Abstract
Background
The Selenium and Vitamin E Chemoprevention Trial (SELECT) is aimed at determining the usefulness of a combination of vitamin E and selenium for Prostate cancer (PCa) prevention in human. The aim of this study is to evaluate the efficacy and mechanistic basis of this combination.
Methods
We determined the effect of vitamin E (+-α-tocopheryl succinate, VES) and selenium (methylselenic acid, MSA), alone and in combination, on the proliferation of LNCaP, DU145, and PC-3 cells as well as normal prostate PrEC cells. We also determined the involvement of Bcl-2 family proteins as a mechanism of the biological effects of vitamin E and selenium combination.
Results
VES or MSA alone led to a modest inhibition in the viability and growth of PCa cells. However, a combination of these two agents resulted in a dramatic increase in growth inhibition of PCa cells. Interestingly, VES and/or MSA were not found to have any effect on the growth or viability of normal PrEC cells. VES and MSA treatment to human PCa cells resulted in i) induction of apoptosis, ii) increase in Bax, Bak, and Bid proteins, and ii) decrease in Bcl-2 protein. Furthermore, Bax knockdown via shRNA and Bcl-2 overexpression via Bcl-2 plasmid resulted in a rescue of PCa cells from apoptosis.
Conclusions
Our study suggested that vitamin E and selenium combination may be more effective than either of these agents alone. Further, our data demonstrated a causal connection between Bax and Bcl-2 modulation and induction of apoptosis by VES and MSA combination.
Keywords: Vitamin E, Selenium, SELECT, apoptosis, Prostate Cancer
INTRODUCTION
Prostate cancer (PCa) is the most prevalent malignancy in men, excluding non-melanoma skin cancers, with an expected 186,320 new cases of PCa and 28,660 deaths due to this cancer in the year 2008 in the United States alone [1]. Despite surgical and diagnostic advances, the incidence of PCa is expected to rise in the near future. The high rate of occurrence and long latency period to clinically significant disease make PCa an ideal disease for pharmacologic or nutritional chemoprevention [2, 3]. Chemoprevention is defined as the use of naturally occurring or synthetic agents to prevent, reverse or inhibit the development of cancer [4-6]. In the recent past, Vitamin E and selenium have received attention for the management of PCa [7-9]. Present in a variety of food products and available as dietary supplements, selenium is an essential micronutrient that occurs predominantly as selenomethionine (SeMet), whereas vitamin E (or α-tocopherol) is a fat-soluble physiological antioxidant, both required for normal health [10-12].
The ongoing Selenium and Vitamin E Chemoprevention Trial (SELECT), sponsored by the National Cancer Institute, is an intergroup Phase III, randomized, double-blind, placebo-controlled, population-based clinical trial designed to test the efficacy of selenium and vitamin E alone and in combination in the prevention of PCa. This 12 years trial has a 2 × 2 factorial design with a target accrual of more than 32,000 healthy men [13].
The rationale for the use of vitamin E and selenium for the prevention of PCa comes from clinical trial data. Vitamin E was shown to be a promising candidate for PCa prevention in the α-Tocopherol β-Carotene Cancer Prevention Study, a controlled smoking trial where α-tocopherol reduced PCa incidence by 32% and mortality by 41% [14]. The SUpplementation en VItamines et Mineraux AntioXidants (SUVIMAX) study found a significant reduction in PCa rates among men receiving a multivitamin containing 30 mg vitamin E, although the protective effect could not be attributed to any specific micronutrient [15]. In contrast, the Heart Outcomes Prevention Evaluation (HOPE) trial, the Heart Protection Study, the NIH-AARP Diet and Health Study and the Cancer Prevention Study II Nutrition Cohort do not support a general protective effect of α-tocopherol supplement use for PCa prevention [16-19].
The Nutritional Prevention of Cancer Trial established selenium as a promising candidate for PCa prevention, it is a large-scale double-blind cancer prevention trial where supplementation was shown to reduce PCa incidence by 63% when examined as a secondary endpoint [20]. In a nested, case-control study within the Health Professionals Follow-Up study, high levels of selenium in toenails, which reflects long-term intake, was correlated with a reduced risk for advanced PCa [21]. Additionally, a meta-analysis using either serum, plasma or toenail selenium levels indicated a possible inverse association between selenium levels and risk of PCa [22].
Although, the SELECT trial is an outstanding effort towards finding novel approaches for the management of PCa, preclinical studies are needed to evaluate the efficacy and mechanistic basis of this combination. Such studies may provide important information regarding the possible outcome of ongoing human SELECT trial and may even provide novel information on which suggestions could be made for the modifications for the ongoing or future trials. The biological effects of selenium in PCa cells include cell cycle arrest [23], DNA synthesis suppression [24], and induction of apoptosis [25]. In vitro studies have demonstrated that vitamin E can inhibit DNA synthesis [26], reduce invasion [27], and induce apoptosis [28]. Only a few studies have evaluated the mechanism of the combination of vitamin E and selenium in PCa [29, 30]. In this study, we tested the hypothesis that a combination of vitamin E and selenium will impart an enhanced anti-proliferative effect in multiple human PCa cell lines via modulations in Bcl-2 family proteins.
MATERIALS AND METHODS
Cell Culture and Treatment
Human prostate carcinoma cell lines LNCaP (lymph node-derived androgen-sensitive cell line; normal for cell cycle-related tumor suppressor genes p53 and retinoblastoma Rb), PC-3 (bone marrow-derived androgen-insensitive cell line; defective for both p53 alleles but normal for both Rb alleles), and DU145 (brain-derived androgen-insensitive cell line; defective for both p53 and both Rb alleles) were purchased from ATCC (Manassas, VA) and maintained in RPMI-1640, MEM, and F12K media, respectively, with 10% Fetal Bovine Serum and 1% Penicillin-Streptomycin (ATCC; Manassas, VA) at standard cell culture conditions (37°C, 5% CO2 in a humidified incubator). Normal human prostate epithelial cells (PrEC) obtained from Clonetics (Walkersville, MD) were cultured in Prostate Epithelial Cell Medium (Clonetics; Walkersville, MD). Because cell lines do not efficiently metabolize selenium and vitamin E, based on published studies [31, 32], we have elected to use methylselenic acid (MSA; Fig. 1A) and vitamin E succinate (VES) (Fig. 1B) in our in vitro experiments. Vitamin E succinate (+-α-tocopheryl acid succinate, VES) and methylselenic acid (MSA) were obtained from Sigma Chemical Co. (St. Louis, MO). Cells (60% confluent) were treated with VES and/or MSA in complete cell culture media for 48 hours before collection.
Figure 1. Effect of VES and/or MSA on growth and viability of human PCa cells and normal human prostate epithelial cells.

The chemical structures of methylselenic acid (MSA) (A) and vitamin E (+α-tocopherol) succinate (VES) (B) are depicted. Following treatment of LNCaP, DU145, and PC-3 cells with VES and/or MSA for 48 hours, the effects on cell viability (C) and cell growth (D) were measured using Trypan Blue Exclusion analysis. Similarly, following treatment of PrEC with VES and/or MSA for 48 hours, the effects on cell viability (E) and cell growth (F) were also measured. Cell viability data is expressed as the percent viable cells out of the total number of cells. The data is expressed as the mean ± SE of three experiments (* p < 0.01 versus untreated control) (# p< 0.01 for combination versus either single agent). Details of the experiments are given in ‘Materials and Methods’.
Trypan Blue Exclusion Assay
Following treatment as described above, cells were trypsinized and collected in a 1.5mL microfuge tube. The cells were pelleted by centrifugation, and the cell pellet was re-suspended in PBS (300 μL). Trypan blue (0.4% in PBS; 10 μL) was added to a 10 μL aliquot of cell suspension, and the number of cells (viable-unstained and non-viable-blue) were counted. Viability is expressed as the percent viable cells out of the total number of cells. Cell growth data is analyzed as percent change of total cell numbers between treatments.
Clonogenic Cell Survival
Cells were treated as described above for 48 hours and were collected by trypsinization. A Trypan Blue assay was performed and cells were replated in triplicate on a 6-well tissue culture plate with 3000 cells/well. The cells were cultured for 12-14 days with growth media being replaced every 3 days. The cells were then stained with 0.5% crystal violet (in methanol:H2O, 1:1) (Sigma; St. Louis, MO) and pictures were taken.
Apoptosis and Cell Cycle Analysis by Flow Cytometry
Apoptosis and cell cycle distribution was assessed with the APO-BrdU TUNEL Apoptosis Assay kit (Molecular Probes; Eugene, OR) as previously reported [33]. Cell Quest software (BD Biosciences; San Jose, CA) was used to quantify apoptosis and ModFit LT software (Verity Software House; Topsham, ME) for cell cycle analysis. When analyzing the cell population with ModFit LT software, the sub-G1 population (apoptotic and cell debris) was excluded.
Western Blot Analysis
Following treatment, cell lysates were prepared as described earlier [33]. The protein concentration of lysates was determined using the BCA Protein Assay (Pierce Biotechnology; Rockford, IL) as per the manufacturer’s protocol. For immunoblot analysis, 30 - 50μg protein was subjected to SDS-PAGE (using 10-15% Tris-HCl gel). The protein was transferred onto a nitrocellulose membrane and blocked with TBS-Tween plus 5% dry milk. The membrane was probed with an appropriate primary antibody followed by a secondary HRP-conjugated antibody. The following antibodies were used: Actin (I-19), Bcl-2 (C-2), and Bid (FL-195) from Santa Cruz (Santa Cruz, CA); Bak (NT) and Bax (NT) from Upstate Biotechnology (Lake Placid, NY); PCNA (PC10) from BioSource International (Camarrillo, CA); and PSA (ER-PR8) from Dako Cytomation (Denmark). The protein was detected by chemiluminescence and the quantification of protein was performed by a digital analysis of protein bands (TIFF images) using UN-SCAN-IT software (Silk Scientific; Orem, UT).
Quantitative Real Time-PCR Analysis
The cells were washed with PBS, trypsinized, collected by centrifugation, washed with DEPC H2O and pelleted. The cell pellet was resuspended in Trizol reagent (Invitrogen; Carlsbad, CA) and RNA was extracted according to the vendor’s recommendation. RNA was treated with DNAse (Invitrogen) and first strand cDNA was transcribed with 300 ng random primers, 10mM dNTPs and 200 units of M-MLV reverse transcriptase (Invitrogen). Quantitative RT-PCR was performed in triplicate, twice, with Premix Ex Taq (Takara; Madison, WI) with 50ng first strand cDNA, 0.2 μM each forward and reverse primers for Bax (Fw 5’AGAGGATGATTGCCGCCGT3’; Rev 5’CAACCACCCTGGTCTTGGATC3’; product size 243bp), Bcl-2 (Fw 5’CATGTGTGTGGAGAGCGTCAA3’; Rev 5’ACAGTTCCACAAAGGCATCCC3’; product size 137bp) or GAPDH (Fw 5’GAAGGTGAAGGTCGGAGTC3’, Rev 5’GAAGATGGTGATGGGATTTC 3’; product size 236bp). The samples were cycled once for 95°C for 2 minutes then 40 cycles of 95°C, 58°C, 72°C for 15, 30 and 45 seconds respectively with a final extension for 5 minutes at 72°C. Relative mRNA for each gene was calculated using the ΔΔCT comparative method using GAPDH as an endogenous control and untreated samples as the calibrator. Purity of product was check with using dissociation curve analysis as well as running the samples on 2-3% agarose gel.
Transfection of Cells
The Bax short hairpin RNA and the full-length Bcl-2 plasmids were purchased from Open Biosystems (Huntsville, AL). The Bax short hairpin RNA and full-length Bcl-2 plasmids were grown up in Luria-Bertani broth and were purified by a Perfectprep Plasmid Mini kit (Eppendorf; Hamburg, Germany) according to the manufacturer’s instructions. For transfection, each plasmid (0.2 μg per 25 μL media) and the LipofectAMINE 2000 reagent (Invitrogen; 0.5 μL per 25 μL media) were diluted with serum-free media for 5 minutes. Then, the diluted LipofectAMINE was added to the diluted plasmid and incubated at room temperature for 20 minutes. Culture media was aspirated, transfection media was added followed by addition of plasmid-LipofectAMINE complex to the cells. The cells were incubated at 37°C for 24 hours followed by treatment with VES and/or MSA as usual for 48 hours. At this time, the cells were collected and processed for further experiments.
Statistical analysis
The results are expressed as the mean plus or minus standard error. Statistical analyses of the data between treated vs. untreated cells (*) or combination vs. single agent (#) were performed by Student’s t-test. A p-value <0.01 was considered statistically significant.
RESULTS
Effect of VES and/or MSA on growth and viability of human PCa cells and normal human prostate epithelial cells
We first determined the effects of VES and MSA (alone or in combination) on growth and viability of human PCa cells viz. LNCaP, DU145, and PC-3 and normal PrEC cells. We found that 1 μM VES was enough to significantly reduce PCa cellular growth while a 5 μM concentration of VES was required to significantly reduce PCa cellular viability 9-13% (Fig. 1C,D). A 2 μM concentration of MSA was required to significantly reduce both growth and viability by 10-30% in PCa cells (Fig. 1C,D). Earlier studies have shown decreases in cell viability in PCa cells treated with VES or MSA alone, albeit at higher concentrations than those in our study [34-37]. Interestingly, a combination of 5 μM VES and 2 μM MSA for 48 hours decreased cellular growth 32-45% in PCa cells with a greater than additive effect in LNCaP cells (Fig. 1D). Further, at 1 μM concentrations of VES and MSA viability was significantly decreased 25-50% and this decrease was further enhanced to 38-70% at 5 μM and 2 μM concentrations which is also greater than additive effect (Fig. 1C). Our data in PC-3 cells differs from Zu and Ip where they found more modest cell growth inhibition by VES and MSA as assessed by MTT assay [30]. Ni et al. found modest decreases in cell growth when LNCaP cells were treated with VES alone, but a 78% cell growth inhibition when LNCaP cells were treated with VES and SeMet for 48 hours [38]. Next, employing the same treatment regimen, we determined the effect of VES and MSA on normal human prostate epithelial PrEC cells. Interestingly, our data demonstrated no effect of VES and/or MSA on PrEC cells on viability or growth as measured by Trypan Blue Exclusion analysis (Fig. 1E,F).
Effect of VES and/or MSA on cell cycle distribution and clonogenic cell survival in human PCa cells
Because earlier studies have shown that vitamin E and or selenium compound cause a cell cycle arrest in certain PCa cells, we next determined the effect of VES and/or MSA on distribution of cells in different phases of cell cycle [27, 30, 34, 38-40]. Our data demonstrated that at selected concentrations, VES and/or MSA treatments did not significantly alter the distribution of PCa cells in different phases of cell cycle (Fig. 2). Further, no observable modulation in cell cycle distribution was evident in PrEC cells treated with VES and/or MSA (data not shown).
Figure 2. Effect of VES and/or MSA on cell cycle distribution and clonogenic cell survival in human PCa cells.
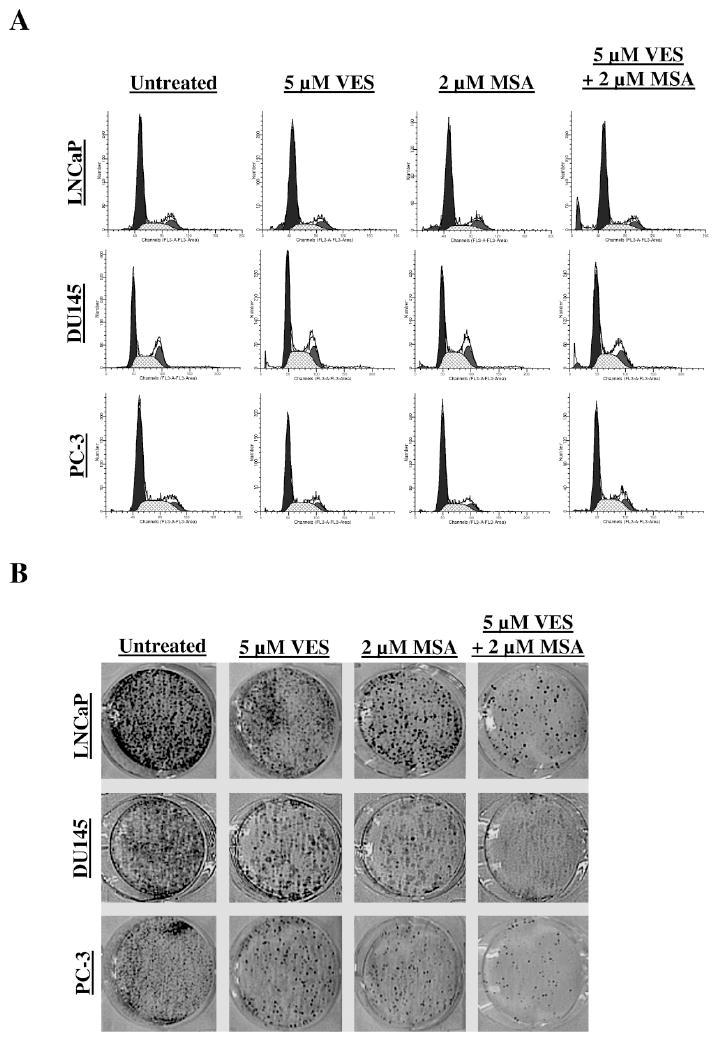
Following treatment of LNCaP, DU145, and PC-3 cells with VES and/or MSA for 48 hours, the cell cycle distribution was assessed using the APO-BrdU TUNEL Assay kit. The FAC profiles indicating the positions of G0/G1, S, and G2/M are shown, with the sub- G0 content excluded as much as possible (A). The effect of treatments on clonogenic survival of PCa cells was determined using colony formation assay (B). Following treatments, the cells were replated in triplicate on a 6-well tissue culture plate with 3000 cells/well. The cells were cultured for 12-14 days with growth media being replaced every 3 days. The cells were then stained with 0.5% crystal violet (in methanol:H2O, 1:1) and pictures were taken using a digital camera. The pictures were enhances using Adobe Photoshop for brightness, contrast, and sharpening for uniformity of appearance. Details of the experiments are given in ‘Materials and Methods’.
Given the observed decreases in cellular growth, proliferation index, and possible delay of cell cycle progression, we examined the long-term effects of VES and/or MSA on PCa cells. We employed a clonogenic cell survival assay where PCa cells were treated with VES and/or MSA (single treatment for 48 hours), re-plated and monitored for the formation of colonies in regular culture media for two weeks. As shown in figure 3A, both VES and MSA resulted in an appreciable decrease in the number of colonies in all the three cell lines. Interestingly, the combination of VES and MSA was found to cause a more pronounced inhibition in colony formation compared to either of the agents alone (Fig. 3C).
Figure 3. Effect of VES and/or MSA on proliferating cell nuclear antigen (PCNA) and prostate specific antigen (PSA) in human PCa Cells.

For determining the effect of treatments on PCNA, following treatment of LNCaP, DU145, and PC-3 cells with VES and/or MSA for 48 hours, the protein level of PCNA was assessed by Western blot analysis (A) and quantified by densitometric analysis of protein bands (B). For determining the effect on PSA protein levels, following treatment of LNCaP cells with VES and/or MSA for 48 hours, the protein level of PSA was assessed by Western blot analysis (C) and quantified by densitometric analysis of protein bands (D). Equal loading was confirmed by stripping the blot and reprobing it for β-actin. The data is expressed as mean ± SE of three experiments (* p < 0.01 versus untreated control) (# p< 0.01 for combination versus either single agent). Details of the experiments are given in ‘Materials and Methods’.
Effect of VES and/or MSA on proliferating cell nuclear antigen (PCNA) and prostate specific antigen (PSA) in human PCa Cells
PCNA serves as a marker for increased proliferation of DNA polymerase-driven DNA synthesis; our data demonstrated that at the concentrations selected, VES and MSA alone significantly decreased PCNA protein levels in DU145 and PC-3 cells but not in LNCaP cells (Fig. 3A,B). However, the combination of VES and MSA treatment significantly reduced PCNA protein levels in all three cell lines (Fig. 3A,B).
We also determined the effect of VES and/or MSA on protein levels of Prostate Specific Antigen (PSA). PSA is expressed in both normal and neoplastic prostatic tissues with prostate cancer causing serum concentrations of PSA to become elevated. Thus, PSA level screening is considered the “gold standard” for detection and progression of PCa [41]. We found that both VES and MSA significantly decreased PSA protein levels in LNCaP cells (Fig. S1). However, the combination was not appreciably more effective in reducing PSA protein levels than the individual agents.
Effect of VES and/or MSA on rate of apoptosis in PCa Cells
To determine if the antiproliferative effects of VES and/or MSA in PCa cells are mediated via apoptotic elimination of the cancer cells, we used the APO-BrdU TUNEL assay kit and flow cytometry to measure and quantify apoptosis. In all three PCa cell lines, 5μM VES and 2μM MSA treatment for 48 hours induced modest levels of apoptosis while the combination of VES and MSA resulted in a significantly more pronounced (37 - 43%) apoptosis of PCa cells (Fig.4).
Figure 4. Effect of VES and/or MSA on rate of apoptosis in PCa Cells.

Following treatment of LNCaP, DU145, and PC-3 cells with VES and/or MSA for 48 hours, the extent of apoptosis was assessed with the APO-BrdU TUNEL Assay kit. BrdU incorporation was analyzed with a flow cytometer (A), followed by a computational analysis (B), of cells staining positive for BrdU. The data is expressed as mean ± SE of three experiments (* p < 0.01 versus untreated control) (# p< 0.01 for combination versus either single agent). Details of the experiments are given in ‘Materials and Methods’.
Involvement of Bcl-2 family proteins in VES and/or MSA-mediated apoptosis of PCa cells
The Bcl-2 family of proteins plays a critical role in controlling apoptotic machinery in mammalian cells via its interacting pro- and anti- apoptotic members (reviewed in [42]). These proteins integrate a wide array of diverse upstream survival and distress signals to decide the fate of the cells. The Bax and Bcl-2 are the key players of this family and a high Bcl-2 expression concurrent with low levels of Bax have been correlated with poor therapeutic response of prostate cancer to therapy [43]. As shown in figure 5A, our data demonstrated that both VES and MSA down-modulated the protein levels of pro-survival Bcl-2 in PCa cells, albeit to a different extent where VSA was more potent. Interestingly, the combination of VES and MSA was found to be the most effective in decreasing Bcl-2 protein levels (Fig. 5A). Further, both VES and MSA treatments resulted in increase in the levels of pro-apoptotic proteins Bax, Bak, and Bid which was further enhanced by the combination of VES and MSA, in all the three PCa cell lines studied (Fig. 5A). Changes in the Bax/Bcl-2 ratio suggest a corresponding change in mitochondrial permeability to begin the induction of apoptosis. We found a significant increase in the protein Bax/Bcl-2 ratio in all three PCa cell lines with the combination of VES and MSA treatment (Fig. 5B). In addition, we found a similar trend in mRNA levels of Bax/Bcl-2 ratio in all three PCa cell lines as measured by qRT-PCR (Fig. 5C).
Figure 5. Effect of VES and/or MSA on modulations in Bcl-2 family proteins in PCa cells.
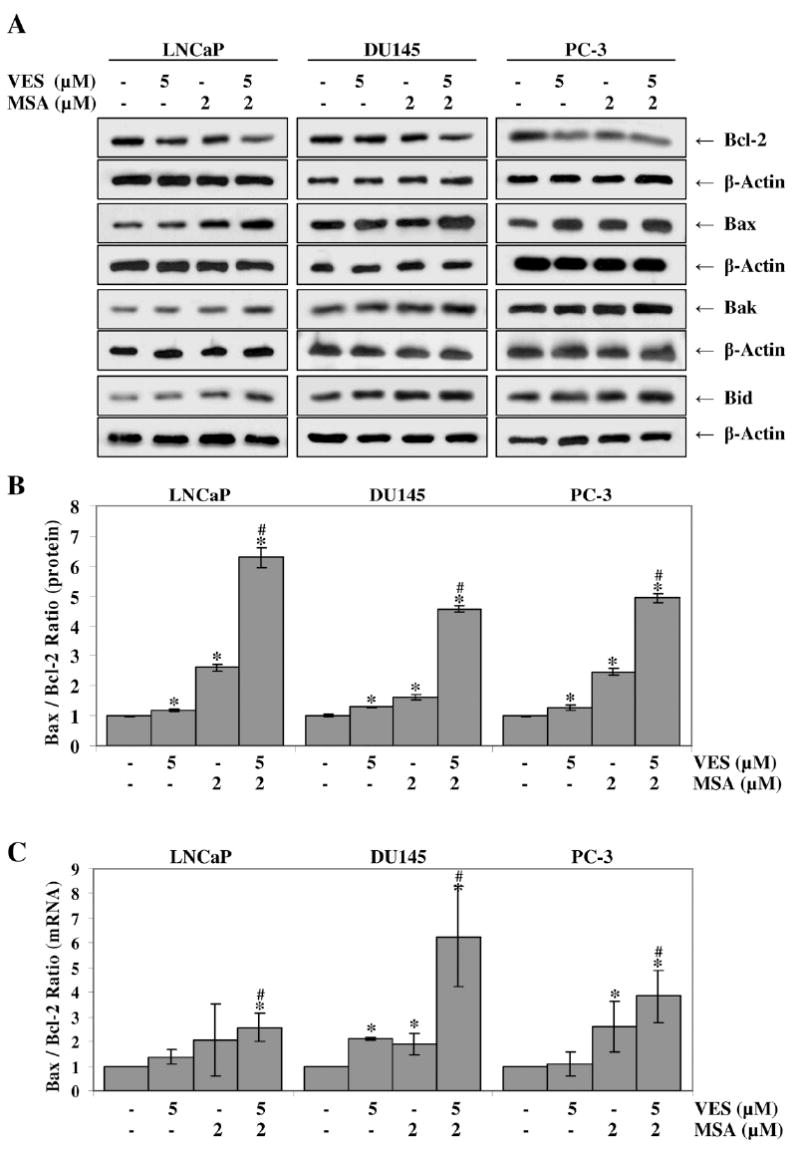
Following treatment of LNCaP, DU145, and PC-3 cells with VES and/or MSA for 48 hours, the protein levels of Bcl-2, Bax, Bak, and Bid were assessed by Western blot analysis (A). Equal loading was confirmed by stripping the blot and reprobing it for β-actin. The effect of treatment on the Bax/Bcl-2 ratio at the protein level (B) was determined by densitometric analysis of the immunoblots relative to β-actin. Quantitative Real-Time PCR analysis was used to calculate the Bax/Bcl-2 ratio at the mRNA level (C) relative to GAPDH. The data is expressed as mean ± SE of three experiments (* p < 0.01 versus untreated control) (# p< 0.01 for combination versus either single agent). Details of the experiments are given in ‘Materials and Methods’.
To determine the cause and effect association between Bax/Bcl-2 and the observed induction of apoptosis by VES and MSA, we performed gene overexpression and knockdown experiments. We first determined the effect of knockdown of Bax using vector-based short hairpin RNA and determined its effect on the observed responses of VES and/or MSA in PCa cells. Immunoblot analysis showed an efficient knockdown of Bax protein levels in PCa cells (Fig. 6A). As shown by TUNEL analysis data (Fig. 6B) the pro-apoptotic response of VES and MSA combination was found to be reversed by Bax knockdown with short hairpin RNA directed against Bax (Fig. 6B). In the next experiment, we determined the effect of a forced overexpression of pro-survival Bcl-2 on the observed proapoptotic response of VES and/or MSA in PCa cells. As shown by Western Blot analysis (Fig. 6C), the transfection of cells with full-length Bcl-2 plasmids resulted in an appreciable increase of Bcl-2 protein levels. Further, as shown in figure 6D, our data demonstrated that Bcl-2 overexpression resulted in a rescue of PCa cells from VES and MSA-mediated apoptosis. These results clearly demonstrated a cause and effect relationship between Bax/Bcl-2 and the induction of apoptosis by VES and MSA combination.
Figure 6. Effect of shRNA-mediated knockdown of Bax and plasmid-mediated overexpression of Bcl-2 on VES and/or MSA-caused apoptosis of PCa cell.
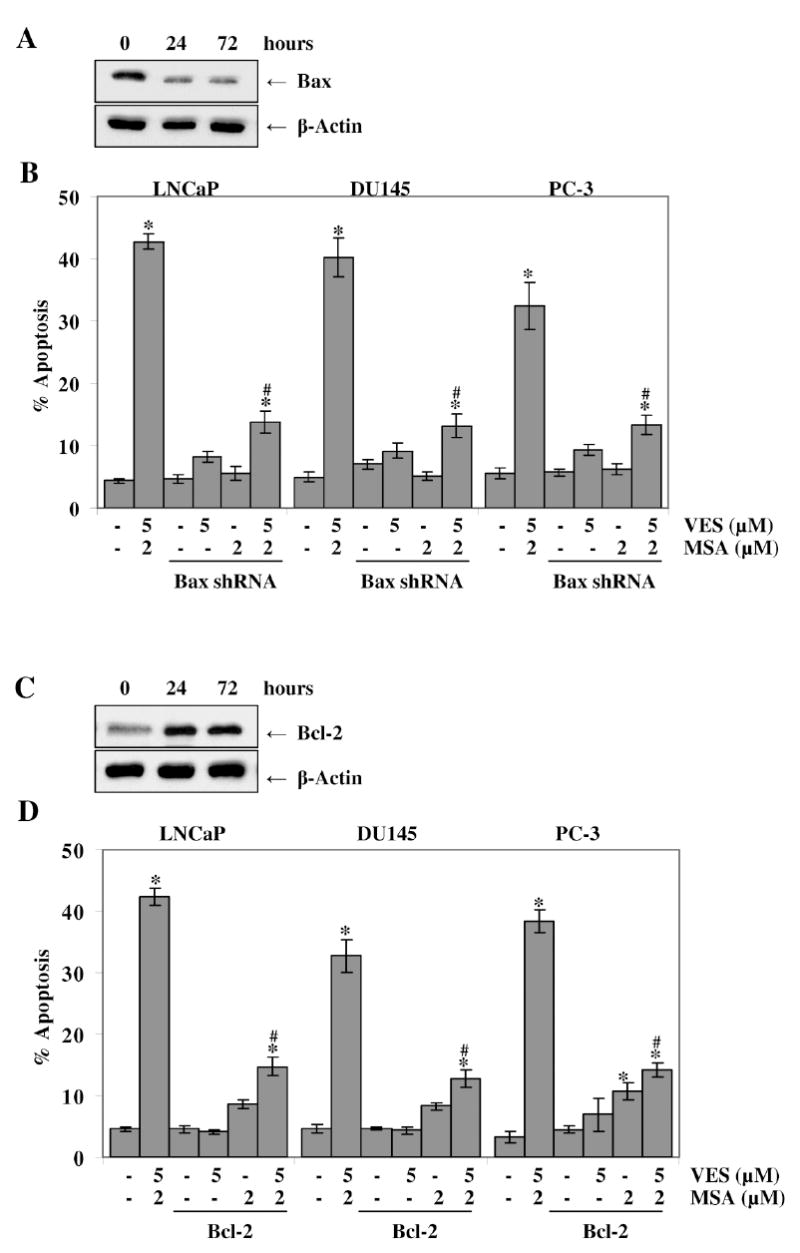
The cells were transiently transfected with Bax shRNA or Bcl-2 overexpression plasmids followed by treatment with VES and/or MSA for 48 hours. The cells were harvested at 0, 24, 72 hours post- transfection and western blot analyses for Bax (in Bax shRNA transfected cells) (A) or for Bcl-2 (in Bcl-2 plasmid transfected cells) were performed (C) transfected cells at representative LNCaP blots are shown. The APO-BrdU TUNEL Assay kit was used to asses the extent of apoptosis in Bax shRNA transfected cells (B) or Bcl-2 plasmid transfected cells (D). The data is expressed as mean ± SE of three experiments (* p < 0.01 versus untreated control) (# p< 0.01 for VES+MSA versus transfected cells treated with VES+MSA). Details of the experiments are given in ‘Materials and Methods’.
DISCUSSION
In this study, employing i) multiple human PCa cell lines representing different stages or factors of disease progression, and ii) normal human prostate epithelial cells; we attempted to determine the antiproliferative effect of a combination of vitamin E and selenium in PCa. We also determined the cause and effect mechanism of the effects of vitamin E and selenium combination. Prostate cancer is an attractive target for prevention due to its age-dependent incidence and disease-related mortality. Over the course of their evolution precancerous and cancerous cells acquire multiple oncogenic defects leading to metastatic spread of disease that ultimately leads to fatal consequences. Therefore, targeting multiple signaling pathways via multiple agents/drugs can offer better clinical payoff. The ongoing SELECT human clinical trial is using a combination of vitamin E and selenium for PCa management. However, limited number of studies has evaluated its efficacy and mechanistic basis in pre-clinical settings. Indeed, it has been shown that selenium taken as an oral supplement accumulates in the human prostate gland [44]. Also, RRR-α-tocopherol is the only stereoisomer of vitamin E that is naturally present in plants or animals and oral supplementation of this stereoisomer has been shown to increase both plasma and total tissue levels of vitamin E [45].
A major finding of this study is that VES and/or MSA causes apoptotic elimination of multiple human PCa cells (differing in status of androgen as well as tumor suppressor p53) without affecting the normal PrEC cells at similar concentrations. This is an important finding because PCa undergoes a transition from an androgen-sensitive, early form of cancer to metastatic, androgen-insensitive metastatic disease and during clinical diagnosis; most PCa represents a mixture of both androgen-sensitive and insensitive cells. Therefore, the key to the control of PCa lies in the elimination of both types of cancer cells (without affecting the normal cells) via mechanism-based preventive/therapeutic approaches.
Apoptosis represents a universal suicide pathway, an ideal way for elimination of damaged cells. We found that VES and/or MSA treatment induced apoptosis in PCa cells (Fig. 4). Data reported from other groups illustrate varying degrees of apoptosis induced by VES or MSA. Zu et al. found a modest increase in apoptotic PC-3 cells when treated with 20 μM VES for 24 hours [46]. Yamaguchi et al. have shown an induction of apoptosis of LNCaP and DU145 cells by MSA [47]. Hu et al. found modest levels of apoptosis in both DU145 and PC-3 cells treated with MSA for 24 hours as measured by histone-associated DNA fragments [48]. Zu and Ip have also shown an induction of apoptosis by 20 μM VES and 5 μM MSA in PC-3 cells [30]. These differences in levels of apoptosis in different studies could be explained by variations in concentrations or treatment protocols, cell passage status and the method used for analysis. We have found that the combination of VES and MSA was much more effective than either of the agents alone. Also, we have used low concentrations of both the agents which makes our finding more relevant to in vivo settings.
We found a significant decrease in proliferation marker PCNA protein levels in all three PCa cell lines treated with the combination of VES and MSA. This decrease in PCNA was found to be correlated with the observed significant decreases in cell viability and cellular growth as well as the long-term clonogenic survival of PCa cells. Further, our data showed that the combination of VES and MSA treatment of PCa cells (a) down-regulates Bcl-2 protein, and (b) up-regulates the protein levels of proapoptotic members of this family, i.e., Bax, Bak, and Bid. We also found that VES and MSA combination treatment resulted in a significant increase in Bax/Bcl-2 ratio, both at protein and RNA levels, which is regarded as a driving force for apoptosis in mammalian cells (Fig.5). Our data, for the first time, has shown a causal connection between Bax and Bcl-2 modulation and induction of apoptosis by a combination of VES and MSA. It has been suggested that Bcl-2 expression may protect prostate cancer cells from many different apoptotic stimuli, including hormone ablation, radiotherapy, and chemotherapy. Therefore, targeting at this survival pathway constitutes a rational approach for the treatment of PCa. Our study suggested that Bcl-2 and Bax are critical to vitamin E and selenium’s anticancer action.
At present, the upstream target of VES and/or MSA combination responsible for the observed modulation in Bcl2 family proteins remains to be elucidated. Studies have suggested that Bcl-2 gene expression in prostate cancer cells is mediated through NF- κB binding sites in the Bcl-2 P2 promoter [49]. Indeed, NF- κB has been found to be activated in 54% of prostate adenocarcinomas while showing little or no activity in normal prostate or PIN tissues [50]. Conversely, down-regulation of NF- κB is associated with suppression of angiogenesis, invasion, and metastasis of PCa in mice [51]. MSA has been shown to inhibit NF- κB DNA binding activity in DU145 cells through inhibition of IKK activity and IκB degradation [52]. VES has been shown to inhibit NF- κB activity in DU145 and PC-3 cells that have been activated by TNF-α [53]. Therefore, it will be interesting to further examine the effect of VES and MSA on NF-κB pathway in PCa cells.
Acknowledgments
We thank undergraduate students Jorien Breur, Iulia Dorneanu, and Stephanie Jones for their technical assistance. This work was supported, in part, by funding from the National Cancer Institute (CA114060).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer Statistics, 2008. CA Cancer J Clin. 2008 doi: 10.3322/CA.2007.0010. Published online before print. [DOI] [PubMed] [Google Scholar]
- 2.Schmid HP, McNeal JE, Stamey TA. Observations on the doubling time of prostate cancer. The use of serial prostate-specific antigen in patients with untreated disease as a measure of increasing cancer volume. Cancer. 1993;71:2031–2040. doi: 10.1002/1097-0142(19930315)71:6<2031::aid-cncr2820710618>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Ries L, Melbert D, Krapcho M, Mariotto A, Miller B, Feuer E, Clegg L, Horner M, Howlader N, Eisner M, Reichman M, Edwards B, editors. SEER Cancer Statistics Review, 1975-2004. Bethesda, MD: National Cancer Institute; 2004. [Google Scholar]
- 4.Mukhtar H, Ahmad N. Cancer chemoprevention: future holds in multiple agents. Toxicol Appl Pharmacol. 1999;158:207–210. doi: 10.1006/taap.1999.8721. [DOI] [PubMed] [Google Scholar]
- 5.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad N, Mukhtar H. Green tea polyphenols and cancer: biologic mechanisms and practical implications. Nutr Rev. 1999;57:78–83. doi: 10.1111/j.1753-4887.1999.tb06927.x. [DOI] [PubMed] [Google Scholar]
- 7.Crispen PL, Uzzo RG, Golovine K, Makhov P, Pollack A, Horwitz EM, Greenberg RE, Kolenko VM. Vitamin E succinate inhibits NF-kappaB and prevents the development of a metastatic phenotype in prostate cancer cells: implications for chemoprevention. Prostate. 2007;67:582–590. doi: 10.1002/pros.20468. [DOI] [PubMed] [Google Scholar]
- 8.Ip C, Thompson HJ, Zhu Z, Ganther HE. In Vitro and in Vivo Studies of Methylseleninic Acid: Evidence That a Monomethylated Selenium Metabolite Is Critical for Cancer Chemoprevention. Cancer Res. 2000;60:2882–2886. [PubMed] [Google Scholar]
- 9.Venkateswaran V, Fleshner NE, Klotz LH. Synergistic effect of vitamin E and selenium in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2004;7:54–56. doi: 10.1038/sj.pcan.4500707. [DOI] [PubMed] [Google Scholar]
- 10.Morris VC, Levander OA. Selenium content of foods. J Nutr. 1970;100:1383–1388. doi: 10.1093/jn/100.12.1383. [DOI] [PubMed] [Google Scholar]
- 11.Schrauzer GN. Nutritional selenium supplements: product types, quality, and safety. J Am Coll Nutr. 2001;20:1–4. doi: 10.1080/07315724.2001.10719007. [DOI] [PubMed] [Google Scholar]
- 12.Harris PL, Quaife ML, Swanson WJ. Vitamin E Content of Foods. J Nutr. 1950;40:367–381. [Google Scholar]
- 13.Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM, Kristal AR, Santella RM, Probstfield JL, Moinpour CM, Albanes D, Taylor PR, Minasian LM, Hoque A, Thomas SM, Crowley JJ, Gaziano JM, Stanford JL, Cook ED, Fleshner NE, Lieber MM, Walther PJ, Khuri FR, Karp DD, Schwartz GG, Ford LG, Coltman CA. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97:94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 14.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, Haapakoski J, Malila N, Rautalahti M, Ripatti S, Maenpaa H, Teerenhovi L, Koss L, Virolainen M, Edwards BK. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90:440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 15.Meyer F, Galan P, Douville P, Bairati I, Kegle P, Bertrais S, Estaquio C, Hercberg S. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int J Cancer. 2005;116:182–186. doi: 10.1002/ijc.21058. [DOI] [PubMed] [Google Scholar]
- 16.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez C, Jacobs EJ, Mondul AM, Calle EE, McCullough ML, Thun MJ. Vitamin E supplements and risk of prostate cancer in U.S. men. Cancer Epidemiol Biomarkers Prev. 2004;13:378–382. [PubMed] [Google Scholar]
- 18.The HOPE and HOPE-TOO Trial Investigators. Effects of Long-term Vitamin E Supplementation on Cardiovascular Events and Cancer: A Randomized Controlled Trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 19.Wright ME, Weinstein SJ, Lawson KA, Albanes D, Subar AF, Dixon LB, Mouw T, Schatzkin A, Leitzmann MF. Supplemental and dietary vitamin e intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2007;16:1128–1135. doi: 10.1158/1055-9965.EPI-06-1071. [DOI] [PubMed] [Google Scholar]
- 20.Clark LC, Dalkin B, Krongrad A, Combs GF, Jr, Turnbull BW, Slate EH, Witherington R, Herlong JH, Janosko E, Carpenter D, Borosso C, Falk S, Rounder J. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81:730–734. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizawa K, Willett WC, Morris SJ, Stampfer MJ, Spiegelman D, Rimm EB, Giovannucci E. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J Natl Cancer Inst. 1998;90:1219–1224. doi: 10.1093/jnci/90.16.1219. [DOI] [PubMed] [Google Scholar]
- 22.Brinkman M, Reulen RC, Kellen E, Buntinx F, Zeegers MP. Are men with low selenium levels at increased risk of prostate cancer? Eur J Cancer. 2006;42:2463–2471. doi: 10.1016/j.ejca.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Zhong W, Oberley TD. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2001;61:7071–7078. [PubMed] [Google Scholar]
- 24.Webber MM, Perez-Ripoll EA, James GT. Inhibitory effects of selenium on the growth of DU-145 human prostate carcinoma cells in vitro. Biochem Biophys Res Commun. 1985;130:603–609. doi: 10.1016/0006-291x(85)90459-0. [DOI] [PubMed] [Google Scholar]
- 25.Zhao R, Domann FE, Zhong W. Apoptosis induced by selenomethionine and methioninase is superoxide mediated and p53 dependent in human prostate cancer cells. Mol Cancer Ther. 2006;5:3275–3284. doi: 10.1158/1535-7163.MCT-06-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigounas G, Anagnostou A, Steiner M. dl-alpha-tocopherol induces apoptosis in erythroleukemia, prostate, and breast cancer cells. Nutr Cancer. 1997;28:30–35. doi: 10.1080/01635589709514549. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Altuwaijri S, Yeh S. RRR-alpha-tocopheryl succinate inhibits human prostate cancer cell invasiveness. Oncogene. 2004;23:3080–3088. doi: 10.1038/sj.onc.1207435. [DOI] [PubMed] [Google Scholar]
- 28.Zu K, Hawthorn L, Ip C. Up-regulation of c-Jun-NH2-kinase pathway contributes to the induction of mitochondria-mediated apoptosis by alpha-tocopheryl succinate in human prostate cancer cells. Mol Cancer Ther. 2005;4:43–50. [PubMed] [Google Scholar]
- 29.Zhang H, Wu Y, Malewicz B, Lu J, Li S, Marshall J, Ip C, Dong Y. Augmented suppression of androgen receptor signaling by a combination of alpha-tocopheryl succinate and methylseleninic acid. Cancer. 2006;107:2942–2948. doi: 10.1002/cncr.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zu K, Ip C. Synergy between selenium and vitamin E in apoptosis induction is associated with activation of distinctive initiator caspases in human prostate cancer cells. Cancer Res. 2003;63:6988–6995. [PubMed] [Google Scholar]
- 31.Prasad KN, Kumar B, Yan XD, Hanson AJ, Cole WC. {alpha}-Tocopheryl Succinate, the Most Effective Form of Vitamin E for Adjuvant Cancer Treatment: A Review. J Am Coll Nutr. 2003;22:108–117. doi: 10.1080/07315724.2003.10719283. [DOI] [PubMed] [Google Scholar]
- 32.Ip C, Thompson HJ, Zhu Z, Ganther HE. In Vitro and in Vivo Studies of Methylseleninic Acid: Evidence That a Monomethylated Selenium Metabolite Is Critical for Cancer Chemoprevention. Cancer Res. 2000;60:2882–2886. [PubMed] [Google Scholar]
- 33.Reagan-Shaw S, Ahmad N. RNA interference-mediated depletion of phosphoinositide 3-kinase activates forkhead box class O transcription factors and induces cell cycle arrest and apoptosis in breast carcinoma cells. Cancer Res. 2006;66:1062–1069. doi: 10.1158/0008-5472.CAN-05-1018. [DOI] [PubMed] [Google Scholar]
- 34.Dong Y, Zhang H, Hawthorn L, Ganther HE, Ip C. Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Cancer Res. 2003;63:52–59. [PubMed] [Google Scholar]
- 35.Malafa M, Fokum F, Andoh J, Neitzel L, Bandyopadhyay S, Zhan R, Iiizumi M, Furuta E, Horvath E, Watabe K. Vitamin E succinate suppresses prostate tumor growth by inducing apoptosis. Int J Cancer. 2006;118:2441–2447. doi: 10.1002/ijc.21689. [DOI] [PubMed] [Google Scholar]
- 36.Shiau CW, Huang JW, Wang DS, Weng JR, Yang CC, Lin CH, Li C, Chen CS. alpha-Tocopheryl succinate induces apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 function. J Biol Chem. 2006;281:11819–11825. doi: 10.1074/jbc.M511015200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Ni J, Messing EM, Chang E, Yang CR, Yeh S. Vitamin E succinate inhibits the function of androgen receptor and the expression of prostate-specific antigen in prostate cancer cells. PNAS. 2002;99:7408–7413. doi: 10.1073/pnas.102014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ni J, Chen M, Zhang Y, Li R, Huang J, Yeh S. Vitamin E succinate inhibits human prostate cancer cell growth via modulating cell cycle regulatory machinery. Biochemical and Biophysical Research Communications. 2003;300:357–363. doi: 10.1016/s0006-291x(02)02851-6. [DOI] [PubMed] [Google Scholar]
- 39.Venkateswaran V, Fleshner NE, Klotz LH. Synergistic effect of vitamin E and selenium in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2004;7:54–56. doi: 10.1038/sj.pcan.4500707. [DOI] [PubMed] [Google Scholar]
- 40.Jiang C, Wang Z, Ganther H, Lu J. Distinct Effects of Methylseleninic Acid versus Selenite on Apoptosis, Cell Cycle, and Protein Kinase Pathways in DU145 Human Prostate Cancer Cells. Mol Cancer Ther. 2002;1:1059–1066. [PubMed] [Google Scholar]
- 41.Stenman UH, Abrahamsson PA, Aus G, Lilja H, Bangma C, Hamdy FC, Boccon-Gibod L, Ekman P. Prognostic value of serum markers for prostate cancer. Scand J Urol Nephrol Suppl. 2005:64–81. doi: 10.1080/03008880510030941. [DOI] [PubMed] [Google Scholar]
- 42.Danial NN. BCL-2 Family Proteins: Critical Checkpoints of Apoptotic Cell Death. Clin Cancer Res. 2007;13:7254–7263. doi: 10.1158/1078-0432.CCR-07-1598. [DOI] [PubMed] [Google Scholar]
- 43.Mackey TJ, Borkowski A, Amin P, Jacobs SC, Kyprianou N. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with prostate cancer. Urology. 1998;52:1085–1090. doi: 10.1016/s0090-4295(98)00360-4. [DOI] [PubMed] [Google Scholar]
- 44.Sabichi AL, Lee JJ, Taylor RJ, Thompson IM, Miles BJ, Tangen CM, Minasian LM, Pisters LL, Caton JR, Basler JW, Lerner SP, Menter DG, Marshall JR, Crawford ED, Lippman SM. Selenium accumulation in prostate tissue during a randomized, controlled short-term trial of l-selenomethionine: a Southwest Oncology Group Study. Clin Cancer Res. 2006;12:2178–2184. doi: 10.1158/1078-0432.CCR-05-0937. [DOI] [PubMed] [Google Scholar]
- 45.Traber MG. Utilization of vitamin E. Biofactors. 1999;10:115–120. doi: 10.1002/biof.5520100205. [DOI] [PubMed] [Google Scholar]
- 46.Zu K, Hawthorn L, Ip C. Up-regulation of c-Jun-NH2-kinase pathway contributes to the induction of mitochondria-mediated apoptosis by alpha-tocopheryl succinate in human prostate cancer cells. Mol Cancer Ther. 2005;4:43–50. [PubMed] [Google Scholar]
- 47.Yamaguchi K, Uzzo RG, Pimkina J, Makhov P, Golovine K, Crispen P, Kolenko VM. Methylseleninic acid sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Oncogene. 2005;24:5868–5877. doi: 10.1038/sj.onc.1208742. [DOI] [PubMed] [Google Scholar]
- 48.Hu H, Jiang C, Ip C, Rustum YM, Lu J. Methylseleninic Acid Potentiates Apoptosis Induced by Chemotherapeutic Drugs in Androgen-Independent Prostate Cancer Cells. Clin Cancer Res. 2005;11:2379–2388. doi: 10.1158/1078-0432.CCR-04-2084. [DOI] [PubMed] [Google Scholar]
- 49.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 50.Domingo-Domenech J, Mellado B, Ferrer B, Truan D, Codony-Servat J, Sauleda S, Alcover J, Campo E, Gascon P, Rovira A, Ross JS, Fernandez PL, Albanell J. Activation of nuclear factor-kappaB in human prostate carcinogenesis and association to biochemical relapse. Br J Cancer. 2005;93:1285–1294. doi: 10.1038/sj.bjc.6602851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 52.Gasparian AV, Yao YJ, Lu J, Yemelyanov AY, Lyakh LA, Slaga TJ, Budunova IV. Selenium compounds inhibit I kappa B kinase (IKK) and nuclear factor-kappa B (NF-kappa B) in prostate cancer cells. Mol Cancer Ther. 2002;1:1079–1087. [PubMed] [Google Scholar]
- 53.Crispen PL, Uzzo RG, Golovine K, Makhov P, Pollack A, Horwitz EM, Greenberg RE, Kolenko VM. Vitamin E succinate inhibits NF-kappaB and prevents the development of a metastatic phenotype in prostate cancer cells: implications for chemoprevention. Prostate. 2007;67:582–590. doi: 10.1002/pros.20468. [DOI] [PubMed] [Google Scholar]


