Abstract
The ligand-activated transcription factor PPARγ is expressed in vascular endothelium where it exerts anti-inflammatory and anti-oxidant effects. However, its role in regulating vascular function remains undefined. We examined endothelial function in transgenic mice expressing dominant negative mutants of PPARγ under the control of an endothelial-specific promoter to test the hypothesis that endothelial PPARγ plays a protective role in the vasculature. Under baseline conditions, responses to the endothelium-dependent agonist acetylcholine (Ach) were not affected in either aorta or the basilar artery in vitro. In response to feeding a high fat diet for 12 weeks, Ach produced dilation that was markedly impaired in the basilar artery of mice expressing dominant negative mutants, but not in mice expressing wildtype PPARγ controlled by the same promoter. Unlike basilar artery, 12 weeks of high fat diet was not sufficient to cause endothelial dysfunction in the aorta of mice expressing dominant negative PPARγ, although it became evident after 25 weeks. The responses to Ach in basilar artery were restored to normal after treatment with a scavenger of superoxide. Baseline blood pressure was only slightly elevated in the transgenic mice, but the pressor response to angiotensin-II was augmented. Thus, interference with PPARγ in the endothelium produces endothelial dysfunction in the cerebral circulation via a mechanism involving oxidative stress. Consistent with its role as a fatty acid sensor, these findings provide genetic evidence that endothelial PPARγ plays a critical role in protecting a range of blood vessels in response to a high fat diet.
Keywords: endothelium, transgenic animals, vascular, oxidative stress, transcription
Introduction
Peroxisome proliferator activated receptor gamma (PPARγ) is a ligand-activated transcription factor targeted by the thiazolidinedione (TZD) class of anti-diabetes medications. Activation of PPARγ by TZDs improves insulin sensitivity and lowers blood pressure in type II diabetes; whereas individuals with dominant negative mutations in PPARγ present with severe insulin resistance, type II diabetes and early onset hypertension 1. Studies in humans and animals suggest that TZDs are generally cardioprotective although their clinical safety has been recently challenged 2. That TZDs can lower blood pressure in the face of weight gain, and water and salt retention by the kidney suggests that the antihypertensive effects may be particularly profound.
Heterozygous mice carrying one normal PPARγ allele and one dominant negative allele (L/+ mice) exhibit a moderate increase in blood pressure 3, 4. We have recently reported that L/+ mice exhibit endothelial dysfunction and hypertrophy and inward remodeling in the cerebral vasculature 4. Oxidative stress was the basis of endothelial dysfunction in the model as superoxide was increased and vascular function was restored to normal by a free radical scavenger. This data provided genetic evidence suggesting a functional role for PPARγ in the vascular wall. However, since this knockin mouse exhibited defective PPARγ signaling in all cells, we were not able to determine whether the abnormalities were due to systemic or vascular-specific interference with PPARγ function.
PPARγ is expressed in both vascular muscle and endothelium. We recently reported that PPARγ plays a critical role in vascular muscle, where it is required to mediate cGMP-dependent signaling from NO released from the endothelium, and is required to inhibit vasoconstriction to endothelin-1 (ET-1) 5. Mice with vascular muscle-specific interference with PPARγ also exhibited mild hypertension. Despite many reports showing that TZDs improve endothelial function in diabetes and hypertension, the importance of PPARγ in the endothelium, independent of ligand-mediated activation, which may have off target effects, has yet to be defined 6. Only one study has utilized an animal model that can avoid the use of exogenous ligand treatment to directly implicate endothelial PPARγ 7. In that model, PPARγ was knocked down in endothelial cells by breeding PPARγ-flox mice with mice expressing cre-recombinase controlled by the Tie-2 promoter. Endothelial PPARγ-null mice exhibited an increase in blood pressure only after feeding a high fat diet. In addition, recent reports suggest that endothelial-specific PPARγ knockout mice also exhibit osteopetrosis 8 and production of a toxic milk in pregnancy 9 suggesting complete loss of PPARγ has pleiotropic effects which may affect multiple organ systems. In the current study, we used endothelium-directed expression of two different clinically relevant dominant negative mutants of PPARγ (P467L and V290M) to test the hypothesis that endothelial PPARγ is a critical mediator of vascular function and its loss through dominant negative interference causes endothelial dysfunction. The use of these mutants prevents problems associated with off-target effects of TZD drugs and the complete loss of PPARγ function which is clearly deleterious.
Methods
Generation of Transgenic Animal Models
The transgene consisted of the VeCad promoter subcloned into a modified form of pStec-1 10. Human PPARγ cDNA was cloned downstream of the promoter with EcoRV. The P467L and V290M mutations were generated using a site directed mutagenesis kit (Stratagene) using the primers: GATCTCCTGCACAGCCTCCATGGAGCGAAACTGGCAGCC, GCTGCCAGTTTCGCTCCATGGAGGCTGTGCAGGAGATC for V290M, and ACAGACATGAGTCTTCACCTGCTCCTGCAGGAGATCTAC, GTAGATCTCCTGCAGGAGCAGGTGAAGACTCATGTCTGT for P467L. The transgene was excised by ClaI, purified, and microinjected into one cell fertilized mouse (C57BL/6J × SJL/J F2) embryos. All transgenic mice were maintained by backcross breeding with C57BL/6J mice for at least 4 generations. Genotyping was performed using the primers CAGCTCACAAAGGAACAATAACAG and CTCCATAGTGAAATCCAGAAG.
All mice were fed standard mouse chow (LM-485; Teklad Premier Laboratory Diets) and water ad libitum. For the high fat diet treatment, male animals were fed D12451 (45 kcal% fat) from Research Diets for 12–25 weeks beginning at 8–10 weeks of age. Experimental animals were 4–8 months of age. Care of the mice used in the experiments met the standards set forth by the NIH in their guidelines for the care and use of experimental animals. All procedures were approved by the University Animal Care and Use Committee at the University of Iowa.
Molecular Assays
Spleen DNA from transgenic and control mice was isolated as described 11. Genomic DNA (10 µg) was digested with BamHI and Southern blotted. The transgene was digested with EcoR1 to generate a 776 bp fragment to label with Rediprime II Random Primer Labeling System (Amersham Biosciences). For RFLP analysis, genomic DNA was amplified using transgene specific primers- GACTTCTCCAGCATTTCTACTCCA and CACTGCATTCTAGTTGTGG to generate a 1408 bp product. The amplified product was digested with BsrBI, NcoI or BamHI. To assay transgene expression, RNA was isolated using Tri-Reagent (Molecular Research Center Inc., Cincinnati, OH) and subjected to RPA using the RPAIII Kit (Ambion, Austin, TX) as described previously 5. Protected fragments were 200 for mouse PPARγ, 280 bases for human PPARγ, 105 nt for Cyclophilin, and 125 nt for 28S as described previously 5.
To assay for endothelial-specificity, mice were given a lethal dose of pentobarbital, the aorta was removed, and cleaned of fat and connective tissue. The vessel was divided into 2 segments and one was denuded of endothelium by rolling on a steel wire. Total RNA was extracted from endothelium-intact and endothelium-denuded samples using TriReagent. RNA samples were treated with DNase-I (Fermentas), cDNA was generated by reverse transcriptase using Superscript III (Invitrogen), and PCR was performed using the Hi-Fidelity Platinum Taq system (Invitrogen). Primer sequences were: SMMHC (CGAAGTTGATGCGAATGAA, TGGAGCCGGAAAGACAGA), VeCAD (CTAGATGTGGATGAGCCCCCTGTC, AATTGGCCTCTGTCACTGGTCTTG), E-PPARγ (TGCAGAAGTTGGTCGTGAGG, AGAAGTCAACAGTAGTGAAGG), and GAPDH (TGCACCACCAACTGCTTAG, GATGCAGGGATGATGTTC).
Aortic Ring Preparation
Male and female mice were given a lethal dose of pentobarbital and the thoracic aorta was quickly removed and prepared for measurements of contraction and relaxation as described in detail 12, 13.
Drugs and Reagents
Ach, SNP, A23187, papaverine, PE, KCl, 5-HT, U-46619, Tempol, lucigenin and NADPH were obtained from Sigma-Aldrich Biochemical and dissolved in physiological saline solution. PGF2α (Lutylase Pfizer pharmaceutical) was from the University of Iowa pharmacy. Endothelin-1 (ET-1) was from Peninsula Laboratories Inc. and dissolved in water.
Studies of cerebral arteries in vitro
Male and female mice were given a lethal dose of pentobarbital, the brain removed and the basilar artery isolated and prepared for measurements of vessel diameter in vitro as described 14. At the end of each experiment papaverine (100 µmol/L) was used to produce maximal vasodilation. Vasodilator responses are expressed as percent dilation (% of induced tone) with 100% representing the difference between the resting value and the constricted value with U46619.
Measurement of oxidative stress
Age matched mice were either fed a normal diet or a high fat diet for 12 weeks as described above. Superoxide was measured by the lucigenin assay in aorta and a pooled sample of cerebral arteries (basilar artery, middle cerebral arteries, and Circle of Willis) as previously described 13, 15.
Microarray analysis
Mouse aortic endothelial cells were cultured by Dominion Pharmakine. Each culture (3 from NT and 3 from E-V290M) was from aortic samples pooled from 2 mice. Each culture was grown in RPMI media supplemented with 50 U/mL penicillin, 50 g/mL streptomycin, 10% FCS, 10U/mL heparin, 1 µg/mL dexamethasone, and 0.1 mg/mL endothelial cell growth supplement (Sigma-Aldrich) on human fibronectin coated plates to the third passage and then frozen. Cultures were considered to not be contaminated with smooth muscle based on α-actin staining (generally less than 10% SMC per culture). RNA was generated using Trizol method. For the microarray hybridizations, 3 independent biological replicates from each experimental group were used. All the microarray procedures were conducted at the University of Iowa DNA Core facility using standard Affymetrix protocols. In brief, approximately 3 µg of total RNA was used as input to a one-step amplification procedure to generate biotin-labeled RNA fragments for hybridization to the Affymetrix GeneChip Mouse Genome 430 2.0 array. This array contains 45,101 probe sets interrogating 22,485 distinct genes. Data from the microarray studies including CEL files have been submitted to the Gene Expression Omnibus (GEO) at NCBI (Accession: GSE11870).
Blood Pressure Analysis
Blood pressure was measured by radiotelemetry as described previously 5. Mice were given 7–10 days to recover, after which time heart rate and arterial pressure were continuously recorded (sampling every 5-minute for 20-second intervals) for one week as a baseline measure of blood pressure. Data were collected and stored using Dataquest ART. Ang-II was infused via minipump (Alzet) at a dose of 1000 ng/kg/min for 5 days as previously described and blood pressure was measured by tail cuff using the Visitech 2000 system 16.
Statistical Analysis
All data are expressed as mean±SEM. Comparisons were made with 2-way repeated measures ANOVA using a Tukey post-hoc test or t-test where appropriate. P<0.05 was considered significant. Data were analyzed by use of SigmaStat (Systat Software).
Results
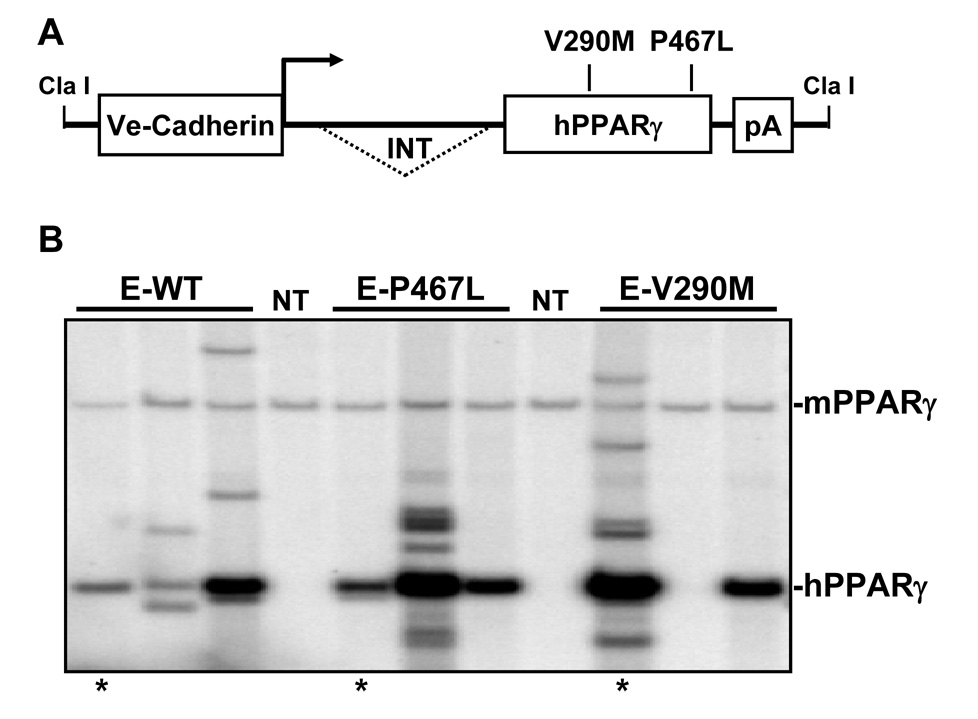
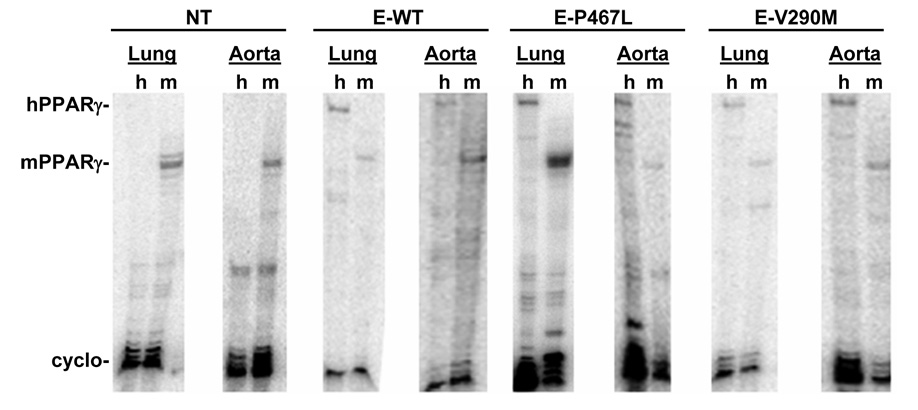
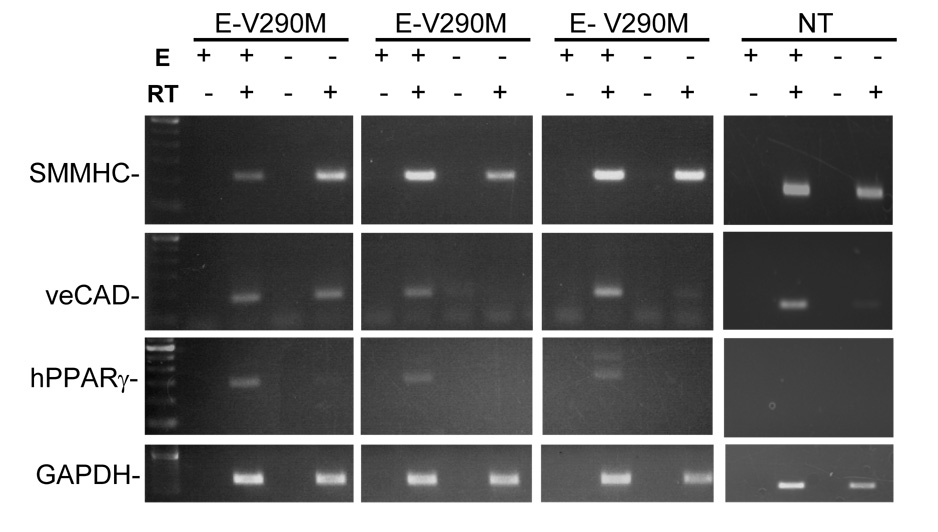
We generated transgenic mice for three constructs all expressing PPARγ under the control of the endothelial-specific vascular cadherin (VeCad) promoter (Figure 1A). The constructs expressed either wildtype (E-WT) or one of two dominant negative mutations (V290M or P467L) previously shown to cause severe hypertension clinically 1. Multiple founders were identified and validated by both Southern blot (Figure 1B) and the presence of the correct mutation by restriction fragment length polymorphism analysis (RFLP) (Figure S1). Expression of PPARγ was assessed in each line of mice by RNase Protection (RPA) and lines were selected on the basis of over-expression compared to endogenous mouse PPARγ in lung and aorta (Figure 2). Although the copy number of the transgene varied we chose lines that expressed approximately equal amounts of the transgene. As expected, the transgene was expressed in all tissues since they all contain endothelial cells (Figure S2). Therefore, in order to demonstrate endothelial-specificity we assayed for expression of smooth muscle myosin heavy chain (SMMHC, a smooth muscle-specific marker), VeCad (an endothelial cell marker), human PPARγ and GAPDH in endothelium intact and endothelium-denuded aorta (Figure 3). The data show a preservation of SMMHC and GAPDH expression in all samples, but a depletion of VeCad and hPPARγ after removal of the endothelium. Consistent with this, there was no change in body weight (26.5±1.0 vs 27.7±1.0 g), brown adipose (0.37±0.1 vs 0.30±0.03 %) or white reproductive adipose tissue (2.2±0.3 vs. 2.0±0.2 %) of E-V290M compared with non-transgenic littermates.
Figure 1. Generation of E-PPARγ Transgenic Mice.
A. A schematic representation of the transgenic construct. B. Southern blot analysis of E-WT, E-P467L and E-V290M lines used in this report. The position of the endogenous mouse PPARγ (mPPARγ) and transgene (hPPARγ) is indicated. The lines marked by * are those used in this report. There was no correlation between transgene copy number and expression.
Figure 2. Expression of PPARγ in the Blood Vessel Wall.
Expression of endogenous mPPARγ with hPPARγ transgene in the lung and aorta assayed by RPA. The aorta RNA is actually a combination of aorta and carotid artery pooled from several transgenic mice of the indicated line and construct. Cyclophilin expression is the internal control. h, human; m, mouse.
Figure 3. Cell-Specific Expression of E-V290M Transgene.
Cell-specificity of transgene expression was analyzed via RT-PCR. SMMHC and VeCad were used as markers for smooth muscle and endothelial cells, respectively. GAPDH was used as an internal control. PPARγ primers were specific for the hPPARγ transgene. The presence of RT and endothelium (E) is indicated by + and −.
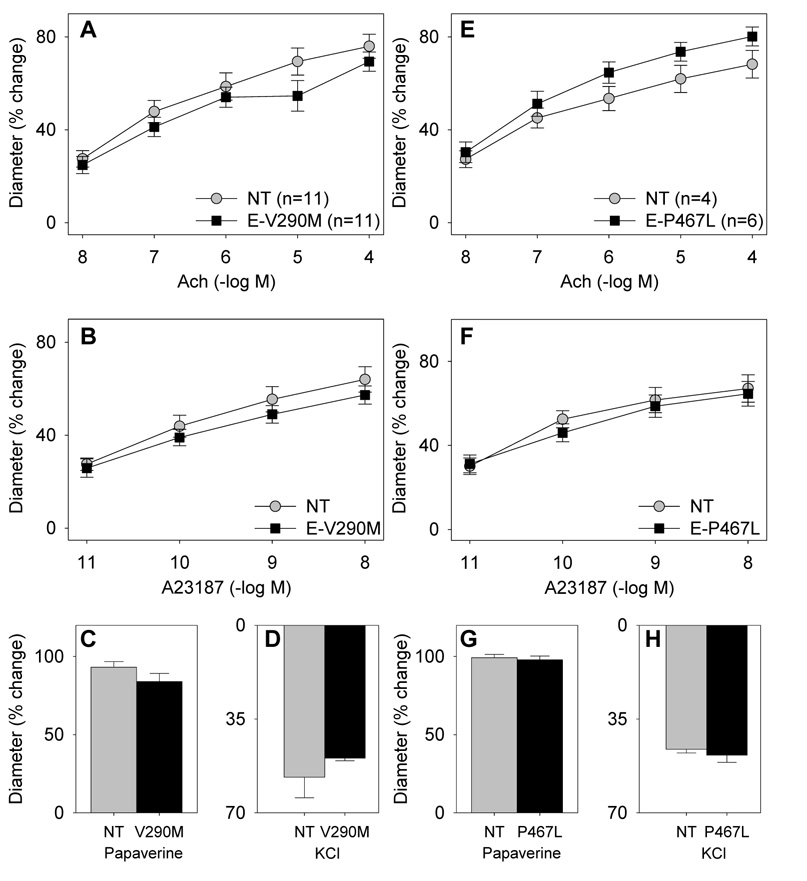
We next examined vascular function in the aorta in vitro by measuring relaxation to acetylcholine (Ach) and sodium nitroprusside (SNP) after precontraction with PGF2α. There was no difference in the response to either agonist in mice expressing either the V290M or P467L mutation in PPARγ (Figure S3). There was also no difference in the contractile response to several receptor-dependent and receptor independent vasoconstrictors (Figure S4). To examine vascular function in a resistance vessel supplying a major organ we studied the basilar artery in vitro. Under normal diet conditions, there were no differences in the dilator response to two endothelial-dependent agonists, Ach and the calcium ionophore A23187, in either E-V290M or E-P467L mice (Figure 4). There was also no difference in the dilator response to the endothelial-independent agonist papaverine, nor the vasoconstrictor KCl.
Figure 4. Endothelial Function in Basilar Artery Under Normal Diet.
Vascular function was measured in E-V290M (A–D) and E-P467L (E–H) mice compared with non-transgenic littermates. Relaxation was measured in response to Ach (A,E), A23187 (B,F), and papaverine (C,G) and contraction was measured in response to KCl (D,H).
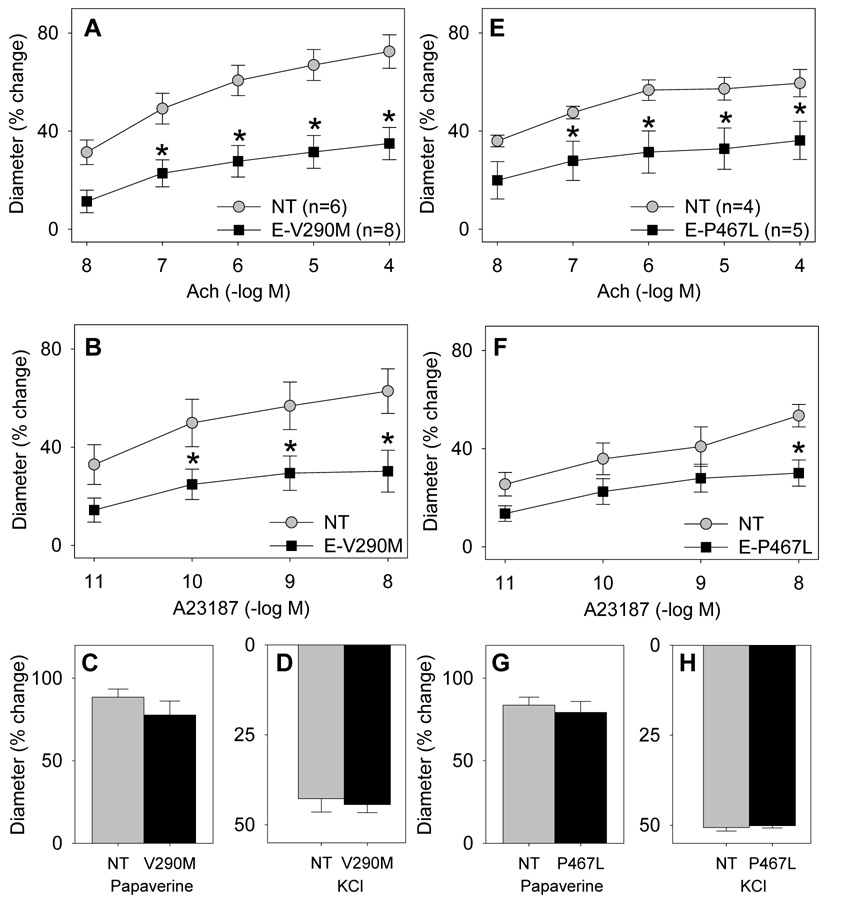
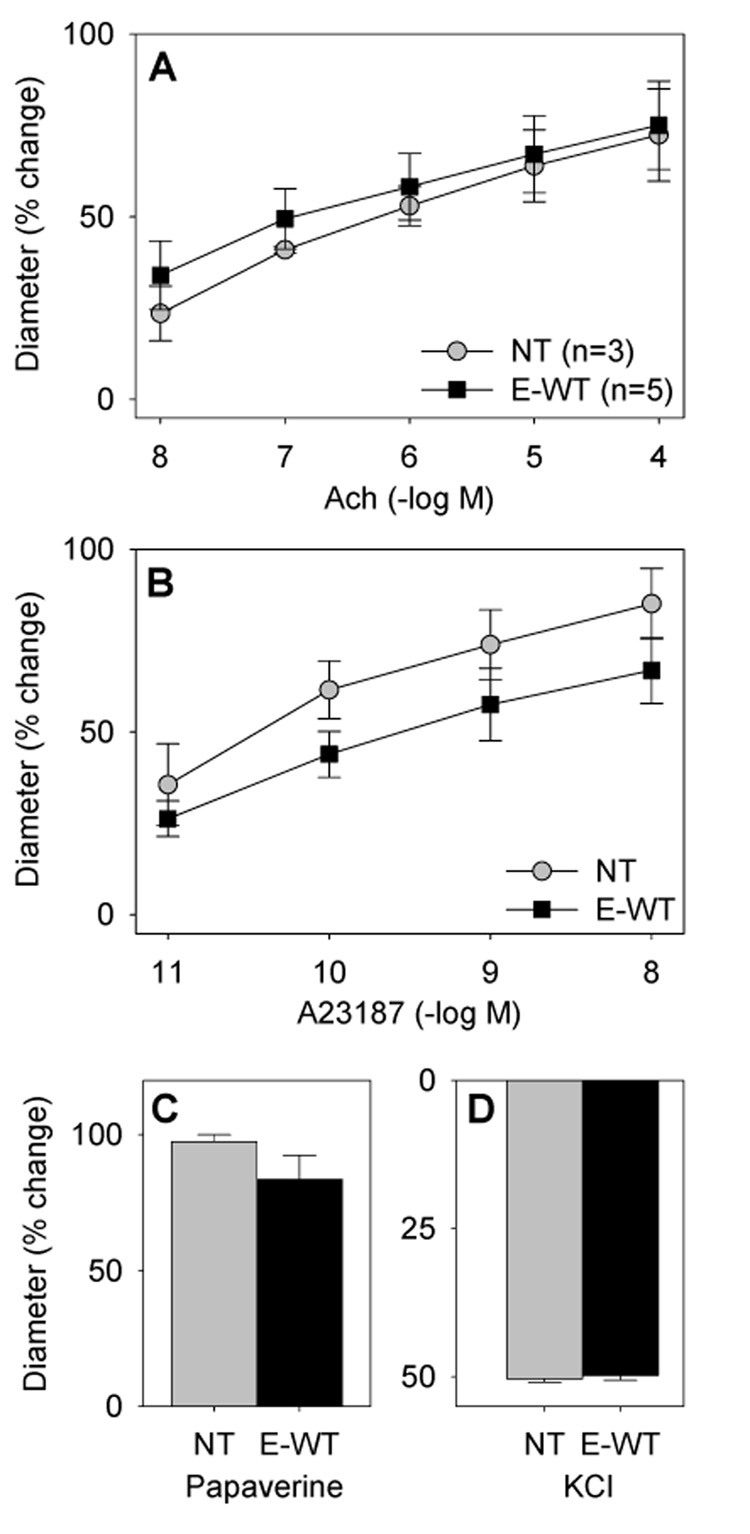
Previous studies suggest that endothelial-PPARγ null mice exhibit an increase in arterial pressure only in response to a high fat diet 7. Consequently, we examined vascular function in mice fed a high fat diet for 12 weeks. The increase in body weight due to the high fat diet was similar in E-V290M (from 26.5±1.0 to 34.1±2.2 g, Δ=7.6 g) and non-transgenic littermates (from 27.7±1.0 to 34.7±1.9 g, Δ=7.0 g). Whereas a high fat diet had no effect on endothelial function in the basilar artery of non-transgenic mice, it caused marked impairment in dilation to both Ach and A23187 in E-V290M or E-P467L mice (Figure 5). Endothelial-independent dilation was not altered nor was the contractile response to KCl. Importantly, the impairment observed in the E-V290M and E-P467L was not evident in high fat diet-fed transgenic mice expressing a wildtype copy of human PPARγ (E-WT) suggesting the impairment was due to dominant negative interference and not to mere over-expression of PPARγ (Figure 6). High fat diet 12 weeks did not cause vascular dysfunction in the aorta (Figure S5A–D), although a modest increase in contraction to ET-1 was noted at the highest concentrations tested (Figure S6). Interestingly, increasing the duration of the high fat diet to 25 weeks caused endothelial dysfunction in the aorta suggesting there is increased susceptibility to dysfunction even in conduit vessels caused by interference with PPARγ in the endothelium (Figure S5E–F).
Figure 5. Endothelial Function in E-V290M and E-P467L Basilar Artery After High Fat Diet.
Vascular function was measured in E-V290M (A–D) and E-P467L (E–H) mice compared with non-transgenic littermates. Relaxation was measured in response to Ach (A,E), A23187 (B,F), and papaverine (C,G) and contraction was measured in response to KCl (D,H). *, P<0.05 vs non-transgenic.
Figure 6. Endothelial Function in E-WT Basilar Artery After High Fat Diet.
Vascular function was measured in E-WT mice compared with non-transgenic littermates. Relaxation was measured in response to Ach (A), A23187 (B), and papaverine (C) and contraction was measured in response to KCl (D).
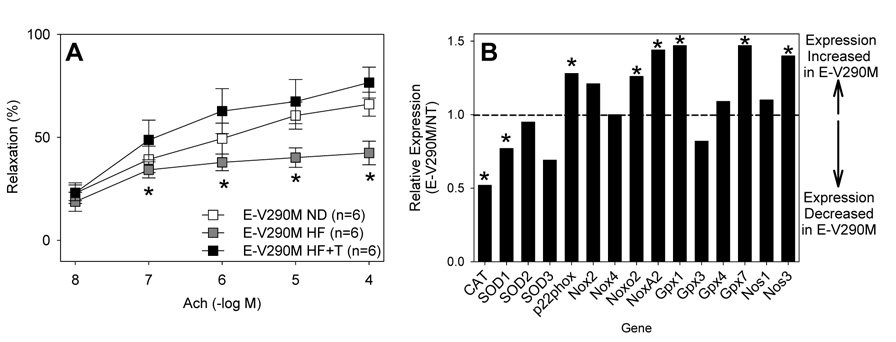
We previously reported that cerebral vascular dysfunction observed in mice systemically expressing the P465L mutation in PPARγ was due to oxidative stress 4. To test if a similar mechanism is operant in E-V290M mice fed a high fat diet, we examined the response to Ach before and after treatment with Tempol, a superoxide dismutase mimetic (Figure 7A). As above, the response to Ach was impaired in basilar artery from mice fed a high fat diet. Tempol significantly improved the Ach response in mice fed a high fat diet. We next measured superoxide in the aorta and cerebral arteries in age matched NT and E-V290M mice fed normal or high fat diet using lucigenin (Figure S7). Surprisingly, there was no difference in baseline lucigenin or the response to increasing doses of NADPH irrespective of diet or vessel. As expected tiron significantly attenuated the lucigenin signal in the presence of NADPH.
Figure 7. Oxidative Stress.
A. Vascular function was measured in E-V290M mice ± high fat diet and Tempol as indicated. *, P<0.05 vs high fat diet plus Tempol. B. Expression of select genes in the oxidative stress pathway as measured by microarray analysis. Each bar reflects the change in gene expression in E-V290M vs NT as a composite of 3 arrays each from NT and E-V290M. In cases where multiple probe sets were available, they were averaged. *, P<0.05 after correction for multiple comparisons.
To obtain a potential molecular explanation for our observations we performed gene expression profiling on aortic endothelial cells cultured from NT and E-V290M mice. Gene set enrichment analysis (GSEA) which examines large sets of genes as a group revealed significant (P=0.019) up-regulation of genes considered to be pro-oxidant including subunits of NADPH oxidase. Individually, there was a modest but significant increase in expression of p22phox, Noxo2 and NoxA2, but not of Nox2 and Nox4 (Figure 7B). Interestingly, there was also a significant decrease in catalase and CuZnSOD (SOD1) expression. We also noted a significant increase in eNOS (NOS3) and Gpx1 expression which may represent compensatory changes.
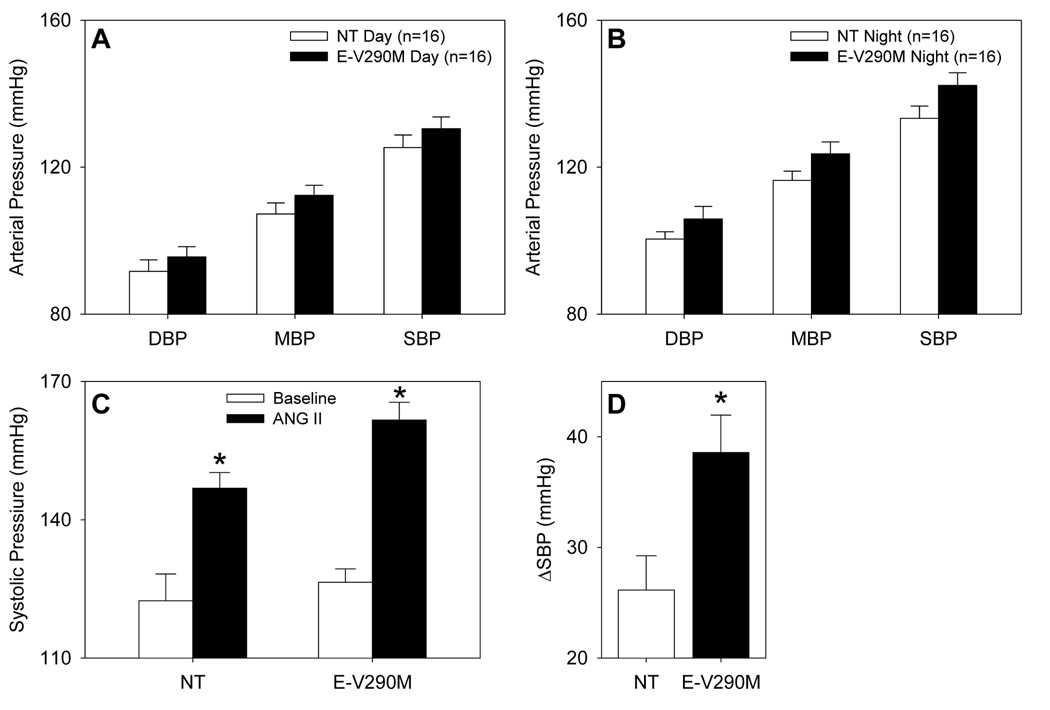
We next measured arterial pressure using radiotelemetry. In mice fed a normal fat diet, we observed a small increase in arterial pressure that was greater during the nighttime hours (9 mmHg, P=0.07) than during the day (5 mmHg) (Figure 8A–B). Interestingly, although the increase in arterial pressure was only modest, the E-V290M mice were more susceptible to the pressor response caused by subcutaneous administration of angiotensin-II via osmotic minipump (ΔSBP: 38.5±3 vs. 26.1±3 mmHg, Figure 8C–D). We did not observe a significant increase in arterial pressure after 12 weeks high fat diet (109.5±1.9 mmHg from 105.2±5.7 mmHg in controls; 112.3±2.7 mmHg from 111.7±3.9 mmHg in E-V290M). For reasons that remain unclear, mice implanted with radiotelemeters during the high fat diet feeding period only gained about 50% of the weight as mice lacking transmitters. Similarly, mice implanted with radiotelemeters after the 12 week high fat diet period lost weight despite being maintained on the high fat diet.
Figure 8. Blood Pressure of E-V290M Mice.
A–B. Arterial blood pressure was measured via radiotelemetry in mice maintained on a normal diet during the day (A) and night (B). C. Systolic blood pressure was measured by tail cuff before (open) and after (closed) angiotensin-II infusion in mice fed a normal diet. *, P <0.01 vs. untreated. D. Increase in SBP after angiotensin-II infusion. *, P=0.013 vs. NT.
Discussion
The importance of PPARγ in the regulation of adipogenesis and type II diabetes is widely accepted, but its role in the vascular wall is not well understood. Although the role of PPARγ in resident macrophages in the vasculature during atherosclerosis has been extensively studied 17, much less is known about the function of PPARγ in the endothelium in vivo. PPARγ is expressed in the endothelium where it has been reported to modulate expression of genes involved in vasoconstriction and oxidative stress 18–21. This is the first study that uses dominant negative mutations of clinical importance to study the role of PPARγ specifically in the vascular endothelium. In humans, naturally occurring mutations interfering with PPARγ function are associated with severe hypertension and metabolic abnormalities 1. Our results provide genetic evidence supporting an important role for PPARγ in the vascular endothelium. The major finding from our study is that interference with PPARγ function specifically in the endothelium results in a significant impairment in endothelial-dependent relaxation in the basilar artery, a resistance vessel in the cerebral circulation. This impairment in basilar artery function was only observed when the mice were fed a high fat diet and was not observed in the aorta (a conduit artery) of mice fed high fat for the same period of time. Like other recent studies, our data suggest that a high fat diet has heterogenous effects on different blood vessels 22. The mechanism accounting for the high fat diet-induced dysfunction appeared to involve oxidative stress as the function of the vessel returned to normal after addition of a scavenger of superoxide. This is consistent with other data showing increased oxidative stress in the vasculature after high fat diet 23.
Our data imply that resistance vessels may be more sensitive to the loss of endothelial PPARγ than conduit vessels, because the basilar artery exhibited dysfunction after 12 weeks on a high fat diet whereas it took 25 weeks before the same effect was observed in aorta. The mechanism for this difference is unclear. Nevertheless, these data are consistent with our recent finding that knockin mice carrying a dominant negative allele of PPARγ (P465L, L/+), expressed in all tissues, exhibit severely impaired cerebral vascular function but only modest aortic dysfunction 4. Interestingly, the knockin mice expressing the dominant negative mutant in both endothelium and vascular muscle exhibit impaired cerebral artery and arteriolar function under baseline conditions without the requirement for high fat diet-induced stress. Consequently, we hypothesize that a stressor, such as a high fat diet, may be required to unmask a phenotype when the function of PPARγ in the endothelium alone is impaired, but not when its function is impaired in both endothelium and vascular muscle. Recall, endothelium-specific PPARγ knockout mice exhibit an increase in blood pressure only after being fed a high fat diet 7. This data is consistent with the role of PPARγ as a fatty acid sensor 24. The link between PPARγ and oxidative stress coupled with our finding that Tempol reverses the high fat diet-induced cerebral vascular dysfunction suggests that free fatty acids or some other PPARγ ligand(s) may increase in the endothelium in response to a high fat diet. It is likely that an increase in PPARγ activity in the endothelium may provide protective mechanisms by increasing synthesis of NO and anti-oxidants 18, 19. The V290M or P467L mutations lie in the ligand binding domain of PPARγ and are thought to destabilize a region of the protein required for co-activator recruitment in response to ligand 25. Thus, the expression of these mutant proteins interferes with this protective mechanism resulting in oxidative stress and impaired vessel function. Along these lines it is particularly interesting that expression of p22phox was increased and expression of catalase and CuZnSOD decreased in cultured endothelial cells from E-V290M mice. Catalase, CuZnSOD, and p22phox genes have been reported to be targets of PPARγ 18, 26–28, and CuZnSOD-deficient mice exhibit oxidative stress and vascular dysfunction 13. Moreover, p22phox over-expressing mice exhibit augmented Ang-II-induced vascular hypertrophy 29. The increase in eNOS expression is interesting in light of its up-regulation in mice over-expressing p22phox 30. Consequently, the upregulation of antioxidant enzymes (CuZnSOD and catalase) which is thought to be part, or indicative of the oxidative stress response, may be PPARγ-dependent. This increase, which would normally be protective, is prevented due to interference with PPARγ function.
Our gene expression data and our finding with tempol are consistent with an increase in oxidative stress in E-V290M mice. We recognize that not being able to detect an increase in superoxide using chemiluminescence does not support a role for an oxidant-dependent mechanism of vascular dysfunction. Considering related work in this area 4, our new microarray findings, and other studies suggesting antioxidant effects of PPARγ 18, 26, 27, it still seems likely that a superoxide-related mechanism contributes to vascular dysfunction following the combination of endothelial-specific interference with PPARγ and a high fat diet. The lack of a detectable increase in superoxide using lucigenin in this model may reflect the sensitivity of that assay in relation to the subcellular localization of superoxide or other factors that may have influenced the results.
The necessity for a high fat diet in the E-V290M and E-P467L mice described herein, but not in the P465L (L/+) knockin mice 4 also suggests a potential contribution of PPARγ in vascular muscle. Indeed, we recently demonstrated the importance of vascular muscle PPARγ by reporting profound aortic dysfunction in mice expressing the same PPARγ mutations (S-P467L and S-V290M) under the control of a smooth muscle specific promoter 5. Like these studies, the effects were only evident in mice expressing the dominant negative mutant but not in mice expressing wildtype PPARγ strongly suggesting the resultant abnormalities were due to dominant negative interference with PPARγ function and not simple over-expression of PPARγ. S-P467L and S-V290M mice exhibited a loss of responsiveness of the aorta to NO and hyper-contraction to endothelin-1. It is unclear why aortic function in the L/+ was only modestly impaired even though the dominant negative was expressed in vascular muscle? The most likely explanation is the level of dominant negative activity. The L/+ mice express one wildtype and one dominant negative allele (a 1:1 ratio), whereas we were able to achieve a higher level of mutant PPARγ expression at both the mRNA and protein level in the aorta by employing the smooth muscle myosin heavy chain promoter. Indeed, we also reported that the magnitude of the aortic dysfunction correlated with the level of dominant negative PPARγ. Therefore, the magnitude of impairment and the type of vessel affected is dependent upon the level of interference garnered and the cell type expressing the mutation.
Male L/+ and male and female S-P467L mice exhibited an approximate 10 mmHg increase in arterial pressure. Baseline blood pressure in the E-V290M mice was elevated by 9 mmHg during the nighttime hours but did not achieve statistical significance. Interestingly, although not overtly hypertensive, these mice exhibited an increased pressor response to angiotensin-II suggesting that, like a high fat diet, they may be sensitive to additional stressors. The increase in angiotensin sensitivity is consistent with studies showing that activation of PPARγ by TZDs prevents the angiotensin-induced pressor response and lowers blood pressure in mice overexpressing angiotensin-II 12, 31. Interestingly, there was no increase in angiotensin-II-mediated contraction in the aorta of E-V290M mice (data not shown).
Endothelial-specific PPARγ knockout mice exhibit normal blood pressure under baseline conditions but become hypertensive after being fed a high fat diet 7. We therefore anticipated a similar finding in our mice. Although we put considerable effort into measuring blood pressure in these mice (both throughout the entire 12 week high fat diet period or after completion of the high fat diet), we observed significant weight loss (about 50% of the gain) after implantation of the radiotelemeters making interpretation of those results difficult. Consequently, further studies are needed to assess if the high fat diet-induced vascular dysfunction translates to an increase in arterial pressure. Interestingly, since the original publication of the endothelial PPARγ knockout 32 there have been other reports which have employed the same methodology of breeding PPARγflox/flox mice with Tie2-Cre mice which express Cre-recombinase in endothelial cells. One study showed that the promoter is active in hematopoietic cells and osteoclasts and resulted in osteopetrosis and increased bone mass 8. In another study, maternal deletion of PPARγ resulted in growth retardation and other defects in nursing pups irrespective of their genotype caused by the production of milk containing elevated inflammatory lipids 9. Therefore, the loss of PPARγ in cells where the Tie2 promoter is active (endothelial cells and other cell types) may have other unidentified consequences.
In summary, our results identify endothelial PPARγ as a critical regulator of endothelial function in the cerebral circulation especially under conditions of high fat-induced stress.
Supplementary Material
Acknowledgments
Transgenic mice were maintained at the University of Iowa Transgenic Animal Facility supported by the Carver College of Medicine. We gratefully acknowledge the generous research support of the Roy J. Carver Trust.
Sources of Funding
This work was supported by grants from the National Institutes of Health (HL48058, HL61446, and HL55006 to CDS; HL38901, HL62984 and NS24621 to FMF). FMF is also supported by a Bugher Foundation Award in Stroke (0575092N). AMB was funded by a Pre-doctoral Fellowship from the American Heart Association Heartland Affiliate (0415460Z) and CMH is funded by a NIH Pre-doctoral Training Program in Genetics (T32 GM008629).
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript accepted for publication in Circulation Research, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circres.ahajournals.org/. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Conflict of Interest No conflict of interest exists with an author on this paper.
Disclosures
NIH funding to Sigmund, Faraci and Halabi
AHA Funding to Faraci and Beyer
References
- 1.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 2.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 3.Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK, Maeda N. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARγ. J Clin Invest. 2004;114:240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, Ghoneim SM, deLange WJ, Keen HL, Tsai Y-S, Maeda N, Sigmund CD, Faraci FM. Interference with PPARγ Signaling Causes Cerebral Vascular Dysfunction, Hypertrophy, and Remodeling. Hypertension. 2008;51:867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARγ Function in Smooth Muscle Causes Vascular Dysfunction and Hypertension. Cell Metabolism. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello RC. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Nicol CJ, Adachi M, Akiyama TE, Gonzalez FJ. PPARγ in endothelial cells influences high fat diet-induced hypertension. Am J Hypertens. 2005;18:549–556. doi: 10.1016/j.amjhyper.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Wan Y, Chong LW, Evans RM. PPARγ regulates osteoclastogenesis in mice. Nat Med. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 9.Wan Y, Saghatelian A, Chong LW, Zhang CL, Cravatt BF, Evans RM. Maternal PPARγ protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev. 2007;21:1895–1908. doi: 10.1101/gad.1567207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stec DE, Morimoto S, Sigmund CD. Vectors for High Level Expression of cDNAs Controlled by Tissue-Specific Promoters in Transgenic Mice. Biotechniques. 2001;31:256–260. doi: 10.2144/01312bm03. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Davis DR, Sigmund CD. The human renin kidney enhancer is required to maintain baseline renin expression but is dispensable for tissue-specific, cell-specific and regulated expression. J Biol Chem. 2006;281:35296–35304. doi: 10.1074/jbc.M608055200. [DOI] [PubMed] [Google Scholar]
- 12.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPARγ agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43:661–666. doi: 10.1161/01.HYP.0000116303.71408.c2. [DOI] [PubMed] [Google Scholar]
- 13.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 14.Faraci FM, Modrick ML, Lynch CM, Didion LA, Fegan PE, Didion SP. Selective cerebral vascular dysfunction in Mn-SOD-deficient mice. J Appl Physiol. 2006;100:2089–2093. doi: 10.1152/japplphysiol.00939.2005. [DOI] [PubMed] [Google Scholar]
- 15.Didion SP, Hathaway CA, Faraci FM. Superoxide levels and function of cerebral blood vessels after inhibition of CuZn-SOD. Am J Physiol Heart Circ Physiol. 2001;281:H1697–H1703. doi: 10.1152/ajpheart.2001.281.4.H1697. [DOI] [PubMed] [Google Scholar]
- 16.Sinn PL, Davis DR, Sigmund CD. Highly Regulated Cell-Type Restricted Expression of Human Renin in Mice Containing 140 Kb or 160 Kb P1 Phage Artificial Chromosome Transgenes. J Biol Chem. 1999;274:35785–35793. doi: 10.1074/jbc.274.50.35785. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, Lee YH, Ricote M, Glass CK, Brewer HB, Jr, Gonzalez FJ. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. 2002;22:2607–2619. doi: 10.1128/MCB.22.8.2607-2619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue I, Goto S, Matsunaga T, Nakajima T, Awata T, Hokari S, Komoda T, Katayama S. The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARγ increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001;50:3–11. doi: 10.1053/meta.2001.19415. [DOI] [PubMed] [Google Scholar]
- 19.Cho DH, Choi YJ, Jo SA, Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) gamma-dependent and PPARgamma-independent signaling pathways. J Biol Chem. 2004;279:2499–2506. doi: 10.1074/jbc.M309451200. [DOI] [PubMed] [Google Scholar]
- 20.Satoh H, Tsukamoto K, Hashimoto Y, Hashimoto N, Togo M, Hara M, Maekawa H, Isoo N, Kimura S, Watanabe T. Thiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPARγ on vascular endothelial function. Biochem Biophys Res Commun. 1999;254:757–763. doi: 10.1006/bbrc.1998.0126. [DOI] [PubMed] [Google Scholar]
- 21.Delerive P, Martin-Nizard F, Chinetti G, Trottein F, Fruchart JC, Najib J, Duriez P, Staels B. Peroxisome proliferator-activated receptor activators inhibit thrombin- induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999;85:394–402. doi: 10.1161/01.res.85.5.394. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharya I, Mundy AL, Widmer CC, Kretz M, Barton M. Regional Heterogeneity of Functional Changes in Conduit Arteries After High-fat Diet. Obesity (Silver Spring) 2008;16:743–748. doi: 10.1038/oby.2007.111. [DOI] [PubMed] [Google Scholar]
- 23.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55:928–934. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Krey G, Braissant O, L'Horset F, Kalkhoven E, Perroud M, Parker MG, Wahli W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 25.Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, Rajanayagam O, Semple R, Luan J, Bath L, Zalin A, Labib M, Kumar S, Simpson H, Blom D, Marais D, Schwabe J, Barroso I, Trembath R, Wareham N, Nagy L, Gurnell M, O'Rahilly S, Chatterjee K. Non-DNA binding, dominant-negative, human PPARγ mutations cause lipodystrophic insulin resistance. Cell Metab. 2006;4:303–311. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girnun GD, Domann FE, Moore SA, Robbins ME. Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter. Mol Endocrinol. 2002;16:2793–2801. doi: 10.1210/me.2002-0020. [DOI] [PubMed] [Google Scholar]
- 27.Yoo HY, Chang MS, Rho HM. Induction of the rat Cu/Zn superoxide dismutase gene through the peroxisome proliferator-responsive element by arachidonic acid. Gene. 1999;234:87–91. doi: 10.1016/s0378-1119(99)00176-6. [DOI] [PubMed] [Google Scholar]
- 28.Calkin AC, Forbes JM, Smith CM, Lassila M, Cooper ME, Jandeleit-Dahm KA, Allen TJ. Rosiglitazone attenuates atherosclerosis in a model of insulin insufficiency independent of its metabolic effects. Arterioscler Thromb Vasc Biol. 2005;25:1903–1909. doi: 10.1161/01.ATV.0000177813.99577.6b. [DOI] [PubMed] [Google Scholar]
- 29.Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;288:H37–H42. doi: 10.1152/ajpheart.00638.2004. [DOI] [PubMed] [Google Scholar]
- 30.Laude K, Cai H, Fink B, Hoch N, Weber DS, McCann L, Kojda G, Fukai T, Schmidt HH, Dikalov S, Ramasamy S, Gamez G, Griendling KK, Harrison DG. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am J Physiol Heart Circ Physiol. 2005;288:H7–H12. doi: 10.1152/ajpheart.00637.2004. [DOI] [PubMed] [Google Scholar]
- 31.Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation. 2002;105:2296–2302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- 32.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPARγ is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.