Abstract
To characterize the impact of gut microbiota on host metabolism, we investigated the multicompartmental metabolic profiles of a conventional mouse strain (C3H/HeJ) (n=5) and its germ-free (GF) equivalent (n=5). We confirm that the microbiome strongly impacts on the metabolism of bile acids through the enterohepatic cycle and gut metabolism (higher levels of phosphocholine and glycine in GF liver and marked higher levels of bile acids in three gut compartments). Furthermore we demonstrate that (1) well-defined metabolic differences exist in all examined compartments between the metabotypes of GF and conventional mice: bacterial co-metabolic products such as hippurate (urine) and 5-aminovalerate (colon epithelium) were found at reduced concentrations, whereas raffinose was only detected in GF colonic profiles. (2) The microbiome also influences kidney homeostasis with elevated levels of key cell volume regulators (betaine, choline, myo-inositol and so on) observed in GF kidneys. (3) Gut microbiota modulate metabotype expression at both local (gut) and global (biofluids, kidney, liver) system levels and hence influence the responses to a variety of dietary modulation and drug exposures relevant to personalized health-care investigations.
Keywords: germ-free, gut microbiota, metabolism, metabonomics
Introduction
The gut microbiota (microbiome) form a complex and dynamic ecosystem that constantly interacts with host metabolism (Dunne, 2001; Hooper and Gordon, 2001; Bourlioux et al, 2003). The microbiome provides trophic (Hooper and Gordon, 2001) and protective (Umesaki and Setoyama, 2000) functions and impact on the host's energy metabolism (Savage, 1986), facilitating the absorption of complex carbohydrates (fiber breakdown) and influencing the homeostasis of amino acids (Hooper et al, 2002). For example in humans, 1–20% of the circulating plasma lysine and threonine are derived from gut bacterial synthesis (Metges, 2000). The microbiota also synthesize essential vitamins such as vitamin K (Hooper et al, 2002) and group B vitamins (Burkholder and McVeigh, 1942). These close symbiotic relationships are the result of co-evolutionary processes, through which natural selection has promoted the host genotypes that provide well-adapted adhesion sites for specific microorganisms (Bäckhed et al, 2005). In total, the mammalian symbiotic superorganism can contain significantly more active DNA in the fan of genes from the microbiome than in the host genome (Nicholson et al, 2005). Indeed, the symbiotic microbiotal speciation of some invertebrates (e.g. plataspid insects) has been shown to be closely connected with host evolution and take control of many metabolic functions resulting in host genome reduction (Hosokawa et al, 2006). The indigenous microbiota of mammals also strongly influences the metabolism of many drugs and nutrients, modifying both their bioavailability and metabolic fate (Nicholson et al, 2005). For example, phytoestrogens are metabolized into active compounds by gut microbiota (Setchell, 1998; Atkinson et al, 2005). But despite their evident important contribution to host biology and function, some bacterial species contained in the gut also have the potential to generate carcinogens or can be the source of opportunistic infections (Berg, 1996). For instance, Helicobacter pylori is well known to be part of the commensal flora of the stomach that can cause gastritis, gastric ulcers and, in some cases, gastric cancer (Amieva and El-Omar, 2008).
We have recently demonstrated a close relationship between the metabolism of gut microbiota and the susceptibility of rodents to insulin resistance in high-fat diet studies (Dumas et al, 2006a). In this context, recent works have shown that even subtle changes in the gut microbiota have an impact on the host phenotype (Holmes and Nicholson, 2005; Robosky et al, 2005; Rohde et al, 2007). Other investigations have demonstrated the close link between obesity and gut microbiota in human and mice (Bäckhed et al, 2004, 2007; Ley et al, 2006; Turnbaugh et al, 2006).
Germ-free (GF) animal studies have been widely used as a source of knowledge on the gut microbiota contributions to host homeostatic controls (Wostmann, 1981). GF mice display unusual gut morphology, i.e. larger cecum, thinner intestinal villi, when compared with conventional animals as well as physiological and immunological abnormalities, i.e. lower peristalsis, decreased inflammatory responses (Berg, 1996). GF animals have also been used to observe the developmental mechanisms of the gastrointestinal tract in interaction with the gut microbiota (Bates et al, 2006). However, despite the extensive use of GF models, the exact mechanisms involved in the morphologic, physiologic and immunologic modifications in GF animals remain unclear. The characterization of the metabolic differences between conventional and GF mice is, therefore, an essential step toward better understanding the interaction between host and gut microbiota.
Metabonomic approaches combining spectroscopic profiling techniques with pattern recognition analysis have proved useful in the assessment of the systemic metabolic responses of organisms to drugs or nutrients (Nicholson et al, 2002; Lindon et al, 2004; Dumas et al, 2006b; Rezzi et al, 2007). This approach has been successfully applied on biofluids and intact intestinal tissues in rodents to demonstrate the involvement of microbiota in the mammalian metabolism (Nicholls et al, 2003; Wang et al, 2005; Martin et al, 2006). In addition, metabonomic approaches have been recently used to demonstrate that hippurate excretion, a marker of gut microbiotal activity in protein catabolism to benzoate, varies between normal and obese rats (Williams et al, 2005) and the close link between gut microbiota and fatty liver phenotype in insulin-resistant mice (Dumas et al, 2006a).
In the current study, we employed a high-resolution 1H NMR spectroscopic approach to investigate the metabolic phenotype, or metabotype (Gavaghan et al, 2000), of GF mice from urine and tissues (gut, liver and kidney) and to determine the biochemical consequences of the absent microbiome on these biological matrices.
Results
Spectra from biofluids and tissue aqueous extracts contain prominent signals from metabolites representing numerous major metabolic pathways. For each analyzed biological matrix, a typical spectrum obtained from a conventional and a GF mouse is displayed (Figures 1A and B, 2A and B, 3A and B, 4A and B and 5A and B) and Table I shows the NMR assignment and corresponding resonance multiplicity. The summaries of all statistical models are shown in Table II. The main metabolic differences in GF group were summarized for all biological matrices in Table III.
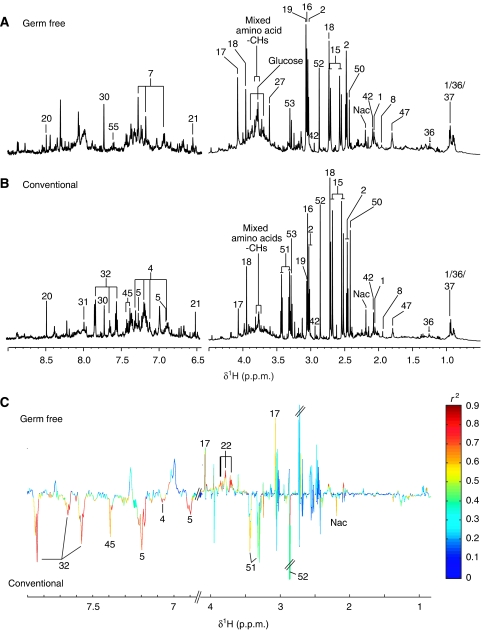
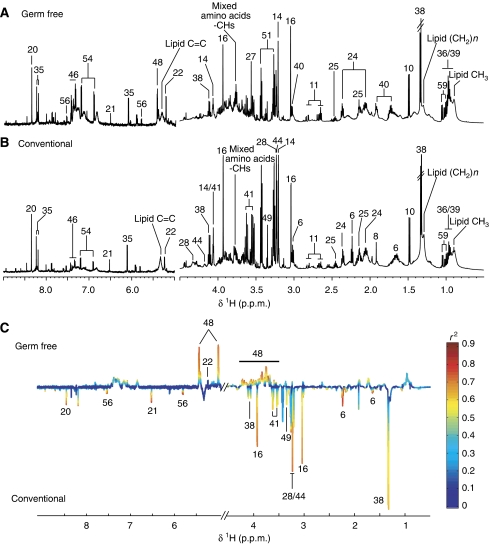
Figure 1.
1H NMR spectra (600 MHz) of urine samples from germ-free (GF) (A) and conventional (B) mice. The aromatic region (δ 6.5–9.0) has been vertically expanded × 4. (C) Plot of O-PLS-DA coefficients related to the discrimination between 1H NMR spectra of urine from GF (top) and conventional (bottom) mice. For identification of the peak numbers, refer to codes in Table II.
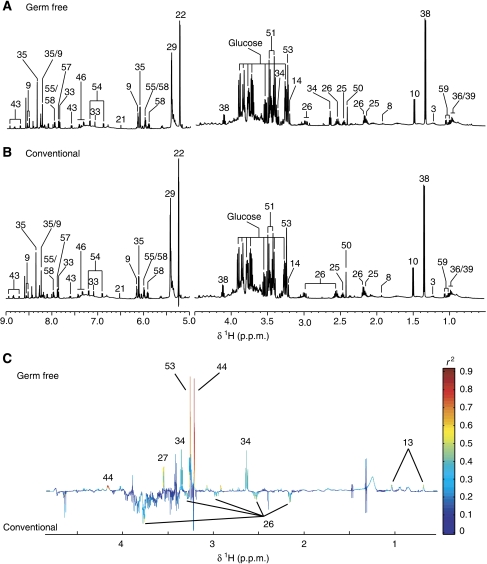
Figure 2.
1H NMR spectra (600 MHz) of liver aqueous extracts of germ-free (GF) (A) and conventional (B) mice. The aromatic region (δ 6.5–9.0) has been vertically expanded × 4. (C) Plot of O-PLS-DA coefficients related to the discrimination between 1H NMR spectra of urine from GF (top) and conventional (bottom) mice. For identification of the peak numbers, refer to codes in Table II.
Figure 3.
1H NMR spectra (600 MHz) of kidney aqueous extracts of germ-free (GF) (A) and conventional (B) mice. The aromatic region (δ 6.5–9.0) has been vertically expanded × 4. (C) Plot of O-PLS-DA coefficients related to the discrimination between 1H NMR spectra of urine from GF (top) and conventional (bottom) mice. For identification of the peak numbers, refer to codes in Table II.
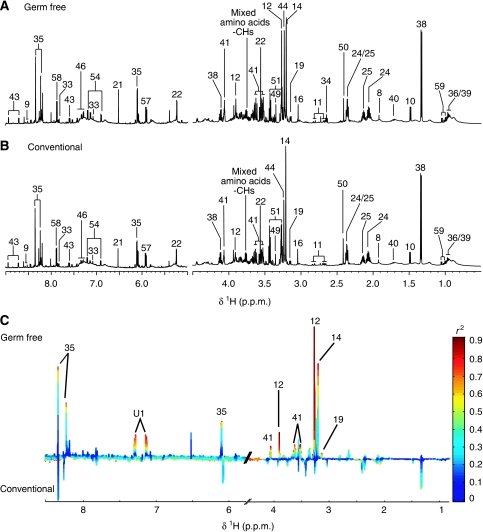
Figure 4.
1H NMR spectra (600 MHz) of ileum aqueous extracts of germ-free (GF) (A) and conventional (B) mice. The aromatic region (δ 6.5–9.0) has been vertically expanded × 4. (C) Plot of O-PLS-DA coefficients related to the discrimination between 1H NMR spectra of urine from GF (top) and conventional (bottom) mice. For identification of the peak numbers, refer to codes in Table II.
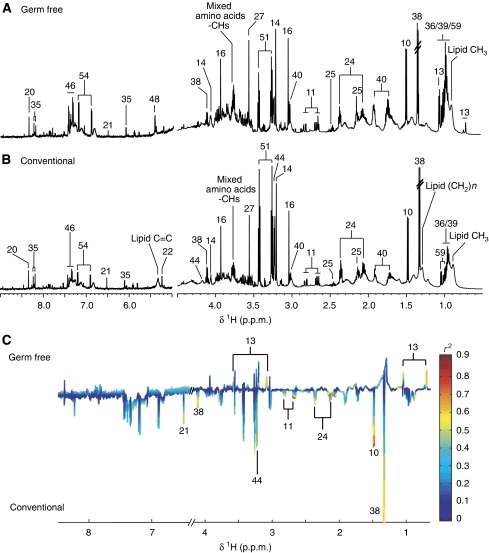
Figure 5.
1H NMR spectra (600 MHz) of colon aqueous extracts of germ-free (GF) (A) and conventional (B) mice. The aromatic region (δ 6.5–9.0) has been vertically expanded × 4. (C) Plot of O-PLS-DA coefficients related to the discrimination between 1H NMR spectra of urine from GF (top) and conventional (bottom) mice. For identification of the peak numbers, refer to codes in Table II.
Table 1.
Full 1H NMR chemical shift data for discriminating metabolites assigned in urine and tissue samples (note that signals for unassigned or non-significantly discriminating metabolites are not reported)
| Code | Metabolite | δ 1H (multiplicity) group | Compartments observed |
|---|---|---|---|
| 1 | 2-Oxoisocaproate | 0.94 (d) CH3, 2.18 (m) CH, 2.64 (d) CH2 | U |
| 2 | 2-Oxoglutarate | 2.47 (t) γCH2, 3.03 (t) βCH2 | U |
| 3 | D-3-Hydroxybutyrate | 1.20 (d) CH3, 2.31 (dd) ½ αCH2, 2.41 (dd) ½ αCH2, 4.16 (dt) CH | L |
| 4 | 3-Hydroxycinnamate | 6.49 (d) αCH, 6.92 (d) H2, 7.09 (s) H6, 7.17 (d) H4, 7.33 (m) H3/β-CH | U |
| 5 | 4-Hydroxyphenylpropionate | 2.52 (t) αCH, 2.91 (t) βCH, 6.92 (d) H2/H6, 7.22 (d) H3/H5 | U |
| 6 | 5-Aminovalerate | 1.64 (m) β/γCH2, 2.25 (t) αCH2, 3.02 (t) δCH2 | C |
| 7 | 5-Hydroxytryptophan | 3.23 (dd) ½ βCH2, 3.41 (dd) ½ βCH2, 4.02 CHNH2, 6.88 H6, 7.14 H2, 7.28, 7.41 H7 | U |
| 8 | Acetate | 1.92 (s) CH3 | U, L, K, D, J, I, C |
| 9 | Adenosine diphosphate | 4.20 (dd) ½ CH2, 4.23 (dd) ½ CH2, 4.27 (dt) H5, 4.50 (m) H4, 4.77 (m) H3, 6.12 (d) H2, 8.18 (s) H7, 8.50 (s) H12, 8.55 (s) H12 | L, K |
| 10 | Alanine | 1.48 (d) βCH3, 3.79 (m) CH | L, K, D, J, I, C |
| 11 | Aspartate | 2.68 (AB of ABX) ½ βCH2, 2.82 (AB of ABX) ½ βCH2, 3.91 (X of ABX) αCH | K, D, J, I, C |
| 12 | Betaine | 3.27 (s) CH3, 3.90 (s) CH2 | K |
| 13 | Bile acids (mixed) | 0.70 (s) CH3, 1.05 (s) CH3 | L, D, J, I |
| 14 | Choline | 3.20 (s) N-(CH3)3, 3.51 (t) βCH2, 4.05 (t) αCH2 | L, K, D; J, I, C |
| 15 | Citrate | 2.69 (AB) ½ CH2, 2.55 (AB) ½ CH2 | U |
| 16 | Creatine | 3.03 (s) N-CH3, 3.94 (s) CH2 | U, K, D, J, I, C |
| 17 | Creatinine | 3.06 (s) N-CH3, 4.05 (s) CH2 | U |
| 18 | Dimethylamine | 2.72 (s) CH3 | U |
| 19 | Ethanolamine | 3.13 (t) NH-CH2, 3.83 (t) HO-CH2 | K |
| 20 | Formate | 8.46 (s) CH | U, D, J, I, C |
| 21 | Fumarate | 6.52 (s) CH | U, L, K, D, J, I, C |
| 22 | α-Glucose | 3.42 (t) H4, 3.54 (dd) H2, 3.71 (t) H3, 3.72 (m) ½ CH2-C6, 3.76 (m) ½ CH2-C6, 3.83 (ddd) H5, 5.23 (d) H1 | L, K, D, J, I, C |
| 23 | β-Glucose | 3.24 (dd) H2, 3.40 (t) H4, 3.47 (ddd) H5, 3.48 (t) H3, 3.84 (m) ½ CH2-C6, 3.90 (dd) ½ CH2-C6, 4.64 (d) H1 | L |
| 24 | Glutamate | 2.08 (m) βCH2, 2.34 (m) γCH2, 3.75 (m) αCH | K, D, J, I, C |
| 25 | Glutamine | 2.15 (m) βCH2, 2.46 (m) γCH2, 3.77 (m) αCH | L, K, D, J, I, C |
| 26 | Glutathione (oxidized) | 2.17 (m) βCH2 Glu, 2.55 (m) γCH2 Glu, 2.98 (AB of ABX, broad) and 3.30 (AB of ABX, broad) βCH2 Cys, 3.78, αCH2 Gly, 4.75 (X of ABX, broad) αCH Cys | L |
| 27 | Glycine | 3.56 (s) αCH | U, D, J, I |
| 28 | Glycerophosphocholine | 3.23 (s) N-(CH3)3, 4.32 (m broad) CH | D, J, C |
| 29 | Glycogen | 3.83 (m broad), 5.41 (m broad) | L |
| 30 | Guanine | 7.72 (s) CH | U |
| 31 | Guanosine | 3.86 (m) CH2, 4.24 (m) H5, 4.41 (t) H4′, 5.91 (d) H2′, 8.00 (s) H8 | U, D, J |
| 32 | Hippurate | 3.97 (d) CH2, 7.56 (t) m-CH, 7.65 (t) p-CH, 7.84 (d) αCH | U |
| 33 | Histidine | 3.14 ½ βCH2 (AB of ABX), 3.25 ½ βCH2 (AB of ABX), 3.99 αCH (X of ABX), 7.08 (s) H5, 7.83 (s) H3 | L, K |
| 34 | Hypotaurine | 2.64 (t) CH2-NH2, 3.37 (t) CH2-SO3 | L |
| 35 | Inosine | 3.85 ½ CH2 (AB of ABX), 3.92 ½ CH2 (AB of ABX), 4.28 H5′ (X of ABX), 6.10 (d) H2′, 8.24 (s) H8, 8.34 (s) H2 | L, K, D, J, I, C |
| 36 | Isoleucine | 0.95 (t) δCH3, 1.01 (d) βCH3, 1.26 (m) ½ γCH2, 1.48 (m) ½ γCH2, 1.98 (m) βCH 3.68 (d) αCH | U, L, K, D, J, I, C |
| 37 | Isovaleric acid | 0.92 (d) CH3, 1.94 (m) CH, 2.05 (d) CH2 | U |
| 38 | Lactate | 1.33 (d) βCH3, 4.12 (q) αCH | L, K, D, J, I, C |
| 39 | Leucine | 0.96 (d) δCH3, 1.71 (m) γCH, 3.73 (t) αCH | L, K, D, J, I, C |
| 40 | Lysine | 1.48 (m) γCH2, 1.73 (m) δCH2, 1.91 (m) βCH2, 3.03 (t) ɛCH2, 3.76 (t) αCH | K, D, J, I, C |
| 41 | myo-Inositol | 3.29 (t) H5, 3.53 (dd) H1/H3, 3.63 (t) H4/H6, 4.06 (t) H2 | K, D, J, C |
| 42 | N-Acetylcysteine | 2.08 (s) CH3, 2.94 (m) CH2, 4.39 (m) CH | U |
| 43 | Nicotinurate | 3.99 (s) CH2, 7.6 (dd) H5, 8.25 (d) H4, 8.71 (d) H6, 8.94 (s) H2 | L, K |
| 44 | Phosphocholine | 3.22 (s) N-(CH3)3, 3.62 (t) βCH2, 4.23 (m) αCH2 | L, I, C |
| 45 | Phenylacetylglycine | 3.67 (s) δCH2, 3.75 (d) αCH2, 7.35 (m) H2/H6, 7.37 (t) H4, 7.42 (m) H3/H5 | U |
| 46 | Phenylalanine | 3.13 ½ βCH2 (AB of ABX), 3.28 ½ βCH2 (AB of ABX), 4.00 αCH (X of ABX), 7.33 (m) H2/H6, 7.39 (t) H4, 7.43 (m) H3/H5 | L, K, D, J, I, C |
| 47 | Putrescine | 1.80 (m broad) βCH2, 3.05 (m broad) αCH2 | U |
| 48 | Raffinose | 3.53 (s), 3.55–3.59 (m), 3.68 (s), 3.70–3.92 (m), 3.96 (t), 4.00–4.07 (m), 4.23 (d) H3 (fructose), 5.00 (d) H21 (galactose), 5.43 (d) H7 (glucose) | C |
| 49 | scyllo-Inositol | 3.35 (s) CH | C |
| 50 | Succinate | 2.41 (s) CH3 | U, L, K |
| 51 | Taurine | 3.27 (t) CH2-SO3, 3.43 (t) CH2-NH | U, L, K, D, J, I, C |
| 52 | Trimethylamine | 2.86 (s) CH3 | U |
| 53 | Trimethylamine N-oxide | 3.27 (s) (CH3)3 | U, L |
| 54 | Tyrosine | 3.06 ½ βCH2 (AB of ABX), 3.16 ½ βCH2 (AB of ABX), 3.94 αCH (X of ABX), 6.87 (d) H2/H6, 7.18 (d) H3/H5 | L, K, D, J, I, C |
| 55 | Uridine diphosphate | 4.21 (dd) ½ CH2, 4.25 (dd) ½ CH2, 4.37 (dt) H5, 4.39 (dd) H4, 4.43 H3, 5.96 (m) H2, 5.98 (d) H10, 7.98 (d) H11 | U, L |
| 56 | Uracil | 5.78 (d) CH, 7.52 (d) CH | I, C |
| 57 | Uridine | 3.81 (dd) ½ CH2, 3.92 (dd) ½ CH2, 4.12 (dt) H5, 4.24 (dd) H4, 4.36 (dd) H3, 5.88 (d) H10, 5.92 (m) H2, 7.88 (d) H11 | L, K, D, J |
| 58 | Uridine triphosphate | 4.25 (dd) ½ CH2, 4.28 (dd) ½ CH2, 4.39 (dt) H5, 4.40 (dd) H4, 4.45(dd) H3, 5.90 (d) H10, 5.98 (m) H2, 7.98 (d) H11 | L |
| 59 | Valine | 0.99 (d) γCH3, 1.05 (d) γ′CH3, 2.28 (m) βCH, 3.62 (d) αCH | L, K, D, J, I, C |
The numbering/nomenclature of compounds follows the IUPAC system.
Key: s, singlet; d, doublet, dd, doublet of doublets; t, triplet; m, multiplet; ABX refers to second-order spin system usually of the form CH2CH where all three protons are non-equivalent; C, colon; D, duodenum; I, ileum; J, jejunum; K, kidney; L, liver; U, urine.
Table 2.
Summaries of O-PLS-DA statistical models
| Sample | Orthogonal component | Q2Y | R2X |
|---|---|---|---|
| Duodenum | 0 | 0.57 | 0.27 |
| Jejunum | 1 | 0.37 | 0.52 |
| Ileum | 0 | 0.46 | 0.26 |
| Colon | 1 | 0.70 | 0.34 |
| Liver | 1 | 0.58 | 0.47 |
| Kidney | 1 | 0.42 | 0.61 |
| Urine | 1 | 0.83 | 0.39 |
Q2Y, cross-validated predicted percentage of the response Y; R2X, variation of X explained by the model.
Table 3.
Summary of variations of metabolite signals with the highest discriminant power for each model
| Metabolite | δ (p.p.m.) | Duodenum | Jejunum | Ileum | Colon | Liver | Kidney | Urine |
|---|---|---|---|---|---|---|---|---|
| 3-HCA | 7.07 | −0.93 | ||||||
| 4-HPP | 6.89 | −0.92 | ||||||
| 5-Aminovalerate | 2.236 | −0.86 | ||||||
| Alanine | 1.476 | +0.84 | −0.83 | |||||
| Aspartate | 2.81 | −0.84 | ||||||
| Betaine | 3.904 | +0.98 | ||||||
| Choline | 3.2052 | +0.94 | ||||||
| Creatine | 3.04 | +0.52 | −0.83 | |||||
| Creatinine | 4.08 | +0.89 | ||||||
| Ethanolamine | 3.1448 | +0.87 | ||||||
| Formate | 8.459 | −0.76 | ||||||
| Fumarate | 6.520 | −0.79 | −0.91 | |||||
| Glutamate | 2.078 | −0.85 | ||||||
| Glutathione | 2.5528 | −0.71 | ||||||
| Glycine | 3.559 | +0.49 | +0.79 | |||||
| GPC | 3.2312 | −0.85 | −0.85 | |||||
| Hippurate | 7.84 | −0.93 | ||||||
| Hypotaurine | 2.645 | +0.60 | ||||||
| Inosine | 8.3468 | +0.88 | ||||||
| Lactate | 1.336 | −0.79 | −0.78 | |||||
| Nac | 2.185 | −0.76 | ||||||
| myo-Inositol | 3.5288 | −0.76 | +0.92 | |||||
| PAG | 7.38 | −0.87 | ||||||
| Phosphocholine | 3.2252 | −0.76 | +0.93 | |||||
| Raffinose | 5.435 | +0.86 | ||||||
| scyllo-Inositol | 3.3485 | −0.72 | +0.78 | |||||
| Tauro-conjugated bile acids | 0.697 | +0.93 | +0.51 | +0.78 | +0.66 | |||
| TMAO | 3.269 | +0.85 | ||||||
| Tyrosine | 6.909 | −0.81 | ||||||
| Uracil | 5.811 | −0.94 |
Full chemical shift data for each metabolite are reported in Table I. The correlation coefficients with the discriminant axis for the metabolites involved in the difference between GF and conventional animals are presented as either higher (+) or lower level (−) compared with the conventional control.
Urine
The urine profile was characterized by high levels of taurine, 2-oxoglutarate, trimethylamine (TMA), citrate and succinate as previously reported (Bollard et al, 2005) (Figure 1A and B). The urinary profile of GF mice was characterized by low levels of hippurate, phenylacetylglycine (PAG), phenolic metabolites, 4-hydroxypropionic acid (4-HPP), 3-hydroxycinnamic acid (3-HCA) and N-acetylated glycoprotein signal and a marked high level of creatinine (Figure 1C).
Liver
Glucose resonances (δ 3.25–3.84) were predominant in the liver profile, which was also dominated by high levels of taurine and trimethylamine-N-oxide (TMAO) (Figure 2A and B). The oxidized glutathione (GSSG) pattern was readily identified in the one-dimensional (1D) spectrum. It is possible to differentiate between the reduced (GSH) and the oxidized (GSSG) forms of glutathione by 2D NMR because the resonances of the magnetically non-equivalent protons of the cysteine β-CH2 residue in GSH (δ 2.95) shift to high frequency in GSSG (δ 3.29 and 2.95) (Koga et al, 1986). The statistical model built from all liver spectra displayed an outlier in the GF group (data not shown). This highly dilute sample was removed from the subsequent analysis and the model was recalculated with four individuals in the GF group against five individuals in the conventional group. The metabolite profile of the liver from GF mice exhibited significant higher levels of TMAO and phosphocholine. Not significant higher levels of tauro-conjugated bile acids and glycine were noted in the GF mouse profile (Figure 2C). In addition, a lower level of GSSG together with a higher level of hypotaurine was observed in the liver of two GF animals.
Kidney
The kidney 1H NMR profiles were dominated by osmoprotectant compounds such as myo-inositol, glycine, betaine, choline and taurine (Yancey, 2005) (Figure 3A and B). The metabolite profile of the kidney from GF mice was characterized by higher levels of betaine, choline, myo-inositol, scyllo-inositol, ethanolamine, inosine and an unknown compound (U1) in the aromatic region of the spectra (δ 7.14 (d) and δ 7.30 (d)) (Figure 3C).
Gut compartments
Duodenum, jejunum and ileum were all characterized by high levels of tyrosine when compared with the other tissue extracts (Figure 4A, B; Supplementary Figures S1A and B, and S2A and B) together with creatine and taurine, a feature shared with the colon (Figure 5A and B). The colonic metabolite profile was characterized by high levels of myo-inositol and scyllo-inositol, as previously described (Martin et al, 2007b) (Figure 5A). Globally, these gut profiles also displayed similar patterns to those observed in human biopsies (Wang et al, 2007). Tauro-conjugated bile acids were observed only in duodenum, ileum and jejunum profiles.
Aqueous extract profiles of gut tissues from GF mice were markedly different to those from conventional mice (Figures 4C and 5C; Supplementary Figures S1C and S2C). The metabolite profile of the duodenum from GF mice was mainly characterized by higher levels of tauro-conjugated bile acids and alanine and lower levels of glycerophosphocholine (GPC) when compared with conventional mice (Supplementary Figure S1C). Two highly diluted samples in the jejunum profiles of the GF group were outliers and hence orthogonal projection on latent structures (O-PLS-DA) correlation coefficients (r2) were not significant (Supplementary Figure S2C). However, the GF group had higher levels of creatine and tauro-conjugated bile acids and lower levels of tyrosine in the jejunal tissue (Supplementary Figure S2C). The ileum of GF mice was also characterized by a higher level of tauro-conjugated bile acids and lower levels of glutamate, fumarate, lactate, phosphocholine and alanine when compared with the ileum from conventional mice (Figure 4C). Finally, when compared with conventional mice, the metabolite profile of the colon from GF mice revealed a higher level in a complex carbohydrate identified as raffinose (Supplementary Figure S3), and lower levels of lactate, creatine, 5-aminovalerate, propionate, glutamine, myo-inositol, scyllo-inositol (Moreno and Arus, 1996), GPC, phosphocholine, choline, formate, uracil and fumarate (Figure 5C).
Discussion
In this study, the metabotypes derived from different biological matrices from GF and conventional mice were characterized (Nicholson et al, 2002; Lindon et al, 2004) and it was showed that the metabolic impact of the microbiota extended beyond the intestinal tissue and biofluids to major organs such as the liver and kidney.
Evidence of gut microbiota re-processing of dietary metabolites
A major source of the intestinal metabolites is produced from dietary nutrients by both the intestinal cells and the gut microbiota. This production occurs mainly in the first 25% of the small intestine for amino acids, and in cecum and colon for fatty acids (Hooper et al, 2002). Here, metabolic variations in response to gut microbial activity are observed in the biochemical profiles of intestinal tissue extracts with increasing effect along the continuous gastrointestinal tract. More specifically, it was observed that duodenum and jejunum displayed fewer metabolic differences between GF and conventional mice, whereas ileum and particularly colon were the most affected (Table III). This reflects the higher microbial loads found in ileum and colon (Dunne, 2001). In particular, 5-aminovalerate was not observed in colon aqueous extract profiles of GF animals, which is consistent with its reported characterization as a product of protein degradation by several anaerobic bacteria, particularly clostridial strains (Figure 5C) (Barker, 1981; Barker et al, 1987). 5-Aminovalerate is degraded to acetate, ammonia and propionate. A higher concentration of propionate also observed in colon profile of conventional mice is consistent with the higher concentration of 5-aminovalerate.
More evidence of the crucial role of gut bacteria in the digestion of dietary nutrients is seen in the lower urinary level of several microbial co-metabolites (hippurate, 4-HPP and 3-HCA) in GF mice (Figure 1C). Indeed, it has been reported that gut microbiota are able to metabolize polyphenols, such as chlorogenic acids, into more absorbable compounds such as 4-HPP, 3-HCA and benzoic acid (Goodwin et al, 1994; Manach et al, 2004). Benzoic acid is then detoxified through conjugation with glycine in the liver and the kidney to form hippurate (benzoylglycine), a more hydrophilic metabolite that is then secreted by the renal tubular cells and excreted in the urine (Goodwin et al, 1994; Williams et al, 2002; Nicholls et al, 2003). Another microbial co-metabolite, PAG, was also found in lower concentration in the urinary profile of GF animals (Figure 1C), illustrating that microorganisms are crucial actors in the production of these urinary metabolites through the modulation of food processing.
Evidence of the host–gut bacterial metabolic interaction: bile acid co-metabolism
The metabolism and synthesis of the major bile acids are another example of mammalian–microbiotal co-metabolism that has been reported recently as crucial in determining the host phenotype (Martin et al, 2007a). In the present study, the metabolite profiles of duodenum, jejunum and ileum (Figure 4C) were all characterized by a higher concentration of tauro-conjugated bile acids in GF mice, which is not apparent in the colon profile (Figure 5C). In conventional animals, tauro- and glycine-conjugated bile acids are deconjugated by gut microbiota, facilitating their fecal elimination. Here, in the absence of microorganisms, primary bile acids are reabsorbed into the enterohepatic cycle, without deconjugation, by passive diffusion in duodenum and jejunum and by active transport in the terminal part of the ileum (Berg, 1996; Houten et al, 2006) (Figure 7). This increased recycling of bile acids is also suggested by the significantly higher level of phosphocholine and by the observed trend of higher concentration of bile acids in the liver metabolic profile of GF mice (Figure 2C). In fact, phosphocholine is the source of phosphatidylcholine, the most common phospholipid in bile, and its secretion is under control of certain bile acids, mainly cholic acid and deoxycholic acid, in hepatocytes (Uchida et al, 1980; Alvaro et al, 1986; Hofmann, 1999). Thus, the observation of the higher level of phosphocholine in liver GF profile may be the result of either a modification of bile acid profile or a higher level of bile acids in hepatocytes.
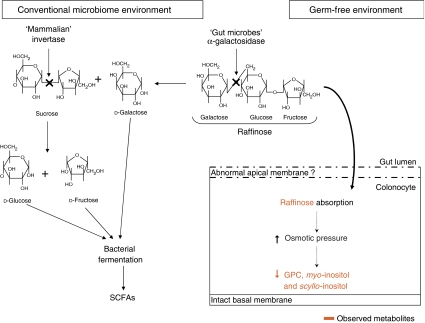
Figure 6.
Variation of raffinose metabolism by colonocytes in germ-free (GF) and conventional microbiome animals. In conventional animals, raffinose is first digested by microbial α-galactosidase to release galactose and sucrose. Then, the mammalian invertase attached to the brush border releases glucose and fructose from sucrose. These monosaccharides are then utilized as a source of carbon for bacterial fermentation. In GF animals, raffinose is not catabolized and passive diffusion into colonocytes may occur contributing to the osmotic pressure that is regulated by decreasing levels of the mobile osmolytes: glycerophosphocholine, myo-inositol and scyllo-inositol. GPC, glycerophosphocholine; SCFAs, short chain fatty acids.
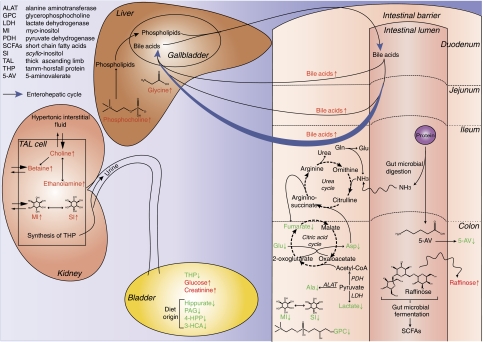
Figure 7.
Summary of some of the major systemic effects of the gut microbiome on mouse metabolism in different compartments. Metabolites observed in this study are shown in red when their level is higher in GF profiles or in green when it is lower. The enterohepatic cycle of bile acids is shown as blue arrows. The citric acid cycle has been simplified for clarity.
Microbial modification of bile acid metabolism may have many biological consequences, as bile acids participate in the regulation of dietary lipid absorption and cholesterol metabolism. They also function as signaling molecules linking to a G-protein-coupled receptor family (Kawamata et al, 2003; Kostenis, 2004) or directly triggering the farnesoid X receptor (FXR), which is a hepatocyte nuclear receptor involved in the regulation of lipid and glucose metabolism (Makishima et al, 1999; Claudel et al, 2005; Modica and Moschetta, 2006). Bile acids exert strong influences on the regulation of the expression of some cytochrome P450 (CYP) detoxification enzymes in the liver (Houten et al, 2006). It is also well known that CYP are key enzymes in the production of bile acids from cholesterol (Russell, 2003). Furthermore, Gram-negative bacteria produce endotoxins (lipopolysaccharides) that affect the expression of some CYP enzymes in the liver (Ueyama et al, 2005). Thereby, the microbiota may have an impact on host energy homeostasis by participating, directly or indirectly, in the control of bile acid metabolism.
Evidence of the modulation of host cell pathways and physiology by gut microbiota
The colonic metabolite profile in GF mice was characterized by lower levels of choline and its phosphorylated derivatives, GPC and phosphocholine. This is likely due to the disturbance of the membrane of colonocytes in GF animals. The observed accumulation of raffinose in these cells is probably also a consequence of this disruption. Raffinose is an oligosaccharide that is only digested by the gut microbiota, as monogastric animals do not express pancreatic α-galactosidase (LeBlanc et al, 2004). In GF animals, it seems that this trisaccharide is able to cross the epithelial membrane and accumulates in colonocytes where it induces a rise in osmotic pressure. This phenomenon provokes a well-described signaling cascade that leads to the release of the mobile osmolytes, GPC, myo-inositol and scyllo-inositol (Wehner, 2003; Alfieri, 2007) (Figure 6). Interestingly, lower levels of these metabolites have previously been associated with human colon adenocarcinoma (Moreno and Arus, 1996), and have also been observed in the brain of patients with hepatic encephalopathy (Lien et al, 1994; Albrecht and Jones, 1999) or associated with osmoregulatory function in the brain in response to atrophy (Tsang et al, 2006). These physiological changes were correlated with significantly lower creatine concentrations that can be associated with lower energy demands and with a lower peristalsis due to an impaired function of the smooth muscle layer in GF mice (Berg, 1996). Furthermore, a number of metabolites involved directly (e.g. fumarate) or indirectly (e.g. glutamate, aspartate, alanine and lactate) in energy pathways were present at lower levels in the ilial and colonic epithelium. Aspartate and fumarate are also key metabolites in the metabolism of urea associated with the citric acid cycle, a pathway that enables the elimination of ammonia produced endogenously from the catabolism of amino acids, and exogenously from the degradation of proteins by gut microflora (Metzler, 2003) (Figure 7). The massive production of exogenous ammonium in colon lumen results in a high intake of ammonium in colonocytes where it is partially detoxified into urea (Mouillé et al, 1999). Thus, it is assumed that the observed lower levels of fumarate, glutamate, aspartate, alanine and lactate in GF profiles reflect the lower input of ammonia and/or the lower smooth muscle activity in these animals. All of these perturbations emphasize the fundamental role of gut microbiota in colonic epithelial metabolism.
Moreover, the liver metabotype of GF animals indicated other bacterial-related changes. A lower level of GSSG, the oxidized form of the powerful antioxidative compound GSH (Meister and Anderson, 1983) and a higher level of hypotaurine were observed in two GF animals of a total of four (Figure 2C). Despite the restricted numbers of individuals included in this study, it is possible that a subgroup of animals may exist. GSSG represents 1% of the total amount of glutathione in vivo (Deneke and Fanburg, 1989). In this study, GSH was not observed because it is readily oxidized to GSSG by exposure to atmospheric oxygen during sample preparation. Thus, it can be considered that the observed GSSG reflects the whole amount of glutathione in the liver extract. Normally, glutathione, rather than hypotaurine, is the predominant antioxidative molecule in the liver. Furthermore, it has been demonstrated that hypotaurine is also a strong antioxidative compound (Aruoma et al, 1988; Yancey, 2005). The observation of a high level of hypotaurine concomitant with low level of glutathione indicates a perturbation of the cell response to oxidative stress. Thus, for these two individuals, the higher level of hypotaurine may compensate for the lack of glutathione in the liver. It is noteworthy that the low total glutathione content was associated in these two animals with high levels of glycine, which is an essential amino acid for glutathione biosynthesis (Meister and Tate, 1976). Taken together, these observations indicate a perturbed γ-glutamyl cycle activity in the liver of two GF mice and this may be suggestive of altered cysteine metabolism (Meister, 1988). The low level of total glutathione in GF animals may impact on many metabolic pathways as it is also a coenzyme involved in the regulation of protein synthesis and degradation, as well as in the mechanism of immune system and in the prostaglandin metabolism (Meister and Anderson, 1983; DeLeve and Kaplowitz, 1991; Uhlig and Wendel, 1992; Wang and Ballatori, 1998).
TMAO variation contributes to the statistical separation between the metabolic profiles of livers from GF and conventional mice (Figure 2C). TMAO was expected to be lower in GF animals as previously observed in urine profiles during re-colonization of GF rats (Nicholls et al, 2003). Here, we observed a significantly higher level of TMAO in GF mice. TMAO in the liver derives either from a direct absorption of TMAO contained in the diet, from the gut microbial processing of choline and carnitine, or is endogenously synthesized by the oxidation of TMA to TMAO by the flavin-containing mono-oxygenase isomer 3 (FMO3) (Smith et al, 1994; Zhang et al, 2007). As the two groups were fed exactly the same diet, it can be deduced that the higher level of TMAO found in the liver of GF animals comes from either a greater uptake of TMA/TMAO contained in the diet, or from a higher endogenous synthesis. In contrast to humans, expression of FMO3 in mouse is sex dependent with a much lower expression in males. However, it has been recently demonstrated that this expression is highly inducible by TCDD (dioxin) in an aryl hydrocarbon receptor-dependent manner (Tijet et al, 2006). Thus, it is possible that FMO3 in male GF animals was induced.
A lower level of N-acetylated glycoprotein (Nac) signal was observed in the urine of GF animals (Figure 1C). This signal comes from the most abundant glycoprotein in urine, the Tamm–Horsfall protein (THP), also known as uromodulin, a small glycoprotein (∼90 kDa) secreted by the thick ascending limb of the Henle's loop of the nephron (Serafini-Cessi et al, 2003). This protein is of particular interest in that its role is associated with the prevention of urinary tract infections. The N-glycans at the protein surface bind to uropathogenic strains of Escherichia coli, preventing the adhesion of these pathogens to the bladder wall (Pak et al, 2001; Mo et al, 2004). Here, the observed lower levels of Nac in GF urine profiles may be caused by a lower amount of THP in the urine or by a lower glycosylation of the protein. The protective function of THP is driven by N-glycans, so this observation reveals that protection against urinary tract pathogens is affected in the absence of gut microbiota. Also, choline, betaine, myo-inositol and scyllo-inositol were elevated in kidneys from GF mice (Figure 3C). All these metabolites are osmoprotectants (Burg, 1995) and it has been shown that their level increases in kidney cells when the environment becomes hypertonic (Yamauchi et al, 1991; Burg, 1995; Beck et al, 1998; Burger-Kentischer et al, 1999). Both decrease in THP concentration in urine and hypertonicity of interstitial fluid in kidney have been associated with renal dysfunction (Seldin and Giebisch, 2000), but it was not possible in this study to establish a link between these two observations.
Finally, the observed increased excretion of creatinine, a biomarker of muscle mass, in the urine of GF mice (Figure 1) is likely related to the lean phenotype of GF mice, as confirmed in a recent study (Bäckhed et al, 2007). Collectively, these data demonstrate that gut microbiota have a function in the control of the metabolic phenotype of the colon and liver and influence the whole-body metabolic homeostasis of the host.
Conclusions
We have demonstrated that gut microbiotal activities have an impact on site-specific intestinal epithelial biochemistry and influence at ‘long-range' hepatic and renal metabolite profiles as well as the global metabolic phenotype of the host (summarized in Figure 7). It would be of considerable interest to correlate the compartmentalized metabolite profiles with the known bacterial strains that compose the microbiota and this is the focus of an ongoing investigation. Gut microbiota seem to be an important regulator of the bile acid metabolism and may have an impact on CYP enzyme induction status. These results also suggest the potential impact of the gut microbiota on antioxidant mechanisms in the liver but further studies are needed. We also show that gut microbiome influences the renal metabolite profile possibly in response to interstitial hypertonicity. By acting directly or indirectly on the metabolism of liver and kidney, key organs of body physiology (i.e. homeostasis of arterial pressure and equilibrium of cholesterol and electrolyte levels), the gut microbiota can be considered as a major contributor of host homeostasis. Improved knowledge of host–microbiome interactions will lead to a better understanding of individual variation in relation to health status and interventional outcomes (Clayton et al, 2006; Nicholson, 2006).
Materials and methods
Animal handling and sample preparation
All studies were conducted according to the Swiss legislation on animal experimentation.
One group of five GF C3H/HeJ mice (Charles River, France) was maintained in isolators on γ-ray-irradiated food (R03-10) and γ-ray-irradiated water, whereas the other group of five conventional C3H/HeJ mice was maintained under identical conditions but in a conventional environment with non-irradiated food and water. Isolators were checked every week for any bacterial contamination throughout the life of GF animals. Throughout the duration of the study, water and food were provided ad libitum. Mice were euthanized when they were 8 weeks old, at which time urine and organs (duodenum, jejunum, ileum, colon, liver and kidney) were collected. Samples were snap frozen in liquid nitrogen and stored at −80°C until analysis.
1H NMR spectroscopy
Urine samples were freeze-dried and dissolved in 50 μL of phosphate buffer 0.2 M (pH 7.4) in D2O plus 0.05% sodium 3-(tri-methylsilyl)propionate-2,3-d4 (TSP) before transferring to capillary tubes for analysis by 1H NMR spectroscopy.
Tissue samples were homogenized and extracted in acetonitrile/water (1:1), as previously described (Waters et al, 2002). The supernatant containing the aqueous phase was collected, freeze-dried and dissolved in 600 μl of D2O. Samples were centrifuged for 10 min at 15 000 g, and 500 μl of the supernatant and 50 μl of water were used for later analysis by NMR spectroscopy.
All 1H NMR spectra were acquired on a Bruker Avance 600 MHz Spectrometer (Bruker Analytische GmbH, Rheinstetten, Germany) operating at 600.13 MHz and using a standard 1D pulse sequence (Nicholson et al, 1995) (recycle delay (RD)–90°–t1–90°–tm–90°–acquire free induction decay (FID)) with water suppression applied during RD of 2 s and mixing time (tm) of 100 ms and a 90° pulse set at 9.75 μs. Spectra were acquired using 256 scans into 32K data points with a spectral width of 12 000 Hz. The FIDs were multiplied by an exponential function corresponding to 0.3 Hz line broadening. Zero filling of a factor of four for all tissue extracts and two for urine samples was also applied to the FIDs. All spectra were manually phased, baseline corrected and calibrated to lactate (δ 1.33) for tissue extracts and to TSP (δ 0.00) for urine samples. Metabolites were assigned using data from literature (Nicholson et al, 1995; Fan, 1996; Garrod et al, 2001) and additional two-dimensional (2D) NMR experiments on selected samples.
The 2D 1H–1H NMR spectra were performed on a Bruker DRX 400 Spectrometer operating at 400.13 MHz (Bruker Analytische GmbH) using 2D correlation spectroscopy (Aue et al, 1975) and total correlation spectroscopy (Glaser et al, 1996) experiments. 2D 1H–13C heteronuclear single quantum coherence NMR (Bodenhausen and Ruben, 1980) was performed on liver aqueous extracts on a Bruker DRX 500 Spectrometer operating at 499.9 MHz (Bruker Analytische GmbH) equipped with a 5 mm 1H–13C inverse cryoprobe.
Data analysis
To eliminate the variability in water resonance presaturation, the chemical shift region between δ 4.66 and 4.88 was removed from all spectra before statistical analysis, except for liver where to avoid bias due to baseline distortion, the region between δ 4.77 and 5.38 was removed. As previously described (Cloarec et al, 2005), all data were analyzed on full-resolution spectra (35 600 data points for liver and 36 500 data points for all other tissue extracts), normalized to the total peak area and models were constructed using O-PLS-DA with unit variance scaling on Matlab 7.0.1 software (The MathWorks Inc.). Despite the use of phosphate buffer, many urine spectra still displayed subtle pH-dependent shifts; therefore, the O-PLS-DA was performed on larger bins of 0.005 p.p.m. (1750 bucketed points) to minimize minor frequency changes in spatial components.
To aid interpretation, the O-PLS coefficients were plotted into a spectral domain using the back-scaling method (Cloarec et al, 2005). Using this method, the weights of each variable are back-scaled to their initial metric of the data and then the shape of NMR spectra and the sign of the coefficients are preserved. However, the weights of the variables can still be compared using a colour code corresponding to the square of the actual O-PLS coefficients. By construction, the O-PLS coefficients are directly proportional to the correlation coefficients between the discriminant axis and the NMR data. For this reason, the square of the coefficients can be represented in terms of correlation after applying the same corrective factor to all coefficients, allowing by this way an estimation of the amount of variance of each NMR variable involved in the discrimination.
Supplementary Material
Supplementary Information
Supplementary Data
Acknowledgments
This study was funded by Nestlé.
References
- Albrecht J, Jones EA (1999) Hepatic encephalopathy: molecular mechanisms underlying the clinical syndrome. J Neurol Sci 170: 138–146 [DOI] [PubMed] [Google Scholar]
- Alfieri R (2007) Hyperosmotic stress response: comparison with other cellular stresses. Pflugers Arch 454: 173–185 [DOI] [PubMed] [Google Scholar]
- Alvaro D, Cantafora A, Attili AF, Ginanni Corradini S, De Luca C, Minervini G, Di Biase A, Angelico M (1986) Relationships between bile salts hydrophilicity and phospholipid composition in bile of various animal species. Comp Biochem Physiol B 83: 551–554 [DOI] [PubMed] [Google Scholar]
- Amieva MR, El-Omar EM (2008) Host–bacterial interactions in Helicobacter pylori infection. Gastroenterology 134: 306–323 [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Hoey BM, Butler J (1988) The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem J 256: 251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson C, Frankenfeld CL, Lampe JW (2005) Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 230: 155–170 [DOI] [PubMed] [Google Scholar]
- Aue WP, Baartholdi E, Ernst RR (1975) Two-dimensional spectroscopy. Application to nuclear magnetic resonance. J Chem Phys 64: 2229–2246 [Google Scholar]
- Bäckhed F, Ding H, Wang T, Hooper LV, Young Koh G, Nagys A, Semenkovich CF, Gordon JI (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 102: 11070–11075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI (2005) Host–bacterial mutualism in the human intestine. Science 307: 1915–1920 [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI (2007) Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 104: 979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker HA (1981) Amino acid degradation by anaerobic bacteria. Annu Rev Biochem 50: 23–40 [DOI] [PubMed] [Google Scholar]
- Barker HA, D'Ari L, Kahn J (1987) Enzymatic reactions in the degradation of 5-aminovalerate by Clostridium aminovalericum. J Biol Chem 262: 8994–9003 [PubMed] [Google Scholar]
- Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, Guillemin K (2006) Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol 297: 374–386 [DOI] [PubMed] [Google Scholar]
- Beck F-X, Burger-Kentischer A, Müller E (1998) Cellular response to osmotic stress in the renal medulla. Eur J Physiol 436: 814–827 [DOI] [PubMed] [Google Scholar]
- Berg RD (1996) The indigenous gastrointestinal microflora. Trends Microbiol 4: 430–435 [DOI] [PubMed] [Google Scholar]
- Bodenhausen G, Ruben DJ (1980) Natural abundance 15N NMR by enhanced heteronuclear spectroscopy. Chem Phys Lett 69: 185–189 [Google Scholar]
- Bollard ME, Stanley EG, Lindon JC, Nicholson JK, Holmes E (2005) NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed 18: 143–162 [DOI] [PubMed] [Google Scholar]
- Bourlioux P, Koletzko B, Guarner F, Braesco V (2003) The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium ‘The Intelligent Intestine', held in Paris, June 14, 2002. Am J Clin Nutr 78: 675–683 [DOI] [PubMed] [Google Scholar]
- Burg MB (1995) Molecular basis of osmotic regulation. Am J Physiol 268: F983–F996 [DOI] [PubMed] [Google Scholar]
- Burger-Kentischer A, Muller E, Marz J, Fraek ML, Thurau K, Beck FX (1999) Hypertonicity-induced accumulation of organic osmolytes in papillary interstitial cells. Kidney Int 55: 1417–1425 [DOI] [PubMed] [Google Scholar]
- Burkholder PR, McVeigh I (1942) Synthesis of vitamins by intestinal bacteria. Proc Natl Acad Sci USA 28: 285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudel T, Staels B, Kuipers F (2005) The farnesoid X receptor—a molecular link between bile acid and lipid and glucose metabolism. Artherioscler Thromb Vasc Biol 25: 2020–2031 [DOI] [PubMed] [Google Scholar]
- Clayton TA, Lindon JC, Cloarec O, Antti H, Charuel C, Hanton G, Provost JP, Le Net JL, Baker D, Walley RJ, Everett JR, Nicholson JK (2006) Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature 440: 1073–1077 [DOI] [PubMed] [Google Scholar]
- Cloarec O, Dumas M-E, Craig A, Barton RH, Lindon JC, Nicholson JK, Holmes E (2005) Evaluation of the O-PLS model limitations caused by chemical shift variability and improved visualization of biomarker changes in 1H NMR spectroscopic metabonomic studies. Anal Chem 77: 517–526 [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Kaplowitz N (1991) Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther 52: 287–305 [DOI] [PubMed] [Google Scholar]
- Deneke SM, Fanburg BL (1989) Regulation of cellular glutathione. Am J Physiol 257: L163–L173 [DOI] [PubMed] [Google Scholar]
- Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, Mitchell SC, Holmes E, McCarthy MI, Scott J, Gauguier D, Nicholson JK (2006a) Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA 103: 12511–12516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas ME, Maibaum EC, Teague C, Ueshima H, Zhou B, Lindon JC, Nicholson JK, Stamler J, Elliott P, Chan Q, Holmes E (2006b) Assessment of analytical reproducibility of 1H NMR spectroscopy based metabonomics for large-scale epidemiological research: the INTERMAP Study. Anal Chem 78: 2199–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne C (2001) Adaptation of bacteria to the intestinal niche: probiotics and gut disorder. Inflamm Bowel Dis 7: 136–145 [DOI] [PubMed] [Google Scholar]
- Fan TWM (1996) Metabolite profiling by one- and two-dimensional NMR analysis of complex mixtures. Prog Nucl Magn Reson Spectrosc 28: 161–219 [Google Scholar]
- Garrod S, Humpher E, Connor SC, Connelly JC, Spraul M, Nicholson JK, Holmes E (2001) High-resolution 1H NMR and magic angle spinning NMR spectroscopic investigation of the biochemical effects of 2-bromoethanamine in intact renal and hepatic tissue. Magn Reson Med 45: 781–790 [DOI] [PubMed] [Google Scholar]
- Gavaghan CL, Holmes E, Lenz E, Wilson ID, Nicholson JK (2000) An NMR-based metabonomic approach to investigate the biochemical consequences of genetic strain differences: application to the C57BL10J and Alpk:ApfCD mouse. FEBS Lett 484: 169–174 [DOI] [PubMed] [Google Scholar]
- Glaser SJ, Schwalbe H, Marino JP, Griesinger C (1996) Direct TOCSY, a method for selection of directed correlations by optimal combinations of isotropic and longitudinal mixing. J Magn Reson B 112: 160–180 [DOI] [PubMed] [Google Scholar]
- Goodwin BL, Ruthven CR, Sandler M (1994) Gut flora and the origin of some urinary aromatic phenolic compounds. Biochem Pharmacol 47: 2294–2297 [DOI] [PubMed] [Google Scholar]
- Hofmann AF (1999) The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med 159: 2647–2658 [DOI] [PubMed] [Google Scholar]
- Holmes E, Nicholson JK (2005) Variation in gut microbiota strongly influences individual rodent phenotypes. Toxicol Sci 87: 1–2 [DOI] [PubMed] [Google Scholar]
- Hooper LV, Gordon JI (2001) Commensal host–bacterial relationships in the gut. Science 292: 1115–1118 [DOI] [PubMed] [Google Scholar]
- Hooper LV, Midtvedt T, Gordon JI (2002) How host–microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 22: 283–307 [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T (2006) Strict host–symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol 4: 1841–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houten SM, Watanabe M, Auwerx J (2006) Endocrine functions of bile acids. EMBO J 25: 1419–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S (2003) A G protein-coupled receptor responsive to bile acids. J Biol Chem 278: 9435–9440 [DOI] [PubMed] [Google Scholar]
- Koga N, Inskeep PB, Harris TM, Guengerich FP (1986) S-[2-(N7-Guanyl)ethyl]glutathione, the major DNA adduct formed from 1,2-dibromoethane. Biochemistry 25: 2192–2198 [DOI] [PubMed] [Google Scholar]
- Kostenis E (2004) A glance at G-protein-coupled receptors for lipid mediators: a growing receptor family with remarkably diverse ligands. Pharmacol Ther 102: 243–257 [DOI] [PubMed] [Google Scholar]
- LeBlanc JG, Silvestroni A, Connes C, Juillard V, Savoy de Giori G, Piard JC, Sesma F (2004) Reduction of non-digestible oligosaccharides in soymilk: application of engineered lactic acid bacteria that produce α-galactosidase. Genet Mol Res 3: 432–440 [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023 [DOI] [PubMed] [Google Scholar]
- Lien YH, Michaelis T, Moats RA, Ross BD (1994) scyllo-Inositol depletion in hepatic encephalopathy. Life Sci 54: 1507–1512 [DOI] [PubMed] [Google Scholar]
- Lindon JC, Holmes E, Bollard ME, Stanley EG, Nicholson JK (2004) Metabonomics technologies and their applications in physiological monitoring, drug safety assessment and disease diagnosis. Biomarkers 9: 1–31 [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B (1999) Identification of a nuclear receptor for bile acids. Science 284: 1362–1365 [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Remesy C, Jimenez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79: 727–747 [DOI] [PubMed] [Google Scholar]
- Martin FP, Dumas ME, Wang Y, Legido-Quigley C, Yap IK, Tang H, Zirah S, Murphy GM, Cloarec O, Lindon JC, Sprenger N, Fay LB, Kochhar S, van BP, Holmes E, Nicholson JK (2007a) A top–down systems biology view of microbiome–mammalian metabolic interactions in a mouse model. Mol Syst Biol 3: 112 10.1038/msb4100153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FP, Verdu EF, Wang Y, Dumas ME, Yap IK, Cloarec O, Bergonzelli GE, Corthesy-Theulaz I, Kochhar S, Holmes E, Lindon JC, Collins SM, Nicholson JK (2006) Transgenomic metabolic interactions in a mouse disease model: interactions of Trichinella spiralis infection with dietary Lactobacillus paracasei supplementation. J Proteome Res 5: 2185–2193 [DOI] [PubMed] [Google Scholar]
- Martin FP, Wang Y, Dumas ME, Cloarec O, Holmes E, Kochhar S, Sprenger N, Nicholson JK (2007b) Biochemical characterization of gut compartments in a germ-free mouse model and effects of Lactobacillus paracasei in mono-associated mice using high-resolution magic angle spinning 1H NMR spectroscopy. J Proteom Res 6: 1471–1481 [Google Scholar]
- Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263: 17205–17208 [PubMed] [Google Scholar]
- Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52: 711–760 [DOI] [PubMed] [Google Scholar]
- Meister A, Tate SS (1976) Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem 645: 559–604 [DOI] [PubMed] [Google Scholar]
- Metges CC (2000) Contribution of microbial amino acids to amino acid homeostasis of the host. J Nutr 130: 1857S–11864 [DOI] [PubMed] [Google Scholar]
- Metzler DE (2003) The metabolism of nitrogen and amino acids. In Biochemistry: the Chemical Reactions of Living Cells, Hayhurst J (ed), pp 1358–1419. USA: Elsevier Science [Google Scholar]
- Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR (2004) Ablation of the Tamm–Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol 286: F795–F802 [DOI] [PubMed] [Google Scholar]
- Modica S, Moschetta A (2006) Nuclear bile acid receptor FXR as pharmacological target: are we there yet? FEBS Lett 580: 5492–5499 [DOI] [PubMed] [Google Scholar]
- Moreno A, Arus C (1996) Quantitative and qualitative characterization of 1H NMR spectra of colon tumors, normal mucosa and their perchloric acid extracts: decreased levels of myo-inositol in tumours can be detected in intact biopsies. NMR Biomed 9: 33–45 [DOI] [PubMed] [Google Scholar]
- Mouillé B, Morel E, Robert V, Guihot-Joubrel G, Blachier F (1999) Metabolic capacity for L-citrulline synthesis from ammonia in rat isolated colonocytes. Biochim Biophys Acta 1427: 401–407 [DOI] [PubMed] [Google Scholar]
- Nicholls AW, Mortishire-Smith RJ, Nicholson JK (2003) NMR spectroscopic-based metabonomic studies of urinary metabolite variation in acclimatizing germ-free rats. Chem Res Toxicol 16: 1395–1404 [DOI] [PubMed] [Google Scholar]
- Nicholson JK (2006) Global systems biology, personalized medicine and molecular epidemiology. Mol Syst Biol 2: 52 msb4100095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JK, Connelly J, Lindon JC, Holmes E (2002) Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov 1: 153–161 [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Foxall PJ, Spraul M, Farrant RD, Lindon JC (1995) 750 MHz 1H and 1H–13C NMR spectroscopy of human blood plasma. Anal Chem 67: 793–811 [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Wilson ID (2005) Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol 3: 431–438 [DOI] [PubMed] [Google Scholar]
- Pak J, Pu Y, Zhang ZT, Hasty DL, Wu XR (2001) Tamm–Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem 276: 9924–9930 [DOI] [PubMed] [Google Scholar]
- Rezzi S, Ramadan Z, Fay LB, Kochhar S (2007) Nutritional metabonomics: applications and perspectives. J Proteome Res 6: 513–525 [DOI] [PubMed] [Google Scholar]
- Robosky LC, Wells DF, Egnash LA, Manning ML, Reily MD, Robertson DG (2005) Metabonomic identification of two distinct phenotypes in Sprague–Dawley (Crl:CD(SD)) rats. Toxicol Sci 87: 277–284 [DOI] [PubMed] [Google Scholar]
- Rohde CM, Wells DF, Robosky LC, Manning ML, Clifford CB, Reily MD, Robertson DG (2007) Metabonomic evaluation of Schaedler altered microflora rats. Chem Res Toxicol 20: 1388–1392 [DOI] [PubMed] [Google Scholar]
- Russell DW (2003) The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72: 137–174 [DOI] [PubMed] [Google Scholar]
- Savage DC (1986) Gastrointestinal microflora in mammalian nutrition. Annu Rev Nutr 6: 155–178 [DOI] [PubMed] [Google Scholar]
- Seldin DW, Giebisch G (2000) The Kidney: Physiology and Pathology. Philadelphia, USA: Lippincott Williams & Wilkins [Google Scholar]
- Serafini-Cessi F, Malagolini N, Cavallone D (2003) Tamm–Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 42: 658–676 [DOI] [PubMed] [Google Scholar]
- Setchell KD (1998) Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 68: 1333S–1346S [DOI] [PubMed] [Google Scholar]
- Smith JL, Wishnok JS, Deen WM (1994) Metabolism and excretion of methylamines in rats. Toxicol Appl Pharmacol 125: 296–308 [DOI] [PubMed] [Google Scholar]
- Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R (2006) Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol Pharmacol 69: 140–153 [DOI] [PubMed] [Google Scholar]
- Tsang TM, Woodman B, Mcloughlin GA, Griffin JL, Tabrizi SJ, Bates GP, Holmes E (2006) Metabolic characterisation of the R6/2 transgenic mouse model of Huntington's disease by high-resolution MAS 1H NMR spectroscopy. J Proteome Res 5: 483–492 [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031 [DOI] [PubMed] [Google Scholar]
- Uchida K, Nomura Y, Takeuchi N (1980) Effects of cholic acid, chenodeoxycholic acid, and their related bile acids on cholesterol, phospholipid, and bile acid levels in serum, liver, bile, and feces of rats. J Biochem (Tokyo) 87: 187–194 [DOI] [PubMed] [Google Scholar]
- Ueyama J, Nadai M, Kanazawa H, Iwase M, Nakayama H, Hashimoto K, Yokoi T, Baba K, Takagi K, Takagi K, Hasegawa T (2005) Endotoxin from various Gram-negative bacteria has differential effects on function of hepatic cytochrome P450 and drug transporters. Eur J Pharmacol 510: 127–134 [DOI] [PubMed] [Google Scholar]
- Uhlig S, Wendel A (1992) The physiological consequences of glutathione variations. Life Sci 51: 1083–1094 [DOI] [PubMed] [Google Scholar]
- Umesaki Y, Setoyama H (2000) Structure of the intestinal flora responsible for development of the gut immune system in a rodent model. Microbes Infect 2: 1343–1351 [DOI] [PubMed] [Google Scholar]
- Wang W, Ballatori N (1998) Endogenous glutathione conjugates occurrence and biological functions. Pharmacol Rev 50: 335–356 [PubMed] [Google Scholar]
- Wang Y, Holmes E, Comelli EM, Fotopoulos G, Dorta G, Tang H, Rantalainen MJ, Lindon JC, Corthesy-Theulaz IE, Fay LB, Kochhar S, Nicholson JK (2007) Topographical variation in metabolic signatures of human gastrointestinal biopsies revealed by high-resolution magic-angle spinning 1H NMR spectroscopy. J Proteome Res 6: 3944–3951 [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang H, Holmes E, Lindon JC, Turini ME, Sprenger N, Bergonzelli G, Fay LB, Kochhar S, Nicholson JK (2005) Biochemical characterization of rat intestine development using high-resolution magic-angle-spinning 1H NMR spectroscopy and multivariate data analysis. J Proteome Res 4: 1324–1329 [DOI] [PubMed] [Google Scholar]
- Waters NJ, Holmes E, Waterfield CJ, Farrant RD, Nicholson JK (2002) NMR and pattern recognition studies on liver extracts and intact livers from rats treated with α-naphthylisothiocyanate. Biochem Pharmacol 64: 67–77 [DOI] [PubMed] [Google Scholar]
- Wehner F (2003) Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol 148: 1–80 [DOI] [PubMed] [Google Scholar]
- Williams RE, Eyton-Jones HW, Farnworth MJ, Gallagher R, Provan WM (2002) Effect of intestinal microflora on the urinary metabolic profile of rats: a 1H-nuclear magnetic resonance spectroscopy study. Xenobiotica 32: 783–794 [DOI] [PubMed] [Google Scholar]
- Williams RE, Lenz EM, Evans JA, Wilson ID, Granger JH, Plumb RS, Stumpf CL (2005) A combined 1H NMR and HPLC-MS-based metabonomic study of urine from obese (fa/fa) Zucker and normal Wistar-derived rats. J Pharm Biomed Anal 38: 465–471 [DOI] [PubMed] [Google Scholar]
- Wostmann BS (1981) The germfree animal in nutritional studies. Annu Rev Nutr 1: 257–279 [DOI] [PubMed] [Google Scholar]
- Yamauchi A, Kwon HM, Uchida S, Preston AS, Handler JS (1991) Myo-inositol and betaine transporters regulated by tonicity are basolateral in MDCK cells. Am J Physiol 261: F197–F202 [DOI] [PubMed] [Google Scholar]
- Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208: 2819–2830 [DOI] [PubMed] [Google Scholar]
- Zhang J, Cerny MA, Lawson M, Mosadeghi R, Cashman JR (2007) Functional activity of the mouse flavin-containing monooxygenase forms 1, 3, and 5. J Biochem Mol Toxicol 21: 206–215 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Data