Abstract
Retinal ganglion cell (RGC) counting is essential to evaluate retinal degeneration especially in glaucoma. Reliable RGC labeling is fundamental for evaluating the effects of any treatment. In rat, about 98% of RGCs is known to project to the contralateral superior colliculus (SC) (Forrester and Peters, 1967). Applying fluoro-gold (FG) on the surface of SC can label almost all the RGCs, so that we can focus on this most vulnerable retinal neuron in glaucoma. FG is taken up by the axon terminals of retinal ganglion cells and bilaterally transported retrogradely to its somas in the retina. Compare with retrograde labeling of RGC by putting FG at stump of transected optic nerve for 2 days, the interference of RGC survival is minimized. Compare with cresyl violet staining that stains RGCs, amacrine cells and endothelium of the blood vessel in the retinal ganglion cell layer, this labeling method is more specific to the RGC. This video describes the method of retrograde labeling of RGC by applying FG on the surface of SC. The surgical procedures include drilling the skull; aspirating the cortex to expose the SC and applying gelatin sponge over entire dorsal surface of SC are shown. Useful tips for avoiding massive intracranial bleeding and aspiration of the SC have been given.
Protocol
Anaesthetize the rat by intra-peritoneal injection of ketamine (80mg/kg) and xylazine (8mg/kg) (volume ratio at 2:1). Apply sterile eye lubricant ointment to prevent drying of the corneas during surgery.
Shave the head fur (from eye to ear level) and fix the head on the head stage by a head clamp and set its incisors in the incisor bar.
Disinfected the operation area with 10% povidone iodine solution followed by 70% alcohol.
Cut the skin and muscles to expose skull and sutures (sagittal, coronal and transverse sutures) (Figure 1). Apply analgesic, bupivicaine as a splash to incision area (not to exceed 2 mg/kg).
Drill a skull hole (ø 2x2 mm, 0.5 mm from both sagittal and transverse sutures) on each side of sagittal suture. Avoid rupture of meninges and the sinus lying beneath the sutures.
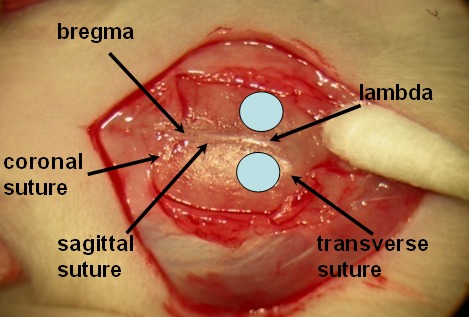 Figure 1. Hallmarks on alive rat
skull.
Figure 1. Hallmarks on alive rat
skull. Cut open the meninges by spring scissors, avoid tearing.
- Aspirate the cerebral content that lies over the dorsal surface of the superior colliculus carefully by the vacuum pump and three check-points can be observed:
- the color of brain tissue changes from grey to white to grey
- stop aspirating when the colorless cerebrospinal fluid come out
- carefully enlarge the lowest suction level horizontally to expose the yellowish superior colliculus covered by the dura
Aspirate the brain content over SC until four edges of SC can be observed directly under microscope. The portion of cerebral cortex removed should be kept to minimum to avoid undesirable side effects (e.g., motor dysfunction). Be careful not to touch the sinus.
Absorb the fluid in the hole with fine sterile cotton stripe to make the surface of the SC as dry as possible. Then, place a thin layer of gelatin sponge pre-soaked with 6% Fluoro-Gold on the surface of SC (FG is taken up by the axon terminals of retinal ganglion cells and bilaterally transported retrogradely to its somas in the retina).
Fill the hole with gelfoam and close the skin wound with suture clips.
The animals were allowed to recover under incandescent lamp to keep warm. Analgesic, buprenorphine (100mcg/kg), is applied orally in the drinking water for 5 days after SC labeling.
Discussion
Why use this surgical method for retinal ganglion cell count? In rat about 98% of retinal ganglion cells (RGC) are known to project to the contralateral superior colliculus (SC). Applying fluoro-gold on the surface of SC can label almost all of the RGCs, so that we can focus on this most vulnerable retinal neuron in glaucoma. Compare with retrograde labeling of RGC by putting FG at stump of transected optic nerve for 2 days, the interference of RGC survival is minimized. Compare with cresyl violet staining that stains the amacrine cell, RGC and endothelia cell of the blood vessel in the retinal ganglion cell layer, this method is more specific to the RGC.
How to avoid excessive bleeding? First, make sure the rat is properly anaesthetized by checking muscle tone and cornea reflex. Insufficient anesthesia will cause extensive bleeding. Second, avoid operations that will damage the sinus such as drilling too close to the suture or tearing of the meninges into the sinus wall. Third, be careful to keep the dura lying superficially on the SC intact especially at the nasal region.
How long does it take until the RGCs are labeled? Four days after the SC labeling, the RGC can be labeled. I normally wait for seven days for better results.
Acknowledgments
The authors would like to thank for the support from National Glaucoma Program of American Health Assistant Foundation.
References
- Ahmed AKMF, Dong K, Sugioka K, Yamadori T. Retrograde double labeling of retinal ganglion cells in the newborn and adult albino rat. Neurosci. Protocols. 1996;1:1–11. [Google Scholar]
- Chan HC, Chang RCC, Ip AKC, Chiu K, Yuen WH, Zee SY, So KF. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Experimental Neurology. 2007;203:269–273. doi: 10.1016/j.expneurol.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Forrester J, Peters A. Nerve fibers in the optic nerve of rat. Nature. 1967;214:245–247. doi: 10.1038/214245a0. [DOI] [PubMed] [Google Scholar]
- Ji JZ, Elyaman W, Yip HK, Lee VWH, Yick LW, Hugon J, So KF. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. European Journal of Neuroscience. 2004;19:265–272. doi: 10.1111/j.0953-816x.2003.03107.x. [DOI] [PubMed] [Google Scholar]
- Li RS, Chen BY, Tay DK, Chan HHL, Pu ML, So KF. Melanopsin-Expressing Retinal Ganglion Cells Are More Injury-Resistant in a Chronic Ocular Hypertension Model. Investigative. Ophthalmology & Visual Science. 2006;47:2951–2958. doi: 10.1167/iovs.05-1295. [DOI] [PubMed] [Google Scholar]


