this effect has come from studies using kittens that, when fed a commercial kitten formula, gained weight at about 50% of the rate of nursed littermates. When the formula was supplemented with human CEL, however, rates of weight gain were nearly equal between the formula-fed and nursed kittens (58).
A role for CEL in fat digestion in infants is further suggested by the findings of several investigators indicating that the pancreas is unable, at birth, to secrete sufficient lipolytic enzymes and/or to respond to the secretagogues cholecystokinin and secretin (19, 34). In premature infants especially, duodenal contents lack pancreas-derived lipolytic activity (32). In dogs, Iverson et al. (30) found that gastric lipase, although highly active in the hydrolysis of TG, did not completely digest the milk fat in the stomach. Diglycerides (DG), free fatty acids, and TG are delivered to the duodenum for further digestion. In neonates with insufficient milk- or pancreas-derived lipase activity, these lipids may transit the small intestine without being completely digested, resulting in the type of lipid-dependent damage seen in the pig models of NEC referred to previously (12, 56, 57).
Thus, in preterm and low birth weight infants, milk-derived CEL may be important not only for proper nutrition but also for protection of the immature intestinal epithelium from the deleterious effects of the otherwise incompletely digested milkfat. This study describes experiments that support this hypothesis. Inhibition or elimination of CEL in neonatal mice for 24 h followed by histological analysis of the villus epithelium revealed the accumulation of neutral lipid droplets, especially in ileal enterocytes, which resulted in cellular and structural damage to the villus epithelium. This lipid accumulation by the distal small intestine suggests that milk-derived CEL may protect the premature or low birth weight infant from damage to the small bowel mucosa.
METHODS
Animals
Mice were housed and cared for by the institution's Department of Laboratory Animal Medicine and were kept in humidified rooms with a 12:12-h light-dark cycle and unrestricted access to food (Teklad LM485, Harlan Teklad, Madison, WI) and water. Mice were either bred in house (CEL knockout animals) or purchased from Taconic Farms (Germantown, NY). The CEL knockout allele was backcrossed onto the C57BL/6 genetic background for seven generations, and then pups nursed by either knockout females or heterozygous females were given the same regimen of triolein administration without inhibitor as described in Inhibitor feeding. CEL knockout mice were genotyped either by PCR or by Southern blot analysis as previously described (28).
Milk collection
Mouse milk was collected from lactating dams by continuous oral suction with the use of a capillary tube and flexible tubing after anesthetizing the animals with 8 µl/g body wt of 5% avertin. After collection, milk was diluted 1:4-1:10 with PBS and was centrifuged to separate the fat. The whey was stored at −80°C until use. Stomach milk was isolated during dissection of neonates and was stored at −80°C until assayed. For enzyme assay, a piece of the frozen milk was weighed, gently resuspended on ice in 10 vol of PBS, and processed as above for fresh milk.
Western blots
Milk protein was fractionated on 7.5% SDS-polyacrylamide gels. After fractionation, gels were either stained with Coomassie brilliant blue or transferred to nitrocellulose (Bio-Rad Laboratories, Melville, NY) by electroblotting essentially as described (51). CEL protein was detected with the use of immunopurified polyclonal rabbit anti-rat CEL antibody and 125I-labeled goat anti-rabbit IgG (Amersham, Arlington Heights, IL) as described (14).
CEL activity
CEL enzyme activity was assayed by measuring the release of [14C]oleate from cholesteryl-[14C]oleate (DuPont NEN, Boston, MA) in the presence of 15 mM sodium taurocholate and 100 µg/ml BSA, with a reaction time of 15 min as described (7). The amount of CEL in milk samples was determined by correlation to the hydrolytic activity from known amounts of purified rat CEL. Activity in intestinal contents from knockout and control mice was similarly assayed. Protein concentrations were determined by the method of Lowry et al. (37).
Inhibitor feeding
The CEL-specific inhibitor, WAY-121,751-5, was a kind gift from Dr. Mar-Lee McKean of Wyeth-Ayerst (Princeton, NJ). A preliminary report of the activity and specificity of this inhibitor has been published (11, 42). Inhibitor was dissolved in triolein (Sigma, St. Louis, MO) at a concentration of 40 mg/ml and was stored at room temperature. Black Swiss × C57BL/6 mouse pups were fed inhibitor at 100 µg/g body wt by oral gavage with a 22-gauge 1.5-in. feeding tube (Roboz Surgical Instrument, Rockville, MD). Control pups were fed an equal volume of vehicle (triolein) alone. Experiments were performed on 4- and 8-day-old neonatal mice, with half the pups in each litter receiving inhibitor and half receiving vehicle. Dosing was repeated every 4-5 h for 20 h beginning in the afternoon to inhibit both the ingested milk CEL and any pancreatic CEL produced endogenously. After dosing, pups were returned to the cage with their mother and allowed to nurse. Litters were monitored for signs of distress and to ascertain that all pups continued to nurse normally throughout the experiment.
Tissue analysis
Four to five hours after the last dose of inhibitor, the pups were killed by CO2 asphyxiation. Blood was collected from the thoracic cavity after the inferior vena cava was severed. Samples were allowed to clot, and serum was collected after centrifugation. The small intestine was carefully removed, rinsed briefly in ice-cold PBS, and then dissected into duodenum, jejunum, and ileum. The junction between duodenum and jejunum was defined at the ligament of Treitz, and the jejunoileal junction was defined as where the diameter of the small intestine reduces markedly. The intestines were not flushed when intended for histological analyses because preliminary experiments showed that doing so denuded the villus epithelium, especially in inhibitor-fed animals. Portions of each segment were immediately frozen in OCT compound (Miles, New Haven, CT) for preparation of frozen sections. Portions of each section were fixed overnight in 10% neutral buffered Formalin and were processed for paraffin embedding. Hematoxylin and eosin (H&E) staining of paraffin sections and oil red O staining of frozen sections were performed according to standard procedures (8).
Morphometric measurements on sections of paraffin-embedded tissues were performed using Image-Pro Plus 1.3 (Media Cybernetics, Silver Spring, MD). Outlines of complete villi from comparable sections of control and affected mice were traced and their lengths compared. In this way, increases in both villus height and diameter were taken into account. Cells per villus length were counted manually. At least 45 villi from each sample were scored. For some animals from the gene-knockout experiments, intestinal contents were collected. The small intestine was opened longitudinally, and the duodenum, jejunum, and ileum were individually washed by agitation in 0.5 ml of 10 mM sodium taurocholate in PBS. The tissue was removed by centrifugation and the supernatant assayed directly for enzyme activity as described in CEL activity or for lipid content as described in Lipid analyses.
Lipid analyses
Glycerolipids present in luminal contents, as well as serum cholesterol and TG, were assayed with commercially available kits according to manufacturers' directions (Sigma or Wako Chemicals USA, Richmond, VA).
Statistics
Data were analyzed with appropriate t-tests and Excel 5.0 (Microsoft, Seattle, WA) or Prism 2.01 (Graphpad Software, San Diego, CA).
RESULTS
Assay of mouse milk
In order to establish the validity of the mouse to study the role of milk CEL, the level of enzyme in mouse milk was determined by both enzymatic and immunoblot analyses. Figure 1 shows the immunoblot results of 45 µg of whey protein after fractionation on a 7.5% SDS-polyacrylamide gel. Individual samples were probed with anti-rat CEL antiserum and 125I-labeled secondary antibody. The protein detected has the expected molecular mass of ∼68 kDa, as seen previously for mouse CEL (28). These gels established that CEL is present in mouse milk.
Fig. 1.
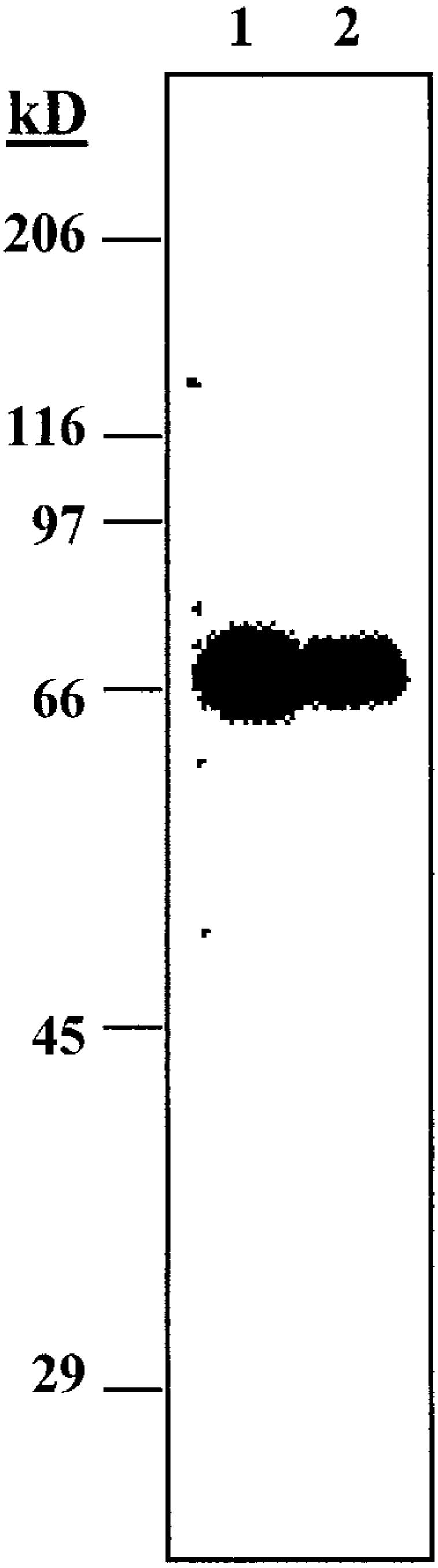
Western blot of whey proteins from mouse milk. Lanes 1 and 2 show milk samples from two different female mice transferred to nitrocellulose, reacted with antibodies against carboxyl ester lipase (CEL), and detected with 125I-labeled secondary antibody.
To determine the amount of CEL in mouse milk, the bile salt-stimulated hydrolysis of cholesteryl oleate by milk preparations from several lactating females was quantitated. Table 1 presents these results as pico-moles of [14C]oleate released per minute per microgram of protein. By comparison with parallel assays of purified rat pancreatic CEL, the mass of CEL protein present per microgram of whey protein in each sample was estimated. As shown in column 3, this assay indicated that CEL constitutes approximately 1-7% of whey protein in mouse milk. CEL content in these same milk samples was also estimated with an immunoblot assay of serial dilutions of milk protein compared with serial dilutions of defined amounts of the purified rat protein. The results (not shown) of this analysis were in agreement with the CEL levels determined from the enzyme activity assay. These high levels of CEL indicate that the mouse is a valid animal model for testing hypotheses regarding the role of milk-derived CEL in neonatal health and nutrition.
Table 1.
CEL activity and quantitation in mouse milk
| Sample | Activity, pmol·min−1·µg−1 | CEL Concentration, ng CEL/µg whey protein |
|---|---|---|
| Activity was measured by the release of [14C]oleate from cholesteryl-[14C]oleate in the presence of bile salt and BSA. Calculation of the mass of carboxyl ester lipase (CEL) present was based on comparison with the purified CEL standard. Values are averages of duplicates performed on 3 different concentrations of enzyme or whey protein. Protein was determined by Lowry assay. | ||
| Purified CEL | 8,889 | |
| Milk | ||
| #1 | 65 | 7 |
| #2 | 394 | 44 |
| B6 | 145 | 16 |
| 2d | 596 | 67 |
| 14d | 277 | 31 |
To further verify that milk CEL and pancreatic CEL are encoded by the same gene and that both activities are ablated in CEL knockout mice (28), samples of stomach milk from pups nursed by wild-type or knockout dams were assayed as above. CEL activity was readily detected in wild-type milk (391 pmol CE hydrolyzed·min−1 ·mg milk−1), whereas essentially no activity was detected in knockout milk (0.12 pmol CE hydrolyzed·min−1 ·mg milk−1). These results provide independent comfirmation that the milk enzyme and the pancreatic enzyme are the same protein encoded by the same gene as has been reported previously based on genetic data (28, 39).
Inhibitor feeding
To test our hypothesis that CEL activity is important in preventing damage to the villus epithelium by the high fat content of milk, a CEL-specific inhibitor or vehicle alone was administered to equal numbers of 4- and 8-day-old neonatal mice. The neonates were allowed to nurse throughout the experiment and were observed to suckle normally, with none showing any overt signs of distress. Four to five hours after the last dose, the pups were killed and analyzed for effects of the inhibitor. As summarized in Table 2, all animals continued to gain weight, with no difference between control and treated animals. Serum TG were measured and were found to vary greatly between individuals, with no statistically significant differences seen between control and treated groups. Because CEL has been implicated in the absorption of dietary cholesterol esters (28), serum cholesterol was determined for all animals as well (Table 2). Only minor differences were found between control and treated pups. These analyses indicated that administration of the inhibitor did not acutely affect serum lipid levels.
Table 2.
Status of animals after treatment with CEL inhibitor
| Animals | Weight Gain, g | Serum Triglycerides, mg/dl | Serum Cholesterol, mg/dl |
|---|---|---|---|
| Values are means ± SE. Number in parentheses is number of animals in each group. ND, not determined. P > 0.05 for all comparisons. | |||
| 4-Day-old | |||
| Control (4) | 0.68 ± 0.28 | 210 ± 37 | 117 ± 4.7 |
| Treated (4) | 0.65 ± 0.13 | 176 ± 18 | 108 ± 4.3 |
| 8-Day-old | |||
| Control (4) | 0.48 ± 0.15 | 146 ± 27 | 165 ± 7.6 |
| Treated (4) | 0.60 ± 0.08 | 177 ± 22 | 151 ± 12 |
| Weanling | |||
| Control (3) | ND | 95 ± 14 | 120 ± 9 |
| Treated (3) | ND | 82 ± 21 | 111 ± 17 |
Histological findings
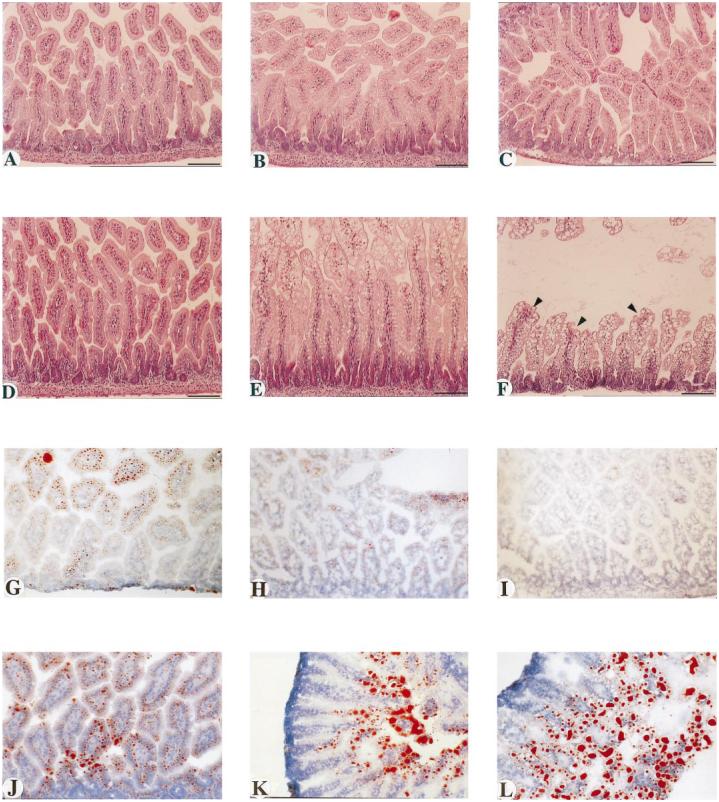
Paraffin sections (10 µm) of each segment of the small intestine were stained with H&E and were examined for signs of injury. The effects of the inhibitor increased with progression distally along the small intestine. As depicted in Fig. 2, A and D, the duodenum was minimally affected. However, striking differences between control and treated animals were apparent in the jejunum (Fig. 2, B and E) and especially in the ileum (Fig. 2, C and F). As shown in Fig. 2E, the jejunal enterocytes of the villus tips of treated animals were enlarged and filled with vacuoles. In the ileum of treated animals (Fig. 2F), a majority of the enterocytes were vacuolated, causing distortion of the enterocyte morphology and of the villus architecture as a whole. Severe distortion of the villus tips was evident in several cases (Fig. 2F, arrowheads). These effects were similar in the 4-day-old and 8-day-old neonates, except that the overall severity and the involvement of the lower jejunum were greater in the 4-day-old pups. Figure 2, A-F, are from 4-day-old mice.
Fig. 2.
Representative histopathology of small intestines from control and treated mice. A-F: hematoxylin and eosin (H&E)-stained sections of paraffin-embedded tissues from 4-day-old pups. G-L: oil red O-stained sections of frozen specimens from 8-day-old pups. A-C and G-I are from control animals, and D-F and J-L are from inhibitor-treated animals. A, D, G, and J are duodenum; B, E, H, and K are jejunum; C, F, I, and L are ileum. Arrowheads in F indicate areas of apparent disruption of villus epithelium in ileum from inhibitor-treated pups. Bars in A-F = 100 µm. Original magnification, × 66 for G, H, I, J, and L and × 50 for K.
Oil red O staining of frozen sections from these same animals was performed to reveal their intestinal lipid content. As can be seen in Fig. 2G, the vehicle-treated animals showed small lipid droplets (red-stained material) in the duodenum, representing absorbed lipid being assembled into chylomicrons and secreted into the lacteals and lymphatic circulation. The distribution of lipid in the duodenum of inhibitor-treated animals is similar, except that the droplet size in the enterocytes is somewhat larger than in control animals (Fig. 2J). Only minimal staining with oil red O is seen in the jejunum and ileum of vehicle-fed animals (Fig. 2, H and I). In contrast, the jejunum and ileum of inhibitor-treated animals showed a very different pattern of oil red O staining. In the jejunum, large lipid droplets were present in the enterocytes of the villus tips, whereas comparatively little lipid was found in the lamina propria or in the central lacteal (Fig. 2K). In the ileum, a majority of the enterocytes were filled with large lipid droplets that had distorted the size and shape of the cells and of the villi as a whole (Fig. 2L). Figure 2, G-L, are from 8-day-old mice.
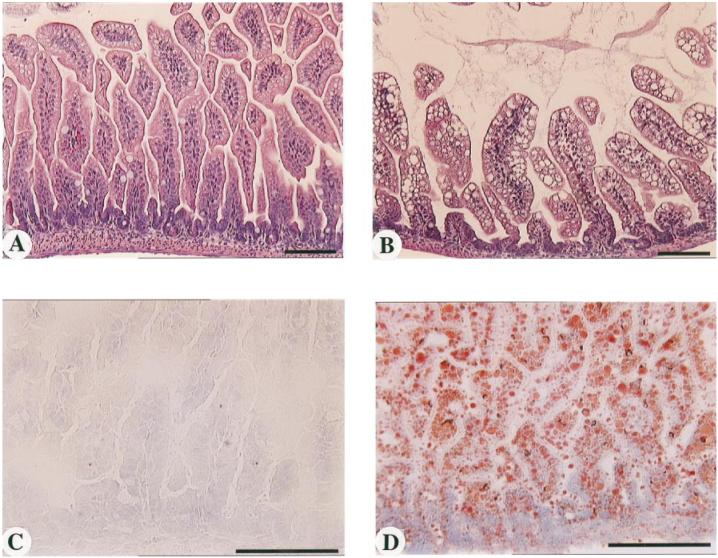
To rule out possible secondary effects of the inhibitor treatment, the above study was repeated without inhibitor administration with the CEL knockout mice (28). When nursed by CEL knockout dams, 6-day-old CEL knockout pups, which fail to receive the enzyme through nursing, develop a pattern of lipid accumulation and villus hypertrophy in the ileum very similar to that seen after inhibitor administration (Fig. 3, B and D). Minimal lipid accumulation is seen when pups (heterozygous or knockout) are nursed by heterozygous females (Fig. 3, A and C). These experiments further indicate that lack of CEL in neonatal mice results in lipid accumulation and gross distortion in the enterocytes of the distal small intestine.
Fig. 3.
Representative histopathology of jejunoileal segments of intestines from 6- to 7-day-old pups nursed by CEL heterozygous females (A and C) or CEL knockout females (B and D). A and B: H&E-stained paraffin sections. C and D: oil red O-stained frozen sections. Bars = 100 µm. Pups were CEL heterozygous in A and C and CEL knockout in B and D.
Lipid accumulation by ileal epithelial cells suggested increased delivery of undigested or partially digested TG to the ileum in the absence of CEL activity. To test this hypothesis, ileal washings from pups nursed by heterozygous or knockout females were analyzed for glycerolipids (TG, DG, monoglyceride, and free glycerol). Knockout pups nursed by a knockout female had 364 ± 145 µg of glycerolipid in the ileum, whereas knockout or heterozygous pups nursed by a heterozygous female had 73 ± 11 µg of glycerolipid in the ileum (P < 0.05). These data support the hypothesis that lack of CEL activity results in less efficient TG digestion and increased delivery of lipid to the distal small intestine, where it accumulates in the villus epithelium.
Because trophic effects of lipids on small intestinal epithelium have been reported (41, 59-61), tissue sections were studied morphometrically to determine if the lack of CEL activity had resulted in any hyperplasia of the intestinal mucosa. The average villus length was analyzed, and the number of cells per villus was counted for the affected regions of the small intestine. Comparable sections were analyzed from control and affected mice. The lower jejunum and middle ileum from the inhibitor experiment and the jejunoileal region from the knockout experiment were analyzed. As summarized in Table 3, no evidence for hyperplasia was found in any of the tissues examined. However, the villus height in ileal tissues was ∼25% greater in the absence of CEL activity compared with control animals (P < 0.001). Because there was no increase in cell number per villus, this increase in length is due entirely to cellular enlargement caused by the accumulation of lipid in the enterocytes.
Table 3.
Effect of CEL activity loss on villus height and cell number
| Villus Height, µm | Cells Per Villus Length | |||
|---|---|---|---|---|
| Animals | Jejunum | Ileum | Jejunum | Ileum |
| Values are means ± SE. Villus heights were measured by tracing microscope images using Image-Pro Plus software. | ||||
| 4-Day-old | ||||
| Control | 213 ± 42a | 146 ± 41c | 19 ± 4 | 17 ± 4 |
| Treated | 240 ± 75a | 187 ± 54c | 19 ± 6 | 16 ± 4 |
| 8-Day-old | ||||
| Control | 193 ± 62b | 149 ± 28d | 18 ± 4 | 17 ± 3 |
| Treated | 253 ± 48b | 175 ± 37d | 17 ± 4 | 17 ± 4 |
| CEL(+/−)pups from CEL(+/−) dam* | 216 ± 66e | 18 ± 5 | ||
| CEL(−/−)pups from CEL(−/−) dam* | 284 ±77 e | 19 ± 4 | ||
Measurements were of the jejunoileal region.
P < 0.001 for each pair.
P < 0.001 for each pair.
P < 0.001 for each pair.
P < 0.001 for each pair.
P < 0.001 for each pair.
To determine whether the effects seen were specific to the neonates, e.g., due to an immature gastrointestinal system, or were a general consequence of lipolysis inhibition, the inhibitor or vehicle alone was administered to weanling mice. In this experiment, no differences were found between the treated and control animals. There was no lipid accumulation and no intestinal damage in any of the inhibitor-treated mice (data not shown). Similarly, examination of knockout mice revealed that villus epithelium from the late neonatal/weaning period is the same in the presence or absence of CEL. These results indicate that lipolytic activity by CEL has a protective effect that is specific to the early postnatal period.
To further assess the relative importance of milk-derived CEL compared with pancreas-derived enzyme, the luminal contents from 5-day-old knockout and heterozygous littermates nursed by the same heterozygous dam were assayed for CEL activity. Enzyme sufficient to hydrolyze 122 ± 5 pmol/min cholesteryl oleate was recovered from the duodenum and jejunum of heterozygous pups compared with 90 ± 9 pmol/min from knockout pups (P < 0.05). These results suggest that ∼75% of the CEL activity in 5-day-old mice is derived from ingested milk. Essentially no activity (1.6 ± 1.6 pmol/min) could be recovered from the lumen of knockout pups nursed by knockout dams, whereas 152 ± 33 pmol/min was recovered from wild-type pups nursed by a wild-type dam.
DISCUSSION
Current work from this laboratory is aimed at determining the physiological significance of CEL in neonatal health and nutrition by utilizing genetically defined mouse models such as the gene knockout mice described previously (28). This is the first quantitative report of CEL in mouse milk. Its presence had been inferred from mRNA studies of lactating mammary glands (35, 39), and Stromqvist et al. (52) reported, but did not quantitate, endogenous milk BSSL (CEL) in transgenic animals. The amount of CEL detected in the current study (∼1-7% of whey protein) shows milk enzyme levels comparable with those found in humans (0.4-4% of total protein; Ref. 26), indicating that the mouse is a valid animal model for studying the role of milk-derived CEL. The wide range in enzyme activity may be due to the lactation period of the females sampled. Indeed, higher levels were found at earlier times of lactation (compare the samples labeled d2 vs. d14 in Table 1).
The results of this study show that when CEL activity is removed from the intestinal lumen, either by chemical inhibition or by genetic ablation, dietary fat digestion and/or transport is altered such that neutral lipid droplets accumulate within the intestinal epithelium of the ileum and lower jejunum. This lipid accumulation was striking and reflected the location of enterocytes along both the horizontal and vertical axes of the small intestine. The villus damage resulting from loss of luminal CEL activity was extensive enough to cause dramatic cellular distortion and to alter the villus architecture, with indications of loss of epithelial integrity. These experiments were performed with mouse pups >4 days old. Although the overall effects were qualitatively similar at different ages, lipid accumulation and villus damage were less severe in 8-day-old pups compared with 4-day-old pups, suggesting a developmental window when CEL activity is most critical.
In experiments with CEL knockout mice, both knockout and wild-type pups <5 days old show similar effects when nursed by a knockout dam. In older pups (≥5 days old), the heterozygous pups nursed by knockout dams are less affected by the lack of milk CEL, suggesting increased synthesis or secretion of the enzyme by the pancreas (data not shown).
Morphometric analysis revealed, on average, ∼20% increase in the cross-sectional circumference of villi from affected areas (Table 3). This measurement translates into ∼70% increase in villus volume (v = 4/3πr3) because of lipid accumulation. Although no obvious signs of inflammation or perforation were seen in this study, the observed dysmorphology and disruption of integrity may predispose affected tissues to further insults. Indeed, a striking observation during removal of tissues was that the small intestines of the treated animals were extremely fragile compared with controls and tended to rupture under even slight tension. Similar fragility is found in young pups nursed by knockout dams and correlates with increased lipid accumulation due to milk-fat load or administered lipid.
A role for milk-derived CEL in the digestion of milk fat has been suggested by several observations. The presence of this enzyme in the milk of many species, its ability to remain active after passing through the stomach, and its efficient lipolysis of all acylglycerols is well established (18, 20, 27). In addition, several studies suggest that the secretion of lipases by the neonatal pancreas (especially that of the premature infant) is insufficient to fully digest the high fat load presented by the neonatal diet (19, 32, 33, 63). Interestingly, a recent report (36) showed reduced milk-fat digestion and absorption in neonates lacking the pancreatic lipase-related protein-2, which is expressed during the neonatal period. An intriguing possibility is that the combined activities of these two lipases is required for proper digestion of milk fat.
Dietary lipids are digested and absorbed in the duodenum and jejunum, with very little fat absorption occurring in the ileum (13). In early experiments by Bennett-Clark et al. (3, 62), when adult rats were challenged with excess luminal triolein, excess lipid reached the ileum, where it accumulated in the intestinal wall. Ileal chylomicron secretion was 40% slower than in the proximal intestine. In the present study, the high fat content of the milk (plus the triolein vehicle) may have approached the limit of the digestive and/or absorptive capacity in the neonates lacking CEL activity, thereby allowing excess lipid to reach the ileum and accumulate in the villus epithelium as seen in the former study. The weanling mice showed no such accumulation, either because the digestive capacity of their pancreatic secretions was not maximally challenged by the administered lipid and their 5% fat diet or because of increased absorptive capacity in their proximal small intestine.
An additional factor that may contribute to the phenotype seen in the present experiments is the immaturity of the distal small intestine in neonates. Maturation of the small intestine occurs in a proximal-to-distal fashion and continues well into the neonatal period (13). Thus one would predict that the most severe effects would be seen in the ileum, with less-severe alterations detected in the jejunum and very little, if any, consequence in the duodenum.
Several investigators have developed neonatal animal models to study the causes of and to develop strategies for the treatment and prevention of NEC and other gastrointestinal diseases of neonates and premature infants. Panigrahi et al. (48) have isolated adherent strains of Escherichia coli from NEC patients and have shown their ability to cause disease in rabbits. Velasquez et al. (55, 56) used perfusion of intestinal loops from neonatal piglets to show the effect of dietary lipids on villus integrity after ischemia. Other evidence suggests a role for PAF in the etiology of NEC and that milk-derived PAF acetyl hydrolase can prevent destruction of the intestinal epithelium (5, 21, 22, 29). In these models, a period of hypoxia or ischemia-reperfusion is necessary to develop the symptoms seen in human NEC. A role for luminal contents in the disease process has also recently been implicated in this latter model (4). Specifically, animals with corn oil or formula in their intestinal lumen during the ischemia-reperfusion-PAF treatment suffered greater degrees of intestinal necrosis than animals with lactose or casein in their lumen.
The experiments described in this report are in agreement with the latter study. Interestingly, the effects observed in this report were most severe in the portion of the small intestine most vulnerable to NEC. Together these studies suggest an additional mechanism by which the intestinal epithelium may become sensitized to various inflammatory or infectious agents. This mechanism involves the accumulation of excess lipid in the epithelium of the distal small intestine because of insufficient lipolytic activity and/or absorptive capacity. We propose that the CEL in breast milk supplies an essential lipolytic activity for premature infants.
These studies represent the first report of a potential physiological consequence of the absence of CEL in neonatal animals. Use of the knockout mice will permit the time course of the observed lipid sensitivity to be determined. They can also be used to test whether lack of CEL results in increased susceptibility to intestinal pathogens. These mice will also be important in cross-breeding experiments with knockout mice for other lipase genes to determine the relative roles of the different pancreatic and preduodenal lipases in lipid digestion and absorption throughout development.
Acknowledgments
We thank Rital Angel, Frost Smith, and the histology core for technical assistance.
This work was supported in part by National Institutes of Health Grants HD-33926 and DK-40917.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Portions of this work have been previously published in abstract form (P. N. Howles, G. Stemmerman, C. Fenoglio-Preiser, and D.Y. Hui. Bile salt-stimulated lipase activity prevents intestinal damage in neonatal mice. FASEB J. 10: A190, 1996).
REFERENCES
- 1.Alemi B, Hamosh M, Scanlon JW, Salzman-Mann C, Hamosh P. Fat digestion in very low-birth-weight infants: effect of addition of human milk to low-birth-weight formula. Pediatrics. 1981;68:484–489. [PubMed] [Google Scholar]
- 2.Barlow B, Santulli TV, Heird WC, Pitt J, Blanc WA, Schullinger JN. An experimental study of acute neonatal enterocolitis—the importance of breast milk. J. Pediatr. Surg. 1974;9:587–595. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 3.Bennett-Clark S, Lawergren B, Martin J. Regional intestinal absorptive capacities for triolein: an alternative to markers. Am. J. Physiol. 1973;225:574–585. doi: 10.1152/ajplegacy.1973.225.3.574. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia AM, Feddersen RM, Musemeche CA. The role of luminal nutrients in intestinal injury from mesenteric reperfusion and platelet-activating factor in the developing rat. J. Surg. Res. 1996;63:152–156. doi: 10.1006/jsre.1996.0239. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia AM, Ramos CT, Scott SM, Musemeche CA. Developmental susceptibility to intestinal injury by platelet-activating factor in the newborn rat. J. Invest. Surg. 1996;9:351–358. doi: 10.3109/08941939609021276. [DOI] [PubMed] [Google Scholar]
- 6.Buescher ES. Host defense mechanisms of human milk and their relations to enteric infections and necrotizing enterocolitis. Clin. Perinatol. 1994;21:247–262. [PubMed] [Google Scholar]
- 7.Camulli ED, Linke MJ, Brockman HL, Hui DY. Identity of a cytosolic neutral cholesterol esterase in rat liver with the bile salt stimulated cholesterol esterase in pancreas. Biochim. Biophys. Acta. 1989;1005:177–182. doi: 10.1016/0005-2760(89)90184-7. [DOI] [PubMed] [Google Scholar]
- 8.Carson FL. Histotechnology: A Self-Instructional Text. ASCP; Chicago: 1990. [Google Scholar]
- 9.Carver JD, Barness LA. Trophic factors for the gastrointestinal tract. Clin. Perinatol. 1996;23:265–285. [PubMed] [Google Scholar]
- 10.Chen Q, Blackberg L, Nilsson A, Sternby B, Hernell O. Digestion of triacylglycerols containing long-chain polyenoic fatty acids in vitro by colipase-dependent pancreatic lipase and human milk bile salt-stimulated lipase. Biochim. Biophys. Acta. 1994;1210:239–243. doi: 10.1016/0005-2760(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 11.Clark DE, Commons TJ, Prozialeck DH, McKean ML, Adelman SJ. Inhibition of hypercholesterolemia by specific pancreatic cholesterol ester hydrolase inhibitors (Abstract) FASEB J. 1992;6:A1388. [Google Scholar]
- 12.Crissinger KD, Burney DL, Velasquez OR, Gonzalez E. An animal model of necrotizing enterocolitis induced by infant formula and ischemia in developing piglets. Gastroenterology. 1994;106:1215–1222. doi: 10.1016/0016-5085(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 13.Davenport HW. Physiology of the Digestive Tract. Year Book Medical; Chicago: 1982. Intestinal digestion and absorption of fat; pp. 211–225. [Google Scholar]
- 14.DiPersio LP, Fontaine RN, Hui DY. Identification of the active site serine in pancreatic cholesterol esterase by chemical modification and site-specific mutagenesis. J. Biol. Chem. 1990;265:16801–16806. [PubMed] [Google Scholar]
- 15.Ellis LA, Hamosh M. Bile salt stimulated lipase: comparative studies in ferret milk and lactating mammary gland. Lipids. 1992;27:917–922. doi: 10.1007/BF02535873. [DOI] [PubMed] [Google Scholar]
- 16.Fanaroff AA, Korones SB, Wright LL, Wright EC, Poland RL, Bauer CB, Tyson JE, Philips JB, III, Edwards W, Lucey JF, Catz CS, Shankaran S, Oh W. A controlled trial of intravenous immune globulin to reduce nosocomial infections in very-low-birth-weight infants. N. Engl. J. Med. 1994;330:1107–1113. doi: 10.1056/NEJM199404213301602. [DOI] [PubMed] [Google Scholar]
- 17.Foglia RP. Necrotizing enterocolitis. Curr. Probl. Surg. 1995;32:757–823. doi: 10.1016/s0011-3840(05)80014-0. [DOI] [PubMed] [Google Scholar]
- 18.Fredrikzon B, Hernell O, Blackberg L, Olivecrona T. Bile salt-stimulated lipase in human milk: evidence of activity in vivo and of a role in the digestion of milk retinol esters. Pediatr. Res. 1978;12:1048–1052. doi: 10.1203/00006450-197811000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Fredrikzon B, Olivecrona T. Decrease of lipase and esterase activities in intestinal contents of newborn infants during test meals. Pediatr. Res. 1978;12:631–634. doi: 10.1203/00006450-197805000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Freed LM, York CM, Hamosh M, Sturman JA, Hamosh P. Bile salt-stimulated lipase in non-primate milk: longitudinal variation and lipase characteristics in cat and dog milk. Biochim. Biophys. Acta. 1986;878:209–215. doi: 10.1016/0005-2760(86)90148-7. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa M, Lee EL, Johnston JM. Platelet-activating factor-induced ischemic bowel necrosis: the effect of platelet-activating factor acetylhydrolase. Pediatr. Res. 1993;34:237–241. doi: 10.1203/00006450-199308000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa M, Narahara H, Yasuda K, Johnston JM. Presence of platelet-activating factor-acetylhydrolase in milk. J. Lipid Res. 1993;34:1603–1609. [PubMed] [Google Scholar]
- 23.Goldman AS, Goldblum RM. Human milk: immunologic-nutritional relationships. Ann. NY Acad. Sci. 1990;587:236–245. doi: 10.1111/j.1749-6632.1990.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman AS, Goldblum RM, Hanson LA. Antiinflammatory systems in human milk. Adv. Exp. Med. Biol. 1990;262:69–76. doi: 10.1007/978-1-4613-0553-8_6. [DOI] [PubMed] [Google Scholar]
- 25.Grosfeld JL, Molinari F, Chaet M, Engum SA, West KW, Rescorla FJ, Scherer LR., III Gastrointestinal perforation and peritonitis in infants and children: experience with 179 cases over ten years. Surgery. 1996;120:650–656. doi: 10.1016/s0039-6060(96)80012-2. [DOI] [PubMed] [Google Scholar]
- 26.Hamosh M. Digestion in the premature infant: the effects of human milk. Semin. Perinatol. 1994;18:485–494. [PubMed] [Google Scholar]
- 27.Hernell O, Blackberg L. Digestion of human milk lipids: physiologic significance of sn-2 monoacylglycerol hydrolysis by bile salt-stimulated lipase. Pediatr. Res. 1982;16:882–885. doi: 10.1203/00006450-198210000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Howles PN, Carter CP, Hui DY. Dietary free and esterified cholesterol absorption in cholesterol esterase (bile salt-stimulated lipase) gene-targeted mice. J. Biol. Chem. 1996;271:7196–7202. doi: 10.1074/jbc.271.12.7196. [DOI] [PubMed] [Google Scholar]
- 29.Hsueh W, Caplan MS, Sun X, Tan X, MacKendrick W, Gonzalez-Crussi F. Platelet-activating factor, tumor necrosis factor, hypoxia and necrotizing enterocolitis. Acta Paediatr. Suppl. 1994;396:11–17. doi: 10.1111/j.1651-2227.1994.tb13234.x. [DOI] [PubMed] [Google Scholar]
- 30.Iverson SJ, Kirk CL, Hamosh M, Newsome J. Milk lipid digestion in the neonatal dog: the combined actions of gastric and bile salt stimulated lipases. Biochim. Biophys. Acta. 1991;1083:109–119. doi: 10.1016/0005-2760(91)90131-z. [DOI] [PubMed] [Google Scholar]
- 31.Kliegman RM, Walker WA, Yolken RH. Necrotizing enterocolitis: research agenda for a disease of unknown etiology and pathogenesis. Pediatr. Res. 1993;34:701–708. doi: 10.1203/00006450-199312000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Lebenthal E, Lee PC. Development of functional responses in human exocrine pancreas. Pediatrics. 1980;66:556–560. [PubMed] [Google Scholar]
- 33.Lebenthal E, Choi TS, Lee PC. The development of pancreatic function in premature infants after milk-based and soy-based formulas. Pediatr. Res. 1981;15:1240–1244. doi: 10.1203/00006450-198109000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Lee PC, Lebenthal E. Prenatal and postnatal development of the human exocrine pancreas. In: Liang V, Go W, editors. The Pancreas: Biology, Pathobiology, and Disease. Raven Press; New York: 1993. pp. 57–73. [Google Scholar]
- 35.Lidmer AS, Kannius M, Lundberg L, Bjursell G, Nilsson J. Molecular cloning and characterization of the mouse carboxyl ester lipase gene and evidence for expression in the lactating mammary gland. Genomics. 1995;29:115–122. doi: 10.1006/geno.1995.1221. [DOI] [PubMed] [Google Scholar]
- 36.Lowe ME, Kaplan MH, Jackson-Grusby L, D'Agostino D, Grusby MJ. Decreased neonatal dietary fat absorption and T-cell cytotoxicity in pancreatic lipase-related protein 2-deficient mice. J. Biol. Chem. 1998;273:31215–31221. doi: 10.1074/jbc.273.47.31215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowry ON, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 38.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 39.Mackay K, Lawn RM. Characterization of the mouse pancreatic/mammary gland cholesterol esterase-encoding cDNA and gene. Gene. 1995;165:255–259. doi: 10.1016/0378-1119(95)00564-m. [DOI] [PubMed] [Google Scholar]
- 40.MacKendrick W, Hill N, Hsueh W, Caplan M. Increase in plasma platelet-activating factor levels in enterally fed preterm infants. Biol. Neonate. 1993;64:89–95. doi: 10.1159/000243976. [DOI] [PubMed] [Google Scholar]
- 41.Maxton DG, Cynk EU, Jenkins AP, Thompson RPH. Effect of dietary fat on the small intestinal mucosa. Gut. 1989;30:1252–1255. doi: 10.1136/gut.30.9.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKean ML, Commons TJ, Berens MS, Hsu PL, Ackerman DM, Steiner KE, Adelman SJ. Effects of inhibitors of pancreatic cholesterol ester hydrolase (PCEH) on 14C-cholesterol absorption in animal models (Abstract) FASEB J. 1992;6:A1388. [Google Scholar]
- 43.Moya FR, Eguchi H, Zhao B, Furukawa M, Sfeir J, Osorio M, Ogawa Y, Johnston JM. Platelet-activating factor acetylhydrolase in term and preterm human milk: a preliminary report. J. Pediatr. Gastroenterol. Nutr. 1994;19:236–239. doi: 10.1097/00005176-199408000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Musemeche CA, Baker JL, Feddersen RM. A model of intestinal ischemia in the neonatal rat utilizing superior mesenteric artery occlusion and intraluminal platelet-activating factor. J. Surg. Res. 1995;58:724–727. doi: 10.1006/jsre.1995.1114. [DOI] [PubMed] [Google Scholar]
- 45.Musemeche CA, Pizzini RP, Andrassy RJ. Intestinal ischemia in the newborn: the role of intestinal maturation. J. Surg. Res. 1993;55:595–598. doi: 10.1006/jsre.1993.1190. [DOI] [PubMed] [Google Scholar]
- 46.Neu J, Walker WR, Engelhardt EL, Wu-Wang CY, Roa MB, Thomas MR, Gimotty PA. Alterations in piglet small intestine after cholesterol deprivation. Pediatr. Res. 1987;22:330–334. doi: 10.1203/00006450-198709000-00018. [DOI] [PubMed] [Google Scholar]
- 47.Olivecrona T, Hernell O. Human milk lipases and their possible role in fat digestion. Paediatr. Paedol. 1976;11:600–604. [PubMed] [Google Scholar]
- 48.Panigrahi P, Gupta S, Gewolb IH, Morris JG., Jr. Occurrence of necrotizing enterocolitis may be dependent on patterns of bacterial adherence and intestinal colonization: studies in Caco-2 tissue culture and weanling rabbit models. Pediatr. Res. 1994;36:115–121. doi: 10.1203/00006450-199407001-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowe MI, Reblock KK, Kurkchubasche AG, Healey PJ. Necrotizing enterocolitis in the extremely low birth weight infant. J. Pediatr. Surg. 1994;29:987–991. doi: 10.1016/0022-3468(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 50.Rudd EA, Brockman HL. Pancreatic carboxyl ester lipase (cholesterol esterase) In: Borgstrom B, Brockman HL, editors. Lipases. Elsevier Science; New York: 1984. pp. 185–204. [Google Scholar]
- 51.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Plainview, NY: 1989. [Google Scholar]
- 52.Stromqvist M, Tornell J, Edlund M, Johansson T, Lindgren K, Lundberg L, Hansson L. Recombinant human bile salt-stimulated lipase: an example of defective O-glycosylation of a recombinant protein produced in milk of transgenic mice. Transgenic Res. 1996;5:475–485. doi: 10.1007/BF01980213. [DOI] [PubMed] [Google Scholar]
- 53.Uceda JE, Laos CA, Kolni HW, Klein AM. Intestinal perforations in infants with a very low birth weight: a disease of increasing survival? J. Pediatr. Surg. 1995;30:1314–1316. doi: 10.1016/0022-3468(95)90493-x. [DOI] [PubMed] [Google Scholar]
- 54.Udall JN, Colony P, Fritze L, Pang K, Trier JS, Walker W. Development of gastrointestinal mucosal barrier. II. The effect of natural versus artificial feeding on intestinal permeability to macromolecules. Pediatr. Res. 1981;15:245–249. doi: 10.1203/00006450-198103000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Velasquez OR, Henninger K, Fowler M, Tso P, Crissinger K. Oleic acid-induced mucosal injury in developing piglet intestine. Am. J. Physiol. 1993;264:G576–G582. doi: 10.1152/ajpgi.1993.264.3.G576. (Gastrointest. Liver Physiol. 27) [DOI] [PubMed] [Google Scholar]
- 56.Velasquez OR, Place AR, Tso P, Crissinger KD. Developing intestine is injured during absorption of oleic acid but not its ethyl ester. J. Clin. Invest. 1994;93:479–485. doi: 10.1172/JCI116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Velasquez OR, Tso P, Crissinger KD. Fatty acid-induced injury in developing piglet intestine: effect of degree of saturation and carbon chain length. Pediatr. Res. 1993;33:543–547. doi: 10.1203/00006450-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Wang CS, Martindale ME, King MM, Tang J. Bile-salt-activated lipase: effect on kitten growth rate. Am. J. Clin. Nutr. 1989;49:457–463. doi: 10.1093/ajcn/49.3.457. [DOI] [PubMed] [Google Scholar]
- 59.Weaver LT, Laker MF, Nelson R, Lucas A. Milk feeding and changes in intestinal permeability and morphology in the newborn. J. Pediatr. Gastroenterol. Nutr. 1987;6:351–358. doi: 10.1097/00005176-198705000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Weaver LT, Landymore-Lim L, Lucas A. Neonatal gastrointestinal growth and function: are they regulated by composition of feeds? Biol. Neonate. 1991;59:336–345. doi: 10.1159/000243369. [DOI] [PubMed] [Google Scholar]
- 61.Weaver LT, Lucas A. Upper intestinal mucosal proliferation in the newborn guinea pig: effect of composition of milk feeds. Pediatr. Res. 1987;22:675–678. doi: 10.1203/00006450-198712000-00012. [DOI] [PubMed] [Google Scholar]
- 62.Wu A-L, Bennett-Clark S, Holt PR. Transmucosal triglyceride transport rates in proximal and distal rat intestine in vivo. J. Lipid Res. 1975;16:251–257. [PubMed] [Google Scholar]
- 63.Zoppi G, Andreotti G, Pajno-Ferrara F, Njai DM, Gaburro D. Exocrine pancreas function in premature and full term neonates. Pediatr. Res. 1972;6:880–886. doi: 10.1203/00006450-197212000-00005. [DOI] [PubMed] [Google Scholar]