Abstract
Objectives
Fusidic acid interferes with the release of elongation factor G (EF-G) after the translocation step of protein synthesis. The objective of this study was to characterize the fusidic acid stimulon of a fusidic acid-susceptible strain of Staphylococcus aureus (SH1000).
Methods
S. aureus microarrays and real-time PCR determined transcriptome alterations occurring in SH1000 grown with fusidic acid. The Staphylococcus aureus microarray meta-database (SAMMD) compared and contrasted the SH1000 fusidic stimulon with 89 other S. aureus transcriptional datasets. Fusidic acid gradient analyses with mutant-parent strain pairs were used to identify genes required for intrinsic fusidic acid susceptibility identified during transcriptional analysis.
Results
Many genes altered by fusidic acid challenge are associated with protein synthesis. SAMMD analysis determined that the fusidic acid stimulon has the greatest overlap with the S. aureus cold shock and stringent responses. Six out of nine peptidoglycan hydrolase genes making up the two component YycFG regulon were also up-regulated by fusidic acid, as were a carboxylesterase gene (est) and two putative drug efflux pump genes (emr-qac1 and macA). Genes down-regulated by fusidic acid induction encoded a putative secreted acid phosphatase and a number of protease genes. Roles for the agr operon, the peptidoglycan hydrolase gene isaA and two proteases (htrA1 and htrA2) in the expression of fusidic acid susceptibility were revealed.
Conclusions
The SH1000 fusidic acid stimulon includes genes involved with two stress responses, YycFG-regulated cell wall metabolism, drug efflux, and protein synthesis and turnover.
Keywords: transcriptomics, qacA efflux, carboxypeptidase, yycFG, staphylococcal secretory antigen
Introduction
During 2005 in the USA, the rate of invasive infection by methicillin-resistant Staphylococcus aureus (MRSA) was 31.8 per 100000.1 The emergence of community-acquired MRSA infections continues to be a major cause for concern.1,2 Fusidic acid is a steroid antibiotic used to treat serious infections caused by S. aureus and is an alternative antimicrobial for treatment of disease caused by MRSA.3 This drug is usually utilized in antimicrobial combination therapies, since resistance to this drug can emerge when used as a monotherapy.3
Fusidic acid inhibits protein synthesis by interfering with the release of elongation factor G (EF-G) after it has functioned in the translocation step on the ribosome.4 Clinical fusidic acid resistance in S. aureus is mediated by two mechanisms, including mutations in the gene that encodes the target of fusidic acid, EF-G (historically referred to as fusA).5,6 Resistance to this drug can also follow the acquisition of horizontally-transferred elements such as the fusidic acid resistance gene 1 (far1 or fusB)7 or other homologues of this gene.8 New evidence suggests that FusB binds to EF-G and protects it from fusidic acid binding.9 Similar to methicillin and fluoroquinolone resistance expression by S. aureus, both fusA- and far1-mediated fusidic acid-resistant strains express heterogeneous resistance to this drug in vitro by producing cell populations that differ in the level of fusidic acid to which they are resistant to.10
One mechanism of laboratory-selected resistance to fusidic acid is conferred by mutation(s) within rplF which encodes the ribosomal protein L6.11 Other factors that contribute to reduced susceptibility of fusidic acid in the laboratory include efflux pumps (e.g. MdeA and NorA)12 and growth in the presence of non-steroidal anti-inflammatories (NSAIDS).13,14 It is unclear whether these mechanisms contribute to the clinical fusidic acid resistance mechanisms discussed above. Growth of S. aureus in the presence of growth-inhibitory fusidic acid concentrations and NSAIDS in the laboratory can also increase the mutation frequency at which fusidic acid-resistant mutants arise.15
Numerous studies have analysed the effect of antibiotics on the bacterial transcriptome in an effort to understand how bacteria respond to mechanistically unrelated antimicrobials.16–21 Analysis of these antimicrobial ‘shock’ stimulons brings us closer to understanding the overall antimicrobial-specific pathways that lead to reduced bacterial growth and potential cidal activity. These studies also reveal intrinsic antimicrobial resistance mechanisms utilized by bacteria to stave off the harmful effects of these toxic compounds, and prepare their population for potential mutational responses.
In order to understand how a staphylococcal cell population responds to fusidic acid, microarray experiments were performed with a fusidic acid-susceptible strain of S. aureus exposed to this unique antimicrobial. This approach has led to the identification of a number of genes that affect intrinsic fusidic acid susceptibility.
Materials and methods
Bacterial strains and gradient plate analyses
Fusidic acid gradient plate analyses were performed in triplicate as previously described.22 S. aureus strains S6C and RN6390 and their agr mutants, S6Cagr::kan, RN3690agr::kan, have been previously described.23 htrA1 and htrA2 single and double knockout mutants, RN6390htrA1::cat, RN6390htrA2::spc, RN6390htrA1::cathtrA2::spc, COLhtrA1::cat, COLhtrA2::spc, and COLhtrA1::cathtrA2::spc and parent strain COL24 were gifts from Candice Rigoulay and Alexandra Gruss of the Institut National de la Recherche Agronomique. Strains SH1000,25 SH1000isaA::tet and SH1000sceD::kan26 were kindly provided by Simon Foster of the University of Sheffield.
Microarray analysis
S. aureus pan-genome microarrays were utilized to determine transcriptome alterations occurring in S. aureus grown in the presence of fusidic acid. Total bacterial RNA was isolated as previously described14 from S. aureus strain SH1000 grown in Mueller–Hinton broth to an OD580 of 1.0 (37°C, 200 rpm) and then induced with 2 mg/L fusidic acid for 15 min. This RNA and RNA isolated from untreated SH1000 cultures were then converted to fluorescently labelled cDNA and hybridized to S. aureus microarrays version 4 (NIAID's Pathogen Functional Genomics Resource Center, http://pfgrc.jcvi.org/index.php/microarray/array_description/staphylococcus_aureus/version4.html) as previously described.14 The transcriptome data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO platform accession number GPL7072, and series accession number GSE12210 (http://www.ncbi.nlm.nih.gov/geo/).
The Staphylococcus aureus microarray meta-database (SAMMD) presently curates data from 89 publicly available microarray experiments (only genes altered 2-fold or more) and allows for the comparison of microarray data obtained from different protocols and experiments.27 SAMMD was used to analyse the transcriptome generated from SH1000 grown in the presence of fusidic acid. The ORF IDs of genes differentially regulated under fusidic acid induction were mapped to strain COL IDs and redundancies were removed. This list of non-redundant IDs was then used as the input to search against SAMMD,27 and the resulting data were prepared as a network file and analysed using Cytoscape.28
Quantitative real-time PCR to confirm microarray data was performed as previously described29 with the primers indicated in Table 1. Following growth of SH1000 with and without fusidic acid addition at various time points, the cultures were serially diluted with fresh MHB, inoculated onto Mueller–Hinton agar and the total surviving cfu/mL were determined after 24 h.
Table 1.
Primer sets used for real-time PCR
| Gene | Sequence 5′→3′ | Nucleotide position based on COL genome | |
|---|---|---|---|
| emr-qac | forward | GGGATTCATGTTAGTAAACGGTATTT | 2406731–2406757 |
| reverse | GTTTTTCAGGTGGATAAATTGTAATAA | 2406943–2406870 | |
| est | forward | AATACTGAAATCTAGTCCTTTCGTTTG | 872408–872435 |
| reverse | ACATCGTTACAATACCCTTTACATCTC | 872575–872548 | |
| yjbG1 | forward | GTCGGTAGAACAAGTATTAGCAACTTT | 1430049–1430076 |
| reverse | AACGATTTGAAACTTTTACGTCTAAAC | 1430250–1430223 | |
| htrA1 | forward | GATCGAAAACTTGATGAAAAA | 1037658–1037679 |
| reverse | TTTGCCGATGTCTTTGTATTTG | 1037843–1037821 | |
| hisS | forward | TATTACATTAAGACCTGAGGGAACAG | 1715271–1715297 |
| reverse | CTAATACTTCTGCATCTACGCTAGGAT | 1715483–1715456 | |
| rrs | forward | TCGTGTCGTGAGATGTTG | 530226–530406 |
| reverse | CTGCCCTTTGTATTGTCC | 530388–530406 |
Results and discussion
Fusidic acid stimulon and SAMMD analysis
We empirically determined the subinhibitory concentrations of fusidic acid to be used in this study by measuring the number of viable cells (cfu/mL) at various exposure time points. Cell viability was not altered after exposure of strain SH1000 to 2 and 4 mg/L fusidic acid for up to 4 h. Based on these data, we exposed the strain SH1000 to 2 mg/L fusidic acid for 15 min to induce the transcriptome in this study. This transcriptome thereby is representative of a cell population that is not experiencing events leading to cell death.
Microarray data demonstrated that fusidic acid induction in SH1000 led to the up-regulation of 272 genes and down-regulation of 272 genes 2-fold or more. The alteration in expression of five of these genes (emr-qac1, est, yjbG1, htrA1 and hisS) was confirmed by real-time PCR (Table 2). Of interest, fusidic acid induction led to the up-regulation of fusA (2.1-fold) and 25 ribosomal protein genes (2–6-fold), and the down-regulation of 21 protein degradation genes (−2- to −11-fold) and 10 tRNA aminoacylation genes (−2- to −4-fold) (Table 2). Since fusidic acid inhibits protein synthesis, it was expected that the expression of genes required for protein synthesis would be altered.
Table 2.
Genes with greatest alteration following growth of SH1000 in the presence of 2 mg/L fusidic acid
| Fold change in gene expression |
||||
|---|---|---|---|---|
| Gene | Function | Locus ID | Microarray | Real-time PCR |
| 20 up-regulated genes | ||||
| ssaA1 | secretory antigen precursor (255 aa in length) | SACOL2581 | 22.2 | |
| emr-qac1 | predicted drug resistance transporter | SACOL2347 | 17.2 | 7.4 |
| hypothetical protein (49 aa in length) | SACOL0674 | 14.7 | ||
| ssaA3 | partial SsaA homologue (166 aa in length) | SACOL2295 | 14.5 | |
| ssaA2 | SsaA homologue (267 aa in length) | SACOL2291 | 14.3 | |
| ssaA4 | partial SsaA homologue (143 aa in length) | SACOL2557 | 13.6 | |
| macA | predicted drug resistance transporter | SACOL2348 | 13.1 | |
| est | putative carboxylesterase | SACOL0845 | 12.2 | 16.8 |
| cation efflux family protein | SACOL2138 | 11.4 | ||
| hypothetical protein | SACOL1845 | 10.4 | ||
| sceD | putative SceD precursor | SACOL2088 | 8.9 | |
| IS1272-related, transposase, degenerate | SACOL1442 | 8.7 | ||
| czrA | zinc metabolism, transcriptional regulator CzrA | SACOL2137 | 8.7 | |
| rnpA | ribonuclease P protein component | SACOL2739 | 8.4 | |
| cspB | cold shock protein, CSD family | SACOL2731 | 8.2 | |
| hypothetical protein | SACOL0406 | 7.8 | ||
| fruA | phosphotransferase system, fructose-IIC component | SACOL2546 | 7.3 | |
| arsB | arsenical pump membrane protein homologue | SACOL1823 | 7.0 | |
| conserved hypothetical protein | SACOL0639 | 6.9 | ||
| similar to ABC transporter (ATP-binding protein) | SACOL2356 | 6.7 | ||
| 20 down-regulated genes | ||||
| sapS | secreted acid phosphatase, e(P4) family | SACOL0303 | −11.8 | |
| yjbG1 | oligoendopeptidase, putative | SACOL1419 | −11.1 | −25.9 |
| yjbG2 | oligoendopeptidase, putative | SACOL1005 | −9.6 | |
| htrA1 | serine protease HtrA | SACOL1777 | −9.4 | −13.0 |
| hypothetical protein | SACOL0851 | −8.0 | ||
| CBS domain protein | SACOL0921 | −7.8 | ||
| lipoate-protein ligase A family protein | SACOL1034 | −7.8 | ||
| purK | phosphoribosylaminoimidazole carboxylase | SACOL1074 | −7.4 | |
| carbon fixation chain hypothetical protein | SACOL2461 | −7.2 | ||
| similar to inosine-uridine preferring nucleoside hydrolase | SACOL0225 | −7.1 | ||
| similar to phytoene dehydrogenase | SACOL2579 | −7.1 | ||
| conserved hypothetical protein | SACOL0922 | −6.4 | ||
| ABC transporter, ATP-binding protein | SACOL2462 | −6.4 | ||
| conserved hypothetical protein | SACOL2241 | −6.1 | ||
| hypothetical protein | SACOL1115 | −6.1 | ||
| ABC transporter, substrate-binding protein | SACOL0688 | −5.9 | ||
| ebpS | elastin binding protein | SACOL1522 | −5.8 | |
| similar to choline transporter ATP-binding protein | SACOL0781 | −5.7 | ||
| hisS | histidyl-tRNA synthetase | SACOL1686 | −5.6 | −14.6 |
| hypothetical protein | SACOL0850 | −5.6 | ||
aa, amino acids.
SAMMD analysis compared the fusidic acid transcriptome with 89 S. aureus transciptomes and revealed that fusidic acid induction up-regulated 20 and down-regulated 6 of the 99 known and predicted transcriptional regulator genes in the S. aureus genome. SAMMD analysis also revealed that this global response to fusidic acid was second only to induction with the protein synthesis inhibitor mupirocin which leads to the alteration of most of these regulators of any condition studied (35/99 total). Mupirocin selectively binds to the bacterial isoleucyl-tRNA synthetase, which leads to a halt in protein synthesis and induction of the stringent response.30–32
The staphylococcal accessory regulator sarA, which when inactivated increases susceptibility to fusidic acid,22 was up-regulated by fusidic acid induction, as were the sarA homologues sarS and sarV (all at 1.7-fold). Genes of the agr operon (agrABD) which controls S. aureus virulence factor production were also up-regulated by fusidic acid induction (1.8–2.7-fold). Fusidic acid gradient plate analysis revealed that both S6Cagr::kan and RN6390agr::kan grew to smaller distances on a fusidic acid gradient compared with their respective parent strains S6C and RN6390 (Table 3). This indicates that a functional agr operon is required to protect the staphylococcal cell against fusidic acid insult. The inactivation of agr function has also been associated with the acquisition of the vancomycin- intermediate susceptibility phenotype by S. aureus33 and leads to reduced methicillin resistance expression as well.34
Table 3.
Mean distances (mm) grown on fusidic acid gradients (±SD)
| Strain | Fusidic acid gradient |
|---|---|
| 0→0.15 mg/L | |
| RN6390 | 16.3 ± 0.6 |
| RN6390agr::kan | 11.6 ± 1.2* |
| S6C | 27 ± 0 |
| S6Cagr::kan | 22.7 ± 0.6* |
| 0→0.1 mg/L | |
| RN6390 | 29.3 ± 1.2 |
| RN6390htrA1::cat | 33.7 ± 1.2* |
| RN6390htrA2::spc | 38.7 ± 1.2* |
| RN6390htrA1::cat htrA2::spc | 31.7 ± 2.9 |
| 0→0.05 mg/L | |
| COL | 60.0 ± 0 |
| COLhtrA1::cat | 23.3 ± 2.9* |
| COLhtrA2::spc | 18.7 ± 1.2* |
| COLhtrA1::cat htrA2::spc | 20.7 ± 1.2* |
| SH1000 | 65.3 ± 0.6 |
| SH1000 isaA::tet | 51.7 ± 1.5* |
| SH1000 sceD::kan | 64.0 ± 1.0 |
*P ≤ 0.02.
A significant portion of the genes altered by fusidic acid induction are also associated with previously well-described bacterial stress stimulons [see Supplementary data, available at JAC Online (http://jac.oxfordjournals.org/)].
Fifty-one genes down-regulated by fusidic acid induction are also down-regulated in the cold shock response32 (see Supplementary data). Genes encoding cold shock proteins cspB (Table 2) and cspC (77% amino acid identity to cspB) were up-regulated by fusidic acid as well (8.2- and 1.8-fold, respectively). cspB is also up-regulated in S. aureus following mupirocin induction.32 A classic study from Neidhardt's laboratory demonstrated the induction of the cold shock response in Escherichia coli by fusidic acid,35 and cold shock proteins are also up-regulated in Bacillus subtilis following chloramphenicol and erythromycin induction.17
The stringent response is mediated by a large gene network geared to respond to starvation conditions created by either the lack of energy or amino acid starvation, such as that induced by mupirocin treatment.32,36 One hundred and three genes down-regulated by mupirocin induction are also down-regulated by fusidic acid induction (see Supplementary data). relA which encodes the (p)ppGpp synthetase of the stringent response30,36,37 is down-regulated by mupirocin32 and fusidic acid induction (−3.4-fold). Previous studies in E. coli have demonstrated that translational inhibitors decrease the synthesis of (p)ppGpp.38,39 Proteomic studies have also demonstrated that exposure of B. subtilis to translation inhibitors can alter the expression level of stringently controlled proteins.17
These findings demonstrate that a large number of the down-regulated genes of the cold shock and stringent response form a major portion of the fusidic acid stimulon. It is well known that the induction of one bacterial stress system can impart on a bacterial cell a cross-protective response against other stressing environments.40 Anderson et al.32 already reported that cold shock and the stringent response share numerous response genes, indicating that these genes may be representative of a generalized S. aureus stress response. Most of the fusidic acid stimulon, cold shock and stringent response (mupirocin induction) overlaps include genes encoding ribosomal proteins, tRNA synthetases and proteins involved with protein degradation, folding and stabilization.
Fusidic acid-induced YycFG controlled regulon
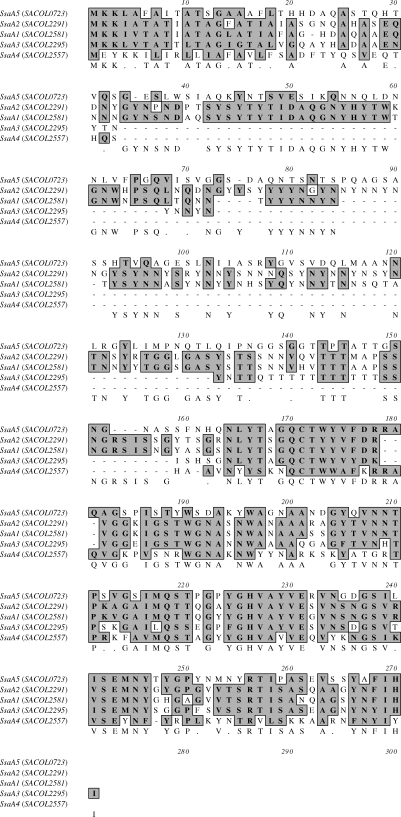
The gene encoding the staphylococcal secretory antigen ssaA1and three ssaA paralogues (ssaA2, ssaA3 and ssaA4) were highly induced in response to fusidic acid (Table 2). ssaA2, which showed the greatest change in expression, is required for the full expression of resistance to MLSB antibiotics41 and ssaA1 is a significant staphylococcal antigen.42 ssaA2 and ssaA3 are transcribed in the same direction and are also found close together on the S. aureus chromosome (separated by 2917 bp) (Table 1). All of these fusidic acid-induced SsaAs, and a fifth SsaA homologue (SACOL0723, ssaA5) not induced by fusidic acid, share the greatest identity at their terminal ends and SsaA3 and SsaA4 are missing an internal sequence (Figure 1). The S. aureus SsaA homologues have also been reported to share a common cysteine, histidine-dependent amidohydrolases/peptidases-amidase domain and amino-terminal signal sequences, suggesting that these paralogues play a role in cell wall metabolism.43 In addition to the ssaA paralogues, other genes associated with cell wall metabolism were also altered in SH1000 following growth in the presence of fusidic acid. Two recently characterized peptidoglycan hydrolases (sceD and isaA)26 were up-regulated by fusidic acid induction (8.9- and 1.9-fold). Interestingly, like SsaA, IsaA also acts as an immunodominant S. aureus antigen.44 While inactivation of sceD did not alter distances grown on the fusidic acid gradient, the inactivation of isaA in SH1000 led to increased susceptibility to fusidic acid (Table 3). Expression of all ssaA paralogues, isaA and sceD are positively regulated by yycFG and represent part of the yycFG-controlled autolytic regulon comprising a total of nine autolysin genes.43
Figure 1.
Clustal alignment of SsaA paralogues.
The yycFGHIJ operon of S. aureus encodes at least one essential two-component regulatory system YycFG, where YycF acts as a response regulator, while YycG acts as the sensor histidine kinase.45 Cells starved of yycFG demonstrate decreased peptidoglycan biosynthesis and turnover, increased peptidoglycan cross-linking and glycan chain length, and increased resistance to Triton X-100- and lysostaphin-stimulated whole cell lysis.43 The S. aureus response regulator YycF has been shown to bind the ssaA1 promoter.46 Growth of SH1000 in the presence of fusidic acid led to the down-regulation of yycF and yycG −2- and −1.8-fold, respectively, indicating that a reduction in yycFG transcription might be required for the up-regulation of the ssaA paralogues as well as isaA and sceD, at least in the presence of fusidic acid insult. We speculate that since fusidic acid is readily incorporated into the cell membrane,47 alterations in cell wall synthesis caused by ssaA paralogues and sceD and isaA up-regulation might contribute to the removal of the drug from the cell's exterior at least.
Other select genes induced by fusidic acid
Another highly up-regulated fusidic acid gene encodes a carboxylesterase (est, 12.2-fold). Interestingly, the fusH gene of Streptomyces lividans 66 actually encodes a fusidic acid modifying carboxylesterase (521 amino acids) which inactivates its antimicrobial activity.48 It is possible that Est (247 amino acids) might contribute to the deactivation of fusidic acid, although Est demonstrates only 10% identity with the N-terminus of FusH.
Two of the most highly fusidic acid-induced genes (erm-qac1 and macA) encode putative drug efflux pumps (Table 2). erm-qac1 (SACOL2347) and macA (SACOL2348) are separated by 13 bp and encode a putative major facilitator protein (643 amino acids) and a putative macrolide transporter subunit (215 amino acids), respectively. Erm-qac1 demonstrates 18% identity across its entire length with the well-characterized S. aureus QacA49 multidrug efflux pump, while MacA demonstrates 15% amino acid identity with the MacA subunit of an E. coli macrolide efflux pump.50 These pumps can protect the cell from multiple mechanistically unrelated toxic compounds including quaternary ammonium compounds, chlorhexidine gluconate, triclosan, ethidium, carbonyl cyanide m-chlorophenylhydrazone, nalidixic acid, erythromycin and thiolactomycin.50–54 Genes encoding these types of efflux pumps can also be induced by antibiotics other than fusidic acid, such as the protein synthesis inhibitor tetracycline and the intercalating agent ethidium.53,54 Further upstream of macA (121 bp) lies a divergently encoded putative tetR regulator gene (183 amino acids) which demonstrates 15% amino acid identity with the well-characterized S. aureus TetR homologue IcaR55 and 12% identity with QacR, which controls qacA transcription.56–58 Like the relationship between macA and tetR, qacR is also separated by 178 bp from qacA and these genes are divergently transcribed on S. aureus plasmid pSK1.59 Another S. aureus erm-qac gene (SACOL2413, 26% identity to Emr-Qac1) was also up-regulated by mupirocin induction.32 It is possible that these efflux gene products contribute to the removal of fusidic acid and mupirocin from inside the cells interior, thereby providing a degree of protection from the toxicity of these drugs.
Genes down-regulated by fusidic acid induction
The most highly down-regulated fusidic acid-induced gene sapS encodes a putative secreted acid phosphatase (−11.8-fold) that has recently been characterized and belongs to the class C family of non-specific acid phosphatases.60 Non-specific acid phosphatases are thought to be involved in cleaving nucleotides and sugar phosphates into dephosphorylated products that can be transported across the cytoplasmic membrane.61 It is possible that the fusidic acid-stressed cell retains phosphorylated organic compounds and reduces the uptake of cleaved compounds since growth and metabolism are altered by fusidic acid exposure.
A number of protease genes were also highly down-regulated by growth in the presence of fusidic acid. Two of these genes yjbG1 and yjbG2 (−11- and −9.6-fold, respectively) demonstrate 54% and 27% identity with a PepF-like oligopeptidase of B. subtilis (YjbG).62 Two other genes expressing surface proteases that contribute to S. aureus pathogenicity24 (htrA1 and htrA2) were also down-regulated by fusidic acid induction (−9.4- and −5.2-fold, respectively) (Table 2). Inactivation of either htrA1 or htrA2 singly in the RN6390 background led to reduced fusidic acid susceptibility, while inactivation of both genes did not alter fusidic acid resistance expression in this strain (Table 3). Inactivation of either htrA1 or htrA2 singly or in combination in the COL background, however, led to increased fusidic acid susceptibility (Table 3). It was previously demonstrated that the inactivation of these genes leads to strain-specific phenotypic differences, so the unique effects of htrA inactivation on fusidic acid resistance in the COL and RN6390 background was not unexpected.24 A reduction in protease production might allow for the preservation of proteins required for cell survival under the influences of fusidic acid, which, under normal circumstances, might be turned over. At the same time, protease gene transcription might be logically repressed when protein biosynthesis is inhibited in a cell.
Conclusions
We have now characterized the fusidic acid stimulon of a fusidic acid-susceptible S. aureus strain. The fusidic acid stimulon includes many genes associated with protein synthesis, and SAMMD analysis revealed that the fusidic acid global response includes alteration in the expression of numerous regulators. Of these, the agr operon is determined to protect against fusidic acid. SAMMD also reveals that the fusidic acid stimulon has the greatest overlap with the cold shock and stringent response. Many autolysin genes making up a large part of the YycFG regulon are also up-regulated by fusidic acid induction, as are a carboxylesterase and two putative drug efflux pumps. One of the YycFG-controlled genes isaA is required for the full expression of fusidic acid susceptibility. Genes down-regulated by fusidic acid induction encode a putative secreted acid phosphatase and a number of protease genes of which htrA1 and htrA2 are proven to affect wild-type fusidic acid susceptibility levels in a strain-specific manner.
Funding
We wish to acknowledge the former and ongoing support from theNational Institutes of Health: S06 GM008136-32 (J. E. G., NMSU SCORE PROGRAM); R25 GM07667-30 (NMSU-MARC PROGRAM); S06 GM61222-05 (A. D., NMSU-MBRS-RISE PROGRAM); and P20RR016480 from the NM-INBRE Program of the National Center for Research Resources. J. E. G. and S. Z. also acknowledge the former and ongoing support of the NMSU Undergraduate Howard Hughes Medical Institute Program (52005881).
Transparency declarations
We do not have any financial conflicts of interest and the funders have not played any decision-making role in the research.
Supplementary data
A Supplementary data file is available at JAC Online (http://jac.oxfordjournals.org/).
Supplementary Material
Acknowledgements
This work was presented in part at a poster session of the Forty-seventh Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 2007. The S. aureus microarrays were obtained through NIAID's Pathogen Functional Genomics Resource Center, managed and funded by the Division of Microbiology and Infectious Diseases, NIAID, NIH, DHHS and operated by The Institute for Genomic Research (TIGR).
References
- 1.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Fridkin SK, et al. Community-associated methicillin-resistant Staphylococcus aureus and healthcare risk factors. Emerg Infect Dis. 2006;12:1991–3. doi: 10.3201/eid1212.060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howden BP, Grayson ML. Dumb and dumber—the potential waste of a useful antistaphylococcal agent: emerging fusidic acid resistance in Staphylococcus aureus. Clin Infect Dis. 2006;42:394–400. doi: 10.1086/499365. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka N, Kinoshita T, Masukawa H. Mechanism of protein synthesis inhibition by fusidic acid and related antibiotics. Biochem Biophys Res Commun. 1968;30:278–83. doi: 10.1016/0006-291x(68)90447-6. [DOI] [PubMed] [Google Scholar]
- 5.Besier S, Ludwig A, Brade V, et al. Compensatory adaptation to the loss of biological fitness associated with acquisition of fusidic acid resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:1426–31. doi: 10.1128/AAC.49.4.1426-1431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagaev I, Bjorkman J, Andersson DI, et al. Biological cost and compensatory evolution in fusidic acid-resistant Staphylococcus aureus. Mol Microbiol. 2001;40:433–9. doi: 10.1046/j.1365-2958.2001.02389.x. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien FG, Price C, Grubb WB, et al. Genetic characterization of the fusidic acid and cadmium resistance determinants of Staphylococcus aureus plasmid pUB101. J Antimicrob Chemother. 2002;50:313–21. doi: 10.1093/jac/dkf153. [DOI] [PubMed] [Google Scholar]
- 8.O'Neill AJ, McLaws F, Kahlmeter G, et al. Genetic basis of resistance to fusidic acid in staphylococci. Antimicrob Agents Chemother. 2007;51:1737–40. doi: 10.1128/AAC.01542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Neill AJ, Chopra I. Molecular basis of fusB-mediated resistance to fusidic acid in Staphylococcus aureus. Mol Microbiol. 2006;59:664–76. doi: 10.1111/j.1365-2958.2005.04971.x. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien FG, Botterill CI, Endersby TG, et al. Heterogeneous expression of fusidic acid resistance in Staphylococcus aureus with plasmid or chromosomally encoded fusidic acid resistance genes. Pathology. 1998;30:299–303. doi: 10.1080/00313029800169486. [DOI] [PubMed] [Google Scholar]
- 11.Norstrom T, Lannergard J, Hughes D. Genetic and phenotypic identification of fusidic acid-resistant mutants with the small-colony- variant phenotype in Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:4438–46. doi: 10.1128/AAC.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, O'Toole PW, Shen W, et al. Novel chromosomally encoded multidrug efflux transporter MdeA in Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:909–17. doi: 10.1128/AAC.48.3.909-917.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price CT, O'Brien FG, Shelton BP, et al. Effects of salicylate and related compounds on fusidic acid MICs in Staphylococcus aureus. J Antimicrob Chemother. 1999;44:57–64. doi: 10.1093/jac/44.1.57. [DOI] [PubMed] [Google Scholar]
- 14.Riordan JT, Muthaiyan A, Van Voorhies W, et al. Response of Staphylococcus aureus to salicylate challenge. J Bacteriol. 2007;189:220–7. doi: 10.1128/JB.01149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price CT, Gustafson JE. Increases in the mutation frequency at which fusidic acid-resistant Staphylococcus aureus arise with salicylate. J Med Microbiol. 2001;50:104–6. doi: 10.1099/0022-1317-50-1-104. [DOI] [PubMed] [Google Scholar]
- 16.Aakra A, Vebo H, Snipen L, et al. Transcriptional response of Enterococcus faecalis V583 to erythromycin. Antimicrob Agents Chemother. 2005;49:2246–59. doi: 10.1128/AAC.49.6.2246-2259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin JT, Connelly MB, Amolo C, et al. Global transcriptional response of Bacillus subtilis to treatment with subinhibitory concentrations of antibiotics that inhibit protein synthesis. Antimicrob Agents Chemother. 2005;49:1915–26. doi: 10.1128/AAC.49.5.1915-1926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirz RT, Jones MB, Gingles NA, et al. Complete and SOS-mediated response of Staphylococcus aureus to the antibiotic ciprofloxacin. J Bacteriol. 2007;189:531–9. doi: 10.1128/JB.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Utaida S, Dunman PM, Macapagal D, et al. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology. 2003;149:2719–32. doi: 10.1099/mic.0.26426-0. [DOI] [PubMed] [Google Scholar]
- 20.Muthaiyan A, Silverman JA, Jayaswal RK, et al. Transcriptional profiling reveals that daptomycin induces the Staphylococcus aureus cell wall stress stimulon and genes responsive to membrane depolarization. Antimicrob Agents Chemother. 2008;52:980–90. doi: 10.1128/AAC.01121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAleese F, Wu SW, Sieradzki K, et al. Overexpression of genes of the cell wall stimulon in clinical isolates of Staphylococcus aureus exhibiting vancomycin-intermediate-S. aureus-type resistance to vancomycin. J Bacteriol. 2006;188:1120–33. doi: 10.1128/JB.188.3.1120-1133.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Leary JO, Langevin MJ, Price CT, et al. Effects of sarA inactivation on the intrinsic multidrug resistance mechanism of Staphylococcus aureus. FEMS Microbiol Lett. 2004;237:297–302. doi: 10.1016/j.femsle.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 23.Blevins JS, Beenken KE, Elasri MO, et al. Strain-dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus. Infect Immun. 2002;70:470–80. doi: 10.1128/IAI.70.2.470-480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rigoulay C, Entenza JM, Halpern D, et al. Comparative analysis of the roles of HtrA-like surface proteases in two virulent Staphylococcus aureus strains. Infect Immun. 2005;73:563–72. doi: 10.1128/IAI.73.1.563-572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horsburgh MJ, Aish JL, White IJ, et al. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol. 2002;184:5457–67. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stapleton MR, Horsburgh MJ, Hayhurst EJ, et al. Characterisation of IsaA and SceD, two putative lytic transglycosylases of Staphylococcus aureus. J Bacteriol. 2007;189:7316–25. doi: 10.1128/JB.00734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagarajan V, Elasri MO. SAMMD: Staphylococcus aureus microarray meta-database. BMC Genomics. 2007;8:351. doi: 10.1186/1471-2164-8-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riordan JT, O'Leary JO, Gustafson JE. Contributions of sigB and sarA to distinct multiple antimicrobial resistance mechanisms of Staphylococcus aureus. Int J Antimicrob Agents. 2006;28:54–61. doi: 10.1016/j.ijantimicag.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassels R, Oliva B, Knowles D. Occurrence of the regulatory nucleotides ppGpp and pppGpp following induction of the stringent response in staphylococci. J Bacteriol. 1995;177:5161–5. doi: 10.1128/jb.177.17.5161-5165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crosse AM, Greenway DL, England RR. Accumulation of ppGpp and ppGp in Staphylococcus aureus 8325-4 following nutrient starvation. Lett Appl Microbiol. 2000;31:332–7. doi: 10.1046/j.1472-765x.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- 32.Anderson KL, Roberts C, Disz T, et al. Characterization of the Staphylococcus aureus heat shock, cold shock, stringent, and SOS responses and their effects on log-phase mRNA turnover. J Bacteriol. 2006;188:6739–56. doi: 10.1128/JB.00609-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakoulas G, Eliopoulos GM, Moellering RC, Jr, et al. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 2002;46:1492–502. doi: 10.1128/AAC.46.5.1492-1502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piriz Duran S, Kayser FH, Berger-Bachi B. Impact of sar and agr on methicillin resistance in Staphylococcus aureus. FEMS Microbiol Lett. 1996;141:255–60. doi: 10.1111/j.1574-6968.1996.tb08394.x. [DOI] [PubMed] [Google Scholar]
- 35.VanBogelen RA, Neidhardt FC. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:5589–93. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens JC, Artz SW, Ames BN. Guanosine 5′-diphosphate 3′-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Natl Acad Sci USA. 1975;72:4389–93. doi: 10.1073/pnas.72.11.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gentry D, Li T, Rosenberg M, et al. The rel gene is essential for in vitro growth of Staphylococcus aureus. J Bacteriol. 2000;182:4995–7. doi: 10.1128/jb.182.17.4995-4997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969;244:3133–41. [PubMed] [Google Scholar]
- 39.Lund E, Kjeldgaard NO. Protein synthesis and formation of guanosinetetraphosphate. FEBS Lett. 1972;26:306–10. doi: 10.1016/0014-5793(72)80599-4. [DOI] [PubMed] [Google Scholar]
- 40.Gustafson JE, Wilkinson BJ. Staphylococcus aureus as a food pathogen: the staphylococcal enterotoxins and stress response systems. In: Griffiths M,, editor. Understanding Pathogen Behaviour. Cambridge, UK: Woodhead Publishing Limited; 2005. pp. 331–50. [Google Scholar]
- 41.Martin PK, Bao Y, Boyer E, et al. Novel locus required for expression of high-level macrolide-lincosamide-streptogramin B resistance in Staphylococcus aureus. J Bacteriol. 2002;184:5810–3. doi: 10.1128/JB.184.20.5810-5813.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang S, Livesley MA, Lambert PA, et al. Identification of a novel antigen from Staphylococcus epidermidis. FEMS Immunol Med Microbiol. 2000;29:213–20. doi: 10.1111/j.1574-695X.2000.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 43.Dubrac S, Boneca IG, Poupel O, et al. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J Bacteriol. 2007;189:8257–69. doi: 10.1128/JB.00645-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorenz U, Ohlsen K, Karch H, et al. Human antibody response during sepsis against targets expressed by methicillin resistant Staphylococcus aureus. FEMS Immunol Med Microbiol. 2000;29:145–53. doi: 10.1111/j.1574-695X.2000.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 45.Winkler ME, Hoch JA. Essentiality, bypass, and targeting of the YycFG (VicRK) two-component regulatory system in gram-positive bacteria. J Bacteriol. 2008;190:2645–8. doi: 10.1128/JB.01682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubrac S, Msadek T. Identification of genes controlled by the essential YycG/YycF two-component system of Staphylococcus aureus. J Bacteriol. 2004;186:1175–81. doi: 10.1128/JB.186.4.1175-1181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falck E, Hautala JT, Karttunen M, et al. Interaction of fusidic acid with lipid membranes: Implications to the mechanism of antibiotic activity. Biophys J. 2006;91:1787–99. doi: 10.1529/biophysj.106.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von der Haar B, Walter S, Schwapenheuer S, et al. A novel fusidic acid resistance gene from Streptomyces lividans 66 encodes a highly specific esterase. Microbiology. 1997;143:867–74. doi: 10.1099/00221287-143-3-867. [DOI] [PubMed] [Google Scholar]
- 49.Tennent JM, Lyon BR, Midgley M, et al. Physical and biochemical characterization of the qacA gene encoding antiseptic and disinfectant resistance in Staphylococcus aureus. J Gen Microbiol. 1989;135:1–10. doi: 10.1099/00221287-135-1-1. [DOI] [PubMed] [Google Scholar]
- 50.Tikhonova EB, Devroy VK, Lau SY, et al. Reconstitution of the Escherichia coli macrolide transporter: the periplasmic membrane fusion protein MacA stimulates the ATPase activity of MacB. Mol Microbiol. 2007;63:895–910. doi: 10.1111/j.1365-2958.2006.05549.x. [DOI] [PubMed] [Google Scholar]
- 51.Smith K, Gemmell CG, Hunter IS. The association between biocide tolerance and the presence or absence of qac genes among hospital-acquired and community-acquired MRSA isolates. J Antimicrob Chemother. 2008;61:78–84. doi: 10.1093/jac/dkm395. [DOI] [PubMed] [Google Scholar]
- 52.Lomovskaya O, Lewis K. Emr, an Escherichia coli locus for multidrug resistance. Proc Natl Acad Sci USA. 1992;89:8938–42. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theis T, Skurray RA, Brown MH. Identification of suitable internal controls to study expression of a Staphylococcus aureus multidrug resistance system by quantitative real-time PCR. J Microbiol Methods. 2007;70:355–62. doi: 10.1016/j.mimet.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Tanabe H, Yamasak K, Furue M, et al. Growth phase-dependent transcription of emrKY, a homolog of multidrug efflux emrAB genes of Escherichia coli, is induced by tetracycline. J Gen Appl Microbiol. 1997;43:257–63. doi: 10.2323/jgam.43.257. [DOI] [PubMed] [Google Scholar]
- 55.Jeng WY, Ko TP, Liu CI, et al. Crystal structure of IcaR, a repressor of the TetR family implicated in biofilm formation in Staphylococcus epidermidis. Nucleic Acids Res. 2008;36:1567–77. doi: 10.1093/nar/gkm1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grkovic S, Brown MH, Schumacher MA, et al. The staphylococcal QacR multidrug regulator binds a correctly spaced operator as a pair of dimers. J Bacteriol. 2001;183:7102–9. doi: 10.1128/JB.183.24.7102-7109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schumacher MA, Miller MC, Grkovic S, et al. Structural basis for cooperative DNA binding by two dimers of the multidrug-binding protein QacR. EMBO J. 2002;21:1210–8. doi: 10.1093/emboj/21.5.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schumacher MA, Miller MC, Grkovic S, et al. Structural mechanisms of QacR induction and multidrug recognition. Science. 2001;294:2158–63. doi: 10.1126/science.1066020. [DOI] [PubMed] [Google Scholar]
- 59.Rouch DA, Cram DS, DiBerardino D, et al. Efflux-mediated antiseptic resistance gene qacA from Staphylococcus aureus: common ancestry with tetracycline- and sugar-transport proteins. Mol Microbiol. 1990;4:2051–62. doi: 10.1111/j.1365-2958.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 60.Thaller MC, Schippa S, Rossolini GM. Conserved sequence motifs among bacterial, eukaryotic, and archaeal phosphatases that define a new phosphohydrolase superfamily. Protein Sci. 1998;7:1647–52. doi: 10.1002/pro.5560070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rossolini GM, Schippa S, Riccio ML, et al. Bacterial nonspecific acid phosphohydrolases: physiology, evolution and use as tools in microbial biotechnology. Cell Mol Life Sci. 1998;54:833–50. doi: 10.1007/s000180050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanamaru K, Stephenson S, Perego M. Overexpression of the PepF oligopeptidase inhibits sporulation initiation in Bacillus subtilis. J Bacteriol. 2002;184:43–50. doi: 10.1128/JB.184.1.43-50.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.