SUMMARY
In Xenopus and zebrafish embryos, elongation of the anterior-posterior body axis depends on convergent extension, a process that involves polarized cell movements and is regulated by non-canonical Wnt signaling. The mechanisms that control axis elongation of the mouse embryo are much less well understood. Here, we characterize the ENU-induced mouse mutation chato, which causes arrest at midgestation and defects characteristic of convergent extension mutants, including a shortened body axis, mediolaterally extended somites and an open neural tube. The chato mutation disrupts Zfp568, a Krüppel Associated Box (KRAB) domain Zinc finger protein. Morphometric analysis reveals that the definitive endoderm of mouse wild-type embryos undergoes cell rearrangements that lead to convergent extension during early somite stages, and that those cell rearrangements fail in chato embryos. Although non-canonical Wnt signaling is important for convergent extension in the mouse notochord and neural plate, the results indicate that chato regulates body axis elongation in all embryonic tissues through a process that is independent of non-canonical Wnt signaling.
INTRODUCTION
In Xenopus and zebrafish, elongation of the anterior-posterior axis from a spherical early embryo depends on the movement and intercalation of lateral cells towards the midline, a process called convergent extension (reviewed in Wallingford et al., 2002). Extensive studies on intact embryos and tissue explants using time-lapse imaging have confirmed that coordinated cell rearrangements mediate convergent extension in fish and frog embryos (Concha and Adams, 1998; Davidson and Keller, 1999; Elul and Keller, 2000; Jessen et al., 2002; Keller and Tibbetts, 1989; Tahinci and Symes, 2003; Wallingford et al., 2000; Wilson and Keller, 1991).
Non-canonical Wnt signaling is required for convergent extension in Xenopus and zebrafish (reviewed in Tada et al., 2002). Genetic and experimental disruptions of this signaling pathway, such as loss of function mutations in zebrafish trilobite/Van Gogh/Strabismus (Hammerschmidt et al., 1996; Jessen et al., 2002) or overexpression of mutated forms of Dishevelled in Xenopus (Goto and Keller, 2002; Moon et al., 1993; Tada and Smith, 2000; Wallingford et al., 2000) cause characteristic convergent extension defects, such as a short anterior-posterior axis, a wide notochord and a broad open neural tube. Other genetic pathways are also important for convergent extension in zebrafish: BMP gradients (von der Hardt et al., 2007), the Zinc finger protein Bloody fingers (Sumanas et al., 2005) and the ERRα orphan nuclear receptor (Bardet et al., 2005) are all required for normal convergent extension.
In the mouse, the morphogenetic events that create the elongated anterior-posterior body axis are not well understood. Elongation of the mouse embryo takes place during late gastrulation (e7.5-9.0), when extensive cell rearrangements/movements generate the germ layers and organ primordia (Kinder et al., 1999). As these cells reorganize and migrate, the embryo grows dramatically, from about 600 cells at pregastrula stages (e6.0) to nearly 14,000 at neurulation (e8.5.) (Lawson, 1999). Recent time-lapse imaging studies showed that cell intercalation takes place in the axial midline of mouse embryos during the lengthening of the node along the anterior-posterior axis (Yamanaka et al., 2007). However, the importance of convergent extension movements to elongation of other embryonic tissues is not clear, in part due to lack of analysis of cell behavior during these stages.
Mouse mutants that lack components of the non-canonical Wnt signaling pathway show some of the features characteristic of Xenopus and zebrafish embryos with disrupted convergent extension, including a wide notochord and open neural tube (Greene et al., 1998; Kibar et al., 2001; Murdoch et al., 2001a). It has been proposed that defects in axial mesendoderm extension in mouse Looptail/Van Gogh2 (Lp/Vangl2) mutant embryos are caused by defective midline cell intercalation in the node area (Ybot-Gonzalez et al., 2007). Although it is clear that non-canonical Wnt signaling contributes to the elongation of the mammalian embryo (Wallingford et al., 2002; Wang et al., 2006a), the phenotypes of mouse mutants that lack non-canonical Wnt signaling are not as severe as those of their zebrafish mutant counterparts. For example, elongation and convergence of non-axial mesoderm is not as severely affected in Lp/Vangl2 embryos (Greene et al., 1998; Kibar et al., 2001; Murdoch et al., 2001a) as in zebrafish trilobite/Vangl mutants (Hammerschmidt et al., 1996; Jessen et al., 2002), even though the mutations disrupt orthologous genes. Mouse mutants that lack non-canonical Wnt signaling die at birth with severe neurulation defects and disruption of planar cell polarity (PCP) in inner ear hair cells (Curtin et al., 2003; Montcouquiol et al., 2003; Wang et al., 2006b), but their trunk length is similar to that of wild type littermates and the contribution of PCP defects to mouse axis elongation is not clear. To date, the results suggest that convergent extension mechanisms controlled by non-canonical Wnt signaling are important for elongation of some embryonic tissues such as the notochord (Ybot-Gonzalez et al., 2007), but the differences between mouse Lp and zebrafish trilobite mutant phenotypes argue that other pathways and/or mechanisms contribute to the elongation of non-axial tissues in the mouse embryo.
Here we report the identification and characterization of Chato, a novel KRAB Zinc finger protein required for mammalian convergent extension. Two independent recessive mutant alleles of chato cause morphogenetic defects similar to those of fish and frog embryos with defective convergent extension, including a shorter and wider body axis, open neural tube and mediolaterally expanded somites. To evaluate whether chato regulates convergent extension mechanisms similar to those seen in fish and frogs, we measured changes in the length and width of wild-type and mutant embryonic tissues during early development. Because of the relative simplicity of its morphogenetic movements, we focused our analysis on the definitive endoderm layer, the precursor of the gut. Morphometric analysis of wild-type embryos shows that the definitive endoderm narrows and elongates during embryogenesis and that convergent extension of this tissue is mediated by cell rearrangements. In chato mutants the definitive endoderm is wider and cell rearrangements do not take place. Genetic experiments indicate that Chato regulates convergent extension events through a novel pathway that is independent of non-canonical Wnt signaling.
MATERIALS AND METHODS
Mouse (Mus musculus) strains
The chato mutation was generated by ENU-mutagenesis of C57BL/6J males, as described previously (Garcia-Garcia et al., 2005; Kasarskis et al., 1998). The chato mutation was analyzed in three different genetic backgrounds: C3H/FeJ, CAST/Ei and 129Sv/ImJ. Mice carrying the nodal-lacZ allele were obtained from Dr. Elizabeth J. Robertson (Collignon et al., 1996b) and Looptail mice (LPT/LeJ strain) were obtained from Jackson Labs. The genotype of mice and embryos at the chato locus was determined by linkage to flanking SSLP markers (see below). Lp mice were outcrossed to C3H/FeJ and SSLP markers D1Mit36 and D1Mit149 were used for Lp genotyping.
Physical mapping and sequencing of candidate genes
Genetic mapping of Zfp568chato was performed by linkage analysis of 981 informative opportunities for recombination with SSLP markers from MIT (www.informatics.jax.org) or generated by us (SKI markers available at http://mouse.ski.mskcc.org). Physical map information was obtained from Ensembl Mouse Genome Sequence (http://www.ensembl.org/Mus_musculus/index.html).
cDNAs of all candidate genes in the chato interval (Zfp27, Zfp74, Zfp568, Zfp14, Zfp82 and Zfp260) were amplified by RT-PCR (Superscript One-Step RT-PRC, Invitrogen) using RNA from e8.5 chato and C57BL/6J (control) embryos. Amplification products were sequenced. A T to C mutation was identified at codon 64 of the Zfp568 ORF. This point mutation generated a MspI restriction fragment length polymorphism that was used to confirm linkage with chato embryos and carrier animals. No mutations were found in any of the other genes in the interval.
Characterization of the Zfp568RRU161 allele
BayGenomics genetrap insertion RRU161 was reported to create an abnormal splicing between the first coding exon of Zfp568 and a splicing acceptor site present in the genetrap vector (http://www.genetrap.org). To test whether the genetrap insertion completely disrupts the normal splicing of Zfp568 transcripts, RRU161 homozygote embryos were tested by RT-PCR using primers located in the first and second coding exons of Zfp568. No amplification was observed in any of the embryos tested, indicating that all Zfp568 transcripts in RRU161 mutants encode truncated proteins. The splicing between Zfp568 and the genetrap vector placed the β-galactosidase encoding sequence out of frame. As a consequence, the RRU161 genetrap fusion protein contains 11 aa from Zfp568 followed by 19 aa that do not contain any recognizable functional domains.
Analysis of mutant embryos
Embryos were dissected in 0.4%BSA-PBS at different developmental stages as assessed by presence of vaginal plugs in mothers. Embryos were fixed overnight in 4% paraformaldehyde at 4°C and stored in methanol at -20°C until used for in situ hybridization. Whole-mount RNA in situ hyridization and X-galactosidase staining were perfomed as described (Belo et al., 1997; Nagy, 2003). All embryos were photographed with a Zeiss AxioCamHRc digital camera mounted on a Leica MZFLIII scope.
Embryos used for length and width measurements were fixed on 4% paraformaldehyde at 4°C for 8-10 hours, then washed and photographed in PBS (dehydratation was avoided to prevent shrinkage of embryos). Measurements were taken with Axiovision AC Zeiss software on pictures taken at the same magnification.
For immunohistochemistry and TUNEL staining, embryos were processed for cryosectioning as previously described (Garcia-Garcia and Anderson, 2003). Sections were taken at 8-10 μm. Antibodies used were anti-E-cadherin (Sigma) at 1/250 and anti-Phospho-Histone H3 (Ser10) (Upstate) at 1/250. TUNEL was performed using Apoptag Fluorescein in situ apoptosis detection kit (Chemicon). Double labeling with anti-E-cadherin antibodies was done according to Apoptag kit instructions. As positive controls for TUNEL staining (not shown), sections treated with Dnase I were used.
Cell counts were collected from embryos processed through: Ttr in situ hybridization, embedding, cryosectioning (8 μm) and counterstaining with Fast Red. Data plots and statistic analysis of measurements were done using Excel software. Statistical significance was calculated using two-tailed t-tests with Prism software.
Scanning electron microscopy was performed at Sloan-Kettering and Cornell Imaging facilities using Jeoul and Hitachi 4500 microscopes respectively. Samples were fixed overnight in 2.5% glutaraldehyde-PBS, washed in PBS, dehydrated in ethanol and then processed for critical point drying and gold-palladium coating.
RESULTS
chato mutants fail to elongate the anterior-posterior axis
The chato mutation was isolated in a mutagenesis screen designed to identify recessive mutations that alter embryonic morphology at midgestation (Garcia-Garcia et al., 2005; Materials and Methods). chato mutant embryos arrested by 9.0 days of development (e9.0) and remained unturned with a short anterior-posterior body axis and an open gut tube (Fig. 1; Suppl. Fig.1).
Figure 1. Morphological defects in mesodermal tissues of chato mutant embryos.
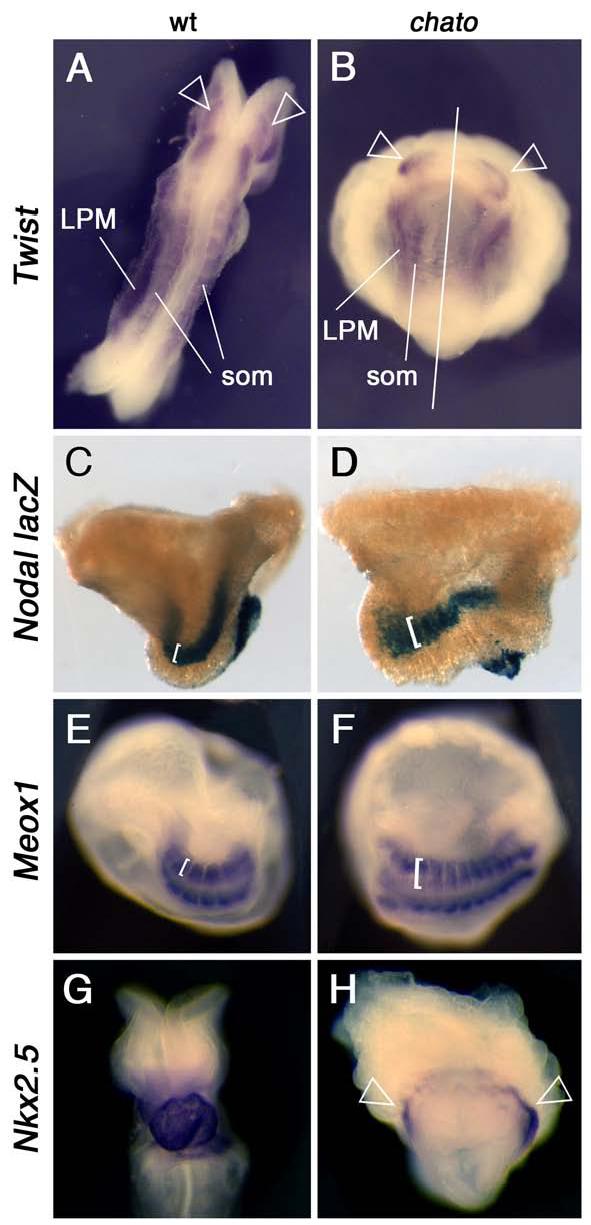
Wild type (A, C, E, G) and chato mutant (B, D, F, H) embryos were assayed by in situ hybridization with markers expressed in head mesenchyme-lateral plate mesoderm-somitic mesoderm (Twist; A-B dorsal and ventral view respectively), somites (Meox1; E-F ventrolateral views) and cardiac mesoderm (Nkx2.5; G-H ventral views). Staining for β-galactosidase activity from a nodal-lacZ reporter labeled the lateral plate mesoderm and node of wild type (C) and chato mutant (D) embryos (lateral views). 33% chato mutants (n=184) had condensed somites that appeared narrow and laterally extended (F). In 52% of chato embryos (n=184) somites were not clearly discernible morphologically, but somite markers Twist and Meox1 were expressed at both sides of the midline and marked some imperfectly shaped somites (B, solid arrowheads and not shown). Only 15% chato mutants showed normal somites. Empty arrowheads in A-B point to head mesenchyme. Brackets in C-D highlight the different width of the lateral plate mesoderm in wild type and chato mutant embryos. Brackets in E-F highlight the different width of the somites. Empty arrowheads in H mark the cardiac mesoderm in chato mutants, which fails to fuse at the midline of the embryo. (LPM) Lateral Plate Mesoderm. (som) somites.
Analysis of mesodermal tissues in chato embryos showed that defects in axis elongation were accompanied by a failure of cells to properly localize with respect to the midline. Analysis of Twist expression, which marks somites and lateral plate mesoderm (Quertermous et al., 1994), showed these mesodermal tissues were located further away from the midline of chato embryos than in wild type littermates (Fig. 1A-B). Expression of a nodal-lacZ reporter (Collignon et al., 1996b) also showed that the lateral plate mesoderm in chato mutants was shorter and wider that in wild type embryos (Fig. 1C-D). Somitic mesoderm was specified in all chato mutants, but it showed defects in morphogenesis (Fig. 1A-B, 1E-F). Many chato embryos (n=61/184) showed condensed somites that were mediolaterally expanded and narrow in the anterior-posterior axis, as shown by expression of Meox1 (Candia et al., 1992) (Fig. 1E-F and Fig. 3C-E). Mesodermal precursors of the heart, which arise from lateral positions, failed to migrate and fuse at the midline of all chato mutants and remained in two separate domains at both sides of the embryo as shown by expression of the heart marker Nkx2.5 (Fig. 1G-H, Lints et al., 1993); this cardia bifida phenotype is presumably responsible for the death of the embryos at e9.5-e10. Altogether, these mesodermal defects are similar to those seen in zebrafish embryos in which convergent extension is disrupted (Matsui et al., 2005), but not in mouse noncanonical Wnt pathway mutants.
Figure 3. The chato mutation disrupts Zfp568.
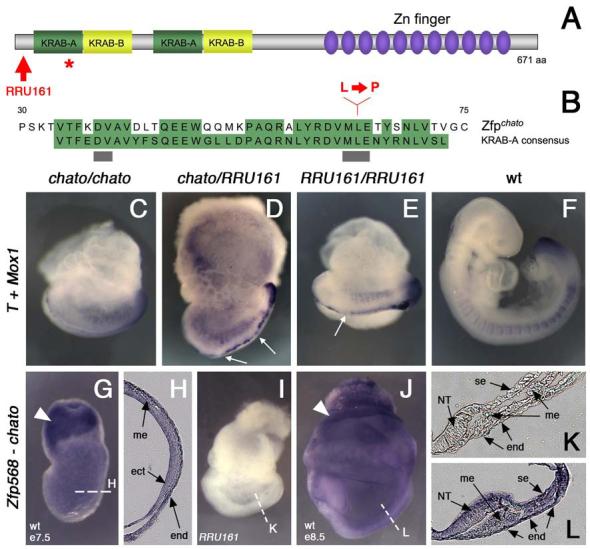
(A) Domain structure of Zfp568, which contains two KRAB-A (green)/ KRAB-B (yellow) domains and eleven Zinc fingers (purple). The red star marks the position of the chato point mutation. Red arrow points to the truncation caused by the RRU161 genetrap allele. (B) Sequence comparison of the first KRAB-A domain of Zfp568/Chato with the KRAB-A consensus sequence. Conserved residues are highlighted in green. Grey bars underline residues required for transcriptional repression (Margolin et al., 1994). Red letters indicate the Leu to Pro change caused by the chato point mutation. (C-F) Complementation test between chato and RRU161 genetrap alleles: wild type (F) and mutant embryos of the allele combinations indicated in the panels (C-E) were assayed by double in situ hybridization with T and Meox1 probes. The overall embryonic morphology, as well as defects in somites and midline, are indistinguishable between the different Zfp568 allele combinations. Notochord expression of T was irregular, showing a variable width and interruptions (arrows in D and E). (G-L) In situ hybridization with a Zfp568 probe on wild type embryos at e7.5 (G-H) and e8.5 (J, L). RRU161 mutant embryos, which generate truncated Zfp568 transcripts, were used as negative controls (I, K). Zfp568/chato is expressed in all embryonic and extraembryonic tissues, as confirmed in transverse embryonic sections (H, K-L). Zfp568 was expressed at higher levels in the extraembryonic ectoderm (solid arrowheads). (me) mesoderm, (ect) ectoderm, (end) endoderm, (NT) neural tube, (se) surface epithelia.
Morphogenetic defects in the chato neural plate and notochord
Epithelial tissues in chato embryos also had morphogenetic defects. The chato headfolds failed to fuse to form a neural tube (Fig. 2A-G). In the open neural plate, markers of specific cell type populations within the head, such as Krox20 (Wilkinson et al., 1989), were expressed in domains that were narrow along the anterior-posterior axis and laterally expanded, when compared with wild-type littermates (Fig. 2A-B), a phenotype similar to zebrafish trilobite/Vangl mutants (Jessen et al., 2002). The neural tube also failed to close normally at more posterior positions of the anterior-posterior axis. In some chato mutants it completely failed to close (55%, Fig. 2E), whereas in others it remained open only at some locations (45%, Fig. 2D), as visualized by expression of the pan-neural marker Sox2 (Collignon et al., 1996a). Failure to close the neural tube is a characteristic phenotype of zebrafish and Xenopus convergent extension conditions (Darken et al., 2002; Goto and Keller, 2002; Wallingford and Harland, 2002), as well as of mouse mutants in components of the non-canonical Wnt signaling pathway (Lp; Fig. 2H; reviewed in Copp et al., 2003).
Figure 2. Defects in the neural epithelium and notochord of chato embryos.
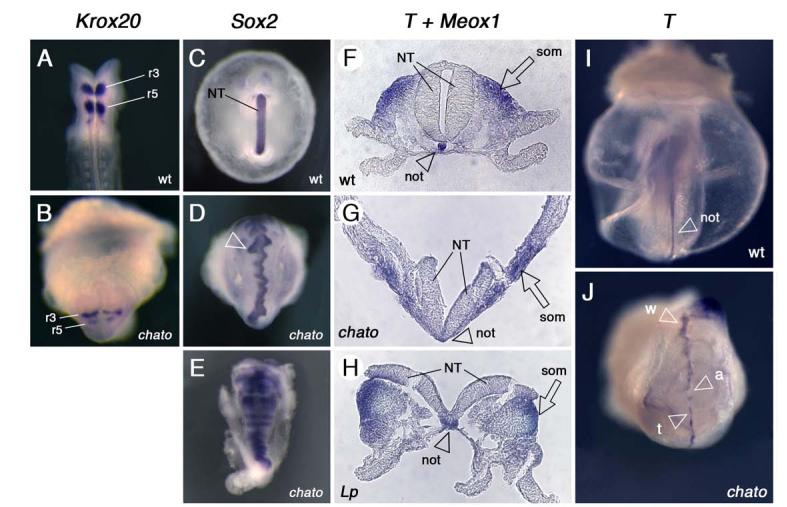
Wild type (A, C, F, I), chato (B, D, E, G, J) and Lp mutant (H) embryos at e8.5 were assayed by in situ hybridization with markers expressed at rhombomeres 3 and 5 (Krox20; A-B dorsal and ventral view respectively), neuroepithelia (Sox2; C-E ventral views), somites (Meox1; F-H) and notochord (T; transverse sections in F-H and posterior and ventral views in I-J respectively). In some chato mutants, parts of the neuroepithelium remained open (arrowhead in D), giving the neural tube a wavy appearance. Transverse sections in F-H were hybridized with probes for both T (arrowheads) and the somitic marker Meox1 (arrows). In chato mutants the notochord tissue was embedded in the mesendoderm layer (arrowhead in G) and never formed an individualized notochord rod (arrowhead in F). The notochord of Lp mutants is wider than that of wild type embryos (F-H arrowheads; Greene et al., 1998). Expression of T in chato mutants (J) shows areas where the notochord was wider (w), thinner (t) or absent (a) as compared to wild type embryos (I, arrowhead). (r3) rhombomere 3, (r5) rhombomere 5, (NT) neural tube, (not) notochord, (som) somitic mesoderm.
However, the basis of the defects in neural tube closure appeared to be different in chato embryos than in mutants in the non-canonical Wnt pathway. It is believed that the underlying cause of neurulation defects in Lp embryos is the abnormally wide floor plate, which might impair the formation of the medial hinge point and the apposition of the neural folds (Greene et al., 1998). The floorplate ventral hinge was morphologically normal in chato mutants (Fig. 2F-G). In addition, other markers of territories along the dorsal-ventral neural axis, including Shh (Echelard et al., 1993), FoxA2 (Ruiz i Altaba et al., 1993), and Olig2 (Zhou et al., 2001) were expressed in regions comparable to those of wild type littermates (not shown).
chato embryos also showed other phenotypic differences from non-canonical Wnt signaling mutants. The notochord, a mesendoderm-derived tissue, is wider in fish, frog and mouse embryos in which the activity of this pathway is disrupted (Goto and Keller, 2002; Greene et al., 1998; Hammerschmidt et al., 1996). Analysis of Brachyury expression (T; Wilkinson et al., 1990) in whole mount chato embryos at e8.5 revealed that the notochord was disrupted, and was wider than in wild type littermates in some regions but narrower or absent in other positions (Fig. 2I-J). In transverse sections, analysis of T expression indicated that the characteristic notochord rod present in wild type embryos at these stages had not been formed in chato mutants and, instead, the notochord was still part of the mesendoderm layer (Fig. 2F-G). Therefore, although the notochord irregularities of chato mutants indicate defects in the reorganization of this tissue, these defects are different than those of non-canonical Wnt signaling mutants (Fig. 2H).
chato does not genetically interact with the non-canonical Wnt signaling pathway
To assess whether chato affected the activity of the non-canonical Wnt pathway, we tested for genetic interactions between chato and Lp. Mouse mutant embryos that lack the transmembrane protein Strabismus/Vangl2 (Lp) display some of the hallmarks of convergent extension mutants, including a wider notochord and failure to close the neural tube (Greene et al., 1998; Murdoch et al., 2001a). Lp mutants show strong genetic interactions with other mutations that affect non-canonical Wnt signaling. For example, embryos that are doubly heterozygous for Lp and Scribble/Circletail (Lp/+; Crc/+; Murdoch et al., 2001b) or for Lp and Ptk7 (Lp/+; Ptk7/+; Lu et al., 2004), as well as Lp+/-; Dvl1+/-; Dvl2-/-embryos (Wang et al., 2006a) all show the same neural tube closure defects seen in Lp homozygous embryos. In contrast, we found that Lp+/-; chato+/- double heterozygous animals were viable and fertile and had the typical curled tail of Lp heterozygotes (Suppl. Fig. 2). We also mated double heterozygous carriers to obtain more severe mutant combinations and evaluated their phenotypes in mesoderm, neural tube and notochord (Suppl. Fig. 2). We did not observe any modification of the Lp mutant phenotype in embryos lacking one dose of chato (Lp-/-; chato+/-). Similarly, the chato mutant phenotype did not change in the absence of one copy of Lp (Lp+/-; chato-/- embryos). Lp-chato double mutant embryos (Lp-/-; chato-/-) showed characteristics of both chato and Lp mutants, including elongated somites and open neural tube (Suppl. Fig. 2). The lack of genetic interaction between the two mutants does not support a role of chato in non-canonical Wnt signaling.
To further test whether chato interferes with non-canonical Wnt signaling, we assayed expression of components of this pathway in chato mutants. We found that Vangl1, Vangl2, Celsr1, Frizzled3, Dvl1, Dvl2 and prickle were all expressed in chato mutants (Suppl. Fig. 3A-H and not shown) in the same tissues than wild type control embryos (Suppl. Fig. 3A-H, Crompton et al., 2007; Torban et al., 2006). Reciprocally, chato expression was unaltered in Lp mutants (Suppl. Fig. 3I-J). Since none of our experiments support an interaction between chato and non-canonical Wnt signaling, we speculate that the morphogenetic defects of chato and Lp mutants might arise through different molecular mechanisms.
The chato mutation disrupts Zfp568, a novel KRAB Zinc finger protein
Meiotic recombination mapping localized the chato mutation to an interval of 209 kb on the proximal region of chromosome 7 (Materials and Methods). Sequence analysis of all six genes in this interval revealed a single change: a missense mutation in Zfp568, which encodes a member of the Krüppel Associated Box (KRAB) domain Zinc finger protein family. KRAB Zinc finger proteins represent one of the largest families of transcriptional regulators in mammals, including ∼290 genes (Urrutia, 2003). Members of this family contain a variable number of Zinc finger domains, which are believed to provide DNA binding specificity to different targets (Gebelein and Urrutia, 2001), and one or several KRAB domains, which function as strong transcriptional repressor domains (Margolin et al., 1994).
The missense mutation in the chato allele causes a Leu to Pro change in the first of the two KRAB domains of Zfp568 (Fig. 3A-B). This change maps to a highly conserved position within the KRAB domain required for transcriptional repression in COS-1 cells (Margolin et al., 1994). To confirm that mutation of Zfp568 is responsible of the chato mutant phenotype and to test whether the missense mutation disrupted activity of Zfp568 completely, we generated mutant mice from the BayGenomics genetrap clone RRU161. This genetrap insertion generates a truncated Zfp568 protein of 11 amino acids that lacks all functional domains, and should represent a null allele of Zfp568 (see Fig. 3A and Materials and Methods). Both Zfp568chato/Zfp568RRU161 and Zfp568RRU161 homozygous embryos recapitulated the chato phenotype (Fig. 3C-F). Thus, the complementation test indicated that the ENU-induced chato mutation is a null allele of Zfp568.
Zfp568 (chato) showed a broad expression pattern during embryogenesis (Fig. 3G-L). At e7.5, chato was expressed in all cell types as assessed by in situ hybridization in whole mount embryos and in sections (Fig. 3G-H). At later stages, expression was also ubiquitous in extraembryonic and embryonic tissues (Fig. 3J, L). Expression was highest in the extraembryonic ectoderm (Fig. 3G,J arrowheads).
chato mutants fail to undergo convergent extension of definitive endoderm
The characterization of the cellular basis of the chato axis elongation defects was complicated by the architecture of the e8.5 mouse embryo, which consists of several cellular layers, some of which are folded (e.g. the neuroepithelium). Compared to other germ layers, we found that the simple epithelial structure of the definitive endoderm made it amenable to straightforward and reliable analysis during the stages of axis elongation. Definitive endoderm cells arise from the primitive streak during gastrulation and form an epithelial monolayer that is continuous with the extraembryonic visceral endoderm (VE) on the exterior of the embryo after e8.0 (reviewed in Lewis and Tam, 2006).
We measured the overall dimensions of the definitive endoderm in wild-type and chato mutant embryos during the stages of anterior-posterior axis elongation. Definitive endoderm and VE cells can be discriminated using markers expressed exclusively in the VE, such as Transthyretin (Ttr; Cereghini et al., 1992). At e7.5 some VE cells were still present in the embryonic region (Fig 4A-B arrowhead). After e8.0 (zero somite stage), the definitive endoderm (Ttr-negative endoderm) covered the entire embryonic region (Fig. 4C-J). Posterior views of wild type embryos marked with Ttr revealed that the definitive endoderm narrowed between e8.0 and e9.5 (Fig. 4). Measurements of definitive endoderm in wild type embryos showed that the total length of the definitive endoderm increased 50% between zero somite and 10 somite stage embryos (Fig. 4K blue columns; 5H). At the same stages, definitive endoderm width, measured as the lateral distance across the center of the embryo (red lines in Fig. 4H), decreased 2.7 fold (Fig. 4K green columns, 5H). These measurements demonstrate that elongation of the definitive endoderm of the wild-type mouse embryo is accompanied by narrowing of the tissue, and thus definitive endoderm undergoes convergent extension.
Figure 4. Convergent extension in the wild-type definitive endoderm.

Whole mount in situ hybridization with Ttr probes to wild type embryos of embryonic stages e7.5 (A, B), e8.0 (C-F) and e8.5 (G-J). Lateral and posterior views respectively illustrate how definitive endoderm (white) grows along the AP axis (length) and narrows laterally (width). Ttr highlights the extraembryonic visceral endoderm (VE). The white tissue covering the embryonic region corresponds to definitive endoderm (DE). Arrows point to white extraembryonic tissue (ext). All pictures were taken at the same magnification. At e7.5, some visceral endoderm cells (arrowhead in A-B) still overlay the exterior of the embryonic region (continuous line in A-B delimits embryonic-extraembryonic parts). Gut closure prevented visualization of all the definitive endoderm in panels I and J. (b’-j’) Representative transverse sections of the embryos in the columns above counterstained with Fast Red. Sections correspond to intermediate levels along the anterior-posterior axis. Only half of the section is shown, with the midline located at the right edge of the panel. (K) Plot of length (blue) and width (green) definitive endoderm measurements in wild type embryos of different stages; data in μm. Dimensions of the endoderm were taken as exemplified by dotted lines in panels G-H. Note that measurements were taken in non-Ttr stained embryos, in which transparency of the tissue allowed for accurate measurements of the whole definitive endoderm. Error bars indicate standard deviation. See Table on Fig. 5H for primary data.
Figure 5. Failure of convergent extension in the definitive endoderm of chato mutants.
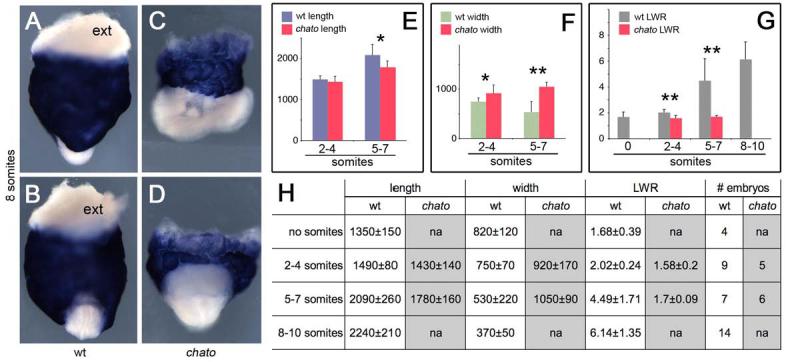
Wild type (A-B), chato (C-D) mutant 8 somite stage embryos hybridized with Ttr probes to highlight extraembryonic visceral endoderm (blue) and definitive endoderm (exterior layer of embryonic tissues in white). ext indicates white extraembryonic tissue. (A, C) lateral; (B, D) anterior views. (E) Plot of wild type (blue) and chato (red) definitive endoderm length. (F) Plot of wild type (green) and chato (red) definitive endoderm width. Data in μm. (G) Plot of definitive endoderm LWR in wild type (grey) and chato mutant (red) embryos. Error bars indicate standard deviation. * p<0.05; ** p<0.01 (H) Length and width average measurements ± standard deviation in μm. The number of embryos analyzed for each stage is indicated (# embryos). LWR, length to width ratio; na, not assayed.
At early e8.5 (2-4 somite stage) the length of the chato definitive endoderm was not significantly different than that of wild-type littermates (p=0.31), but its width was 1.23 times that of wild type (p=0.019, Fig. 5F, H). At the 5-7 somite stage, the definitive endoderm of chato mutants was 14% shorter (p=0.031) and twice as wide (p=0.0002) as that of wild-type embryos of the same stage (Fig. 5 E-F, H). The length and width measurements indicated that the chato mutant endoderm grew in both dimensions (Fig. 5 E-H). However, the length-to-width ratio (LWR) of chato embryonic endoderm did not significantly change between 2-4 and 5-7 somite stages (p= 0.23, Fig. 5G, red columns), whereas the wild type LWR more than doubled (p=0.0007, Fig. 5G, grey columns). By the 5-7 somite stage, the LWR of wild-type definitive endoderm was 2.6-fold greater than that of chato mutants (p=0.0022, Fig. 5G). Thus, convergent extension of the mouse definitive endoderm requires the activity of the Chato protein.
Elongation and narrowing of the wild-type definitive endoderm is coupled to cell rearrangements
Convergent extension in zebrafish and Xenopus embryos depends on cell rearrangements, including mediolateral cell intercalation and polarized cell migration, which contribute to decrease the number of cells across the width of the embryo and increase the number of cells along the anterior-posterior axis (reviewed in Wallingford et al., 2002). We therefore evaluated variations in the number of cells across the width of the mouse definitive endoderm to assess the contribution of cell rearrangements to convergent extension of the mouse endoderm.
We quantified the number of cells across the width of the definitive endoderm at different developmental stages by counting the number of Fast Red stained nuclei in the outermost layer of transverse sections from e8.0 (0 somites) to e9.0 (10 somite) wild type embryos (Fig. 4b’, d’, f’, h’, j’). In headfold stage embryos (e8.0; 0-4 somites), the definitive endoderm layer was 47±6 cells wide at intermediate positions of the anterior-posterior axis (Fig. 6A-B). In 5-10 somite embryos, the number of definitive endoderm cells across the width of the embryo decreased to 32±6 cells (p<0.0001, Fig. 6A-B). This decrease in cell number correlated with the dramatic narrowing of the definitive endoderm that occurred between these stages (Compare Fig. 4D, F, H and J).
Figure 6. Cell number changes across the width of the definitive endoderm.
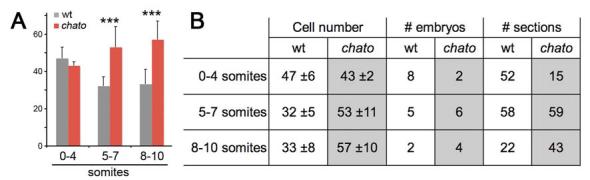
(A) Plot of the average definitive endoderm cell number in wild type (grey) and chato mutants (red). Cells were counted in sections of the definitive endoderm stained with Fast Red at medial levels along the anterior-posterior axis (Fig. 4). Error bars represent standard deviation. (B) Table shows average number of cells ± standard deviation. Number of sections indicates the total number of sections counted for each condition. na, not assayed. *** p<0.0001
A decrease in the number of cells across the width of the definitive endoderm could be the result of mediolateral cell intercalation. However, this number could also be influenced by proliferation, apoptosis and delamination of cells from the primitive streak. To evaluate the contribution of cell proliferation, we assayed the frequency of mitotic cells in transverse sections of the definitive endoderm using Phosphohistone H3 antibodies (green signal Fig. 7A, C). Between e8.0 (0 somites) and e9.0 (10 somites), the definitive endoderm contained 0-3 proliferating cells per section at all levels along the anterior-posterior axis where the gut remained open (n=21embryos/380sections, Fig. 7A, C and not shown). In contrast, other embryonic tissues such as the mesoderm or neuroepithelia showed a higher mitotic index (Fig. 7A, C). Our results confirm previous reports indicating that the definitive endoderm is a relatively quiescent tissue during early developmental stages (Tremblay and Zaret, 2005) and indicate that the rate of proliferation in the endoderm plays a relatively minor role in the growth of the definitive endoderm during these stages. We did not observe any apoptotic cells in the definitive endoderm at any of the stages analyzed (data not shown). Delamination of cells from the primitive streak has been shown to play important roles in the growth of the definitive endoderm at gastrulation stages (Lewis and Tam, 2006). Therefore, during the stages of anterior-posterior axis elongation, the number of cells in the endoderm might increase due to the continued delamination of cells from the primitive streak, with a minor contribution from cell proliferation. Because we did not observe apoptosis in the endoderm at this stage, we conclude that cellular rearrangements (mediolateral cell intercalation or polarized cell migration) must be responsible for the observed decrease in the number of cells across the width of the definitive endoderm and the decrease in the width of the tissue.
Figure 7. Proliferation in definitive endoderm.
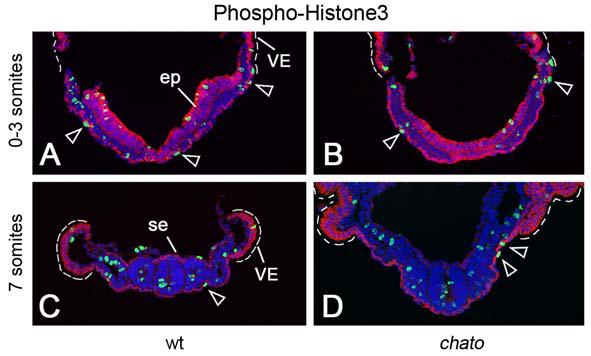
Cryosections of wild type (A, C) and chato mutant (B,D) embryos at different embryonic stages were labeled using anti-E-cadherin antibodies (red) and Phospho-Histone H3 antibodies (green). Mitotic cells (green in A-D) in the definitive endoderm (highlighted in red by localization of E-cadherin) are indicated by empty arrowheads. E-cadherin is also present in e7.5 epithelia (ep), embryonic surface ectoderm (se) and extraembryonic visceral endoderm (VE, dashed line). Proliferation of mesoderm and epithelial tissues was not significantly affected between wild type (n=21embryos/380 sections) and chato mutant embryos (n=3 embryos/56 sections) at these stages.
chato mutants fail to undergo the cell rearrangements required for definitive endoderm convergent extension
We also analyzed the rearrangements of cells in the definitive endoderm of chato mutants, using the approaches described above. Headfold stage (0-4 somites) chato mutants had approximately the same cell number across the width of the definitive endoderm as wild-type littermates (Fig. 6A-B). At the 5-7 and 8-10 somite stages, chato embryos contained on average ∼70% more cells across the width of the definitive endoderm than wild type embryos (p<0.0001, Fig. 6A-B), paralleling the increased width of the definitive endoderm in chato mutants (Fig. 5B, D). The rate of cell proliferation (n=3 embryos/56 sections) in the definitive endoderm of chato mutants was similar to that of wild type (Fig. 7B-D). Also, no apoptosis was observed in the chato mutant endoderm and we did not detect any abnormality in the delamination of definitive endoderm from the primitive streak or in the migration of definitive endoderm cells at gastrulation (Suppl. Fig. 1C-F). Therefore, we conclude that the definitive endoderm is wide in chato mutants because normal function of the chato gene is required for the cells to rearrange into a longer, narrower structure.
DISCUSSION
Convergent extension in the definitive endoderm of the mouse embryo depends on Chato
Although the contribution of convergent extension mechanisms to the elongation of zebrafish and Xenopus embryos has been well studied, evidence for a role for convergent extension in mammalian embryogenesis has been limited to the notochord and neural tube (Wang et al., 2006a; Yamanaka et al., 2007; Ybot-Gonzalez et al., 2007). Based on embryo morphology and the pattern of expression of molecular markers, chato mutants appear to have global defects in elongation of the body axis, with abnormalities in the neural plate, paraxial mesoderm, lateral plate mesoderm and definitive endoderm.
To test definitively whether chato affects convergent extension, we examined the morphogenesis of the definitive endoderm, a single-layered cell sheet that can be analyzed reliably. Our morphometric analysis provides evidence that the wild-type mouse definitive endoderm undergoes convergent extension. The definitive endoderm begins to narrow and elongate in headfold stage embryos and continues to do so until approximately 14 somite stage embryos, when definitive endoderm closes to form the gut tube. From 0 somite to 10 somite stages, the width of the wild-type definitive endoderm narrows 2.6 fold (from 820mm to 310mm), at the same time it elongates two-fold. Although delamination of cells from the primitive streak probably contributes to the elongation of the definitive endoderm, the cell rearrangements that we observed are likely to account for the narrowing of the definitive endoderm and to contribute to the anterior-posterior elongation of this tissue (Suppl. Fig. 5). In contrast, the definitive endoderm does not narrow in chato embryos. The most dramatic change in dimensions of the definitive endoderm of wild-type embryos occurs between the 2-4 and 5-7 somite stages, when the length-to-width ratio more than doubles; at the same stages, the length-to-width ratio of the chato definitive endoderm does not change significantly. In parallel with the abnormal dimensions of the tissue, the chato mutation disrupts cell rearrangements in the definitive endoderm. We therefore conclude that the cell rearrangements that depend on Chato are responsible for convergent extension of the definitive endoderm.
The mechanisms that underlie the cell rearrangements of convergent extension have been studied in both vertebrate and invertebrate embryos. Mediolateral cell intercalation has been shown to mediate the elongation of Xenopus embryos and animal cap explants (Elul et al., 1997; Keller and Tibbetts, 1989; Wilson and Keller, 1991), polarized cell migration is also important for zebrafish convergent extension (Concha and Adams, 1998; Jessen et al., 2002; Warga and Kimmel, 1990), and germ band elongation of Drosophila embryos is propelled by the directional generation and resolution of multicellular rosettes (Bertet et al., 2004; Blankenship et al., 2006). One or more of these mechanisms may mediate convergent extension of the mouse definitive endoderm. Because mouse definitive endoderm has an epithelial organization, where cells are hold together by adherent apical complexes (Fig. 7, E-cadherin in red), we favor the hypothesis that mediolateral cell intercalation and/or multicellular rosettes, rather than cell migration, mediate definitive endoderm convergent extension. Development of new methods to observe live mouse embryos at a cellular resolution will be required to elucidate the precise mechanisms and dynamics involved.
Chato is likely to act in convergent extension of all germ layers
Although our studies of convergent extension in chato focused on the definitive endoderm, the chato phenotype suggests that it also acts in other tissues to regulate convergent extension. Both the chato lateral plate and the somitic mesoderm are shorter in the anterior-posterior axis and wider in the mediolateral dimension than in wild-type embryos, similar to the phenotypes characterized in zebrafish convergent extension mutants (Hammerschmidt et al., 1996; Jessen et al., 2002). The neural plate in chato fails to close normally, which could be due to defects in cell rearrangement in this tissue layer. Because chato is broadly expressed, it seems likely that it may act autonomously in these tissues to control cell rearrangements. It is, however, possible that convergent extension of the definitive endoderm is required for the migration and/or reorganization of epithelial and mesenchymal tissues.
Most chato mutants (n=156/184) also show extraembryonic defects, including a ruffled visceral endoderm (Suppl. Fig. 1A-B). It is therefore possible that these extraembryonic defects could influence the reorganization of definitive endoderm, epithelial and mesenchymal tissues in chato mutants. However, the defects in embryonic morphogenesis precede the appearance of extraembryonic phenotypes in chato mutants (see Suppl Fig. 1C-F). In addition, 16% of e8.5 chato mutants do not show obvious extraembryonic abnormalities but have strong convergent extension phenotypes. Therefore, we favor the hypothesis that the embryonic and extraembryonic defects in chato embryos represent distinct, autonomous requirements for chato. Further experiments assessing the phenotype of chato chimeric embryos or using conditional alleles will define the tissue requirements of this novel KRAB zinc finger protein.
The role of the Chato KRAB zinc finger protein in morphogenesis
The chato mutation defines the role of a novel KRAB Zinc finger protein in mammalian convergent extension. Although KRAB domain Zinc finger proteins represent one of the largest gene families in mammals, represented by ∼290 different genes (Urrutia, 2003), only a few mutants have been described. These mutants affect diverse processes, including fertility, pigmentation and embryonic growth (Casademunt et al., 1999; Krebs et al., 2003), but Chato is the first member of this family shown to be required for embryonic development. Although the high degree of sequence conservation among members of the family suggests that the genes might be functionally redundant, the severity and specificity of the chato phenotype indicates that some KRAB domain proteins have distinct functions. Since members of such a large gene family would not have been good candidates for targeted mutagenesis, our findings highlight the value of forward mutagenesis screens for the discovery of gene function.
The KRAB domain seems to be a relatively recent evolutionary feature, as it has only been found in the genomes of tetrapod vertebrates (Urrutia, 2003; www.ensembl.org). Nevertheless, the C-terminal zinc finger-containing region of chato shows homology to genes found in other animals. The closest homologue of chato in Drosophila is crooked legs (crol), with 39% identity and 53% similarity to the Chato zinc finger domain. crol mutant pupae die with twisted legs that fail to elongate (D’Avino and Thummel, 1998). Although the zebrafish genome does not encode any KRAB domain proteins, morpholinos that disrupt activity of zebrafish zinc finger gene Bloody fingers (Blf) display shortened and widened axial tissue due to defective convergent extension (Sumanas et al., 2005). Blf and Chato share similar zinc finger domains, but, based on synteny, it is unlikely that Blf is the zebrafish ortholog of Chato. Therefore, it is possible that the Chato, Crol and Blf derived from a common ancestral Zinc finger protein that controlled tissue elongation during morphogenesis.
Our results suggest that the Chato KRAB zinc finger protein acts through a molecular pathway that is independent of non-canonical Wnt signaling. Although mutations in both the mouse chato and non-canonical Wnt signaling genes affect convergent extension, their phenotypes are fundamentally different. The defects in axis elongation in the chato mesoderm are more profound than those reported in mouse non-canonical Wnt signaling mutants (Fig.1-2; Greene et al., 1998; Wang et al., 2006a). Most clearly, our analysis shows that chato mutants fail to close the gut endoderm and fail to undergo convergent extension in the gut, phenotypes that are not present in Lp mutants (Suppl. Fig. 3). In contrast, Lp mutants have more dramatic defects in neural tube closure and in convergent extension of the notochord than chato mutants (Greene et al., 1998; Wang et al., 2006a; Ybot-Gonzalez et al., 2007). A specific role for non-canonical Wnt signaling in morphogenesis of axial tissues is supported by the high level of expression of Lp/Vangl2 and Vangl1 in the mouse neural tube (Torban et al., 2006; Torban et al., 2008). Altogether, the observations suggest that Chato and non-canonical Wnt signaling act in different tissues and regulate convergent extension through different molecular mechanisms.
Because chato mutants are blocked in both convergent extension of definitive endoderm convergent extension and the accompanying cell rearrangements, we conclude that these cell rearrangements drive convergent extension of the mammalian endoderm. KRAB Zinc finger proteins are believed to act as transcriptional repressors (Bellefroid et al., 1991), so Chato may regulate transcription of genes that regulate specific aspects of cytoskeleton dynamics, components of the extracellular matrix (ECM) or chemotactic clues. Because mutations in mouse genes that have global effects on cytoskeleton organization or the extracellular matrix (Garcia-Garcia and Anderson, 2003; George et al., 1993; Rakeman and Anderson, 2006) cause phenotypes dramatically different from those of chato mutants, we infer that chato controls cellular processes that are specific to convergent extension.
Chato may act in a common molecular pathway with Hand1 and Yap65
While the molecular mechanisms that implement Chato function remain to be discovered, additional information may come from analysis of two other mouse genes that produce phenotypes similar to chato. Mutants that lack Hand1, which encodes a bHLH transcription factor, arrest development at the 9-14 somite stage, fail to close the gut endoderm, have a kinked neural plate and show extraembryonic defects similar to those of chato embryos (Firulli et al., 1998). Loss of mouse Yap65 (which encodes a protein with a proline rich domain, WW domains, SH3 binding motifs, a coiled-coil and a PDZ binding motif) also causes the same set of developmental phenotypes (Morin-Kensicki et al., 2006). Similar studies to those described here could test whether these mutants have convergent extension defects in epithelia, mesenchyme and endoderm and if cell rearrangements underlie Hand1 and Yap65 abnormalities. Future genetic and molecular experiments will be able to test whether chato, Hand1 and Yap65 act in a common biochemical process that regulates convergent extension in the mouse.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Andrew K. Recknagel for technical support and maintenance of the chato colonies, to Maegan V. Harden for help with experiments and to Nina Lampen and Carole Daugherty for assistance with scanning electron microscopy. We thank Elizabeth J. Robertson, Scott Weatherbee, Tristan Rodriguez, Philippe Gros, Andre Goffinet, Tudorita Tumbar and Tony Bretscher for providing mouse strains, reagents and/or use of lab equipment. We thank Holger Sondermann, Jeffrey Lee and Isabelle Migeotte for helpful discussions and comments on the manuscript. This work was supported by NIH grant HD035455 to KVA and a Basil O’Connor March of Dimes award to MJGG.
REFERENCES
- Bardet PL, Horard B, Laudet V, Vanacker JM. The ERRalpha orphan nuclear receptor controls morphogenetic movements during zebrafish gastrulation. Dev. Biol. 2005;281:102–11. doi: 10.1016/j.ydbio.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bellefroid EJ, Poncelet DA, Lecocq PJ, Revelant O, Martial JA. The evolutionarily conserved Kruppel-associated box domain defines a subfamily of eukaryotic multifingered proteins. Proc Natl Acad Sci USA. 1991;88:3608–12. doi: 10.1073/pnas.88.9.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–71. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–70. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Candia AF, Hu J, Crosby J, Lalley PA, Noden D, Nadeau JH, Wright CV. Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development. 1992;116:1123–36. doi: 10.1242/dev.116.4.1123. [DOI] [PubMed] [Google Scholar]
- Casademunt E, Carter BD, Benzel I, Frade JM, Dechant G, Barde YA. The zinc finger protein NRIF interacts with the neurotrophin receptor p75(NTR) and participates in programmed cell death. EMBO J. 1999;18:6050–61. doi: 10.1093/emboj/18.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S, Ott MO, Power S, Maury M. Expression patterns of vHNF1 and HNF1 homeoproteins in early postimplantation embryos suggest distinct and sequential developmental roles. Development. 1992;116:783–97. doi: 10.1242/dev.116.3.783. [DOI] [PubMed] [Google Scholar]
- Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow PN, Lovell-Badge R. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development. 1996a;122:509–20. doi: 10.1242/dev.122.2.509. [DOI] [PubMed] [Google Scholar]
- Collignon J, Varlet I, Robertson EJ. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature. 1996b;381:155–8. doi: 10.1038/381155a0. [DOI] [PubMed] [Google Scholar]
- Concha ML, Adams RJ. Oriented cell divisions and cellular morphogenesis in the zebrafish gastrula and neurula: a time-lapse analysis. Development. 1998;125:983–94. doi: 10.1242/dev.125.6.983. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 2003;4:784–93. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Crompton LA, Du Roure C, Rodriguez TA. Early embryonic expression patterns of the mouse Flamingo and Prickle orthologues. Dev Dyn. 2007;236:3137–43. doi: 10.1002/dvdy.21338. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- D’Avino PP, Thummel CS. crooked legs encodes a family of zinc finger proteins required for leg morphogenesis and ecdysone-regulated gene expression during Drosophila metamorphosis. Development. 1998;125:1733–45. doi: 10.1242/dev.125.9.1733. [DOI] [PubMed] [Google Scholar]
- Darken RS, Scola AM, Rakeman AS, Das G, Mlodzik M, Wilson PA. The planar polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J. 2002;21:976–85. doi: 10.1093/emboj/21.5.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Keller RE. Neural tube closure in Xenopus laevis involves medial migration, directed protrusive activity, cell intercalation and convergent extension. Development. 1999;126:4547–56. doi: 10.1242/dev.126.20.4547. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–30. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Elul T, Keller R. Monopolar protrusive activity: a new morphogenic cell behavior in the neural plate dependent on vertical interactions with the mesoderm in Xenopus. Dev Biol. 2000;224:3–19. doi: 10.1006/dbio.2000.9746. [DOI] [PubMed] [Google Scholar]
- Elul T, Koehl MA, Keller R. Cellular mechanism underlying neural convergent extension in Xenopus laevis embryos. Dev Biol. 1997;191:243–58. doi: 10.1006/dbio.1997.8711. [DOI] [PubMed] [Google Scholar]
- Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat Genet. 1998;18:266–70. doi: 10.1038/ng0398-266. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia MJ, Anderson KV. Essential role of glycosaminoglycans in Fgf signaling during mouse gastrulation. Cell. 2003;114:727–37. doi: 10.1016/s0092-8674(03)00715-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia MJ, Eggenschwiler JT, Caspary T, Alcorn HL, Wyler MR, Huangfu D, Rakeman AS, Lee JD, Feinberg EH, Timmer JR, et al. Analysis of mouse embryonic patterning and morphogenesis by forward genetics. Proc Natl Acad Sci USA. 2005;102:5913–9. doi: 10.1073/pnas.0501071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebelein B, Urrutia R. Sequence-specific transcriptional repression by KS1, a multiple-zinc-finger-Kruppel-associated box protein. Mol. Cell. Biol. 2001;21:928–39. doi: 10.1128/MCB.21.3.928-939.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–91. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev. Biol. 2002;247:165–81. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- Greene ND, Gerrelli D, Van Straaten HW, Copp AJ. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech Dev. 1998;73:59–72. doi: 10.1016/s0925-4773(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, et al. Mutations affecting morphogenesis during gastrulation and tail formation in the zebrafish, Danio rerio. Development. 1996;123:143–51. doi: 10.1242/dev.123.1.143. [DOI] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol. 2002;4:610–5. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasarskis A, Manova K, Anderson KV. A phenotype-based screen for embryonic lethal mutations in the mouse. Proc Natl Acad Sci USA. 1998;95:7485–90. doi: 10.1073/pnas.95.13.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Tibbetts P. Mediolateral cell intercalation in the dorsal, axial mesoderm of Xenopus laevis. Dev Biol. 1989;131:539–49. doi: 10.1016/s0012-1606(89)80024-7. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–5. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A, Tam PP. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development. 1999;126:4691–701. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- Krebs CJ, Larkins LK, Price R, Tullis KM, Miller RD, Robins DM. Regulator of sex-limitation (Rsl) encodes a pair of KRAB zinc-finger genes that control sexually dimorphic liver gene expression. Genes Dev. 2003;17:2664–74. doi: 10.1101/gad.1135703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA. Fate mapping the mouse embryo. Int J Dev Biol. 1999;43:773–5. [PubMed] [Google Scholar]
- Lewis SL, Tam PP. Definitive endoderm of the mouse embryo: formation, cell fates, and morphogenetic function. Dev Dyn. 2006;235:2315–29. doi: 10.1002/dvdy.20846. [DOI] [PubMed] [Google Scholar]
- Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:419–31. doi: 10.1242/dev.119.2.419. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–8. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Margolin JF, Friedman JR, Meyer WK, Vissing H, Thiesen HJ, Rauscher FJ. Kruppel-associated boxes are potent transcriptional repression domains. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4509–13. doi: 10.1073/pnas.91.10.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Raya A, Kawakami Y, Callol-Massot C, Capdevila J, Rodriguez-Esteban C, Belmonte Izpisua J. C. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev. 2005;19:164–75. doi: 10.1101/gad.1253605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–7. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Moon RT, Campbell RM, Christian JL, McGrew LL, Shih J, Fraser S. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development. 1993;119:97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O’Neal W, Milgram SL. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001a;10:2593–601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Rachel RA, Shah S, Beermann F, Stanier P, Mason CA, Copp AJ. Circletail, a new mouse mutant with severe neural tube defects: chromosomal localization and interaction with the loop-tail mutation. Genomics. 2001b;78:55–63. doi: 10.1006/geno.2001.6638. [DOI] [PubMed] [Google Scholar]
- Nagy A. Manipulating the mouse embryo : a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2003. [Google Scholar]
- Quertermous EE, Hidai H, Blanar MA, Quertermous T. Cloning and characterization of a basic helix-loop-helix protein expressed in early mesoderm and the developing somites. Proc Natl Acad Sci USA. 1994;91:7066–70. doi: 10.1073/pnas.91.15.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakeman AS, Anderson KV. Axis specification and morphogenesis in the mouse embryo require Nap1, a regulator of WAVE-mediated actin branching. Development. 2006;133:3075–83. doi: 10.1242/dev.02473. [DOI] [PubMed] [Google Scholar]
- Altaba A. Ruiz i, Prezioso VR, Darnell JE, Jessell TM. Sequential expression of HNF-3 beta and HNF-3 alpha by embryonic organizing centers: the dorsal lip/node, notochord and floor plate. Mech Dev. 1993;44:91–108. doi: 10.1016/0925-4773(93)90060-b. [DOI] [PubMed] [Google Scholar]
- Sumanas S, Zhang B, Dai R, Lin S. 15-zinc finger protein Bloody Fingers is required for zebrafish morphogenetic movements during neurulation. Dev. Biol. 2005;283:85–96. doi: 10.1016/j.ydbio.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Tada M, Concha ML, Heisenberg CP. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol. 2002;13:251–60. doi: 10.1016/s1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol. 2003;259:318–35. doi: 10.1016/s0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Torban E, Wang HJ, Patenaude AM, Riccomagno M, Daniels E, Epstein D, Gros P. Tissue, cellular and sub-cellular localization of the Vangl2 protein during embryonic development: effect of the Lp mutation. Gene Expr. Patterns. 2006;7:346–54. doi: 10.1016/j.modgep.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Torban E, Patenaude AM, Leclerc S, Rakowiecki S, Gauthier S, Andelfinger G, Epstein DJ, Gros P. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc Natl Acad Sci USA. 2008;105:3449–54. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Urrutia R. KRAB-containing zinc-finger repressor proteins. Genome Biol. 2003;4:231. doi: 10.1186/gb-2003-4-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Hardt S, Bakkers J, Inbal A, Carvalho L, Solnica-Krezel L, Heisenberg CP, Hammerschmidt M. The Bmp Gradient of the Zebrafish Gastrula Guides Migrating Lateral Cells by Regulating Cell-Cell Adhesion. Curr Biol. 2007 doi: 10.1016/j.cub.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–25. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbächer U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–5. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006a;133:1767–78. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 2006b;26:2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warga RM, Kimmel CB. Cell movements during epiboly and gastrulation in zebrafish. Development. 1990;108:569–80. doi: 10.1242/dev.108.4.569. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Chavrier P, Bravo R, Charnay P. Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature. 1989;337:461–4. doi: 10.1038/337461a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–9. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Wilson P, Keller R. Cell rearrangement during gastrulation of Xenopus: direct observation of cultured explants. Development. 1991;112:289–300. doi: 10.1242/dev.112.1.289. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Tamplin OJ, Beckers A, Gossler A, Rossant J. Live imaging and genetic analysis of mouse notochord formation reveals regional morphogenetic mechanisms. Dev. Cell. 2007;13:884–96. doi: 10.1016/j.devcel.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, Greene ND, Copp AJ. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–99. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


