Abstract
Objectives
The aim of this study was to assess whether non-specific clinical signs or biological results can identify patients with a high probability of infective endocarditis (IE) to improve outcome.
Patients and methods
All patients tested for IE were included in a cohort and classified according to the modified Duke criteria. Patients with rejected endocarditis served as controls. Univariate and multivariate analyses were performed, and a score was calculated by adding 1 when a variable independently associated with IE (excluding major Duke criteria) was present and 0 when the variable was absent. A second score for patients with prior valvular damage (PVD) was also used. Scores were evaluated using the ROC curve method.
Results
IE was diagnosed in 402 of 2039 participants (19.7%). By multivariate analysis, PVD, fever, emboli, stroke, splenomegaly, finger clubbing, leucocytosis and erythrocyte sediment rate >50 were independently associated with IE. The rate of IE increased significantly from 4% (10/254) for a score of 0 to 83% (10/12) for a score of 6 in all patients, and from 9.5% (23/241) to 100% (10/10) in patients with PVD. The area under the ROC curve was 0.75 for the first score and 0.7 for the second. In a prospective study of 117 patients with suspicion of IE, the proportion of confirmed IE was 19% and the area under the ROC curve was 0.72.
Conclusions
This simple score can be used to identify patients with a high probability of IE, in the emergency room or on admission, to speed up diagnosis, or to initiate empirical antimicrobial therapy without replacing the modified Duke criteria.
Keywords: treatment, diagnosis, IE
Introduction
Infective endocarditis (IE) remains associated with high morbidity and mortality, despite progress in diagnosis and treatment. One difficulty encountered in the diagnosis of endocarditis is related to the fact that all or some of the classical clinical manifestations of endocarditis such as bacteraemia, fungaemia, evidence of active valvulitis, emboli or immunological vascular signs may be absent, while only non-specific clinical signs are present. This may delay the diagnosis and subsequently the treatment, making the prognosis poorer. In addition, major modified Duke criteria, such as positive blood culture for IE or positive endocardial involvement, which are indispensable to classify patients, are not usable on admission to identify patients at high risk of endocarditis when this infection is suspected because they can be used only at the end of an investigation, requiring several days for completion.1 In addition, this investigation requires imaging and microbiological resources that are not always available, especially in developing countries.
The prognosis of IE could be further improved if it was possible to identify earlier patients at high risk for IE when such infection is suspected in order to shorten the interval between such suspicion and therapy. Since 1994, we have used a diagnostic kit for IE, which has made it possible to evaluate the incidence of IE in a cohort of patients with suspected IE.2 Therefore, based on these data, we performed the present study to assess whether or not clinical signs and biological results available in the emergency room could enable us to identify patients at high risk of IE.
Patients and methods
A structured standardized questionnaire was used, by a resident in infectious diseases based in the microbiology laboratory, to prospectively collect the following data on all patients with suspicion of IE subjected to the diagnostic kit: age, sex, signs and symptoms, duration of symptoms, biological results, history of antimicrobial therapy for the current illness that prompted the patient to seek medical attention, antecedent disease, predisposing factors for IE including systemic disease, prosthetic valve, intravenous drug abuse, dental or surgical manipulation, treatment received during the course of hospitalization and outcome.
Each patient consulting or hospitalized in any of the Assistance Publique of Marseille hospitals (AP-HM) with suspicion of IE underwent testing guided by the diagnostic kit after informed consent was obtained.2 Each kit contained written guidelines for testing requirements and the informed consent form. The kit consisted of three units. The first, to be used immediately, included a set of two blood culture vials for aerobic and anaerobic cultures (Bactec®, Becton–Dickinson, Sparks, MD, USA), and a tube to collect a serum sample used for rheumatoid factor detection (Rapitex® RF, Dade Behring Inc., Newark, NJ, USA) and estimation of specific antibodies directed against Coxiella burnetii, Bartonella spp., Brucella spp., Chlamydia spp., Mycoplasma pneumoniae, Legionella pneumophila and Aspergillus spp.3–9 The second and third units of the diagnosis kit each contained a set of two blood culture vials to be used 2 and 4 h, respectively, after the first one. All diagnostic kits were processed at the Microbiology Laboratory of La Timone Hospital (AP-HM).
Data on patients with suspicion of IE who are subjected to the diagnostic kit are routinely prospectively collected, and were used for this epidemiological study. The approval of our Institutional Research Ethics Committee was not required.
Bacterial identification was performed according to the clinical microbiology procedures handbook.10 When usual methods were inconclusive, PCR amplification followed by sequencing of the 16 rDNA gene was performed.11–13 DNA extracts were prepared from suspect colonies for use as templates in PCR amplification using QIAmp Blood Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions.14
Valvular surgical samples included valvular and periprosthetic tissue, vegetations, valve prostheses and tissue fragments obtained from abscess debridement. A smear of each sample was prepared and stained using Gram and Ziehl methods. As described above for bacterial identification, a part of each tissue sample was inoculated into brain heart medium and blood agar medium as well as cell culture. A DNA extract, suitable for use as a template for PCR as above, was prepared from each sample using a QIAmp Tissue Kit (Qiagen), according to the manufacturer's instructions.14 For pathological examinations, formalin-fixed and paraffin-embedded tissue samples were cut to a thickness of 5 µm and stained with routine haematoxylin–eosin. Valvular tissue examination enabled us to recognize consistent patterns of tissue damage associated with IE, namely vegetation and valvular inflammation.15 Special stains were used to detect bacteria and fungi: Giemsa, Brown-Brenn and Brown-Hopps tissue Gram, periodic acid-Schiff, Gimenez,16 Grocott-Gomori methenamine silver, Ziehl-Neelsen and Warthin-Starry.17,18
The modified Duke criteria were used to define cases of endocarditis.19 All patients assessed as having possible IE received antibiotic treatment if they had one major Duke criterion or transoesophageal echocardiographic abnormalities or a microbiologically proven infection. All cases of possible IE were followed up for a minimum period of 6 months.
Definitions
Leucocytosis was defined as a white blood cell count >10 000 mm−3 (10 × 109 L−1). Anaemia was defined as haemoglobin level <13 g/dL for men and <12 g/dL for women, and thrombocytopenia as platelet count <150 000 mm−3.
Cohort study
All patients with a suspicion of IE were included in a cohort study whose objective was to assess the clinical and biological features associated and/or predictive of IE. Criteria used to reject the diagnosis of IE were the modified Duke criteria proposed by Li et al.19 Patients with rejected endocarditis served as control patients, and patients with possible endocarditis were not included in the study. Major modified Duke criteria (positive blood culture and positive echocardiogram for IE) were excluded from the analysis since those criteria had been used to classify the patients.
To validate the scoring system, a prospective study was implemented in November 2007. The score was calculated for all patients with a suspicion of IE for whom a diagnostic kit was used and the preliminary results are presented in this article.
Data analysis
The results of the diagnosis kits were considered redundant when more than one kit was used for the diagnosis of one episode of IE and those results were deleted from the database.
An EXCEL spread sheet was used to enter clinical and biological data. Univariate and multivariate analyses were performed by using SPSS software version 10 (Chicago, IL, USA). Scores were calculated by adding 1 each time a variable significantly associated with IE in the logistic regression analysis was present and 0 when the variable was absent. χ2 for trend was calculated, as well as the odds ratio, with the Statcalc program of EpiInfo version 3.4.1 (Centers for Disease Control and Prevention, Atlanta, GA, USA), for each score level compared with the score of 0. Positive predictive values (PPVs) and negative predictive values (NPVs) were also calculated for each score level by using the UBC Bayesian calculator type 2 (http://www.healthcare.ubc.ca/calc/bayescalc2.html). Due to the small number of patients with the highest scores, scores from 6 to 8 were combined in the first scoring system and from 5 to 8 in the second scoring system. ROC curves were drawn with SPSS for Windows (version 10.0.5).
Results
During the study period from 1 October 1999 to 31 January 2006, a total of 1870 patients aged from 4 to 103 years admitted at any of the Assistance Publique-Hôpitaux de Marseille healthcare facilities with clinical suspicion of IE were subjected to the diagnostic kit and included in the study. These patients were subjected to 2039 diagnosis kits (1.09 kit/patient). The highest proportion of diagnosis kits was sent by the cardiology and cardiac surgery services (48.3%, 985) followed by the internal medicine services (21.3%, 434). The characteristics of the patients with a suspicion of IE are shown in Table 1. The mean age of the patients was 62 years, 60% were male, 59.4% had prior valvular damage (PVD), 11.4% had a bio-prosthesis, 12.3% a mechanical prosthesis and 13% a pacemaker. The most frequently damaged valve was the mitral valve (37.3%), followed by the aortic valve (34%) and the tricuspid valve (4.3%).
Table 1.
Characteristics of the patients with suspicion of IE included in the study
| Characteristic | Number |
|---|---|
| Sex, male | 1222 (60%) |
| Mean age (range, SD, median) | 62 years (4–103, 17, 66) |
| males | 61.4 years (4–93, 17, 65) |
| females | 63 years (5–103, 18, 67) |
| Prior valvular damage | 1206 (59.4%) |
| Bio-prosthesis | 231 (11.4%) |
| Mechanical prosthesis | 250 (12.3%) |
| Pace maker | 267 (13%) |
| Aortic valve damage | 544 (34%) |
| Mitral valve damage | 595 (37.3%) |
| Tricuspid valve damage | 64 (4.3%) |
| History of acute articular rheumatism | 156 (9.8%) |
| Fever | 1214 (65.6%) |
| Services | |
| intensive care unit | 86 (4.4%) |
| cardiology | 853 (43.7%) |
| internal medicine | 441 (22.6%) |
| cardiac surgery | 165 (8.5%) |
| infectious diseases | 104 (5.3%) |
As categorized by the modified Duke criteria, following the use of the diagnostic kits and the performance of echocardiograms, a diagnosis of definite IE was made for 19.7% (402/2039) of the patients and of possible IE for 3.2% (66/2039) of the patients and a diagnosis of IE was rejected in 77% (1571/2039). Seventeen patients presented two episodes of IE and one patient presented four episodes.
Following the exclusion of the 66 patients with possible endocarditis, 1152 of the remaining patients had PVD (this category included patients with prosthetic heart valves, a pacemaker or congenital heart disease) and 30.2% of them (348) were diagnosed with IE. With regard to the presence of foreign bodies, the highest proportion of IE was diagnosed in patients with pacemakers (37.5%, 94/251), followed by patients with bio-prostheses (30.5%, 68/223) and patients with mechanical prostheses (19.7%, 47/239).
Two hundred and eighty patients (69.7%) with IE were male and their ages ranged from 4 to 95 years with a mean age of 63 years ±17 and a median age of 67. The proportion of patients with IE was significantly higher from cardiology services (28.3%, 279/985) than from internal medicine services (6.5%, 28/434) [relative risk (RR): 1.4, 95% confidence interval (95% CI): 1.35–1.5, P < 0.0000001].
Of all IE, 88.8% (357/402) were microbiologically documented. The 15 microorganisms most commonly considered responsible for endocarditis were as follows: Staphylococcus aureus (17.2%, 69/402), Streptococcus bovis (13.4%, 54/402), Staphylococcus epidermidis (8.5%, 34/402), Enterococcus faecalis (8%, 32/402), C. burnetii (5%, 20/402), Streptococcus mitis (3.2%, 13/402), Escherichia coli (2%, 8/402), Streptococcus agalactiae (1.5%, 6/402), Streptococcus oralis (1.5%, 6/402), Bartonella spp. (1.5%, 6/402), Candida spp. (1.5%, 6/402), Staphylococcus lugdunensis (1.5%, 6/402), Streptococcus anginosus (1.2%, 5/402), Streptococcus sanguinis (1%, 4/402) and Corynebacterium spp. (1%, 4/402).
Retrospective cohort study
The purpose of the cohort study was to assess which clinical signs and biological results were associated with or predictive of IE in order to identify patients at high risk of IE at the time of admission. When, by univariate analysis, patients with endocarditis were compared with patients with rejected endocarditis, non-specific clinical signs and biological results including male sex, hepatomegaly, splenomegaly, stroke, emboli, spondylodiscitis, diarrhoea, anaemia, leucocytosis, thrombocytopenia and erythrocytes sediment rate (ESR) > 50 were significantly associated with and predictive of IE (Tables 2 and 3). Spondylodiscitis was excluded as the diagnosis of this clinical manifestation requires additional imaging procedures.
Table 2.
Clinical signs: comparison between IE and rejected cases
| Variable | Infective endocarditis (n = 402) | Rejected cases (n = 1571) | Relative risk (95% CI) | P |
|---|---|---|---|---|
| Sex, male | 280 (69.7%) | 899 (57.3%) | 1.6 (1.3–1.9) | <0.000001 |
| Age, mean ± SD | 63 ± 17 | 61.7 ± 17 | 0.2 | |
| Stroke | 50 (14.4%) | 55 (4.3%) | 2.5 (2–3) | <0.0000001 |
| Arthralgia | 38 (10%) | 121 (9%) | 1.1 (0.8–1.5) | 0.6 |
| Splenomegaly | 41 (11%) | 42 (3%) | 2.4 (1.9–3) | <0.0000001 |
| Skin rash | 14 (4%) | 64 (4.7%) | 0.8 (0.5–1.3) | 0.5 |
| Hepatomegaly | 50 (13.3%) | 98 (7.3%) | 1.6 (1–3.2) | 0.0003 |
| Finger clubbing | 25 (6.7%) | 19 (1.4%) | 2.7 (2–3.6) | <0.0000001 |
| Janeway lesion | 1 (0.3%) | 1 (0.1%) | 2.2 (0.6–9) | 0.9 |
| Multiple adenopathies | 7 (2%) | 29 (2.2%) | 0.9 (0.5–1.7) | 0.9 |
| Spondylodiscitis | 14 (4.4%) | 16 (1.4%) | 2.2 (1.5–3.3) | 0.002 |
| Spleen infarction | 22 (6.7%) | 6 (0.5%) | 4 (3.2–5) | <0.0000001 |
| Diarrhoea | 25 (6.6%) | 52 (4%) | 1.5 (1–2) | 0.03 |
| Pneumonia | 23 (6.2%) | 117 (8.8%) | 0.7 (0.5–1) | 0.1 |
95% CI, 95% confidence interval.
Table 3.
Biological results: comparison between IE and rejected cases
| Variable | Infective endocarditis (n = 402) | Rejected cases (n = 1571) | Relative risk (95% CI) | P |
|---|---|---|---|---|
| Anemia | 199 (54.4%) | 507 (39.5%) | 1.6 (1.3–1.9) | 0.000002 |
| Leucocytosis | 171 (45.8%) | 399 (30.8%) | 1.6 (1.4–1.9) | 0.000001 |
| Thrombocytopenia | 67 (18.7%) | 152 (12%) | 1.5 (1.2–2) | 0.002 |
| Erythrocyte sedimentation rate >50 | 204 (64.4%) | 492 (43.3%) | 2.4 (1.6–2.4) | <0.0000001 |
95% CI, 95% confidence interval.
Therefore, a second analysis was performed in which only variables corresponding to clinical signs and biological results available or present on admission and significantly associated with IE in univariate analysis were used in a model tested by binary logistic regression analysis. By multivariate analysis, male sex, PVD, fever, emboli, stroke, splenomegaly, finger clubbing, leucocytosis and ESR > 50 were independently associated with IE, and the model fitted the observed data according to the Hosmer–Lemeshow goodness-of-fit test (Table 4).
Table 4.
Multivariate analysis
| Variable | Odds ratio | 95% CI | P |
|---|---|---|---|
| Sex, male | 2.5 | 1.7–3.6 | <0.00001 |
| Prior valvular damage | 8.2 | 5–13.3 | <0.00001 |
| Fever | 2.1 | 1.4–3 | 0.003 |
| Stroke | 4.3 | 2.2–8 | <0.00001 |
| Emboli | 3.6 | 1.5–8.6 | 0.004 |
| Finger clubbing | 2.7 | 1.1–6.7 | 0.03 |
| Splenomegaly | 6 | 1.4–25 | 0.02 |
| Leucocytosis | 1.6 | 1.1–2.2 | 0.01 |
| Thrombocytopenia | 2.3 | 1.4–3.8 | 0.002 |
| Erythrocyte sedimentation rate >50 | 1.9 | 1.3–2.7 | 0.0006 |
95% CI, 95% confidence interval.
Hosmer–Lemeshow goodness of fit test: 0.9.
Scoring system
We then designed a very simple scoring system in order to quantify the probability for a given patient to have IE according to the number of predictive factors present identified by multivariate analysis. The score was calculated by adding 1 each time a predictive factor was present and 0 when it was absent. Patients were classified into six score categories ranging from 0 when no predictive factor was present to 6 when six predictive factors or more were present. The rate of endocarditis increased progressively and significantly from 4% (10/256) when no predictive factor was present (score of 0) to 83% (10/12) when six predictive factors or more were present (score of 6). The probability that an IE was present, as measured by the odds ratio, increased from 1.7 for a score of 1 (the score of 0 being considered as baseline) to 123 for a score of 6 (Table 5). In parallel, the PPV rose from 0.06 for a score of 1 to 0.8 for a score >6. The NPV was 0.96 for a score of 1. The scoring system gave even better results when it was applied to patients with PVD. In this subset of patients, the proportion of patients with IE rose from 9.5% for a score of 0 to 100% for a score ≥5, and the probability of having IE increased from 2.8 for a score of 1 to 95 for a score of ≥5 (Table 6). The PPV also rose in parallel from 0.2 for a score of 1 to 1 for a score ≥5 and the NPV was 0.9 for a score of 1.
Table 5.
Predictive score of IE: overall cases
| Score | Proportion of IE | PPV | NPV | Odds ratio |
|---|---|---|---|---|
| 0 | 10 (4%) | 0.04 | 0.93 | baseline 1 |
| 1 | 30 (6.5%) | 0.06 | 0.96 | 1.7 |
| 2 | 99 (18%) | 0.2 | 0.93 | 5.4 |
| 3 | 113 (28%) | 0.28 | 0.82 | 9.5 |
| 4 | 97 (44%) | 0.44 | 0.72 | 19 |
| 5 | 43 (67%) | 0.67 | 0.56 | 50 |
| ≥6 | 10 (83%) | 0.83 | 0.32 | 123 |
PPV, positive predictive value; NPV, negative predictive value.
χ2 for trend: P < 0.0000001.
Table 6.
Predictive score of IE: score excluding PVD applied to patients with PVD
| Score | Endocarditis in patients with prior valvular damage | PPV | NPV | Odds ratio |
|---|---|---|---|---|
| 0 | 23 (9.5%) | 0.045 | 0.9 | baseline 1 |
| 1 | 81 (24%) | 0.24 | 0.9 | 2.8 |
| 2 | 97 (32.7%) | 0.32 | 0.76 | 4.6 |
| 3 | 95 (45.7%) | 0.46 | 0.67 | 8 |
| 4 | 42 (67.7%) | 0.68 | 0.5 | 20 |
| ≥5 | 10 (100%) | 1 | 0.3 | 95 |
PPV, positive predictive value; NPV, negative predictive value.
χ2 for trend: P < 0.0000001.
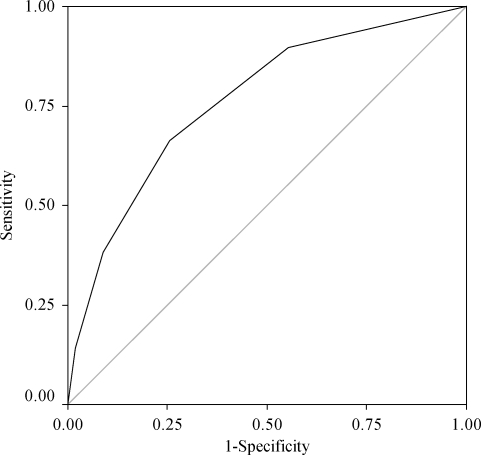
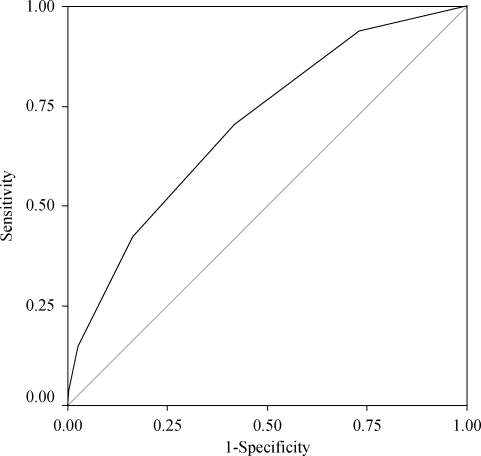
Finally, in order to evaluate the diagnosis value of the score, we drew ROC curves for all cases and for patients with PVD (Figures 1 and 2). The areas under the ROC curves were 0.753 and 7.03, respectively. Both curves were statistically significant (P < 0.0001).
Figure 1.
ROC curve including all confirmed and excluded cases of IE.
Figure 2.
ROC curve including only patients with PVD.
Prospective cohort study
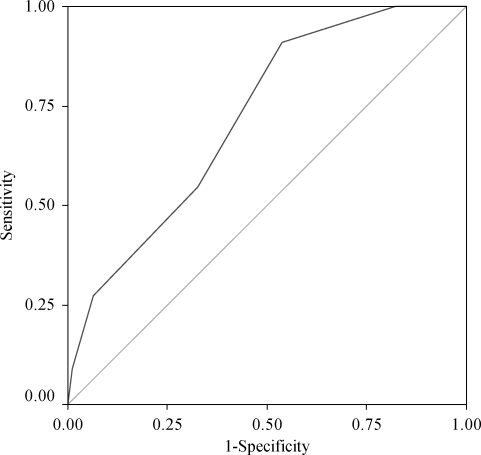
One hundred and seventeen patients with a suspicion of IE have been prospectively included in an ongoing cohort study. The diagnosis of IE was confirmed in 22 patients (19%). The proportion of IE ranged from 0% for a score of 0 to 50% for a score ≥5. The area under the ROC curve was 0.72 and the curve was statistically significant (P = 0.01) (Figure 3).
Figure 3.
ROC curve of the prospective study.
Discussion
The diagnosis of IE remains problematic despite the development of standard diagnostic schemes first proposed by von Reyn et al.20 and subsequently replaced by the modified Duke criteria. The incorporation of imaging diagnostic features of IE by echocardiography in the Duke criteria has greatly improved the sensitivity and specificity of these criteria. Another important progress has been the standardization of the aetiological diagnosis procedures including systematic serologic testing. Such strategy has enabled our team to obtain an aetiological diagnosis within 5 days for 94% of all patients with definite IE.2 As a matter of fact, diagnosis of IE is easy when echocardiographic and microbiological evidences are present (major Duke criteria), but the delay in obtaining these data may in turn delay the start of empirical therapy that may be critical for this deadly disease. However, it is important to note that in the same study the diagnosis of IE was rejected in 77% of the 2039 diagnosis procedures performed, leading to extra-costs associated with unnecessary diagnosis procedures, days of hospitalization and treatment for patients at a very low risk of endocarditis. For this reason, and in order to further improve the prognosis of IE by shortening the delay between suspicion of IE and initiation of antimicrobial therapy, we performed this cohort study.
Our study shows that several features obtained on admission including Duke minor criteria, such as predisposing heart condition, vascular phenomena and fever as well as others such as spleen enlargement or finger clubbing, and trivial biological results such as thrombocytopenia, leucocytosis or ESR >50, were also independently associated with and/or predictive of IE and may be used to differentiate high risk patients from low risk patients. Our study also showed that emboli events such as spondylodiscitis, splenomegaly, intracranial haemorrhage or members’ arterial emboli were independently associated with IE. Our model fitted very well with the observed data even if the major modified Duke criteria were not included. In a recent study, Todd et al.21 have shown that splenomegaly was an independent predictor of IE, and the proposal by Lamas et al.22 to add increased ESR to Duke criteria as well as our work fuels this hypothesis.
In addition, our study also shows that the greater the number of predictive factors present, the higher the probability of IE, and we developed a scoring system enabling us to quantify the probability of IE. The proportion of IE increased steadily and concomitantly with the score for both scoring systems, with even better PPVs when the scoring system was applied to patients with PVD. Almost one out of every two patients with a score of 3 had IE and all the patients with a score of ≥5 had IE. The simple and easy to calculate score we have developed can be used to decide in the office of the general practitioner or on admission, for instance, when to speed up diagnosis procedures, or even initiate empirical antimicrobial therapy. It is also important to note that despite the fact that our cohort was heterogeneous in terms of risk factors for IE, as patients with biological prostheses, mechanical prostheses or pacemakers were included, this heterogeneity had no impact on the ability of the scoring system to identify patients with a high probability of endocarditis. The ROC curves confirmed that our scoring system accurately separates patients with and without IE. Therefore, our scoring system, which is based on non-specific clinical signs and biological results, may be used to identify patients at high risk of having IE.
Finally, our results were partly unexpected due to the design of our study. Because we chose as a control group patients with rejected endocarditis who were in some way suspected to have IE, we thought that our results would be biased towards the null hypothesis, and it was surprising to identify such differences between patients with IE and patients with rejected IE. For example, in this study, a male patient presenting with fever and previous valve lesion having leucocytosis, thrombocytopenia and ESR >50 has a 74% probability of having IE. Using Duke criteria at this stage, however, it is impossible to make a possible IE diagnosis. Therefore, when confirmation of the diagnosis of IE may take time, a prompt empirical therapy after blood sampling for culture may help to cure the patient.
The Duke scoring system has been approved and is widely used. It appears specific, but its sensitivity depends on the range and quality of microbiological testing and echocardiography.2 According to the American Heart Association, the variability in the clinical presentation of IE requires a diagnostic strategy that will be both sensitive for disease detection and specific for its exclusion across all the forms of the disease.23 The results of our study suggest that this aim could be achieved by first using a combination of non-specific clinical and biological criteria that should be tested in order to identify both low and high risk patients, and thereafter specifically validate or eliminate the diagnosis made using the major Duke criteria.
However, our study has some limitations, including the fact that although the collection of the data was prospective, the study itself was retrospective as it used data that had not been collected for this purpose. Therefore, the next step will be to prospectively validate the scoring system in an independent cohort of patients with suspicion of IE, as the preliminary results of our prospective study confirmed the results of the retrospective study.
Funding
No funding was sought or obtained for this study.
Transparency declarations
No conflicts of interest to declare.
H. R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
We acknowledge Christophe Rogier, MD, PhD, for reviewing the manuscript prior to submission.
References
- 1.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardographic findings. Am J Med. 1994;96:200–9. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 2.Raoult D, Casalta JP, Richet H, et al. Contribution of systematic serological testing in the diagnosis of infective endocarditis. J Clin Microbiol. 2005;43:5238–42. doi: 10.1128/JCM.43.10.5238-5242.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz LJ, Folds D. Evaluation of the Rhema-lex latex agglutination test for detection of rheumatoid factor. J Clin Lab Immunol. 1993;40:187–93. [PubMed] [Google Scholar]
- 4.Tissot-Dupont H, Thirion X, Raoult D. Q-fever serology: cutoff determination for immunofluorescence. Clin Diagn Lab Immunol. 1994;1:189–96. doi: 10.1128/cdli.1.2.189-196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fournier PE, Lelievre H, Eykyn SJ, et al. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine. 2001;80:245–51. doi: 10.1097/00005792-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Black CM, Johnson JE, Farskey CE, et al. Antigenic variation among strains of Chlamydia pneumoniae. J Clin Microbiol. 1991;29:1312–6. doi: 10.1128/jcm.29.7.1312-1316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander TS, Gray LD, Kraft JA, et al. Performance of Meridian ImmunoCard Mycoplasma test in a multicenter clinical trial. J Clin Microbiol. 1996;34:1180–3. doi: 10.1128/jcm.34.5.1180-1183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson HW, Reingold AL, Brake BJ, et al. Reactivity of serum from patients with suspected legionellosis against 29 antigens of Legionellaceae and Legionella-like organisms by indirect immuno-fluorescence assay. J Infect Dis. 1983;147:23–31. doi: 10.1093/infdis/147.1.23. [DOI] [PubMed] [Google Scholar]
- 9.Tonder O. Indirect haemagglutination for demonstration of antibodies to Aspergillus fumigatus. Acta Pathol Microbiol Scand Microbiol Immunol. 1974;82:167–70. doi: 10.1111/j.1699-0463.1974.tb02385.x. [DOI] [PubMed] [Google Scholar]
- 10.Krieg NR, Holt JG. Bergey's Manual of Systematic Bacteriology, Volume 1. Baltimore, MD, USA: Williams & Wilkins; 1984. [Google Scholar]
- 11.Drancourt M, Raoult D. Characterization of mutations in the rpoB gene in naturally rifampicin-resistant Rickettsia species. Antimicrob Agents Chemother. 1999;43:2400–3. doi: 10.1128/aac.43.10.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fournier PE, Raoult D. Non-culture laboratory methods for the diagnosis of infectious endocarditis. Curr Infect Dis Rep. 1999;1:136–41. doi: 10.1007/s11908-996-0020-x. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg D, Kunzli P, Vogt R, et al. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J Clin Microbiol. 1997;35:2733–9. doi: 10.1128/jcm.35.11.2733-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greub G, Lepidi H, Rovery C, et al. Diagnosis of infectious endocarditis in patients undergoing valve surgery. Am J Med. 2005;118:230–8. doi: 10.1016/j.amjmed.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. A case–control study. Am J Clin Pathol. 2000;114:880–9. doi: 10.1309/R0KQ-823A-BTC7-MUUJ. [DOI] [PubMed] [Google Scholar]
- 16.Bruneval P, Choucair J, Paraf F, et al. Detection of fastidious bacteria in cardiac valves in cases of blood culture negative endocarditis. J Clin Pathol. 2001;54:238–40. doi: 10.1136/jcp.54.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepidi H, Durack DT, Raoult D. Diagnostic methods current best practices and guidelines for histologic evaluation in infective endocarditis. Infect Dis Clin North Am. 2002;16:339–61. doi: 10.1016/s0891-5520(02)00005-3. [DOI] [PubMed] [Google Scholar]
- 18.Woods GL, Walker DH. Detection of infection or infectious agents by use of cytologic and histologic stains. Clin Microbiol Rev. 1996;9:382–404. doi: 10.1128/cmr.9.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 20.Von Reyn CF, Levy BS, Arbeit RD, et al. Infective endocarditis: an analysis based on strict case definitions. Ann Intern Med. 1981;94:505–17. doi: 10.7326/0003-4819-94-4-505. [DOI] [PubMed] [Google Scholar]
- 21.Todd AJ, Leslie SJ, MacDougall M, et al. Clinical features remain important for the diagnosis of infective endocarditis in the modern era. QJM. 2006;99:22–31. doi: 10.1093/qjmed/hci150. [DOI] [PubMed] [Google Scholar]
- 22.Lamas CC, Eykyn SJ. Suggested modifications of the Duke criteria for the clinical diagnosis of native valve and prosthetic valve endocarditis: analysis of 118 pathologically proven cases. Clin Infect Dis. 1997;25:713–9. doi: 10.1086/513765. [DOI] [PubMed] [Google Scholar]
- 23.Bayer AS, Bolger AF, Taubert KA, et al. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998;98:2936–48. doi: 10.1161/01.cir.98.25.2936. [DOI] [PubMed] [Google Scholar]