Abstract
While production of reactive nitrogen and reactive oxygen species (RNS and ROS) is a component of innate defense against viral infection, their overproduction in the brain may also lead to deleterious consequences. To investigate potential immunopathologic roles of oxidative stress during herpes encephalitis, we examined the expression kinetics of inducible nitric oxide synthase (iNOS) as well as heme oxygenase-1 (HO-1), a marker of oxidative stress, and evaluated infection-induced oxidative brain damage. Results from these studies showed that both iNOS and HO-1 gene expression were highly elevated in the brain within 7 d post-infection (p.i.) and remained elevated through 21 d p.i. Real-time bioluminescence imaging of HO-1 promoter-luciferase transgenic mice confirmed HO-1 promoter activity in the brains of HSV-1-infected animals within 3 d p.i., which peaked between 5 and 7 d p.i. Immunohistochemical staining for both 3-nitrotyrosine and 8-hydroxydeoxyguanosine (8-OH-dG), as well as quantitative assessment of 8-isoprostane levels, demonstrated the presence of viral infection-induced oxidative brain damage. In addition, when brain leukocytes obtained from animals with experimental herpes encephalitis were sorted using FACS and the individual cell populations analyzed, CD45(int)/CD11b(+) resident microglia were found to be the major cellular source of iNOS expression.
Keywords: microglia, oxidative stress, iNOS, HO-1, HSV
Introduction
Previous studies from our laboratory have shown that vigorous pro-inflammatory immune responses occur in the brains of HSV-infected BALB/c mice (Marques et al, 2006). The kinetics of these neuroimmune responses, taken together with the kinetics of mortality, suggest that early events in the course of infection play a pivotal role in the progression of experimental herpes encephalitis. We have also previously identified microglial cells as key players in orchestrating these neuroimmune responses (Marques et al, 2004). Microglia are a potent cellular source of pro-inflammatory cytokines, chemokines, and oxidative enzymes in the brain. The production of these innate immune mediators implies both protective as well as potentially detrimental roles during viral brain infection.
Regarded as a critical component of the innate immune response, nitric oxide (NO) has been shown to mediate an array of physiological functions. It is produced endogenously in various cell types by a one of three nitric oxide synthases (neuronal NOS, endothelial NOS, and iNOS). Neurons, endothelial cells, and macrophages are the best characterized producers of NO (Croen, 1993). NO is known to modulate neuronal function, regulate vasomotor tone, and more pertinent to this study, mediate antimicrobial activity, inflammatory responses, and cytotoxicity (Bredt and Snyder, 1992; Garthwaite, 1991; Moncada et al, 1991; Nathan and Hibbs, 1991).
The role of NO during the course of viral brain infection is still unclear. While NO has been shown to inhibit viral replication, overproduction of this reactive species has also been shown to induce cellular apoptosis and promote tissue damage. Evidence suggests that localized production of NO early during HSV infection, may be responsible for decreased pathogenesis (Croen, 1993; Gamba et al, 2004; MacLean et al, 1998). In contrast, several studies also suggest a pathological role for NO during acute HSV infections. In two different models of HSV-induced disease, it has been reported that treatment of infected animals with the non-selective NOS inhibitor L-NMMA resulted in significantly improved survival rates, despite no decrease in viral titers (Adler et al, 1997; Fujii et al, 1999). Further studies indicate that NO induces proinflammatory responses by stimulating vasodilatation and changes in vascular permeability, which modify leukocyte adhesion and thereby alter immune cell trafficking (Adler et al, 1997; Martin et al, 1997). Based on such findings, it is likely that brain damage resulting from HSV infection may be partially attributed to host-mediated defense mechanisms, specifically the release of reactive species.
In light of conflicting evidence reported regarding the role of NO during HSV brain infection, it appears that the reported discrepancies may be mediated by alterations in the physiological balance necessary to keep this immune defense mediator from producing deleterious consequences. The immune system has developed mechanisms which regulate the over-production of NO. The most impressive negative feedback regulator for NO production is the antioxidant enzyme heme oxygenase (HO)-1 (Turcanu et al, 1998a; Turcanu et al, 1998b). Under inflammatory conditions, HO-1 is the rate-limiting enzyme in the degradation of cellular heme, producing equimolar amounts of iron, carbon monoxide (CO), and biliverdin. This cytoprotective anti-inflammatory stress response gene is well known as a marker for oxidative stress and is induced in most cell types by various stimuli such as proinflammatory cytokines, hypoxia, heavy metals, and, most notably, by RNS and ROS. Of particular relevance to this study, HO-1 is induced by NO and this induction is also known to inhibit subsequent NO production. The induction of HO-1 mRNA has previously been used as a marker of oxidative stress during HSV-1-induced encephalitis in rat brains following intranasal infection (Fujii et al, 1999). Taken together with mounting evidence that shows HO-1 is a potent mediator of both anti-inflammatory and anti-apoptotic effects, it is plausible that this enzyme plays an important role in protecting NO-secreting cells and the surrounding tissue from oxidative damage (Baranano and Snyder, 2001; Chora et al, 2007; Sato et al, 2001; Turcanu et al, 1998b). Given the intimate relationship between NO and HO-1, we set out to investigate the expression of these mediators in murine brains during experimental herpes encephalitis.
Results
iNOS mRNA induction during herpes encephalitis
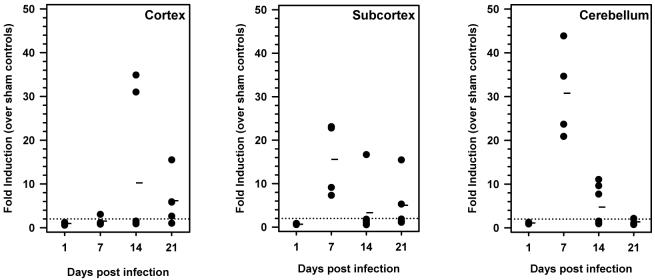
We first investigated the kinetics of iNOS mRNA induction in the brains of animals with herpes encephalitis. In these experiments, susceptible BALB/c mice were infected via the intranasal (i.n.) route and the infected brains were collected at 1, 7, 14, and 21 d p.i. Upon harvesting, the brains were divided into three regions: cortex, subcortex, and cerebellum. Total RNA was extracted from each brain region, DNAse-treated, and reverse transcribed. The cDNA obtained was analyzed by real-time PCR using iNOS-specific primers (Figure 1). RNA levels were normalized to hypoxanthine guanine phosphoribosyl transferase-1 (HPRT) and fold induction was calculated by comparison to uninfected controls. Data obtained from these experiments indicate that iNOS mRNA expression was elevated in the subcortex and cerebellum of HSV-infected mice by 7 d p.i. and remained elevated at 21 and 14 d p.i.. Interestingly, while iNOS levels in the cortex peaked at a later time point (i.e., 14 d p.i.), increased expression was still detected at 21 d p.i. in this region.
Figure 1.
Kinetics of iNOS mRNA induction in the brains of animals with herpes encephalitis. BALB/c mice were intranasally infected with 2.5 × 105 PFU HSV-1 17syn+ and brain tissue was harvested at 1, 7, 14, and 21 d p.i. The cortex, subcortex, and cerebellum were separately collected and total RNA was isolated and reverse-transcribed. Quantitative real-time PCR was performed using primers specific for iNOS. Sample variation was controlled by normalization to hypoxanthine phosphoribosyltransferase-1 (HPRT-1) and fold induction was calculated by comparison to uninfected controls. Three to five mice were sacrificed for each time point and each closed circle represents one animal. Dashes represent the mean per group at each time point.
Kinetics of HO-1 mRNA induction
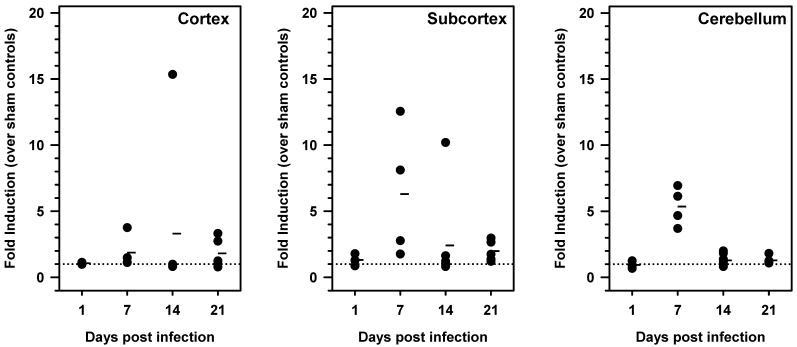
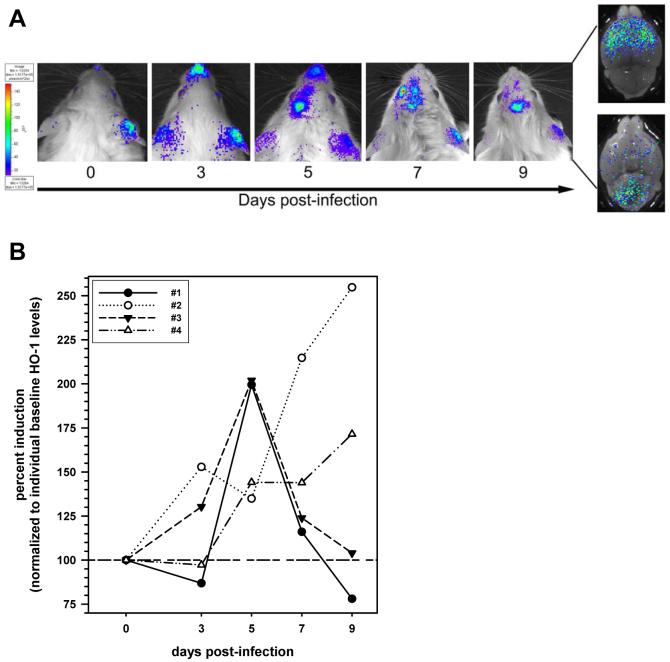
Because HO-1 induction is a widely used indicator of oxidative stress, we next investigated the kinetics of HO-1 mRNA expression throughout the course of viral brain infection. HO-1 mRNA expression levels were quantified at 1, 7, 14, and 21 d p.i. For these experiments, cortex, subcortex, and cerebellum were harvested and total RNA was isolated and reverse-transcribed. cDNA obtained was analyzed by real-time PCR using primers specific for HO-1 (Figure 2). Transcript levels were normalized to HPRT and data are presented as fold induction over uninfected controls. Results obtained during these experiments show that, similar to the kinetics of iNOS expression, HO-1 mRNA levels were highly elevated in the subcortex and cerebellum of mice as early as 7 d p.i. Levels in the cortex were also highest at d 14 p.i. To further confirm that the HO-1 gene was induced in the HSV-infected brain, bioluminescence imaging studies were performed using HSV-infected HO-1 promoter-luciferase transgenic mice. For these experiments, the transgenic mice were infected via the i.n. route and subsequently imaged at 3, 5, 7, and 9 d p.i. (Figure 3A and B). Baseline levels of expression were also determined prior to infection. Data obtained from these experiments show that viral infection stimulated HO-1 promoter activity in the brains of infected animals, between 3 and 9 d p.i.
Figure 2.
Kinetics of HO-1 mRNA induction in the brains of animals with herpes encephalitis. BALB/c mice were intranasally infected and brain tissue was harvested at 1, 7, 14, and 21 d p.i. The cortex, subcortex, and cerebellum were separately collected and total RNA was isolated and reverse-transcribed. RT-qPCR was performed using primers specific for HO-1. Sample variation was controlled by normalization to HPRT-1 and fold induction was calculated by comparison to sham-infected controls. Three to five mice were sacrificed for each time point and each closed circle represents one animal. Dashes represent the mean per group at each time point.
Figure 3.
Bioluminescence imaging of HO-1 induction during herpes encephalitis. HO-1 promoter-luciferase expressing transgenic mice were infected via the i.n. route with 5 × 105 PFU of HSV strain 17syn+. For bioimaging, the infected animals were injected intraperitoneally with D-luciferin (a bioluminescent substrate for luciferase) and live images were acquired on a Xenogen IVIS® Imaging System (Alameda, CA) 5-10 min after the luciferin injection. (A) Time-course of HO-1 promoter activity in the HSV-infected murine brain: images of a representative animal are shown. Right panel insert represents direct imaging of (top) dorsal and (bottom) ventral sides of brain extracted from the skull (d 9). (B) Signal intensity of the HO-1 promoter-luciferase expression in the brain was quantified as photons/sec/cm2 at each indicated time-point and expressed as percent induction normalized to each individual animal’s (n=4) baseline level (100%).
Oxidative damage in the HSV-infected brain
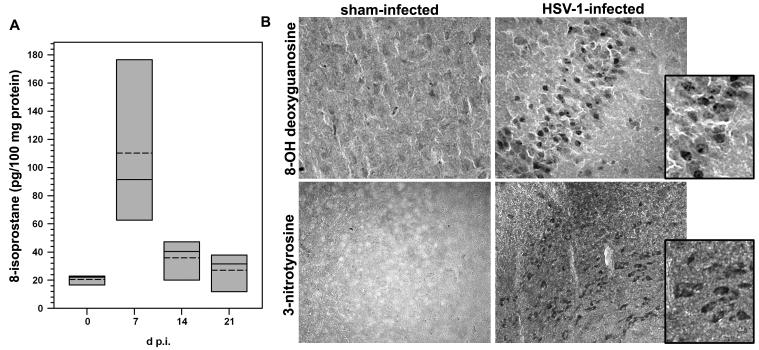
To investigate the implications of oxidative stress during viral encephalitis, levels of 8-isoprostane in infected brains were quantified using ELISA (Cayman Chemical, Ann Arbor, MI). Isoprostanes are produced by the random oxidation of tissue phospholipids through free radical-catalyzed peroxidation of arachidonic acid. 8-Isoprostane, in particular, appears in tissue that has undergone oxidative degradation and is used as a marker of antioxidant deficiency and oxidative stress (Morrow et al, 1995; Morrow et al, 1990; Reilly et al, 1997). Brains were removed from HSV-infected animals (n=3-4 mice/group) at 0, 7, 14, and 21 d p.i. and the tissue was processed for isolation of 8-isoprostanes. This analysis revealed significantly higher 8-isoprostane levels in the brains of HSV-infected mice at 7 d p.i. as compared to sham-infected controls; P < 0.05, Student’s t-test (Figure 4A). HSV-induced oxidative brain damage was further confirmed using immunohistochemical staining for the presence of 8-hydroxydeoxyguanosine (8-OH-dG) and 3-nitrotyrosine. 8-OH-dG detection is a sensitive and widely used biomarker of in vivo oxidative damage to cellular DNA because its formation reflects a guanosine modification that occurs due to attack by hydroxyl radicals that are formed as byproducts of oxidative stress. Likewise, production of highly reactive nitrogen species derived from NO, such as peroxynitrite, leads to the nitration of tyrosine residues in tissue proteins. Therefore, nitrotyrosine formation indicates the presence of RNS as well as cellular damage. For these experiments, thinly sliced sections cut from frozen brain tissue obtained from HSV-infected murine brains (6 d p.i.), along with sham-infected controls, were probed with either goat-anti-8-OH-dG antiserum or anti-3-nitrotyrosine antibodies. Results from these experiments demonstrate infection-induced oxidative brain, as positive staining for both 8-OH-dG and nitrotyrosine were observed (Figure 4B).
Figure 4.
Infection-induced oxidative brain damage. (A) Quantitative assessment of lipid peroxidation in the brains of animals with herpes encephalitis using ELISA for 8-isoprostanes. Brains were removed from animals infected with HSV via the intranasal route (n= 3-4 mice/group) at 0, 7, 14, and 21 d p.i. and prepared for analysis according to the protocol provided by the manufacturer (8-Isoprostane Affinity Purification Kit, Cayman Chemicals). 8-isoprostane levels were subsequently assessed using an ELISA (8-Isoprostane EIA Kit, Cayman) and the data are presented as box plots showing the mean (dashed line), median (solid line), and range (shaded box) expressed as pg/100 mg protein. Significantly higher levels of 8-isoprostane were found in the brains of HSV-infected mice at 7 d p.i. as compared to sham-infected controls; P < 0.05, Student’s t-test. (B) Immunohistochemical staining for the products of oxidative brain damage during herpes encephalitis. Twenty micron sections were cut from frozen brain tissues of mice collected 6 d following infection with HSV via direct intracerebral inoculation. Oxidative damage to DNA was probed using goat-anti-8-OH-dG antiserum (Chemicon) in both sham-infected and HSV-infected animals. Tissues were pretreated with 10 μg/mL of proteinase K for 40 min at 37°C. Brain sections from sham-infected and HSV-infected mice were also stained with anti-3-nitrotyrosine Abs demonstrating NO-induced protein damage. For these experiments, sections of brain tissue were pretreated using heat-induced epitope retrieval. Incubation with a rabbit polyclonal anti-nitrotyrosine Ab (Chemicon) was overnight, and the reaction was developed using DAB.
CD45(int)/CD11b(+) resident microglia are the cellular source of iNOS mRNA
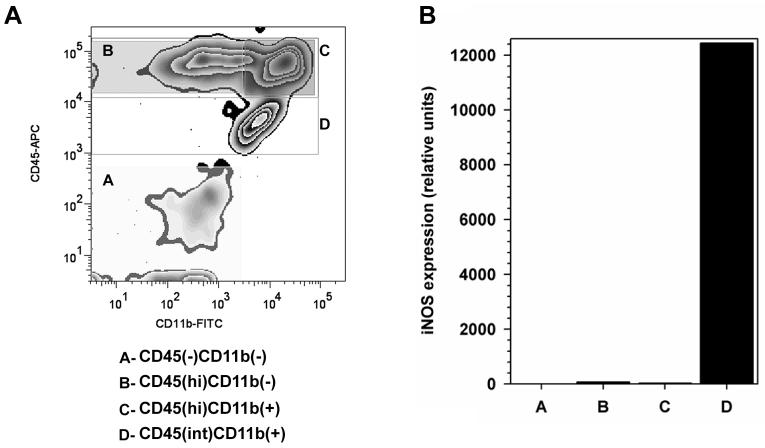
To identify the cellular source of iNOS, and thereby the cell type mediating oxidative injury in the virus-infected brain, leukocytes were pooled from 5 HSV-infected murine brains (7 d p.i.), isolated by centrifugation on a Percoll cushion, and sorted into four distinct populations (Zhang et al) using FACS, with anti-mouse CD45 and CD11b Abs (Figure 5A). These sorted populations were then individually assayed for iNOS mRNA expression by real time RT-PCR, as described for infected brain tissue. Results from these experiments indicated that the CD45int/CD11b+ microglia were the major cellular source of iNOS during experimental murine herpes encephalitis (Figure 5B).
Figure 5.
Expression of iNOS mRNA by CD45(int)/CD11b(+) resident microglial cells. (A) Single cell suspensions of brain tissue obtained from HSV-infected mice (n=5 animals; 7 d p.i.) were banded on a 70% Percoll cushion. Brain leukocytes at the 30-70% Percoll interface were collected, labeled with mouse Abs specific for CD45 and CD11b, and sorted into four populations (A through D) using FACS. (B) Total RNA extracted from each of the separated populations (A through D) was analyzed by real-time RT-PCR for expression of iNOS. Relative transcript levels in cells isolated from brain tissues of five animals normalized to HPRT expression are presented.
Discussion
Previously published studies have shown that both acute and latent HSV-1 brain infections are associated with oxidative damage. During the acute phase, HSV-infected mice developed encephalitic symptoms associated with severe inflammation, viral protein expression, and significantly elevated levels of F4-neuroprostanes and F2-isoprostanes, which are sensitive in vivo biomarkers of oxidant injury, in the brain (Milatovic et al, 2002). Additional studies investigating oxidative brain damage during the latent phase of herpes virus infection have demonstrated the presence of 8-hydroxyguanosine immunoreactivity, indicative of hydroxyl radical attack on RNA or DNA, a result which suggests that inflammatory mediators could be responsible for damage found in the absence of HSV proteins (Valyi-Nagy et al, 2000). Recently, it has been shown in vitro that HSV-infection increases reactive oxygen species in neural cell cultures as early as 1 h p.i. and these levels remain elevated at 24 h p.i. (Kavouras et al, 2007). Results reported in this paper show that microglial cells are the major source of iNOS produced in the brain during herpes encephalitis. While there is little doubt oxidative mediators are produced to protect the brain and control viral infection, in vivo evidence presented here suggests an additional consequence of this innate neuroimmune response: infection-induced RNS formation and oxidative damage within the virus-infected brain. The presence of oxidative mediators in the HSV-infected brain was further corroborated by the detection of HO-1 mRNA, as HO-1 expression is known to be a sensitive marker of oxidative stress (Chen and Regan, 2007).
Comparisons between the kinetics of HSV-induced neuroimmune responses and those of mortality in our murine encephalitis model are especially intriguing. In this model, experimentally infected mice typically succumb to infection between 7 and 10 d p.i. We have previously described a robust microglia-driven cascade of pro-inflammatory neuroimmune responses which peaks at this time (Marques et al, 2006). While the effect of oxidative stress in the brain on the survival of the host cannot be assessed from the present study, this damage was observed at the same time HSV infection-induced mortality began. In any event, the results of this study further emphasize the important role of microglial cells during the progression of herpes encephalitis.
While microglial activation is necessary for host defense, the activated state of this cell type has also been linked to neurotoxicity, neurodegeneration, and chronic neuroinflammation in several disorders including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and HIV-associated dementia (Reynolds et al, 2007a). In the case of HSV-brain infection, microglia are not only a source of pro-inflammatory cytokines and chemokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and CXCL10 (Lokensgard et al, 2001; Marques et al, 2006), but, as we describe here, also a major source of NO. Recent studies investigating interactions between NO and cytokines in the murine brain demonstrate that NO together with TNF-α is a critical combination that leads to intense neurodegeneration and demyelination in vivo (Blais and Rivest, 2004). In support of this notion, results from another study investigating the effects of macrophage-depletion on the pathogenesis of HSV hepatitis suggest that overproduction of free radicals in combination with cytokines, such as TNF-α, IL-6, and IFN-α may result in hepatic cell apoptosis (Irie and Shiga, 2005). The connection between HSV infection and oxidative stress is especially interesting since it has been recently reported that infection increases ROS in cultured neuronal cells and that this induction is actually required for efficient viral replication (Kavouras et al, 2007). The authors suggest that lipid-peroxidation by-products generated in infected neuronal cells may be important regulators of HSV pathogenesis in the brain. Such collateral evidence indicates that in addition to suppressing aberrant pro-inflammatory cytokine and chemokine production, along with immune cell infiltration; regulating oxidative stress in the infected brain may be important in controlling the severity and long-term sequelae following herpes encephalitis. Interestingly, a recent report regarding the protective effects of regulatory T-cells (Tregs) on toxicity-induced neuronal loss, in an experimental murine model of Parkinson’s disease, showed that Tregs mediated neuroprotection through suppression of microglial oxidative stress and inflammation (Reynolds et al, 2007b). Another study investigating the effects of glucorticoid treatment on progression of herpes encephalitis in mice revealed that the timing of treatment is critical to survival. These experiments revealed that anti-inflammatory treatment concomitant with viral infection exacerbated the disease, while the same treatment began at 3 d p.i. significantly improved survival and was associated with decreased neurodegeneration (Sergerie et al, 2007). The authors suggested that by restraining inflammation and limiting microglial activation, glucocorticoids could decrease cerebral damage and increase survival, thereby protecting the host from fatal disease. Potential benefits of anti-inflammatory therapy in herpes encephalitis treatment are further supported by a recent clinical case report describing remarkable improvement as a result of high-dose steroid therapy in a case of severely progressive herpes encephalitis, despite appropriate course of antiviral medication and decreased viral load (Musallam et al, 2007).
It is also interesting to consider how modulating the production of HO-1 may benefit the CNS. It has previously been reported that upregulation of HO-1 mRNA paralleled the time profile of viral replication and iNOS expression in HSV-1-infected rat brains (Fujii et al, 1999). In addition to being well known as a marker for oxidative stress, HO-1 is also regarded as a potent cytoprotective, anti-inflammatory stress response gene. Mounting evidence suggests that HO-1 induction protects both vascular cells and astrocytes from oxidative injury (Chen-Roetling et al, 2005; Ono et al, 2002; Regan et al, 2000). HO-1 has been shown to mediate its anti-inflammatory effects by targeting p38α mitogen-activated protein kinase (MAPK) for degradation (Silva et al, 2006). This is particularly relevant to viral-microglial interactions, as we have previously shown that HSV-induced cytokine and chemokine production from human microglial cells is dependent on the p38 MAPK pathway (Lokensgard et al, 2001). The full range of anti-inflammatory effects of HO-1 in the brain remains to be determined, and these studies may elucidate new targets for therapeutic treatment of diseases induced by activated microglia.
Materials and Methods
Virus and infection
HSV-1 strain 17syn+ was propagated and titrated using plaque assay on rabbit skin fibroblasts (CCL68; American Type Culture Collection, Manassas, VA). Eight to 10-week-old BALB/c female mice (Charles River Laboratories, Boston, MA) were anesthetized and infected via the i.n. route with 1.25 × 105 HSV-1 PFU/nostril (2.5 × 105 PFU/mouse). Experiments assessing oxidative damage by immunochemical staining used 8-10 week BALB/c female mice that were anesthetized and given an intracerebral (i.c.) injection of 1 × 104 PFU/mouse of sucrose-purified HSV-1 17 syn+ into the left cerebral hemisphere. Control animals were inoculated with vehicle solution (saline). Bioluminescent imaging experiments were performed with i.n. infected (5 × 105 PFU/mouse) FVB HO-1-luc Xen mice (Taconic Farms, Germantown, NY). This light producing animal model carries a mouse HO-1-luciferase fusion containing a 15 kb mouse HO-1 promoter and modified firefly luciferase cDNA (Promega pGL-3), (Zhang et al, 2002; Zhang et al, 1999; Zhang et al, 2001).
Isolation of brain leukocytes and sorting using FACS
Leukocytes were isolated from HSV-infected murine brains using a previously described procedure with minor modifications (Ford et al, 1995; Marten et al, 2003). Briefly, brain tissues harvested from 4-6 animals were minced finely in RPMI (2 g/L D-glucose and 10mM HEPES) and digested in 0.25% trypsin (Ca/Mg free HBSS) at room temperature for 20 min. The single cell suspension was resuspended in 30% Percoll and banded on a 70% Percoll cushion at 900 xg at 15°C. For sorting, brain leukocytes obtained from the 30-70% Percoll interface were stained with anti-mouse CD45-Allophycocyanin (APC) (eBioscience, San Diego, CA) and anti-mouse CD11b-FITC for 45 min at 4°C. Non-overlapping populations were separated using a fluorescence-activated cell sorter (FACS; BD FACSAria, BD Biosciences). Total RNA, isolated from the sorted cell populations, was analyzed by real-time RT-PCR for iNOS expression.
Assessment of iNOS and HO-1 mRNA expression
Upon sacrifice, murine brains were dissected into three regions: cortex subcortex, and cerebellum to elucidate any regional differences in mRNA expression of oxidative and anti-oxidative enzyme mRNA. Total RNA was extracted from brain tissue homogenates using the TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. cDNA was synthesized using 1-5 μg of total RNA, SuperScript II reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA) and oligo dT6-12 primers (Sigma-Genosys, The Woodlands, TX). Quantitative real-time PCR was performed using the FullVelocity SYBR Green QPCR master mix (Stratagene, La Jolla, CA) following the manufacturer’s specifications. The 25 μl final reaction volume consisted of pre-made reaction mix (SYBR Green I dye, reaction buffer, Taq DNA polymerase, and dNTPs), 0.3 mM of each primer, and 0.5 ng cDNA in water. Reaction conditions for PCR for the Mx3000P QPCR System (Stratagene) were as follows: polymerase activation at 95° C for 5 min, 40 denaturation cycles of 95° C for 10 s and annealing/elongation at 60° C for 30 s. Primers used in the amplification of iNOS and HO-1 were designed from the murine genes (GenBank accession nos. NM_010927, 5′-TGGCCACCTTGTTCAGCTACG-3′ and 5′-GCCAAGGCCAAACACAGCATA-3′, 211 bp product and NM_010442, 5′-CACGCATATACCCGCTACCT-3′ and 5′-CCAGAGTGTTCATTCGAGCA-3′, 176 bp product, respectively). Primers recognizing the housekeeping gene, hypoxanthine guanine phosphoribosyl transferase-1 (HPRT) were designed from the murine HPRT mRNA sequence (GenBank accession no. NM_013556, 5′-TGCTCGAGATGTCATGAAGG-3′ and 5′-AATCCAGCAGGTCAGCAAAG-3′, 294 bp product). A melting curve analysis was performed to assess primer specificity and product quality by denaturation at 95°C, annealing at 55°C and melting at a rate of 0.1°C/sec to 95°C. The relative levels of PCR product were quantified using the 2(-Delta Delta CT) method (Livak and Schmittgen, 2001).
Quantitative assessment of lipid peroxidation
Brains from sham-or HSV-infected mice were quickly harvested, weighed and homogenized in 1-ml of ice-cold 0.1M phosphate buffer (pH 7.4) containing 1 mM EDTA and 10 μM indomethacin for 30 sec. An equal volume of 15% KOH was added to the homogenized mixture and incubated at 40 °C for 60 min, followed by the addition of 2 ml 100% ethanol containing 0.01% BHT. After vortexing, the mixture was incubated for 5 min at 4 °C and centrifuged at 1,500 rpm for 10 min (4 °C). The supernatants were then diluted with 2 ml 0.1 M phosphate buffer containing 0.5 M NaCl and 0.05% sodium azide. Each sample was purified by passage through an 8-isoprostane affinity sorbent column (Cayman, Ann Arbor, MI). The purified samples were then subjected to vacuum centrifugation until lyophilized (about 4h). The lyophilized samples were reconstituted with enzyme immunoassay buffer (50 μl) and reaction buffer (50 μl containing 8-isoprostane antiserum and tracer), and added to 96-well plate for 8-isoprostane assay (Cayman). The plate was incubated at RT for 18h followed by washing (5x, 3 min each) with washing buffer. One hundred μl of Ellman’s reagent was added and the plate was shaken in the dark for 90 to 120 min. The plate was read at 405 nm using a microplate reader (Molecular Devices, Sunnyvale, CA) and the corrected sample and standard optical density was calculated according to the manufacturer’s protocol. The final total 8-isoprostane concentrations were normalized to each sample’s total protein concentration and expressed as pg/mg protein.
Immunohistochemical staining for oxidative brain damage
Immunohistochemical staining was performed using a previously described protocol (Lokens gard et al, 1999; Marques et al, 2006). Briefly, sham- or HSV-infected mice were sacrificed and perfused with 2% sodium nitrate and phosphate buffered saline (PBS), followed by 4% paraformaldehyde. Murine brains were harvested, submerged in 4% paraformaldehyde for 24h, and transferred to a 30% sucrose solution for 2 d. Infected brains were then cryosectioned (20 μm) and stained for 1) oxidative damage to DNA using goat anti-8-hydroxyoxyguanosine (8-OH-dG) antiserum (Chemicon-Millipore, Billerica, MA); and 2) cellular damage induced by reactive nitrogen species using rabbit anti-nitrotyrosine antibody (Chemicon-Millipore). Murine brain sections were either pre-treated with 10 μg/ml proteinase K (Invitrogen, Carlsbad, CA) for 40 min at 37 °C (for 8-OH-dG staining) or subjected to heat-induced epitope retrieval (for nitrotyrosine staining) according to manufacturer’s protocol. After washing (3x, 5 min each) with TBS, slides were incubated in blocking solution (TBS containing 1% BSA, 0.05% triton X-100, and 10% serum: normal rabbit serum (for 8-OH-dG) or goat serum (for nitrotyrosine) for 60 min. After washing, the slides were incubated overnight at 4 °C with each primary Ab diluted in blocking solution (anti-8-OH-dG serum, 1:200; anti-nitrotyrosine, 1:500). After washing (4x, 5 min each), slides were incubated in biontinylated secondary antibodies (for 8-OH-dG: rabbit anti-goat antibody, 1:200; for nitrotyrosine: goat anti-rabbit antibody, 1:200) for 60 min. After washing (4x, 5 min each), the slides were incubated for 60 min in Vectastain ABC reagents diluted according to the manufacturer’s protocol. After washing (4x, 5 min each), the bound complex was visualized by incubating the slides in 0.05% 3,3′-diaminobenzidine (DAB) and 0.03% hydrogen peroxide for 5-10 min at room temperature (RT). Color development was terminated by washing and slides were visualized and analyzed using light microscopy.
Bioluminescence imaging
HO-1 promoter activity was assessed in the brains of i.n. infected (5 × 105 PFU) HO-1-luciferase transgenic mice (Malstrom et al, 2004; Zhang et al, 2002; Zhang et al, 1999; Zhang et al, 2001). Imaging of firefly luciferase in mice was performed on a charge-coupled device camera (Xenogen Corp., Alameda, Calif.) as previously described with minor modifications (Luker et al, 2003). Briefly, 150 μg (100 μl) of D-luciferin (a bioluminescent substrate for luciferase; Gold Biotechnology, St. Louis, MO) was administered to anesthetized mice by intraperitoneal injection. The anesthetic cocktail was a combination of Ketamine (100mg/ml) and Xylazine (16mg/ml); 50μl/mouse was injected i.p. and the anesthetic effects lasted approximately 20 min. Imaging began 5 min after administration of D-luciferin. Images were acquired for 5 min. Signal intensity of luciferase expression, measured by the amount of transmitted light, was represented as a pseudocolor image ranging from violet (least intense) to red (most intense). Corresponding gray-scale photographs and color luciferase images were superimposed and photon levels were quantified as photons/sec/cm2 from the cranial region of the mouse with LivingImage (Xenogen) and Igor (Wavemetrics, Lake Oswego, Oreg.) image analysis software. Data are reported as individual values from 4 mice at different time points p.i. as described in the figure legends. No animals died as a result of imaging or chemical treatment.
Acknowledgements
The authors thank Paul Marker, University of Minnesota Stem Cell Institute, for assistance in sorting brain leukocyte populations. This work was supported by NIH grant MH-066703 and a University of Minnesota Graduate School Doctoral Dissertation Fellowship.
References
- Adler H, Beland JL, Del-Pan NC, Kobzik L, Brewer JP, Martin TR, Rimm IJ. Suppression of herpes simplex virus type 1 (HSV-1)-induced pneumonia in mice by inhibition of inducible nitric oxide synthase (iNOS, NOS2) J Exp Med. 1997;185:1533–40. doi: 10.1084/jem.185.9.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranano DE, Snyder SH. Neural roles for heme oxygenase: contrasts to nitric oxide synthase. Proc Natl Acad Sci U S A. 2001;98:10996–1002. doi: 10.1073/pnas.191351298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais V, Rivest S. Effects of TNF-alpha and IFN-gamma on nitric oxide-induced neurotoxicity in the mouse brain. J Immunol. 2004;172:7043–52. doi: 10.4049/jimmunol.172.11.7043. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Cultured astrocytes from heme oxygenase-1 knockout mice are more vulnerable to heme-mediated oxidative injury. J Neurosci Res. 2005;82:802–10. doi: 10.1002/jnr.20681. [DOI] [PubMed] [Google Scholar]
- Chen M, Regan RF. Time course of increased heme oxygenase activity and expression after experimental intracerebral hemorrhage: correlation with oxidative injury. J Neurochem. 2007;103:2015–21. doi: 10.1111/j.1471-4159.2007.04885.x. [DOI] [PubMed] [Google Scholar]
- Chora AA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L, Soares MP. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest. 2007;117:438–47. doi: 10.1172/JCI28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen KD. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Invest. 1993;91:2446–52. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–21. [PubMed] [Google Scholar]
- Fujii S, Akaike T, Maeda H. Role of nitric oxide in pathogenesis of herpes simplex virus encephalitis in rats. Virology. 1999;256:203–12. doi: 10.1006/viro.1999.9610. [DOI] [PubMed] [Google Scholar]
- Gamba G, Cavalieri H, Courreges MC, Massouh EJ, Benencia F. Early inhibition of nitric oxide production increases HSV-1 intranasal infection. J Med Virol. 2004;73:313–22. doi: 10.1002/jmv.20093. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991;14:60–7. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Irie H, Shiga J. Pathogenesis of herpes simplex hepatitis in macrophage-depleted mice: possible involvement of tumor necrosis factor-alpha and inducible nitric oxide synthase in massive apoptosis. Anat Sci Int. 2005;80:199–211. doi: 10.1111/j.1447-073X.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- Kavouras JH, Prandovszky E, Valyi-Nagy K, Kovacs SK, Tiwari V, Kovacs M, Shukla D, Valyi-Nagy T. Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. J Neurovirol. 2007;13:416–25. doi: 10.1080/13550280701460573. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lokensgard JR, Hu S, Sheng W, vanOijen M, Cox D, Cheeran MC, Peterson PK. Robust expression of TNF-alpha, IL-1beta, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J Neurovirol. 2001;7:208–19. doi: 10.1080/13550280152403254. [DOI] [PubMed] [Google Scholar]
- Luker GD, Prior JL, Song J, Pica CM, Leib DA. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J Virol. 2003;77:11082–93. doi: 10.1128/JVI.77.20.11082-11093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean A, Wei XQ, Huang FP, Al-Alem UA, Chan WL, Liew FY. Mice lacking inducible nitric-oxide synthase are more susceptible to herpes simplex virus infection despite enhanced Th1 cell responses. J Gen Virol. 1998;79(Pt 4):825–30. doi: 10.1099/0022-1317-79-4-825. [DOI] [PubMed] [Google Scholar]
- Malstrom SE, Jekic-McMullen D, Sambucetti L, Ang A, Reeves R, Purchio AF, Contag PR, West DB. In vivo bioluminescent monitoring of chemical toxicity using heme oxygenase-luciferase transgenic mice. Toxicol Appl Pharmacol. 2004;200:219–28. doi: 10.1016/j.taap.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Marques CP, Hu S, Sheng W, Cheeran MC, Cox D, Lokensgard JR. Interleukin-10 attenuates production of HSV-induced inflammatory mediators by human microglia. Glia. 2004;47:358–66. doi: 10.1002/glia.20045. [DOI] [PubMed] [Google Scholar]
- Marques CP, Hu S, Sheng W, Lokensgard JR. Microglial cells initiate vigorous yet non-protective immune responses during HSV-1 brain infection. Virus Res. 2006;121:1–10. doi: 10.1016/j.virusres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Marten NW, Stohlman SA, Zhou J, Bergmann CC. Kinetics of virus-specific CD8+ -T-cell expansion and trafficking following central nervous system infection. J Virol. 2003;77:2775–8. doi: 10.1128/JVI.77.4.2775-2778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LD, Krunkosky TM, Dye JA, Fischer BM, Jiang NF, Rochelle LG, Akley NJ, Dreher KL, Adler KB. The role of reactive oxygen and nitrogen species in the response of airway epithelium to particulates. Environ Health Perspect. 1997;105(Suppl 5):1301–7. doi: 10.1289/ehp.97105s51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Zhang Y, Olson SJ, Montine KS, Roberts LJ, 2nd, Morrow JD, Montine TJ, Dermody TS, Valyi-Nagy T. Herpes simplex virus type 1 encephalitis is associated with elevated levels of F2-isoprostanes and F4-neuroprostanes. J Neurovirol. 2002;8:295–305. doi: 10.1080/13550280290100743. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–7. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musallam B, Matoth I, Wolf DG, Engelhard D, Averbuch D. Steroids for deteriorating herpes simplex virus encephalitis. Pediatr Neurol. 2007;37:229–32. doi: 10.1016/j.pediatrneurol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Nathan CF, Hibbs JB., Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Ono S, Komuro T, Macdonald RL. Heme oxygenase-1 gene therapy for prevention of vasospasm in rats. J Neurosurg. 2002;96:1094–102. doi: 10.3171/jns.2002.96.6.1094. [DOI] [PubMed] [Google Scholar]
- Regan RF, Guo Y, Kumar N. Heme oxygenase-1 induction protects murine cortical astrocytes from hemoglobin toxicity. Neurosci Lett. 2000;282:1–4. doi: 10.1016/s0304-3940(00)00817-x. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Delanty N, Roy L, Rokach J, Callaghan PO, Crean P, Lawson JA, FitzGerald GA. Increased formation of the isoprostanes IPF2alpha-I and 8-epi-prostaglandin F2alpha in acute coronary angioplasty: evidence for oxidant stress during coronary reperfusion in humans. Circulation. 1997;96:3314–20. doi: 10.1161/01.cir.96.10.3314. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Laurie C, Mosley RL, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007a;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leukoc Biol. 2007b;82:1083–94. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001;166:4185–94. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- Sergerie Y, Boivin G, Gosselin D, Rivest S. Delayed but not early glucocorticoid treatment protects the host during experimental herpes simplex virus encephalitis in mice. J Infect Dis. 2007;195:817–25. doi: 10.1086/511987. [DOI] [PubMed] [Google Scholar]
- Silva G, Cunha A, Gregoire IP, Seldon MP, Soares MP. The antiapoptotic effect of heme oxygenase-1 in endothelial cells involves the degradation of p38 alpha MAPK isoform. J Immunol. 2006;177:1894–903. doi: 10.4049/jimmunol.177.3.1894. [DOI] [PubMed] [Google Scholar]
- Turcanu V, Dhouib M, Poindron P. Heme oxygenase inhibits nitric oxide synthase by degrading heme: a negative feedback regulation mechanism for nitric oxide production. Transplant Proc. 1998a;30:4184–5. doi: 10.1016/s0041-1345(98)01385-2. [DOI] [PubMed] [Google Scholar]
- Turcanu V, Dhouib M, Poindron P. Nitric oxide synthase inhibition by haem oxygenase decreases macrophage nitric-oxide-dependent cytotoxicity: a negative feedback mechanism for the regulation of nitric oxide production. Res Immunol. 1998b;149:741–4. doi: 10.1016/s0923-2494(99)80050-9. [DOI] [PubMed] [Google Scholar]
- Valyi-Nagy T, Olson SJ, Valyi-Nagy K, Montine TJ, Dermody TS. Herpes simplex virus type 1 latency in the murine nervous system is associated with oxidative damage to neurons. Virology. 2000;278:309–21. doi: 10.1006/viro.2000.0678. [DOI] [PubMed] [Google Scholar]
- Zhang W, Contag PR, Hardy J, Zhao H, Vreman HJ, Hajdena-Dawson M, Wong RJ, Stevenson DK, Contag CH. Selection of potential therapeutics based on in vivo spatiotemporal transcription patterns of heme oxygenase-1. J Mol Med. 2002;80:655–64. doi: 10.1007/s00109-002-0375-x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Contag PR, Madan A, Stevenson DK, Contag CH. Bioluminescence for biological sensing in living mammals. Adv Exp Med Biol. 1999;471:775–84. doi: 10.1007/978-1-4615-4717-4_89. [DOI] [PubMed] [Google Scholar]
- Zhang W, Feng JQ, Harris SE, Contag PR, Stevenson DK, Contag CH. Rapid in vivo functional analysis of transgenes in mice using whole body imaging of luciferase expression. Transgenic Res. 2001;10:423–34. doi: 10.1023/a:1012042506002. [DOI] [PubMed] [Google Scholar]