Abstract
Aims
We investigated the role of the Akt-glycogen synthase kinase (GSK)-3β signalling pathway in mediating the protective effects of tissue kallikrein on myocardial injury by promoting angiogenesis and blood flow in rats after myocardial infarction (MI).
Methods and results
Human tissue kallikrein gene in an adenoviral vector, with or without co-administration of dominant-negative Akt (Ad.DN-Akt) or constitutively active GSK-3β (Ad.GSK-3βS9A), was injected into rat myocardium after MI. The expression of recombinant human kallikrein in rat heart significantly improved cardiac function and reduced infarct size 10 days after gene delivery. Kallikrein administration significantly increased myocardial blood flow as well as capillary and arteriole densities in the infarcted myocardium. Kallikrein increased cardiac Akt and GSK-3β phosphorylation in conjunction with decreased GSK-3β activity and the upregulation of vascular endothelial growth factor (VEGF) and VEGF receptor-2 (VEGFR-2). All of kallikrein’s effects on the myocardium were abrogated by Ad.DN-Akt and Ad.GSK-3βS9A. Moreover, in cultured human aortic endothelial cells, tissue kallikrein stimulated capillary tube formation and promoted cell migration; however, these effects were blocked by Ad.DN-Akt, Ad.GSK-3βS9A, icatibant (a kinin B2 receptor antagonist), Tki (a VEGF receptor tyrosine kinase inhibitor), and a neutralizing VEGF antibody. In addition, tissue kallikrein decreased GSK-3β activity via the phosphatidylinositol 3-kinase-Akt pathway and enhanced VEGF and VEGFR-2 expression in endothelial cells.
Conclusion
These data provide the first direct evidence that tissue kallikrein protects against acute-phase MI by promoting neovascularization, restoring regional blood flow and improving cardiac function through the kinin B2 receptor-Akt-GSK-3β and VEGF signalling pathways.
Keywords: Tissue kallikrein, Angiogenesis, Myocardial infarction, GSK-3β, VEGF
1. Introduction
Most common cardiovascular diseases are accompanied by endothelial dysfunction and cell loss. After myocardial infarction (MI), the restoration of cardiac function requires not only the replacement of lost cardiomyocytes, but also the revascularization of the injured region. The development of new vessels and remodelling of pre-existing collaterals at the border zone can improve local perfusion and enhance function of the ischaemic but otherwise viable myocardium. The restoration of blood supply to the ischaemic area prevents cardiomyocyte apoptosis and subsequent development of heart failure. In addition, promoting angiogenesis in the injured heart after MI can improve cardiac function and reduce the mortality after MI. Therefore, therapeutic angiogenesis is an exciting concept with significant clinical potential in post-infarction heart failure.
Tissue kallikrein processes low molecular weight kininogen to produce potent vasoactive kinin peptides.1 Intact kinins trigger the activation of the kinin B2 receptor and exert a wide range of biological activities.2 Kallikrein gene delivery has been observed to accelerate spontaneous angiogenesis in a mouse model of hindlimb ischaemia.3 In streptozotocin-induced diabetic mice, local delivery of the human tissue kallikrein gene halted the progression of microvascular rarefaction in hindlimb skeletal muscle by inhibiting apoptosis and promoting neovascularization.4 Kallikrein administration by gene transfer also attenuated cardiac remodelling and promoted neovascularization in animal models with hypertension and MI.5,6 Moreover, the disruption of the kinin B2 receptor gene results in myocardial capillary rarefaction.7 However, the signalling mechanisms by which tissue kallikrein promotes blood flow and angiogenesis after MI have not yet been investigated.
Tissue kallikrein has been shown to inhibit cardiomyocyte apoptosis and promote the phosphorylation of glycogen synthase kinase (GSK)-3β after acute myocardial ischaemia/reperfusion injury.8 However, tissue kallikrein protects against pressure overload-induced cardiac hypertrophy via decreased GSK-3β phosphorylation.9 GSK-3β functions at a convergence of multiple signalling pathways to coordinate endothelial cell responses to different angiogenic inputs. GSK-3β inactivation by phosphorylation can increase β-catenin levels, which induce capillary formation by the upregulation of vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR-2, KDR/Flk-1) expression in endothelial cells.10 Phosphatidylinositol-3 kinase (PI3K)-Akt-GSK-3β signalling can be further upregulated by GSK-3β/VEGF signalling.10 Kinin has been shown to enhance capillary tube formation in cultured endothelial cells by the transactivation of VEGF receptor via the kinin B2 receptor.11,12 However, kallikrein gene transfer was found to induce angiogenesis in an animal model of hindlimb ischaemia by Akt and endothelial nitric oxide synthase (eNOS) signalling without affecting VEGF expression or VEGFR-2 activation.13 In this study, we investigated the role and signalling mechanisms of the Akt-GSK-3β-VEGF pathway in mediating kallikrein’s effects on cardiac performance, neovascularization, and myocardial blood flow in the ischaemic myocardium after MI, and in cultured coronary artery endothelial cells.
2. Methods
2.1. Preparation of replication-deficient adenoviral vectors
Adenovirus harbouring the human tissue kallikrein cDNA (Ad.TK), under the control of the cytomegalovirus enhancer/promoter, or adenoviral vector alone (Ad.Null) was constructed and prepared as described previously.14 Adenoviruses expressing dominant-negative mutant Akt (Ad.DN-Akt) or non-phosphorylatable, constitutively active mutant GSK-3β (Ad.GSK-3βS9A) were kindly provided by Dr Kenneth Walsh, St Elizabeth’s Medical Center in Boston, MA, USA.
2.2. Animals and treatments
Wistar rats (male, 220–250 g body weight, Harlan–Sprague–Dawley) were employed in this study. The study complied with the standards stated in the Guides for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). MI was established by the ligation of the left coronary artery as described previously.8 Briefly, a thoracotomy was performed via the fourth intercostal space, the heart was exposed, and ECG was then monitored. A 6-0 polypropylene suture (Ethicon) was passed loosely around the left anterior descending (LAD) coronary artery near its origin. Once haemodynamics were stabilized, LAD coronary artery occlusion was performed by the irreversible tightening of the suture loop. Acute myocardial ischaemia was deemed successful on the basis of regional cyanosis of the myocardial surface distal to the suture, accompanied by the elevation of the ST segment on ECG. One hour after surgery, rats with MI were randomly divided into four groups (n = 8–10 rats per group) and administered intramyocardial injection of Ad.Null, Ad.TK, Ad.TK and Ad.DN-Akt, or Ad.TK and Ad.GSK-3βS9A in a total volume of 200 µL (2 × 109 plaque-forming units in PBS) at five sites in the left ventricular free wall. The expression and localization of recombinant human tissue kallikrein in rat heart after gene delivery were determined by immunohistochemistry, enzyme-linked immunosorbent assay (ELISA), and reverse transcriptase–polymerase chain reaction (RT–PCR).15
2.3. Haemodynamic parameters
At 10 days after infarction, haemodynamics and cardiac function were measured by the advancement of a 2.5 French micro-manometer (Millar Instrument) from the carotid artery into the left ventricle.8 Mean arterial pressure (MAP), left ventricular end-diastolic pressure (LVEDP), left ventricular end-systolic pressure (LVESP), cardiac contractility (dP/dt maximum and dP/dt minimum) were recorded and analysed by a model 7E polygraph (BIOPAC).
2.4. Histological analyses
At the end of the procedure, cardiac tissues were fixed in 4% paraformaldehyde and embedded. The left ventricle was cut into three transverse slices on basal, middle, and apex levels. Four-micron sections were obtained for morphological analyses. Masson’s trichrome staining was performed to determine the percentage of scar length to total LV circumference. Immunohistochemical analyses were performed to visualize capillaries and small arteries in the border zone using a kit according to the manufacturer’s instructions (Universal Elite ABC, Vector). Primary antibodies against CD-31 (endothelial cell antigen; Santa Cruz; 1:200 dilution) and α-smooth muscle actin (Dako; 1:200) were used. Capillaries were identified as having a diameter <20 µm and a layer of endothelial cells without smooth muscle cells. Arterioles were identified as having a diameter in the range of 20–100 µm with a layer of smooth muscle cells. To determine capillary density and arteriole density, the number of positive staining was counted in a double-blind fashion from 20 different fields of each section (n = 8). The average number of the vessels in one section was used for the assessment of vascular density.
2.5. Myocardial regional blood flow
Myocardial blood flow was measured using fluorescent microspheres as described previously with modifications.16 Briefly, PE-50 tubing was inserted through the right carotid artery to the left ventricle by cannulation. The left carotid was opened to insert a withdrawal catheter. Blood was withdrawn at a rate of 1–2 mL/min for 2 min, starting 5 s before the injection of fluorescent microspheres into the left atrium. Microspheres, consisting of a mixture of equal amounts of blue–green, green, and yellow–green beads (Molecular Probes; Invitrogen Corp.), were injected in a total volume of 30 µL. Animals were sacrificed 2 min after injection. The infarcted myocardium was isolated, and a similar size of non-infarcted myocardium served as a control. The blood and myocardium were then digested and washed. The pellet was dissolved with 0.5 mL of xylene and then incubated at 50°C for 3 h. The supernatant was collected and subjected to the measurement of fluorescence intensity (Victor2, Perkin-Elmer) with triplicates for each sample. After normalizing with weight, the regional blood flow was calculated and expressed as millilitre/minute/gram.
2.6. Cell culture
Human coronary artery endothelial cells (HCAECs) were purchased from Cambrex Bio Science and were used between the third and sixth passages. The cells were cultured in endothelial basal medium (EBM)-2 containing EGM-2-MV SingleQuots (BioWhittaker). For migration and tube formation, HCAECs were grown to subconfluence in six-well plates and transduced at a multiplicity of infection of 50 overnight with replication-deficient adenoviral vectors (Ad.Null, Ad.DN-Akt, or Ad.GSK-3βS9A). Tissue kallikrein was purified from rat salivary glands according to previously described procedures.17 The expression of human tissue kallikrein levels in rat serum samples was determined by ELISA as described previously.6
2.7. Tube formation assay
Growth factor-reduced Matrigel (BD Biosciences) was added to 24-well culture plates (200 µL per well) following overnight incubation on ice. After polymerization at 37°C for 30 min, the gels were overlaid with 500 µL of EBM-2 and supplemented with 0.1% foetal bovine serum (FBS). A total of 4 × 104 HCAECs were then added. The cells were incubated with tissue kallikrein (TK, 0.1 µmol/L) in the presence or absence of icatibant (a kinin B2 receptor antagonist, 1 µmol/L), Tki (VEGF receptor tyrosine kinase inhibitor, 10 µmol/L), or neutralizing anti-VEGF antibody (10 ng/mL) at 37°C for 18 h. VEGF (50 ng/mL) was used as the positive control. To test the effect of Ad.DN-Akt and Ad.GSK-3βS9A on kallikrein-induced tube formation, cells were transduced with adenoviral vectors at a multiplicity of infection of 50 overnight in EBM-2 and then trypsinized. Tube images were obtained using an inverted microscope, and the degree of tube formation was quantified by measuring the length of tubes in three random fields from each well using Scion image software (Scion Corporation).
2.8. Cell migration
The migration of HCAECs was performed as described previously.18 A total of 3 × 105 cells were seeded into each well of a 24-well plate and incubated with complete medium at 37°C and 5% CO2. After 24 h of incubation, cells were starved for 18 h in medium with low serum (0.5% FBS). The cells were then scraped horizontally in each well using a P100 pipette tip and allowed to migrate for 20 h. Three randomly selected views along the scraped line were photographed in each well using a fluorescent-inverted microscope, with a CCD camera attached to the microscope at ×50 magnification as soon as the medium was changed to fresh medium and incubated with tissue kallikrein in the presence or absence of icatibant, Tki, or neutralizing anti-VEGF antibody. To test the effect of Ad.DN-Akt and Ad.GSK-3βS9A on kallikrein-induced endothelial cell migration, cells were transduced with adenoviral vectors at a multiplicity of infection of 50 overnight in EBM-2 and then trypsinized. After incubation for 13 h, another set of images was taken using the same method.
2.9. Induction of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 expression
HCAECs were seeded in six-well plates (1 × 105 cells/well). After incubation overnight with EBM-2 supplemented with 0.1% FBS, tissue kallikrein was added to the medium at a final concentration of 0.05 µmol/L. After another 24 h, cells were harvested for mRNA extraction. The expression of VEGF and VEGFR-2 in HCAECs was confirmed by real-time PCR.
2.10. Quantitative real-time polymerase chain reaction
Total RNA was extracted from myocardium or cultured HCAECs using Trizol reagent (Invitrogen). cDNA was transcribed using a cDNA Archive Kit (Applied Biosystems). The quantitative real-time PCR reaction was carried out using the gene expression assay on a 7300 real-time PCR system (Applied Biosystems). The transcription of the housekeeping genes, rat glyceraldehyde 3-phosphate dehydrogenase, and human 18S were used as internal controls (Applied Biosystems). The final quantification was determined by Relative Quantification software (Applied Biosystems).
2.11. Vascular endothelial growth factor and vascular endothelial growth factor receptor-2 levels by enzyme-linked immunosorbent assay and flow cytometry
For the determination of VEGF levels in endothelial cell culture medium, HCAECs were seeded into 10 cm dishes and incubated in EBM-2 supplemented with 0.1% FBS for 16 h. Tissue kallikrein (0.05 µmol/L) was added and cells were incubated for another 24 h. Cell media were harvested and lyophilized and VEGF levels were detected using an ELISA kit (R&D Systems). For flow cytometric analysis of VEGF and VEGFR-2 expression, HCAECs were seeded in 60 mm dishes. After overnight incubation in EBM-2 supplemented with 0.1% FBS, tissue kallikrein (0.05 µmol/L) was added and cells were incubated for another 24 h. Brefeldin A (eBioscience) was added to the medium at a final concentration of 3 µg/mL 2 h after tissue kallikrein treatment. After washing once, the cells were resuspended in PBS containing 1% BSA and incubated with 3 µg/mL anti-human VEGF (R&D Systems) or VEGFR-2 (Cell Signalling) antibody, or anti-rat VEGF (R&D Systems) or VEGFR-2 (Abcam) antibody, followed by 10 µg/mL FITC-conjugated anti-goat IgG at 4°C for 1 h. After washing and centrifugation, the cells were resuspended in 0.4 mL PBS for immediate acquisition by flow cytometry.
2.12. Cultured primary cardiomyocytes
Neonatal cardiomyocytes were isolated from 1–3-day-old Sprague–Dawley rats by four rounds of digestion using collagenase II (Worthington) and pancreatin (Sigma).8 Cardiomyocytes were collected after 1 h of differential plating of myofibroblasts and identified by immunohistochemical staining for sarcomeric α-actinin. Cardiomyocytes were incubated with tissue kallikrein (0.05 µmol/L) for 24 h prior to determining VEGF and VEGFR-2 expression as mentioned earlier.
2.13. Western blot analysis
Quiescent HCAECs or left ventricle extracts were homogenized in lysis buffer (10 mmol/L HEPES, 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L Na3VO4, 1 mmol/L EDTA, 1% NP-40). Western blots were performed for total and phosphorylated Akt and GSK-3β as described previously.8
2.14. Assay for GSK-3β activity
HCAECs and left ventricular tissues were pulverized under liquid nitrogen and homogenized in ice-cold lysis buffer as described previously.19 For GSK-3β activity assay, 10 µg of protein from cardiac tissue or cell extracts were incubated at 37°C for 30 min with reaction buffer [8 mmol/L MOPS, 0.2 mmol/L EDTA, 10 mmol/L magnesium acetate, 62.5 mmol/L GSK-3β substrate peptide (Upstate) and 1 mCi/10 mL [γ-32P]-ATP (DuPont NEN)]. Samples were transferred to P81 paper and washed three times with 0.75% phosphoric acid followed by a final rinse with acetone. Radioactivity was measured in a scintillation counter.
2.15. Statistical analysis
Data are expressed as mean ± SEM and were compared between experimental groups with the use of ANOVA and unpaired student’s t-test. Differences were considered statistically significant at a value of P < 0.05.
3. Results
3.1. Expression of human tissue kallikrein in rat heart reduces infarct size after myocardial infarction
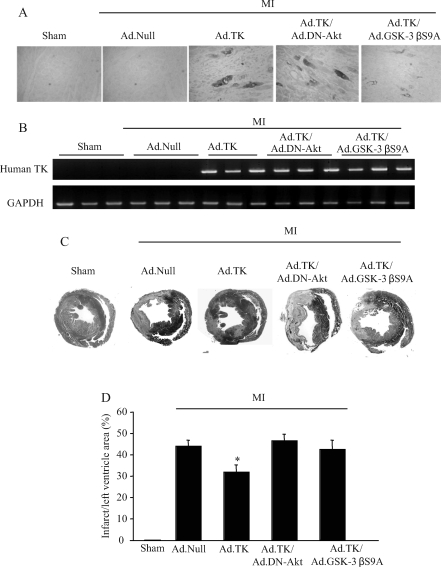
The expression and localization of recombinant human tissue kallikrein were identified by immunohistochemistry, ELISA, and RT–PCR in rat heart 10 days after gene delivery (Figure 1A and B). Human tissue kallikrein immunostaining and mRNA were detected in the heart after local injection of Ad.TK with or without the co-administration of Ad.DN-Akt or Ad.GSK-3βS9A, but not in sham rats or rats injected with control Ad.Null. Immunoreactive human tissue kallikrein levels (2.3 ± 0.2 ng/mg protein, n = 7) in the heart after kallikrein gene delivery were measured by ELISA. These results demonstrate the expression of recombinant human tissue kallikrein in rat heart after local kallikrein gene delivery. Moreover, kallikrein gene delivery significantly reduced infarct size in the left ventricle compared with the MI/Ad.Null control group, as determined by Masson’s trichrome staining and quantitative analysis (Figure 1C and D). Kallikrein’s effect on MI was abolished by Ad.DN-Akt and Ad.GSK-3βS9A.
Figure 1.
Expression and localization of recombinant human tissue kallikrein in rat heart reduced infarct size at 10 days after acute myocardial infarction (MI). (A) Representative immunohistochemistry photographs of recombinant human tissue kallikrein in the infarcted region. Original magnification is ×200. (B) Representative reverse transcriptase–polymerase chain reaction products of human tissue kallikrein mRNA. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as an internal control. (C) Representative Masson’s trichrome staining. Original magnification is ×10. (D) Quantitative analysis of infarct size. Infarct size is expressed as percentage of the infarct/left ventricle area. Values are expressed as mean ± SEM (n = 6–8, *P < 0.01 vs. other myocardial infarction groups).
3.2. Kallikrein gene delivery improves cardiac function after myocardial infarction
Table 1 shows the effect of kallikrein gene transfer on haemodynamic parameters 10 days after MI. MAP was markedly reduced after MI, but was partially restored by kallikrein. Characteristic impairments in cardiac contractility (dP/dt max) and diastolic function (LVEDP and dP/dt min) after MI were significantly improved after kallikrein gene injection. However, kallikrein’s protective actions were abrogated by Ad.DN-Akt and Ad.GSK-3βS9A, implicating a role for Akt and GSK-3β in mediating cardiac protection by kallikrein.
Table 1.
Effects of kallikrein gene delivery on cardiac function 10 days after myocardial infarction
| Variable | Sham | MI/Ad.Null | MI/Ad.TK | MI/Ad.TK/Ad.DN-Akt | MI/Ad.TK/Ad.GSK-3βS9A |
|---|---|---|---|---|---|
| MAP (mmHg) | 104.5 ± 2.5 | 71.8 ± 3.0 | 81.4 ± 5.2* | 70.6 ± 4.6 | 66.5 ± 3.8 |
| LVEDP (mmHg) | 1.2 ± 0.7 | 11.0 ± 1.0 | 7.7 ± 0.8* | 10.8 ± 1.1 | 10.4 ± 1.0 |
| dP/dt max (mmHg/s) | 3796.8 ± 112.7 | 2445.7 ± 73.6 | 2889.9 ± 78.1* | 2459.1 ± 68.9 | 2393.4 ± 72.6 |
| dP/dt min (mmHg/s) | 3184.5 ± 156.8 | 1873.2 ± 66.0 | 2315.4 ± 75.2* | 1863.1 ± 66.3 | 1761.0 ± 57.9 |
Values are expressed as mean ± SEM.
dP/dt max, maximum first derivative of pressure; dP/dt min, minimum first derivative of pressure.
*P < 0.05 vs. other MI groups.
3.3. Kallikrein increases regional blood flow, angiogenesis, and arteriogenesis in the peri-infarct area
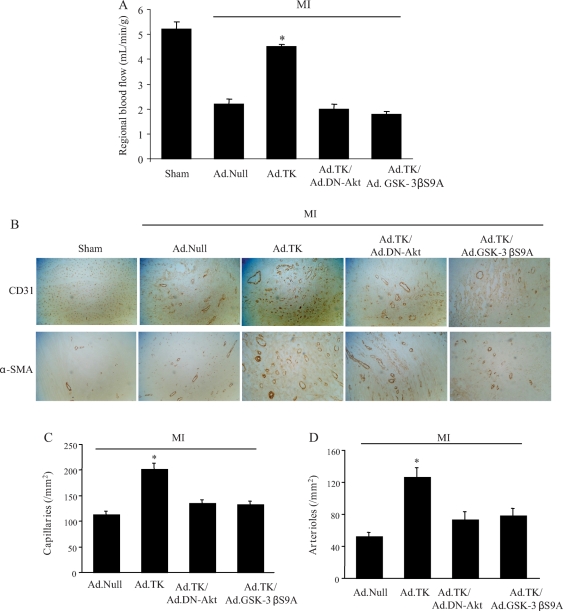
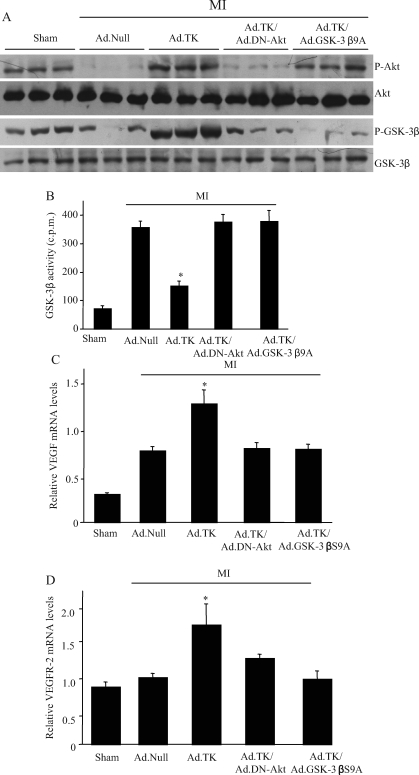
Myocardial blood flow was significantly reduced in rats after MI (Figure 2A). Although kallikrein treatment restored regional blood flow, Ad.DN-Akt and Ad.GSK-3βS9A abolished kallikrein’s effects. Capillary density was determined by immunohistochemical staining for CD31 in the peri-infarct area (Figure 2B). Tissue kallikrein gene transfer increased capillary density compared with the MI/Ad.Null group, and Ad.DN-Akt and Ad.GSK-3βS9A completely blocked kallikrein’s effect. Quantification indicated that capillary density was significantly higher in the kallikrein group compared with the MI/Ad.Null group (1805 ± 221 vs. 703 ± 35 capillaries/mm2, P < 0.05) (Figure 2C). Moreover, small arterioles were clearly visible in the tissue sections stained with an antibody against α-smooth muscle actin (Figure 2B). Quantitative analysis showed that arteriolar density was significantly increased in the tissue kallikrein treatment group compared with the MI/Ad.Null group (126.6 ± 11.0 vs. 52.5 ± 5.0, P < 0.05) (Figure 2D). Both Ad.DN-Akt and Ad.GSK-3βS9A blocked kallikrein’s effects on capillary and arteriolar density. Kallikrein’s effect on angiogenesis in the ischaemic myocardium was associated with increased Akt and GSK-3β phosphorylation and reduced GSK-3β activity (Figure 3A and B). Dominant-negative Akt abolished kallikrein’s effects on both Akt and GSK-3β phosphorylation. However, constitutively active GSK-3β mutant had no effect on kallikrein’s effect on Akt phosphorylation but blocked kallikrein’s effects on GSK-3β phosphorylation and activity. Moreover, tissue kallikrein treatment significantly increased the expression of VEGF and VEGFR-2 mRNA levels compared with the control group (Figure 3C and D). Both dominant-negative Akt and constitutively active GSK-3β mutant abolished kallikrein’s effects on VEGF and VEGFR-2 expression. These findings suggest that kallikrein-induced angiogenesis in the injured myocardium is mediated through the Akt-GSK-3β-VEGF signalling pathway.
Figure 2.
Effects of kallikrein gene delivery on regional blood flow and capillary and arteriole densities. (A) Myocardial blood flow. (B) Representative immunostaining for CD31 and α-SMA identifies capillaries and arterioles, respectively. (C) Quantitative analysis of capillary density in the peri-infarct myocardium. Original magnification is ×200. (D) Quantitative analysis of arteriolar density in the peri-infarct myocardium. Original magnification is ×200. Values are expressed as mean ± SEM [n = 6–7, *P < 0.01 vs. other myocardial infarction (MI) groups].
Figure 3.
Effect of kallikrein on Akt and GSK-3β activation and vascular endothelial growth factor and vascular endothelial growth factor receptor-2 expression. (A) Western blots for Akt and GSK-3β. (B) GSK-3β activity. (C) Vascular endothelial growth factor (VEGF) and (D) vascular endothelial growth factor receptor-2 (VEGFR-2) mRNA levels determined by real-time polymerase chain reaction. Values are expressed as mean ± SEM [n = 6–7, *P < 0.05 vs. other myocardial infarction (MI) groups].
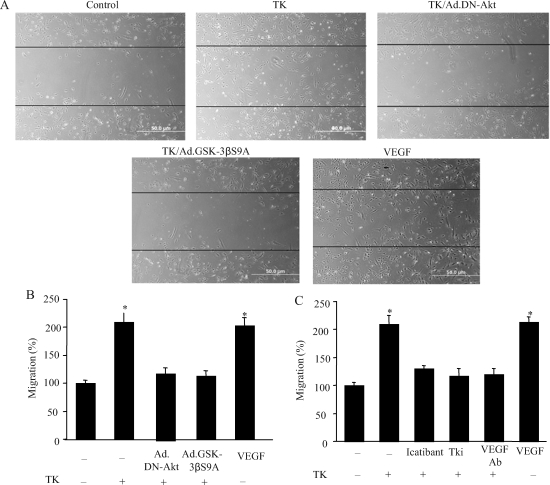
3.4. Tissue kallikrein stimulates tube formation and migration of cultured endothelial cells
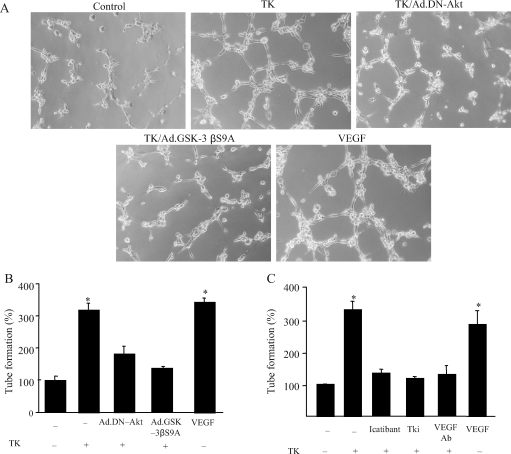
On the basis of the results from the in vivo studies, we further elucidated the role of Akt-GSK-3β in mediating tissue kallikrein’s angiogenic action in cultured endothelial cells. As shown in Figure 4A, tissue kallikrein formed capillary-like tubes in Matrigel, but not control, and kallikrein’s effect was blocked by dominant-negative Akt and constitutively active GSK-3β. Quantitative analysis demonstrated that tissue kallikrein significantly increased tube formation, but kallikrein’s effect was abolished by Ad.DN-Akt, Ad.GSK-3βS9A, icatibant, VEGF-neutralizing antibody, and VEGF receptor tyrosine kinase inhibitor (Tki) (Figure 4B and C).
Figure 4.
Effect of tissue kallikrein (TK) on capillary tube formation in cultured endothelial cells. (A) Representative photographs of Matrigel-coated cultured endothelial cells after incubation with tissue kallikrein with or without prior transduction with Ad.DN-Akt or Ad.GSK-3βS9A. (B and C) Quantitative analysis of tube formation. Cells were incubated with tissue kallikrein (0.1 mmol/L) with or without Ad.DN-Akt, Ad.GSK-3βS9A, icatibant, Tki, or vascular endothelial growth factor (VEGF)-neutralizing antibody. Tube formation was assessed using a colorimetric assay in 24-well plates. Each column represents mean ± SEM of three independent experiments compared with control (n = 3, *P < 0.05 vs. other groups).
Cell migration was assessed using a scrape method. Representative images of migrating endothelial cells showed that kallikrein increased endothelial cell migration, and that the effect was blocked by dominant-negative Akt and the constitutively active GSK-3β mutant (Figure 5A). Quantitation indicated that tissue kallikrein significantly increased the number of migrating cells compared with the control. Ad.DN-Akt and Ad.GSK-3βS9A, icatibant, VEGF-neutralizing antibody, and Tki abrogated kallikrein’s effect (Figure 5B and C).
Figure 5.
Effect of tissue kallikrein on migration of cultured endothelial cells. (A) Representative photographs of endothelial cell migration after incubation with tissue kallikrein (0.1 mmol/L) with or without prior transduction with Ad.DN-Akt or Ad.GSK-3βS9A. (B and C) Quantitative analysis of tube formation. Cells were incubated with tissue kallikrein with or without Ad.DN-Akt, Ad.GSK-3βS9A, icatibant, Tki, or vascular endothelial growth factor (VEGF)-neutralizing antibody. Migratory ability was assessed on Matrigel-coated plates. Each column represents mean ± SEM of three independent experiments compared with control (n = 3, *P < 0.05 vs. other groups).
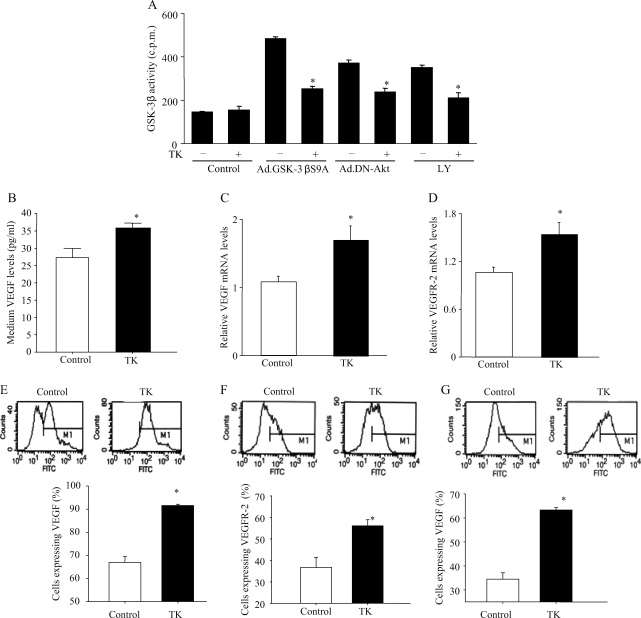
3.5. Tissue kallikrein inhibits GSK-3β activity and stimulates vascular endothelial growth factor and vascular endothelial growth factor receptor-2 expression in cultured endothelial cells
Increased GSK-3 activities were observed in cultured endothelial cells by treatment with constitutively active GSK-3β mutant, dominant-negative Akt, and LY294002 (a PI3K inhibitor). However, these effects were significantly reduced by tissue kallikrein (Figure 6A). Moreover, tissue kallikrein significantly increased VEGF and VEGFR-2 levels compared with vehicle as determined by real-time PCR, ELISA, and flow cytometry (Figure 6B–F). These results are consistent with the in vivo data. Taken together, these studies indicate that tissue kallikrein-induced capillary tube formation and the migration of endothelial cells are mediated by kinin B2 receptor-Akt-GSK-3β and VEGF signalling cascades.
Figure 6.
Effect of tissue kallikrein on GSK-3β activity and vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor-2 (VEGFR-2) expression in cultured cells. (A) Effect of tissue kallikrein (0.05 µmol/L) on GSK-3β activity induced by Ad.DN-Akt, Ad.GSK-3βS9A, and LY294002 (25 mmol/L, a PI3K inhibitor) in endothelial cells. (B) Enzyme-linked immunosorbent assay of vascular endothelial growth factor levels in culture media. Real-time polymerase chain reaction for the measurement of (C) vascular endothelial growth factor mRNA levels and (D) vascular endothelial growth factor receptor-2 mRNA levels in endothelial cells. Flow cytometric analysis of (E) vascular endothelial growth factor levels and (F) vascular endothelial growth factor receptor-2 levels in endothelial cells. (G) Flow cytometric analysis of vascular endothelial growth factor expression in cardiomyocytes. (n = 3–4, *P < 0.05 vs. other groups).
4. Discussion
This is the first study to demonstrate that tissue kallikrein protects against cardiac dysfunction by promoting angiogenesis and arteriogenesis through the Akt-GSK-3β-VEGF pathway, thus increasing blood flow in the ischaemic myocardium. Our results showed that kallikrein supplementation by gene delivery reduced infarct size and improved cardiac function and neovascularization in rat hearts 10 days after MI. Moreover, kallikrein stimulated endothelial cell migration and tube formation in cultured endothelial cells. In both in vivo and cell culture studies, tissue kallikrein treatment increased VEGF and VEGFR-2 expression. Using dominant-negative Akt and constitutively active GSK-3β mutant, our studies indicate a role of Akt-GSK-3β in mediating kallikrein’s pro-angiogenic effect. This study underscores the therapeutic potential of tissue kallikrein in cardiac protection by promoting neovascularization and improving cardiac function in post-infarction heart failure.
Arterioles provide the bulk of blood flow to tissue. Thus, the interconnection of the arterial system is necessary to give relief to ischaemic organs. Since myocardial perfusion is mainly dependent on the arteriolar tree, vascular growth strategies should be directed to both angiogenesis and arteriogenesis. We showed that the expression of human tissue kallikrein in the heart at 10 days after local gene delivery led to increases in both angiogenesis and arteriogenesis at the border zone of the ischaemic myocardium. In conjunction with increased neovascularization, tissue kallikrein also increased myocardial blood flow, resulting in improved cardiac function and decreased infarct size. Our results showed that kallikrein’s effects on arteriolar number and capillary density in the ischaemic myocardium were blocked by dominant-negative Akt and constitutively active GSK-3β. Similarly, in cultured endothelial cells, Akt and GSK-3β blockade abrogated kallikrein’s stimulatory effects on endothelial cell migration and tube formation. GSK-3β has been shown to have the novel function of coordinating endothelial cell responses to different angiogenic stimuli.10 GSK-3β functions as a downstream target of PI3-kinase-Akt signalling in endothelial cells to promote vessel growth.20 These combined results indicate that kallikrein’s pro-angiogenic effects were mediated by Akt-GSK-3β activation.
Akt has previously been reported to promote endothelial cell migration and differentiation.21 In cultured endothelial cells, kinins were shown to activate the PI3K-Akt pathway,22 although dominant-negative Akt inhibited the proliferative effect induced by kinin.13 Our present study showed that kallikrein-induced neovascularization in the ischaemic heart was blocked by dominant-negative Akt and non-phosphorylatable, constitutively active GSK-3β mutant. Furthermore, the inhibition of PI3K, Akt, and GSK-3β phosphorylation abolished kallikrein’s effects on capillary formation and migration in cultured endothelial cells. These findings indicate that kallikrein promotes angiogenesis through PI3K-Akt-GSK-3β signalling pathway.
PI3K-Akt activation plays an important role in angiogenesis by regulating VEGF expression.23 VEGF is a well-known endothelial cell-specific angiogenic factor that regulates angiogenesis through the stimulation of proteolytic activity as well as endothelial cell proliferation and migration.24 Our current study showed that kallikrein significantly upregulates VEGF and VEGFR-2 expression in the ischaemic myocardium and in cultured endothelial cells. However, kallikrein’s stimulatory effect on VEGF and VEGFR-2 expression was abrogated by dominant-negative Akt and constitutively active GSK-3β, suggesting that VEGF/VEGFR-2 is downstream of the Akt-GSK-3β signalling pathway. Moreover, tissue kallikrein markedly increased VEGF levels in primary cardiomyocytes as determined by flow cytometry (Figure 6G). Flow cytometry also revealed that tissue kallikrein induced a small but significant elevation in VEGFR-2 expression in these cells (data not shown). Therefore, kallikrein-induced neovascularization in the ischaemic myocardium, in part, is attributed to the elevated expression of VEGF and its receptor. Kallikrein’s stimulating effects on tube formation and cell migration were abolished by VEGF-neutralizing antibody in cultured endothelial cells. Whether VEGF-neutralizing antibody abolishes kallikrein’s effect on promoting angiogenesis in this animal model awaits further investigation. These results indicate an important role of VEGF/VEGFR-2 in kallikrein-induced angiogenesis after MI. Consistent with our data, studies by other investigators have shown that kallikrein/kinin can directly stimulate VEGF expression.25 However, Emanueli et al.13 reported that tissue kallikrein gene transfer increased angiogenesis in the ischaemic hindlimb by improving blood flow via the upregulation of Akt and eNOS, independent of VEGF. These conflicting data might be explained by the use of different animal models and different experimental approach.
Tissue kallikrein-induced angiogenesis is mediated by the kinin B2 receptor, as the inhibition of B2 receptor activation by icatibant blocked kallikrein’s effect on both tube network formation and endothelial cell migration. A previous study reported that the activation of the kinin B1 receptor promotes angiogenesis by the upregulation of endogenous basic fibroblast growth factor (bFGF) in endothelium.26 Our unpublished results showed that bFGF expression increased in all the MI groups regardless of treatment, indicating that the B1 receptor/bFGF signalling may not make a significant contribution to kallikrein-mediated angiogenesis in the ischaemic myocardium after MI.
In conclusion, this study demonstrated that tissue kallikrein improves cardiac performance by promoting angiogenesis and arteriogenesis, and thus blood flow, in a rat model of MI. Kallikrein’s effects on cardiac protection and blood vessel growth were found to be mediated by the kinin B2 receptor-Akt-GSK-3β and VEGF signalling pathways. This information provides important insights regarding the role and mechanisms of the tissue kallikrein–kinin system in protection against cardiovascular diseases.
Funding
This work was supported by National Institutes of Health grants HL-29373 and DK-066350, and C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources.
Conflict of interest: none declared.
References
- 1.Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- 2.Regoli D, Rhaleb NE, Drapeau G, Dion S. Kinin receptor subtypes. J Cardiovasc Pharmacol. 1990;15(Suppl. 6):S30–S38. [PubMed] [Google Scholar]
- 3.Emanueli C, Minasi A, Zacheo A, Chao J, Chao L, Salis MB, et al. Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Circulation. 2001;103:125–132. doi: 10.1161/01.cir.103.1.125. [DOI] [PubMed] [Google Scholar]
- 4.Emanueli C, Salis MB, Pinna A, Stacca T, Milia AF, Spano A, et al. Prevention of diabetes-induced microangiopathy by human tissue kallikrein gene transfer. Circulation. 2002;106:993–999. doi: 10.1161/01.cir.0000027104.33206.c8. [DOI] [PubMed] [Google Scholar]
- 5.Agata J, Chao L, Chao J. Kallikrein gene delivery improves cardiac reserve and attenuates remodeling after myocardial infarction. Hypertension. 2002;40:653–659. doi: 10.1161/01.hyp.0000036035.41122.99. [DOI] [PubMed] [Google Scholar]
- 6.Bledsoe G, Chao L, Chao J. Kallikrein gene delivery attenuates cardiac remodeling and promotes neovascularization in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2003;285:H1479–H1488. doi: 10.1152/ajpheart.01129.2002. [DOI] [PubMed] [Google Scholar]
- 7.Maestri R, Milia AF, Salis MB, Graiani G, Lagrasta C, Monica M, et al. Cardiac hypertrophy and microvascular deficit in kinin B2 receptor knockout mice. Hypertension. 2003;41:1151–1155. doi: 10.1161/01.HYP.0000064180.55222.DF. [DOI] [PubMed] [Google Scholar]
- 8.Yin H, Chao L, Chao J. Kallikrein/kinin protects against myocardial apoptosis after ischemia/reperfusion via Akt-glycogen synthase kinase-3 and Akt-Bad.14-3-3 signaling pathways. J Biol Chem. 2005;280:8022–8030. doi: 10.1074/jbc.M407179200. [DOI] [PubMed] [Google Scholar]
- 9.Li HJ, Yin H, Yao YY, Shen B, Bader M, Chao L, et al. Tissue kallikrein protects against pressure overload-induced cardiac hypertrophy through kinin B2 receptor and glycogen synthase kinase-3beta activation. Cardiovasc Res. 2007;73:130–142. doi: 10.1016/j.cardiores.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HS, Skurk C, Thomas SR, Bialik A, Suhara T, Kureishi Y, et al. Regulation of angiogenesis by glycogen synthase kinase-3beta. J Biol Chem. 2002;277:41888–41896. doi: 10.1074/jbc.M206657200. [DOI] [PubMed] [Google Scholar]
- 11.Miura S, Matsuo Y, Saku K. Transactivation of KDR/Flk-1 by the B2 receptor induces tube formation in human coronary endothelial cells. Hypertension. 2003;41:1118–1123. doi: 10.1161/01.HYP.0000064345.33807.57. [DOI] [PubMed] [Google Scholar]
- 12.Thuringer D, Maulon L, Frelin C. Rapid transactivation of the vascular endothelial growth factor receptor KDR/Flk-1 by the bradykinin B2 receptor contributes to endothelial nitric-oxide synthase activation in cardiac capillary endothelial cells. J Biol Chem. 2002;277:2028–2032. doi: 10.1074/jbc.M109493200. [DOI] [PubMed] [Google Scholar]
- 13.Emanueli C, Salis MB, Van Linthout S, Meloni M, Desortes E, Silvestre JS, et al. Akt/protein kinase B and endothelial nitric oxide synthase mediate muscular neovascularization induced by tissue kallikrein gene transfer. Circulation. 2004;110:1638–1644. doi: 10.1161/01.CIR.0000142051.36244.83. [DOI] [PubMed] [Google Scholar]
- 14.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida H, Zhang JJ, Chao L, Chao J. Kallikrein gene delivery attenuates myocardial infarction and apoptosis after myocardial ischemia and reperfusion. Hypertension. 2000;35:25–31. doi: 10.1161/01.hyp.35.1.25. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao J, Margolius HS. Isozymes of rat urinary kallikrein. Biochem Pharmacol. 1979;28:2071–2079. doi: 10.1016/0006-2952(79)90226-0. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Rifkin DB. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988;107:1199–1205. doi: 10.1083/jcb.107.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, et al. Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–130. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K. Glycogen-synthase kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res. 2005;96:308–318. doi: 10.1161/01.RES.0000156273.30274.f7. [DOI] [PubMed] [Google Scholar]
- 21.Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, et al. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587–16591. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 23.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruyama K, Mori Y, Murasawa S, Masaki H, Takahashi N, Tsutusmi Y, et al. Interleukin-1 beta upregulates cardiac expression of vascular endothelial growth factor and its receptor KDR/flk-1 via activation of protein tyrosine kinases. J Mol Cell Cardiol. 1999;31:607–617. doi: 10.1006/jmcc.1998.0895. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda Y, Hayashi I, Kamoshita E, Yamazaki A, Endo H, Ishihara K, et al. Host stromal bradykinin B2 receptor signaling facilitates tumor-associated angiogenesis and tumor growth. Cancer Res. 2004;64:5178–5185. doi: 10.1158/0008-5472.CAN-03-3589. [DOI] [PubMed] [Google Scholar]
- 26.Morbidelli L, Parenti A, Giovannelli L, Granger HJ, Ledda F, Ziche M. B1 receptor involvement in the effect of bradykinin on venular endothelial cell proliferation and potentiation of FGF-2 effects. Br J Pharmacol. 1998;124:1286–1292. doi: 10.1038/sj.bjp.0701943. [DOI] [PMC free article] [PubMed] [Google Scholar]