Abstract
Aims
We investigated the role of src-family kinases (srcFKs) in hypoxic pulmonary vasoconstriction (HPV) and how this relates to Rho-kinase-mediated Ca2+ sensitization and changes in intracellular Ca2+ concentration ([Ca2+]i).
Methods and results
Intra-pulmonary arteries (IPAs) were obtained from male Wistar rats. HPV was induced in myograph-mounted IPAs. Auto-phosphorylation of srcFKs and phosphorylation of the regulatory subunit of myosin phosphatase (MYPT-1) and myosin light-chain (MLC20) in response to hypoxia were determined by western blotting. Translocation of Rho-kinase and effects of siRNA knockdown of src and fyn were examined in cultured pulmonary artery smooth muscle cells (PASMCs). [Ca2+]i was estimated in Fura-PE3-loaded IPA. HPV was inhibited by two blockers of srcFKs, SU6656 and PP2. Hypoxia enhanced phosphorylation of three srcFK proteins at Tyr-416 (60, 59, and 54 kDa, corresponding to src, fyn, and yes, respectively) and enhanced srcFK-dependent tyrosine phosphorylation of multiple target proteins. Hypoxia caused a complex, time-dependent enhancement of MYPT-1 and MLC20 phosphorylation, both in the absence and presence of pre-constriction. The sustained component of this enhancement was blocked by SU6656 and the Rho-kinase inhibitor Y27632. In PASMCs, hypoxia caused translocation of Rho-kinase from the nucleus to the cytoplasm, and this was prevented by anti-src siRNA and to a lesser extent by anti-fyn siRNA. The biphasic increases in [Ca2+]i that accompany HPV were also inhibited by PP2.
Conclusion
Hypoxia activates srcFKs and triggers protein tyrosine phosphorylation in IPA. Hypoxia-mediated Rho-kinase activation, Ca2+ sensitization, and [Ca2+]i responses are depressed by srcFK inhibitors and/or siRNA knockdown, suggesting a central role of srcFKs in HPV.
Keywords: Hypoxia, Tyrosine kinase, src-family kinase, Rho-kinase, Pulmonary, Vasoconstriction
1. Introduction
Hypoxic pulmonary vasoconstriction (HPV) is the acute localized adaptive process whereby blood flow is directed away from poorly ventilated areas of the lung in order to maximize ventilation–perfusion matching. In isolated pulmonary arteries, HPV is typically biphasic, characterized by a large but transient first phase and a sustained and often gradually developing second phase.1,2 The first phase is associated with a sizeable transient elevation in intracellular Ca2+ ([Ca2+]i), whereas during the second phase, there is a relatively small and rapidly stabilizing increase in [Ca2+]i, 2 the effect of which is greatly enhanced by a Rho-kinase- dependent Ca2+ sensitization pathway.3
src-family kinases (srcFKs) are a group of closely related non-receptor tyrosine kinases, of which three (src, fyn, and yes) are highly expressed in pulmonary artery.4 Although tyrosine kinases are essential components of cell growth and proliferation signalling, they have also been implicated in vascular smooth muscle contraction.5,6 We have recently shown in rat pulmonary artery, as have others in porcine coronary artery, that agonist-induced Rho-kinase-mediated Ca2+ sensitization and force generation is dependent on srcFKs.4,7
Ca2+ sensitization of the contractile apparatus occurs through inhibition of myosin light-chain phosphatase (MLCP), leading to increased phosphorylation of the 20 kDa myosin light-chain (MLC20), independently of changes in [Ca2+]i.8 MLCP inhibition occurs through phosphorylation of the myosin-binding subunit MYPT-19 via Rho-kinase.10,11 We have shown that the second phase of HPV is selectively inhibited by the Rho-kinase blocker Y27632 but not by the PKC blocker Ro31-8220.2,3 In addition, hypoxia enhances phosphorylation of MYPT-1 and MLC20 in cultured pulmonary artery smooth muscle cells (PASMCs).12,13 Rho-kinase- mediated Ca2+ sensitization is also a key factor in the pathogenesis of chronic hypoxia-induced pulmonary hypertension.14,15
Two studies using broad-spectrum tyrosine kinase blockers (genistein and tyrphostin) have suggested the involvement of tyrosine kinases in HPV.16,17 src and fyn are both activated by hypoxia in cardiac myocytes,18 and src plays a crucial role in hypoxia-inducible factor (HIF-1) induction in vascular smooth muscle cells.19 However, a specific role of srcFKs in HPV and the contractile pathways affected remains to be determined. In the present study, therefore, we evaluated the role of srcFKs in HPV of rat small distal pulmonary arteries and determined how this relates to the hypoxia-mediated Rho-kinase-dependent Ca2+ sensitization and [Ca2+]i responses.
2. Methods
2.1. Animals, solutions, drugs, and chemicals
This study conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Male Wistar rats (200–250 g) were killed by lethal overdose of pentobarbital (ip), with approval from local Ethics Review Board. Lungs were excised, then first- to third-order branches of the intra-pulmonary artery (IPA) were dissected free of surrounding parenchyma and placed in physiological salt solution (PSS), containing in mM: NaCl 118; NaHCO3 24; KCl 4; CaCl2 1.8; MgSO4 1; NaH2PO4 0.43; and glucose 5.56. A normal pH of 7.4 was maintained by gassing samples with air containing 5% CO2. For hypoxia, gassing in different test chambers was adjusted in each case to achieve a pO2 of ∼15 Torr. SU6656, PP2, PP3, and Y27632 were all obtained from Calbiochem (Merck Biosciences Nottingham, UK). Fura-PE3/AM was purchased from Sigma (Poole, UK). PGF2α (tromethamine salt) was purchased from Biomol (Exeter, UK). All other reagents were obtained from Calbiochem, Sigma, Invitrogen (Paisley, UK), or Fisher (Loughborough, UK).
2.2. Measurement of force and intracellular Ca2+
Intra-pulmonary arteries (second- to third-order; 150–400 µm diameter) were mounted on a Mulvany–Halpern wire myograph (DMT A/S, Aarhus, Denmark), bathed in PSS at 37°C, pH 7.4, and stretched to give a wall tension equivalent to a transmural pressure of 30 mmHg.1 All tension experiments were preceded by equilibration with three 3 min contractions to 80 mM K+-substituted PSS (K+PSS). In order to elicit a full contractile response to hypoxia, IPAs were pre-constricted with a concentration of PGF2α necessary to produce tension equivalent to 10–15% of that produced by 80 mM K+PSS (typically 5 µM), and then made hypoxic for 40 min. Two HPV responses were elicited in each artery; the second of which in treated arteries was preceded by a 10 min pre-incubation with inhibitor. In the presence of inhibitor, the concentration of PGF2α required to achieve a 10–15% pre-constriction was adjusted accordingly.20
Isolated IPAs were mounted either on a modified Cambustion AM-10 myograph (Cambustion Ltd, Cambridge, UK) or on a confocal wire myograph (Danish Myo Technology). After stretching and equilibration as described above, IPAs were incubated for 1 h at 37°C in PSS containing 4 µM Fura PE-3/AM, and for a further 30 min to allow for de-esterification. Myograph modules were mounted on an inverted microscope (Nikon Diaphot, Nikon UK Ltd, Kingston-upon-Thames, UK) with a ×10 or ×20 Fluor objective combined with a double-excitation microfluorimeter (CairnResearch Ltd, Faversham, UK). Tension was recorded simultaneously with light emitted by the whole artery at >510 nm at excitation wavelengths 340 and 380 nm. The ratio of the emission intensities (R340/380) was taken as a measure of [Ca2+]i.
2.3. Western blot
Intra-pulmonary artery samples (half main IPA plus 2–3 second-order side-branches per sample) were subjected to a 15 min equilibration period in normoxic PSS at 37°C, followed by a 10 min pre-incubation with pharmacological agents where appropriate. IPAs were then made hypoxic for 1, 2 5, 10, or 30 min and snap frozen in liquid N2, prior to homogenization. Protein was extracted in 50 µL of Tris–SDS sample buffer containing phosphatase and protease inhibitor cocktails (Sigma). Protein extracts (12–15 µL per lane) were run on SDS–PAGE gels (4–12% gradient, Invitrogen), transferred to nitrocellulose membrane and blocked with 5% skimmed milk for 1 h at room temperature. Membranes were probed with primary antibody overnight at 4°C (1:500–1:5000 dilution where appropriate) in Tris-buffered saline containing 0.05% Tween 20 and 1% skimmed milk, followed by horseradish-peroxidase-conjugated anti-IgG secondary antibody for 1 h at room temperature (1:5000 dilution with 1% milk). Protein bands were visualized with chemiluminescence reagent (Pierce, Cramlington, UK, or Amersham, Bucks., UK) and then exposed to photographic film. Membranes were first probed with anti-phospho-antibodies, stripped for 1 h (Pierce stripping buffer), re-blocked, and re-probed with corresponding anti-total antibodies. Band intensity was expressed as a ratio of phospho/total for each protein, and values from each treated sample were normalized to those from control (untreated) samples run on the same gel. Two to three control samples were run on each gel and averaged. Antibodies were obtained from Cell Signalling, Upstate (UK), Santa Cruz Biotechnology (CA, USA), or Sigma.
2.4. siRNA design and transfection
siRNAs were designed as described previously.21,22 The 19 nucleotide target sequences (src-siRNA: position 614–632, GenBank accession no. AF157016; and fyn-siRNA: position 1096–1114, GenBank accession no. U35365) were synthesized into 64–65 mer oligonucleotides with BamHI/HindIII overhangs (Eurofins MWG Operon) and cloned into the expression vector pSilencer 3.0-H1 (Ambion Inc.). All clones were purified using an EndoFree Plasmid Maxi Kit (Qiagen Ltd) and sequenced (Geneservice Ltd) (see Supplementary material online for more details).
Pulmonary artery smooth muscle cells were dispersed enzymatically and grown in DMEM with 10% FCS to passage 3 or 4. Cells were plated on 13 mm coverslips and then growth arrested for 24 h. Identification of cells as smooth muscle was verified by positive staining with anti-smooth muscle α-actin and anti-calponin antibodies (as described previously4). Cells were transfected using the Basic Nucleofector® Kit for primary smooth muscle cells and a nucleofector device (Amaxa Biosystems). After 72 h, the transfection efficiency was >90%, determined using pmaxGFP (green fluorescent protein expressing vector) provided in the kit and confirmed by fluorescence microscopy. Efficiency and selectivity of knockdown was confirmed by western blot (see Supplementary material online).
2.5. Rho-kinase translocation
Pulmonary artery smooth muscle cells were treated in DMEM at 37°C under either normoxic or hypoxic conditions for 40 min. Reactions were terminated by addition of paraformaldehyde fixative. Fixed cells were permeabilized with Triton-100 and stained overnight with anti-ROCK-2 primary antibody (1:100) at 4°C following with incubation with Alexa Fluor 488-labelled secondary antibody (1:300) for 2 h at room temperature. Specificity of anti-ROCK-2 was confirmed by western blot (single band at 160 kDa), and staining was negative with secondary antibody alone (as described previously4).
2.6. Data analysis and statistics
Western blot images were quantified by ImageJ software (rsb.info.nih.gov). Stained cells were photographed and analysed with Zeiss Axiovert-200 microscope with CARV II confocal imager (BD Biosciences) and MetaMorph software (Molecular Devices). Statistical analysis was performed with SigmaStat (Systat Software Inc., San Jose, CA, USA). Time-dependent effects of hypoxia on tension or [Ca2+]i or of hypoxia and/or PGF2α on phosphorylation were examined by two-way repeated measures ANOVA with appropriate post hoc tests. Comparisons of the effects of different drugs against hypoxia or PGF2α were performed by one-way ANOVA with Holm–Sidak post hoc tests or by Kruskal–Wallis one-way ANOVA on ranks, where appropriate.
3. Results
3.1. srcFK inhibitors block hypoxic pulmonary vasoconstriction
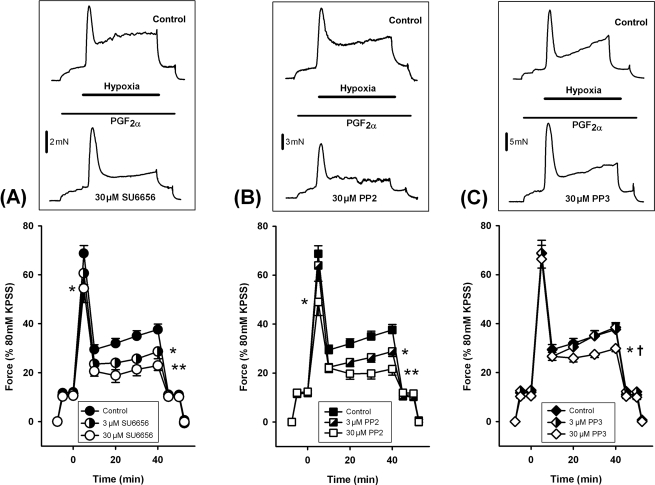
Hypoxic pulmonary vasoconstriction was induced by pre-constricting IPA with a low concentration of PGF2α (typically 5–10 µM) then making the chamber hypoxic for 40 min. Hypoxia induced a biphasic contractile response comprising a rapid transient phase followed by a slower sustained phase which was fully reversed upon re-oxygenation, as described previously.1–3 Two hypoxic exposures were performed in each artery. Drugs were applied during the second exposure and their effects were compared with the second exposure in untreated time controls. In time control experiments, the second HPV response was consistently larger than the first (n = 16, data not shown). HPV was inhibited by pre-incubation with the srcFK inhibitors SU665623 and PP224,25 (Figure 1A and B). We used concentrations of SU6656 and PP2 previously shown to be required for inhibition of srcFK auto-phosphorylation and protein tyrosine phosphorylation in IPAs.4 The sustained phase was diminished by both 3 and 30 µM of each drug, whereas the transient phase was only partially blocked by 30 µM. PP3, the inactive analogue of PP2, had no effect at 3 µM and caused only a slight inhibition of the sustained phase at 30 µM (Figure 1C).
Figure 1.
Effects of src-family kinase (srcFK) inhibitors on hypoxic pulmonary vasoconstriction in intra-pulmonary arteries. Intra-pulmonary arteries were pre-constricted with PGF2α before being made hypoxic. Example traces (top panels) show the effects of hypoxia first in the absence (upper trace), and then in the presence (lower trace) of inhibitors. The sustained phase contraction was inhibited by the srcFK inhibitors SU6656 [(A) 3 µM, 33 ± 10% block, *P < 0.05, n = 8 arteries; 30 µM, 56 ± 6% block, **P < 0.001, n = 8 arteries] and PP2 [(B) 3 µM, 37 ± 6% block, *P < 0.01, n = 10 arteries; 30 µM, 64 ± 9% block, **P < 0.001, n = 10 arteries]. The inactive analogue PP3 also partially inhibited the sustained phase at 30 µM [(C) 28 ± 6% block, *P < 0.05, n = 8 arteries], but this effect was significantly less than for PP2 (†P < 0.01 vs. 30 µM PP2); PP3 had no effect at 3 µM. The transient phase was partially inhibited by SU6656 (30 µM, 23 ± 10% block, *P < 0.01) and PP2 (30 µM, 35 ± 9% block, *P < 0.01).
3.2. Hypoxia enhances srcFK auto-phosphorylation and PP2-sensitive protein tyrosine phosphorylation
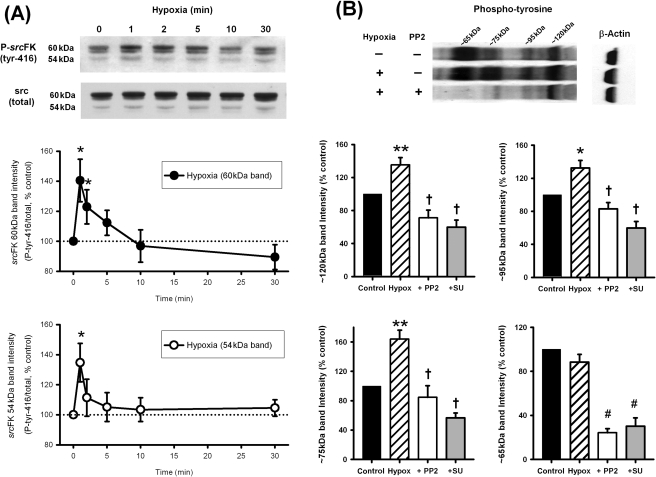
To determine whether srcFKs were activated by hypoxia or whether the effects of inhibitors reflected suppressed constitutive activity, we first examined srcFK auto-phosphorylation (an indication of srcFK activation26) and protein-tyrosine phosphorylation in response to hypoxia in IPAs. As shown previously,4 three protein bands were visualized with anti-srcFK antibodies: a doublet at 59/60 kDa and another at ∼54 kDa, corresponding to the src-family members src, fyn, and yes, respectively. Figure 2A shows that hypoxia caused a significant and transient increase in srcFK (tyr-416) phosphorylation at the 59/60 kDa band (upper panel) and 54 kDa band (lower panel). Several protein bands or collections of bands were visualized with anti-phospho-tyrosine in IPAs. Four of these, at ∼120, ∼95, ∼75, and 65 kDa are shown in Figure 2B. Hypoxia enhanced immunoreactivity at three of these bands, and this enhancement was reversed by prior incubation with 30 µM PP2 or 30 µM SU6656.
Figure 2.
Effects of hypoxia on src-family kinase tyr-416 auto-phosphorylation and protein tyrosine phosphorylation in intra-pulmonary artery. (A) Hypoxia enhanced phospho-src (tyr-416) immunoreactivity at 60 and 54 kDa in a time-dependent manner (*P < 0.05 vs. control, n = 9–11 rats). (B) Hypoxia (30 min) also enhanced phospho-tyrosine immunoreactivity at several protein bands (*P < 0.05, **P < 0.01 vs. control, n = 20 rats). At ∼120, ∼95, and ∼75 kDa, this enhancement was reversed by either PP2 or SU6656 (30 µM, †P < 0.001 vs. hypoxia alone, n = 8–11 rats). At ∼65 kDa, although not enhanced by hypoxia, basal immunoreactivity was also greatly inhibited by both inhibitors (#P < 0.0001 vs. control).
3.3. Hypoxia enhances MYPT-1 and MLC20 phosphorylation
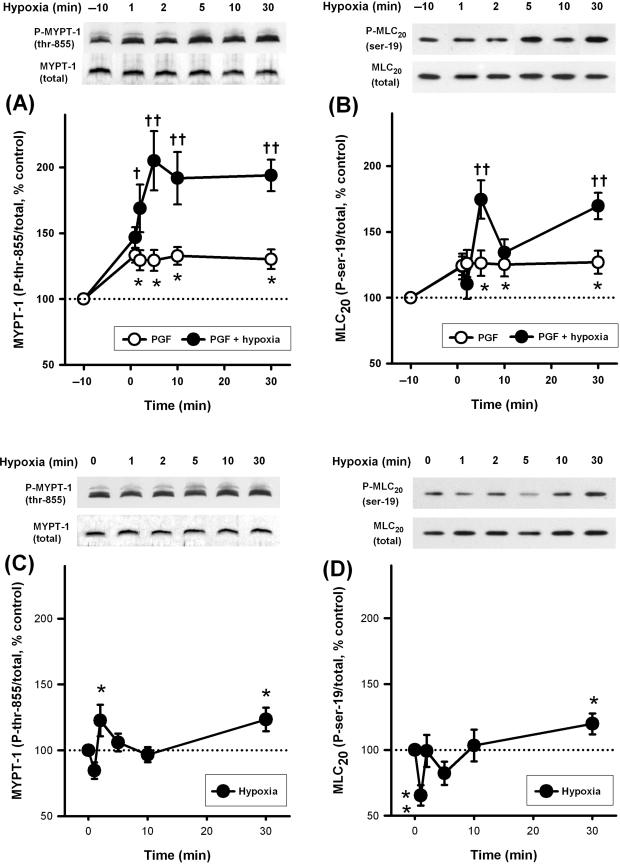
Since a small degree of pre-constriction is required for a full HPV response, we evaluated the effects of hypoxia on MYPT-1 and MLC20 phosphorylation in IPAs both in the presence and absence of PGF2α. Five micromolar PGF2α was used throughout as this approximates the concentration required to achieve the correct level of pre-constriction. The effects of 5 µM PGF2α itself were also evaluated. Five micromolar PGF2α alone caused a small but sustained increase in phosphorylation at both sites (Figure 3A and B). As is also shown in Figure 3A, hypoxia in the presence of PGF2α caused a large sustained enhancement of MYPT-1 phosphorylation (at thr-855, equivalent to thr-850 in human) that was substantially greater than that caused by PGF2α alone. Hypoxia in the presence of PGF2α also caused significant enhancement of MLC20 phosphorylation (at ser-19, target of myosin light-chain kinase) above that of PGF2α alone. This response was biphasic, with a peak at 5 min and another increase towards 30 min (Figure 3B). Hypoxia alone, however, produced a complex response in both MYPT-1 and MLC20 phosphorylation. This was characterized by a reduction at 1 min, a small peak at 2 min, another dip at 5–10 min, and a final small increase towards 30 min, which rose significantly above control levels for both phosphorylation sites (Figure 3C and D).
Figure 3.
Effects of hypoxia and PGF2α on phosphorylation of MYPT-1 (120–130 kDa) and MLC20 (18 kDa) in intra-pulmonary artery. (A and B) Phosphorylation at both sites was enhanced by 5 µM PGF2α (open circles, *P < 0.05 vs. control, n = 12–13 rats). In the continued presence of 5 µM PGF2α, hypoxia caused substantial further enhancement at both sites (filled circles, †P < 0.05, ††P < 0.01 vs. PGF2α alone, n = 12–17 rats). (C and D) In the absence of PGF2α, hypoxia caused a small sustained increase in phosphorylation (*P < 0.05, **P < 0.01 vs. control, n = 12–17 rats).
3.4. srcFK and Rho-kinase inhibitors block hypoxia-mediated MYPT-1 and MLC20 phosphorylation
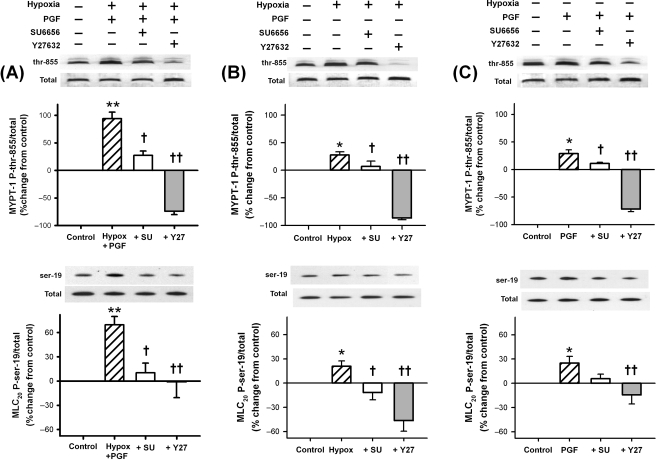
In order to determine the contribution of Rho-kinase to phosphorylation of MYPT-1 and MLC20 during hypoxia and the possible upstream involvement of srcFK, we performed additional 30 min exposures to hypoxia and 5 µM PGF2α, both together and individually, with the addition of the Rho-kinase inhibitor Y27632 and the srcFK blocker SU6656. Both in the presence (Figure 4A) and absence (Figure 4B) of PGF2α, the hypoxia-induced enhancement of phosphorylation at both sites was inhibited by both SU6656 and Y27632. The small enhancement mediated by 5 µM PGF2α was inhibited by Y27632 at both phosphorylation sites, but only the phosphorylation of MYPT-1 was significantly reduced by SU6656 (Figure 4C). We have previously shown that basal phosphorylation of MYPT-1 at thr-855 and of MLC20 at ser-19 is unaffected by SU6656 but that basal MYPT-1 phosphorylation at thr-855 is almost abolished by Y27632.4 Inhibition of MYPT-1 phosphorylation at thr-855 by SU6656, therefore, suggests that srcFK may be regulating the activity of Rho-kinase.
Figure 4.
Effects of inhibition of src-family kinase and Rho-kinase on MYPT-1 (120–130 kDa) and MLC20 (18 kDa) phosphorylation. (A) In the presence of PGF2α, hypoxia greatly enhanced phosphorylation (**P < 0.001 vs. control, n = 17 rats for both sites), and this enhancement was reversed by SU6656 (†P < 0.01, n = 10 rats for both sites) and Y27632 (††P < 0.001, n = 6 rats for both sites). (B) In the absence of PGF2α, a smaller but still significant increase was apparent (*P < 0.01, n = 17 rats for both sites), and this increase too was inhibited by SU6656 (†P < 0.01, n = 10 rats for both sites) and Y27632 (††P < 0.001, n = 6 rats for both sites). (C) Five micromolar PGF2α also enhanced phosphorylation (*P < 0.01, n = 13 rats for both sites). This increase was significantly reduced by both SU6656 [†P < 0.01 (MYPT-1 only), n = 9 rats for both sites] and Y27632 (††P < 0.001, n = 6 rats for both sites).
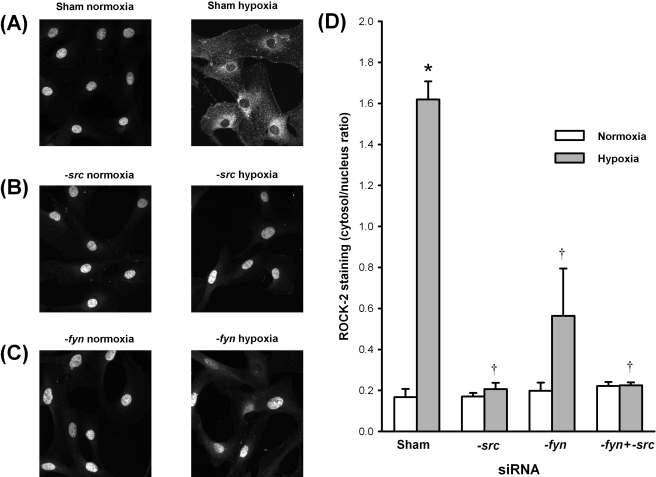
3.5. Hypoxia triggers srcFK-dependent translocation of Rho-kinase in pulmonary artery smooth muscle cells
In order to confirm that srcFKs were acting upstream of Rho-kinase activation, we examined hypoxia-mediated translocation of Rho-kinase (ROCK-2) in PASMCs transfected with siRNAs directed against src, fyn, and a combination thereof, compared with sham-transfected cells. Basally, ROCK-2 was localized to the nucleus, and this was unaffected by either siRNA. Upon exposure to hypoxia, ROCK-2 translocated to the cytosol and/or cytoskeleton (Figure 5A and D). In cells transfected with src-siRNA and fyn-siRNA, however, translocation was completely and partially prevented, respectively (Figure 5B–D). Double transfection with both src- and fyn-siRNAs also abolished translocation (Figure 5D).
Figure 5.
Hypoxia-mediated translocation of Rho-kinase in pulmonary artery smooth muscle cells: effects of src and fyn knockdown. (A–C) Examples of ROCK-2 staining in pulmonary artery smooth muscle cells either sham-transfected (A) or transfected with src-siRNA (B) or fyn-siRNA (C), under either normoxic (left panels) or hypoxic conditions (right panels). (D) Hypoxia-induced ROCK-2 translocation, as determined by the ratio of cytosol/nuclear staining intensity. *P < 0.01 vs. normoxia; †P < 0.01 vs. sham-transfected. n = 4 experiments (in cells from four different rats).
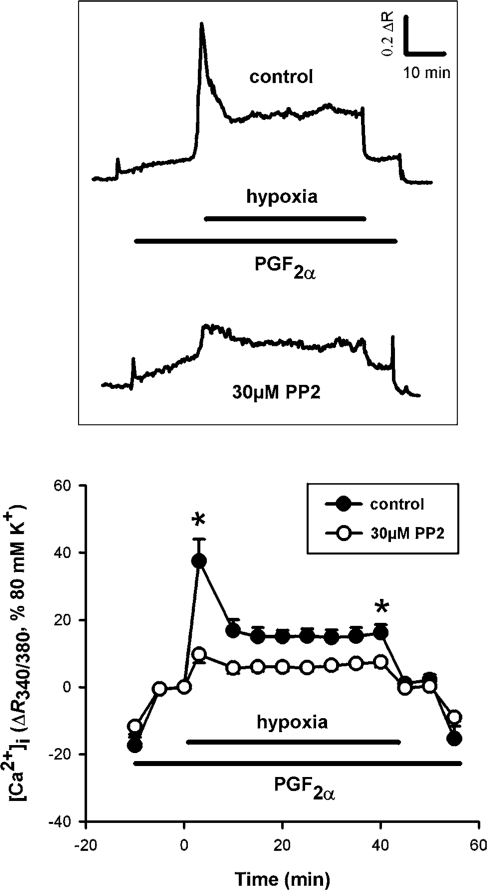
3.6. Hypoxia-induced [Ca2+]i response is PP2 sensitive
In addition to Ca2+ sensitization pathways, a rise in [Ca2+]i is almost certainly also essential for a full HPV response.20 In order to determine whether srcFKs are also involved in changes in [Ca2+]i during hypoxia, we examined the effects of the srcFK inhibitor PP2 in Fura-PE3-loaded IPA. As shown in Figure 6, hypoxia induced a biphasic [Ca2+]i response, comprising a large rapid transient phase followed by a small sustained phase, superimposed on the smaller PGF2α-mediated response. PP2 suppressed both the transient and sustained phases of this response. These experiments were not repeated with the other srcFK blocker SU6656 because of the overlap of this drug’s yellow colour with the emission spectra of Fura-PE3.
Figure 6.
Effects of hypoxia and srcFK inhibition on [Ca2+]i in intra-pulmonary arteries (IPAs). Example traces (upper panel) of the ratio of light emitted at >510 nm from excitation at 340 nm over that at 380 nm (R340/380) against time, and average effects with time (lower panel): 30 µM PP2 inhibited both the first phase (taken at 5 min; 58 ± 6% block, *P < 0.01 vs. control, n = 7 arteries) and the second phase (taken at 40 min; 43 ± 7% block, *P < 0.01 vs. control, n = 7 arteries). Note: the level of [Ca2+]i immediately before IPAs were made hypoxic was set to zero in order to distinguish the effect of PP2 on the underlying PGF2α-induced response from that of hypoxia per se.
4. Discussion
This study provides the first direct evidence for a role or roles of srcFKs in HPV and firm supportive evidence for the importance of Ca2+ sensitization in the sustained phase of the contraction. Our observations also forge a clear link between the actions of Y27632 on hypoxic force generation3,12 and on hypoxia-mediated enhancement of Rho-kinase-dependent MYPT-1 and MLC20 phosphorylation. Our findings that hypoxia both activates srcFK and enhances PP2-sensitive tyrosine phosphorylation of multiple protein targets, coupled with the inhibition of HPV by both SU6656 and PP2, are suggestive of an active role of these kinases in HPV. Hypoxia has been previously shown to activate src and the closely related kinase fyn in cardiac myocytes,18 and to enhance srcFK- specific tyrosine phosphorylation and activation of focal adhesion kinases p125FAK and paxillin.27,28 p125FAK may be the ∼120 kDa protein whose tyrosine phosphorylation was enhanced by hypoxia and inhibited by PP2 in the present study. HPV is believed to involve a change in the generation of reactive oxygen species (ROS) in the mitochondria.29,30 Many protein kinases, including srcFK, are activated by ROS,31,32 indeed src appears to play an essential role in many of the intracellular pathways activated by hypoxia and ROS in the systemic vasculature.19 Exactly how srcFK activity may be induced by ROS, assuming ROS production is enhanced during hypoxia in IPAs, remains to be determined.
The time course of the phosphorylation responses to hypoxia was complex. In the absence of PGF2α, hypoxia caused a multi-phasic phosphorylation response ending in sustained enhancement at the maximum time point of 30 min. The initial drop at 1 min was large enough to become significant with regards to MLC20 at ser-19. A similar drop (although not significant) was also apparent with MLC20 in the presence of PGF2α, and indeed, in isolated IPAs, we sometimes observe a small very transient relaxation immediately after inducing hypoxia that precedes the first-phase contraction (unpublished observations). The causes of these fluctuations are unknown. In the presence of PGF2α, hypoxia caused a large sustained increase in MYPT-1 phosphorylation. This, however, was not exactly mirrored by MLC20 phosphorylation, the time course of which resembled the time course of contraction, with a distinct transient first phase at 5 min followed by a sustained phase of up to 30 min. This is not surprising since the first phase of HPV is clearly associated with a large transient increase in [Ca2+]i, whereas the sustained phase of HPV is primarily associated with a Ca2+ sensitization pathway, as shown previously.2,3,20
Prostanoids cause vascular smooth muscle contraction in part via Rho-kinase-mediated phosphorylation of MYPT-1.33 In addition, we have recently demonstrated a direct involvement of srcFK in PGF2α-mediated Ca2+ sensitization and contraction in rat IPAs, in which we showed that srcFKs were activated by PGF2α and provided evidence that srcFKs were upstream mediators of Rho-kinase translocation, and phosphorylation of MYPT-1 and MLC20.4 Similarly, in this study, Rho-kinase-mediated MYPT-1 phosphorylation was inhibited by SU6656, and hypoxia-induced translocation of Rho-kinase in PASMCs was completely prevented by siRNA knockdown of src and strongly inhibited by knockdown of fyn. Therefore, srcFKs are clearly involved in the upstream regulation of hypoxia-mediated Rho-kinase activity.
Since both hypoxia and PGF2α4 activate srcFKs and enhance MYPT-1 and MLC20 phosphorylation, we confirmed that the action of hypoxia on this phosphorylation or of srcFK blockers on HPV was independent of any action on the underlying pre-constriction. Hypoxia caused enhancement of Rho-kinase-mediated phosphorylation of MYPT-1 in the absence as well in the presence of PGF2α, and this enhancement was sensitive to an inhibitor of srcFKs (Figure 4B). However, the relative effectiveness of hypoxia in the presence of PGF2α at enhancing phosphorylation (MYPT-1 at 30 min: 94%; and MLC20 at 30 min: 70%) was apparently greater than the sum of the individual effects of hypoxia alone and PGF2α alone (MYPT-1 at 30 min: 28 + 29 = 57%; and MLC20 at 30 min: 21 + 25 = 46%), suggesting a synergistic action of the two stimuli. This is mirrored by force generation, where hypoxia in the absence of pre-constriction produced very small contractions of only 3–4% the size of that caused by 80 mM K+PSS,1 and yet in the presence of 5 µM PGF2α which produces contractions of 10–15% of K+PSS, hypoxia caused additional sustained contractions of up to 30% of K+PSS. Thus, stimulation of srcFKs by both PGF2α and hypoxia may explain at least part of the enhancement of HPV seen in the presence of this agonist.
In addition to an involvement in Ca2+ sensitization, we have also found that the srcFK inhibitor PP2 blocks the hypoxia-mediated [Ca2+]i response in IPAs. It is well established that the net phosphorylation status of MLC20 at ser-19 involves a balance between Ca2+-calmodulin-dependent phosphorylation by MLCK and constitutive de-phosphorylation by MLCP.8 Concomitant phosphorylation of MYPT-1 by Rho-kinase together with even a small elevation of [Ca2+]i will, therefore, greatly enhance the phosphorylation of MLC20. Inhibition of either MYPT-1 phosphorylation or [Ca2+]i-mediated MLCK activation, or both will thus inhibit force generation. In addition to triggering Rho-kinase-mediated Ca2+ sensitization, both hypoxia and PGF2α cause an elevation of [Ca2+]i, which in both cases is comprised of transient and sustained components.20,34–36 In our hands, the transient component of the hypoxic [Ca2+]i response is partly due to voltage-dependent Ca2+ influx and partly due to capacitative Ca2+-influx triggered by release from thapsigargin-sensitive stores, whereas the sustained component is probably due to influx through another as yet uncharacterized voltage-independent pathway.20 Other studies propound the importance of influx through voltage-gated Ca2+ channels.37,38 In any case, srcFKs have been implicated in both voltage-dependent and -independent mechanisms of Ca2+ mobilization as triggered by either agonists or ROS.39,40
In summary, our results suggest a direct role of srcFKs in the acute contractile response to hypoxia in small pulmonary arteries of rat. Since srcFK inhibitors block both hypoxia-mediated Rho-kinase-dependent MYPT-1 phosphorylation and the hypoxic [Ca2+]i response in IPAs, we may speculate that srcFKs may act upstream of both key pathways to MLC20 phosphorylation and contraction. The specific phosphorylation targets of srcFKs as activated by hypoxia, whether they be guanine exchange factors41 or guanine dissociation inhibitors42 leading to RhoA/Rho-kinase activation, or L-type Ca2+ channels40 or phospholipase-Cγ,43 resulting in [Ca2+]i mobilization, remain to be determined.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
British Heart Foundation (FS/06/003 to G.A.K.); Wellcome Trust (078075 to V.A.S.).
Conflict of interest: none declared.
Supplementary Material
References
- 1.Leach RM, Robertson TP, Twort CH, Ward JP. Hypoxic vasoconstriction in rat ulmonary and mesenteric arteries. Am J Physiol. 1994;266:L223–L231. doi: 10.1152/ajplung.1994.266.3.L223. [DOI] [PubMed] [Google Scholar]
- 2.Robertson TP, Aaronson PI, Ward JP. Hypoxic vasoconstriction and intracellular Ca2+ in pulmonary arteries: evidence for PKC-independent Ca2+ sensitization. Am J Physiol. 1995;268:H301–H307. doi: 10.1152/ajpheart.1995.268.1.H301. [DOI] [PubMed] [Google Scholar]
- 3.Robertson TP, Dipp M, Ward JP, Aaronson PI, Evans AM. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol. 2000;131:5–9. doi: 10.1038/sj.bjp.0703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knock GA, Shaifta Y, Snetkov VA, Vowles B, Drndarski S, Ward JP, et al. Interaction between src family kinases and rho-kinase in agonist-induced Ca2+ sensitization of rat pulmonary artery. Cardiovasc Res. 2008;77:570–579. doi: 10.1093/cvr/cvm073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen LJ, Lu-Chao H, Netherton S. Excitation-contraction coupling in pulmonary vascular smooth muscle involves tyrosine kinase and Rho kinase. Am J Physiol Lung Cell Mol Physiol. 2001;280:L666–L674. doi: 10.1152/ajplung.2001.280.4.L666. [DOI] [PubMed] [Google Scholar]
- 6.Ohanian J, Ohanian V, Shaw L, Bruce C, Heagerty AM. Involvement of tyrosine phosphorylation in endothelin-1-induced calcium sensitization in rat small mesenteric arteries. Br J Pharmacol. 1997;120:653–661. doi: 10.1038/sj.bjp.0700950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakao F, Kobayashi S, Mogami K, Mizukami Y, Shirao S, Miwa S, et al. Involvement of Src family protein tyrosine kinases in Ca2+ sensitization of coronary artery contraction mediated by a sphingosylphosphorylcholine- Rho-kinase pathway. Circ Res. 2002;91:953–960. doi: 10.1161/01.res.0000042702.04920.bf. [DOI] [PubMed] [Google Scholar]
- 8.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 9.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa K, Ito M, Hartshorne DJ. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. J Biol Chem. 1996;271:4733–4740. doi: 10.1074/jbc.271.9.4733. [DOI] [PubMed] [Google Scholar]
- 11.Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett. 2002;527:101–104. doi: 10.1016/s0014-5793(02)03175-7. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Jin N, Ganguli S, Swartz DR, Li L, Rhoades RA. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2001;25:628–635. doi: 10.1165/ajrcmb.25.5.4461. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Lanner MC, Jin N, Swartz D, Li L, Rhoades RA. Hypoxia inhibits myosin phosphatase in pulmonary arterial smooth muscle cells: role of Rho-kinase. Am J Respir Cell Mol Biol. 2003;29:465–471. doi: 10.1165/rcmb.2002-0157OC. [DOI] [PubMed] [Google Scholar]
- 14.Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2008;294:L797–L806. doi: 10.1152/ajplung.00253.2007. [DOI] [PubMed] [Google Scholar]
- 15.McNamara PJ, Murthy P, Kantores C, Teixeira L, Engelberts D, van Vliet T, et al. Acute vasodilator effects of Rho-kinase inhibitors in neonatal rats with pulmonary hypertension unresponsive to nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2008;294:L205–L213. doi: 10.1152/ajplung.00234.2007. [DOI] [PubMed] [Google Scholar]
- 16.Uzun O, Demiryurek AT, Kanzik I. The role of tyrosine kinase in hypoxic constriction of sheep pulmonary artery rings. Eur J Pharmacol. 1998;358:41–47. doi: 10.1016/s0014-2999(98)00592-5. [DOI] [PubMed] [Google Scholar]
- 17.Uzun O, Tuncay DA. Involvement of tyrosine kinase pathway in acute hypoxic vasoconstriction in sheep isolated pulmonary vein. Vascul Pharmacol. 2003;40:175–181. doi: 10.1016/s1537-1891(03)00051-x. [DOI] [PubMed] [Google Scholar]
- 18.Seko Y, Tobe K, Takahashi N, Kaburagi Y, Kadowaki T, Yazaki Y. Hypoxia and hypoxia/reoxygenation activate Src family tyrosine kinases and p21ras in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1996;226:530–535. doi: 10.1006/bbrc.1996.1389. [DOI] [PubMed] [Google Scholar]
- 19.Sato H, Sato M, Kanai H, Uchiyama T, Iso T, Ohyama Y, et al. Mitochondrial reactive oxygen species and c-Src play a critical role in hypoxic response in vascular smooth muscle cells. Cardiovasc Res. 2005;67:714–722. doi: 10.1016/j.cardiores.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000;525 Pt 3:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A, et al. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 22.Ui-Tei K, Naito Y, Takahashi F, Haraguchi T, Ohki-Hamazaki H, Juni A, et al. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res. 2004;32:936–948. doi: 10.1093/nar/gkh247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, et al. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and Fyn-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 25.Karni R, Mizrachi S, Reiss-Sklan E, Gazit A, Livnah O, levitski A. The pp60c-Src inhibitor PP1 is non-competitive against ATP. FEBS Lett. 2003;537:47–52. doi: 10.1016/s0014-5793(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 26.Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 27.Salazar EP, Rozengurt E. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. J Biol Chem. 1999;274:28371–28378. doi: 10.1074/jbc.274.40.28371. [DOI] [PubMed] [Google Scholar]
- 28.Seko Y, Takahashi N, Sabe H, Tobe K, Kadowaki T, Nagai R. Hypoxia induces activation and subcellular translocation of focal adhesion kinase (p125FAK) in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1999;262:290–296. doi: 10.1006/bbrc.1999.1185. [DOI] [PubMed] [Google Scholar]
- 29.Killilea DW, Hester R, Balczon R, Babal P, Gillespie MN. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L408–L412. doi: 10.1152/ajplung.2000.279.2.L408. [DOI] [PubMed] [Google Scholar]
- 30.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 31.Abe J, Takahashi M, Ishida M, Lee JD, Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J Biol Chem. 1997;272:20389–20394. doi: 10.1074/jbc.272.33.20389. [DOI] [PubMed] [Google Scholar]
- 32.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 33.Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J. 2005;389:763–774. doi: 10.1042/BJ20050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang TM, Park MK, Uhm DY. Characterization of hypoxia-induced [Ca2+]i rise in rabbit pulmonary arterial smooth muscle cells. Life Sci. 2002;70:2321–2333. doi: 10.1016/s0024-3205(02)01497-2. [DOI] [PubMed] [Google Scholar]
- 35.Snetkov VA, Aaronson PI, Ward JP, Knock GA, Robertson TP. Capacitative calcium entry as a pulmonary specific vasoconstrictor mechanism in small muscular arteries of the rat. Br J Pharmacol. 2003;140:97–106. doi: 10.1038/sj.bjp.0705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, et al. Increases in mitochondrial reactive oxygen species trigger hypoxia- induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- 37.Cornfield DN, Stevens T, McMurtry IF, Abman SH, Rodman DM. Acute hypoxia causes membrane depolarization and calcium influx in fetal pulmonary artery smooth muscle cells. Am J Physiol. 1994;266:L469–L475. doi: 10.1152/ajplung.1994.266.4.L469. [DOI] [PubMed] [Google Scholar]
- 38.Olschewski A, Hong Z, Nelson DP, Weir EK. Graded response of K+ current, membrane potential, and [Ca2+]i to hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1143–L1150. doi: 10.1152/ajplung.00104.2002. [DOI] [PubMed] [Google Scholar]
- 39.Touyz RM, Wu XH, He G, Park JB, Chen X, Vacher J, et al. Role of c-Src in the regulation of vascular contraction and Ca2+ signaling by angiotensin II in human vascular smooth muscle cells. J Hypertens. 2001;19:441–449. doi: 10.1097/00004872-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Wijetunge S, Hughes AD. pp60c-src increases voltage-operated calcium channel currents in vascular smooth muscle cells. Biochem Biophys Res Commun. 1995;217:1039–1044. doi: 10.1006/bbrc.1995.2874. [DOI] [PubMed] [Google Scholar]
- 41.Chikumi H, Fukuhara S, Gutkind JS. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J Biol Chem. 2002;277:12463–12473. doi: 10.1074/jbc.M108504200. [DOI] [PubMed] [Google Scholar]
- 42.DerMardirossian C, Rocklin G, Seo JY, Bokoch GM. Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol Biol Cell. 2006;17:4760–4768. doi: 10.1091/mbc.E06-06-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marrero MB, Paxton WG, Duff JL, Berk BC, Bernstein KE. Angiotensin II stimulates tyrosine phosphorylation of phospholipase C-γ1 in vascular smooth muscle cells. J Biol Chem. 1994;269:10935–10939. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.