Abstract
Background
Chronic intermittent alcohol vapor exposure and selective breeding procedures have been used separately for many years to model specific aspects of alcohol dependence. The purpose of the present investigation was to combine these 2 approaches by exposing alcohol-preferring (P) rats to chronic intermittent alcohol vapor for extended periods of time and then testing them for operant alcohol responding in parallel with a group of outbred Wistar rats at multiple time points following the termination of vapor exposure.
Methods
P rats (n = 20) and Wistar rats (n = 18) were trained to respond for 10% (w/v) ethanol in an operant situation, then divided into groups matched for intake levels. Animals were then exposed to chronic intermittent alcohol vapor (14 hours ON/10 hours OFF) or air for 8 weeks. Rats were then tested for operant alcohol responding under various conditions and at multiple time points during alcohol withdrawal (6 hours) and protracted abstinence (1 to 15 days).
Results
Chronic alcohol vapor exposure produced similar increases in operant alcohol responding in P rats and Wistar rats during acute withdrawal and protracted abstinence.
Conclusions
These results illustrate the separate and combined effects of genetic selection for high alcohol preference and dependence on alcohol drinking behavior. Furthermore, these results confirm past findings that dependent rats consume more alcohol than nondependent controls well into abstinence following extended periods of alcohol vapor exposure.
Keywords: Dependence, P Rats, Alcohol Vapor, Withdrawal, Abstinence
An alcohol vapor inhalation procedure has been validated for its ability to produce somatic (Rogers et al., 1979) and motivational (Roberts et al., 1996, 2000) aspects of alcohol dependence that parallel those seen in humans. This animal model was initially developed to address some of the methodological concerns with liquid diet and intragastric intubation procedures (Rogers et al., 1979). The model has since been refined to include daily 10-hour withdrawal periods between daily 14-hour alcohol vapor exposure periods; this exposure schedule accelerates and accentuates the development of increased alcohol drinking induced by dependence (O'Dell et al., 2004).
There are multiple rodent lines that have been selectively bred for high and low alcohol preference, including the alcohol-preferring/-nonpreferring (P/NP) lines (Lumeng et al., 1977). Rats with high and low alcohol preference have been bred over generations in order to produce lines that are either highly prone or highly resistant to voluntary consumption of alcohol. High alcohol-drinking lines are characterized not only by their voluntary intake of high amounts of alcohol (>5 g ethanol/kg body weight/d), but also their preference for alcohol in the presence of other solutions (Murphy et al., 2002).
P rats do not consume alcohol simply for its taste (Bice and Kiefer, 1990), smell, or caloric properties, as they consume alcohol even in the presence of other palatable solutions (Lankford et al., 1991). P rats also self-administer alcohol via nonoral routes (e.g., intracranially; Gatto et al., 1994) and work for a wide range of alcohol concentrations in an operant situation, even when water and food are concurrently available (Murphy et al., 1989). P rats also voluntarily drink amounts of alcohol sufficient to achieve blood–alcohol levels (BALs) that are known to be reinforcing in rodents (Li et al., 1979; Lumeng and Li, 1986). Finally, following free-choice alcohol drinking, P rats exhibit both functional (Gatto et al., 1987) and metabolic tolerance (Lumeng and Li, 1986) and, following many weeks of access, they exhibit signs of physical dependence (Kampov-Polevoy et al., 2000; Waller et al., 1982).
The primary purpose of the present investigation was to test the hypothesis that P rats will self-administer higher levels of alcohol following dependence induction via chronic alcohol vapor exposure. In order to determine the effects of genetic susceptibility to high alcohol preference on the motivational aspects of alcohol dependence, a control group of genetically heterogeneous Wistar rats was trained and tested in parallel with P rats. Wistar rats are the parent line from which P rats were initially derived. A variety of parametric manipulations were employed to determine the effects of this genetic susceptibility on dependence-induced drinking during both acute withdrawal and protracted abstinence. Such gene-environment interactions have not been adequately explored to this point.
A second goal of this study was to determine the long-lasting effects of alcohol dependence on alcohol-drinking behavior in P and Wistar rats. The motivational factors that drive relapse alcohol drinking in humans last well into protracted abstinence (Hershon, 1977; Voltaire-Carlsson et al., 1996). Many alcohol vapor studies have examined dependence-induced drinking in animals allowed to self-administer alcohol during acute withdrawal (2 to 12 hours), usually following approximately 4 weeks of alcohol vapor inhalation (Funk et al., 2006, 2007; O'Dell et al., 2004; Richardson et al., 2008; Roberts et al., 1996; Walker and Koob, 2007). Recently, it was shown that longer periods (≥8 weeks) of chronic intermittent alcohol vapor exposure produce dependence-induced increases in alcohol drinking that persist well into the abstinence period (Sommer et al., 2008), in agreement with an earlier study that showed protracted elevations in alcohol responding following a shorter period of vapor exposure (∼3 weeks; Roberts et al., 2000). Examination of drinking during protracted abstinence allows for complete dissociation of the somatic and motivational aspects of alcohol dependence, as well as separation of their respective effects on dependence-induced increases in alcohol drinking.
The main hypothesis for the current study was that alcohol vapor inhalation would produce increases in alcohol self-administration behavior in all rats, and that these increases would be larger and last longer in selectively bred P rats than in genetically heterogeneous Wistar rats. Therefore, it was hypothesized that selective breeding for high alcohol preference (i.e., enrichment of genes that underlie alcohol preference) and dependence induced by alcohol vapor inhalation would produce a greater enhancement of alcohol-drinking behavior than either procedure alone. A secondary hypothesis was that the interaction effect of these 2 models, one genetic and the other environmental, would produce increases in alcohol drinking that last well into protracted abstinence.
Method
Subjects
Adult male Wistar rats obtained from Charles River (n = 18; Kingston, NY) and adult male alcohol-preferring (P) rats (n = 20; Indiana University School of Medicine) were used in the experiments. P rats weighed between 427 and 607 g (∼13 weeks of age) and Wistar rats weighed between 350 and 435 g (∼8 weeks of age) at the start of operant training. P rats generally weighed more than Wistar rats throughout the experiment since they were quarantined for 10 weeks upon arrival at The Scripps Research Institute (TSRI). Rats were group-housed in standard plastic cages with wood chip bedding under a 12-hour light/12-hour dark cycle (lights on at 8 pm) and given ad libitum access to food and water throughout except during experimental drinking sessions. All procedures were conducted in the dark cycle and met the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Operant Chambers
The operant chambers (Coulbourn Instruments, Allentown, PA) utilized in the present study had 2 retractable levers located 4 cm above a grid floor and 4.5 cm to either side of a 2-well acrylic drinking cup. Operant responses and resultant fluid deliveries were recorded by custom software running on a PC computer. A single lever-press activated a 15 rpm Razel syringe pump (Stanford, CT) that delivered 0.1 ml of fluid to the appropriate well over a period of 0.5 seconds. Lever presses that occurred during the 0.5 seconds of pump activation were not recorded and did not result in fluid delivery. Operant chambers were individually housed in sound-attenuated ventilated cubicles to minimize environmental disturbances.
Operant Ethanol Self-Administration Training
Upon arrival at TSRI, P rats were quarantined for 10 weeks. During that time, P rats were allowed 30-minute 2-bottle choice drinking sessions of 10% (w/v) ethanol versus water 3 to 4 days per week to allow them to habituate to the ethanol solution (data not shown). P rats were then delivered to the research facility colony room and allowed several days to habituate to the new housing conditions before operant training began.
P rats were then trained to orally self-administer 10% (w/v) ethanol or water in a concurrent, 2-lever, free-choice contingency. Lever presses were reinforced on a continuous fixed ratio-1 (FR1) schedule such that each response resulted in delivery of 0.1 ml of fluid. P rats were initially allowed 4 extended sessions in operant chambers in order to learn the lever-pressing procedure. Then sessions were shortened to the standard 30-minute length, and P rats were allowed 11 sessions of operant responding for 10% (w/v) versus water. Operant responding was stable and reliable by this 11th day of operant responding. P rats were divided into dependent and nondependent groups based on mean intakes across the final 5 days of the baseline period.
Wistar rats were treated similarly except for several key differences. They were initially trained to respond for a “supersaccharin” solution (3% glucose and 0.125% saccharin; Valenstein et al., 1967) versus water, following which 10% (w/v) ethanol was added and sweeteners removed from the experimental solution. Upon completion of this 12-day fading procedure, Wistar rats were allowed 16 sessions of operant responding for 10% (w/v) versus water. Operant responding was stable and reliable by this 16th day of operant responding. Wistar rats were divided into dependent and nondependent groups based on mean intakes across the final 5 days of the baseline period.
Ethanol Vapor Inhalation
To induce ethanol dependence, standard rat cages were housed in separate, sealed, clear plastic chambers into which ethanol vapor was intermittently introduced. This procedure has been described in detail elsewhere (O'Dell et al., 2004). Briefly, 95% ethanol was evaporated and vapor was delivered at rates between 22 and 27 mg/l. Ethanol vapor was turned on (6 pm) for 14 hour per day and off (8 am) for 10 hour per day (O'Dell et al., 2004) for 4 consecutive weeks, and the target range for BALs during vapor exposure was 150 to 200 mg%. Nondependent control rats were treated in parallel except they were exposed to control air. Tail blood samples were collected periodically at 8 am for BAL determination and vapor adjustments during vapor exposure. This chronic intermittent vapor exposure produces somatic and motivational aspects of alcohol dependence during alcohol withdrawal (O'Dell et al., 2004).
Postdependence Testing for Operant Alcohol Responding
Following 4 weeks of alcohol vapor or ambient air exposure, dependent and nondependent P rats and Wistar rats were tested at various withdrawal and abstinence time points, as described below, and as illustrated in Fig. 1. In general, rats were tested for operant ethanol self-administration either on consecutive days or intermittently, either with or without vapor exposure between tests, and at various time points following termination of vapor exposure (i.e., acute withdrawal versus protracted abstinence). Intermittent operant tests with vapor exposure between tests occurred on days 28, 32, 35, 39, and 47 (all tests occurred 6 hours into withdrawal) of vapor exposure (data from tests on vapor days 35 and 39 not shown because vapor BALs for both lines of animals were higher than 200 mg%). Consecutive-days operant tests with vapor exposure between tests occurred on days 47, 48, 49, and 50 (all tests occurred 6 hours into withdrawal) of vapor exposure. Consecutive-days operant tests without vapor exposure between tests occurred on days 55 (6 hours withdrawal), 56 (24 hours withdrawal), 57 (48 hours withdrawal), and 58 (72 hours withdrawal) of vapor exposure (i.e., ethanol drip terminated on day 55, 6 hours prior to operant test; rats only exposed to air vapor from this point forward). Finally, operant tests without vapor exposure between tests also occurred on days 62, 63, 64, 69, and 70 (tests occurred 7, 8, 9, 14, and 15 days into abstinence, respectively) of vapor exposure (i.e., ambient air for all rats) to examine operant responding at various time points during protracted abstinence from alcohol. Tests of abstinence-induced ethanol responding were conducted at a time of day that corresponded to prevapor baseline and acute withdrawal tests.
Fig. 1.
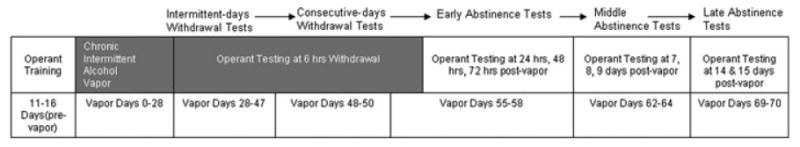
Timeline of operant training, alcohol vapor exposure, and operant tests for P rats and Wistars. Rats were trained to respond for 10% (w/v) ethanol in an operant situation for between 11 and 16 days, divided into groups based on baseline intake levels, and exposed to either alcohol vapor or air vapor for 28 days prior to the next operant test. Operant tests began on day 28 of vapor exposure, and continued on an intermittent basis (approximately twice per week) as described in the Method section. Darkened portions of the top row in the timeline represent periods when rats were being exposed to vapor; white portions of the top row in the timeline represent periods when rats were not being exposed to vapor.
Blood–Alcohol Level Determinations
Tail blood was sampled at the end of 14-hour ethanol vapor exposure periods and also following representative operant ethanol self-administration sessions. Rats were gently restrained while the tip of the tail (2 mm) was cut off with a clean razor blade. Tail blood (0.2 ml) was collected and centrifuged. Plasma (5 μl) was used for measurement of BALs using an Analox AM 1 analyzer (Analox Instruments LTD, Lunenberg, MA). The reaction is based on the oxidation of alcohol by alcohol oxidase in the presence of molecular oxygen (alcohol + O2 → acetaldehyde + H2O2). The rate of oxygen consumption is directly proportional to the alcohol concentration. Single point calibrations are done for each set of samples with reagents provided by Analox Instruments (0.025 to 0.400 g%).
The BAL target range in dependent P rats and Wistars was 150 to 200 mg% during vapor exposure. Mean ± SEM BALs for P rats (145.27 ± 15.60 mg% < range < 205.46 ± 12.24 mg%) and Wistar rats (173.11 ± 7.86 mg% < range < 219.39 ± 18.70 mg%) were successfully maintained in this range for the duration of the 55 days of alcohol vapor exposure. Following operant test sessions, ranges of BALs were also exhibited by individual dependent (30.1 mg% < range < 194.7 mg%) and nondependent (20.4 mg% < range < 79.1 mg%) Wistar rats, as well as dependent (27.5 mg% < range < 206.4 mg%) and nondependent (20.1 mg% < range < 111.9 mg%) P rats. Following the 6-hour withdrawal test on vapor day 55, ethanol vapor-exposed rats were removed from vapor and maintained with zero BALs for the remainder of the experiment.
Statistical Analysis
Ethanol and water responses and intakes are expressed as mean ± SEM, and ethanol intake is normalized for body weight (i.e., g ethanol/kg body weight). Self-administration data were initially analyzed using a series of 3-way mixed-design analyses of variance (ANOVAs), with genetic line (P rats vs. Wistar rats) and ethanol dependence history (dependent vs. nondependent) as between-subjects factors and day (baseline vs. consecutive test days) as the within-subjects factor. A separate series of 3-way mixed-design ANOVAs were also used to analyze self-administration data based on the a priori hypothesis that intakes would differ on individual test days versus baseline, but not necessarily between multiple test days; these ANOVAs contained the same 3 factors except that day contained only 2 levels (prevapor baseline vs. single test day) in all analyses. Post-hoc comparisons were conducted using the Student Newman–Keuls test. Statistical significance was set at p < 0.05.
Results
Blood–Alcohol Levels Following Operant Alcohol Responding
Dependent P rats consumed significantly more ethanol (g/kg), t = 2.79, p = 0.012, and exhibited significantly higher BALs, t = 2.67, p = 0.016, than nondependent P rats (left and right panels of Fig. 2A, respectively). Dependent Wistar rats consumed significantly more ethanol (g/kg), t = 2.74, p = 0.015, and exhibited significantly higher BALs, t = 3.38, p = 0.004, than nondependent controls (left and right panels of Fig. 2A, respectively). Across lines of rats and dependence histories, there was a significant correlation between ethanol intake (g/kg) and BALs, r(36) = 0.73, p < 0.001. Across dependence histories, P rats exhibited a significant correlation between ethanol intake (g/kg) and BALs, r(18) = 0.87, p < 0.001. Across dependence histories, Wistar rats also exhibited a significant correlation between ethanol intake (g/kg) and BALs, r(16) = 0.64, p = 0.004. Figure 2B shows scatter plots of ethanol intake (g/kg) versus resultant BALs in dependent and nondependent Wistar rats (left panels), as well as dependent and nondependent P rats (right panels).
Fig. 2.
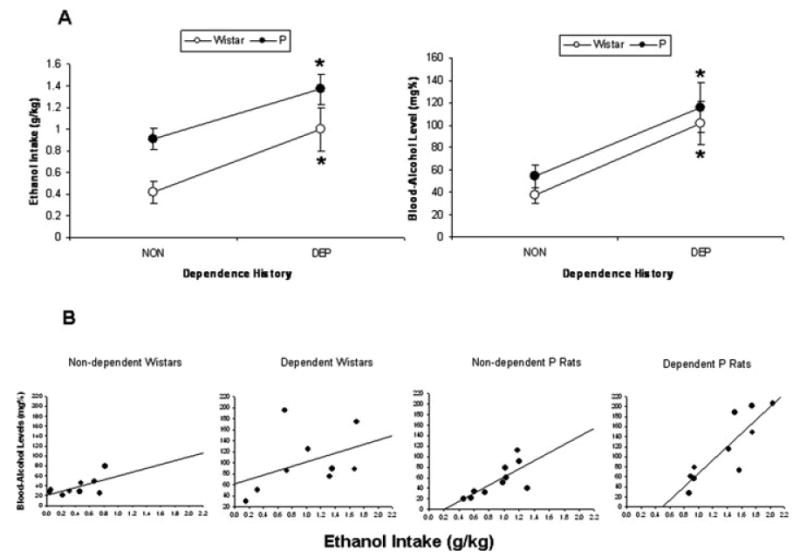
(A) Mean ± SEM ethanol intake (g/kg) by dependent and nondependent P rats and Wistars (left panel) and resultant mean ± SEM blood–alcohol levels (mg%) in those rats (right panel); and (B) scatter plots of blood–alcohol levels (BALs) produced by ethanol intake (g/kg) by dependent and nondependent P rats and Wistars during a representative postvapor (6 hours withdrawal) operant test session. Ethanol intake (g/kg) and BAL data are from an operant test that occurred 6 hours into withdrawal on day 55 of vapor exposure (i.e., 6 hours into the final abstinence period), and are representative of acute withdrawal operant test sessions. *p < 0.05 significantly higher ethanol intake (g/kg) and BALs relative to nondependent controls.
Intermittent Tests of Operant Alcohol Responding During Acute Withdrawal
Figure 3 shows ethanol intake (g/kg) by dependent and nondependent P rats and Wistars during 30-minute operant sessions conducted 6 hours into withdrawal on 3 test days each separated by 3 to 4 days of intermittent vapor exposure. In 3 separate 3-way ANOVAs (Line × Vapor treatment × Test day), 6-hour withdrawal ethanol intakes (g/kg) for each of the 3 test days were separately compared to prevapor baseline intakes. There was a significant vapor treatment × test day interaction effect on ethanol intake (g/kg) for the first, F(1,34) = 31.81, p < 0.001, second, F(1,34) = 23.95, p < 0.001, and third, F(1,34) = 4.36, p = 0.044, intermittent 6-hour withdrawal tests. Post-hoc analyses indicated that dependent rats consumed more ethanol (g/kg) during the first 2 of these 6-hour withdrawal tests relative to nondependent controls (p < 0.001 in both cases) and also relative to their own baseline (p < 0.001 in both cases). There were no significant 3-way interaction effects on ethanol intake (g/kg), indicating that the effects of chronic intermittent vapor exposure on ethanol intake by P rats during intermittent testing was similar to the effects of vapor on outbred Wistar rats. Analyses of operant ethanol responses yielded similar results. Relative to P rats, Wistars responded significantly more for water across test days and treatment histories, F(1,34) = 8.18, p = 0.007, and exhibited significantly lower preference for ethanol across test days and treatment histories, F(1,34) = 22.27, p < 0.001. Tables 1 and 2 display ethanol and water lever presses and ethanol preference ratios exhibited by dependent and nondependent P rats and Wistars during intermittent withdrawal tests.
Fig. 3.
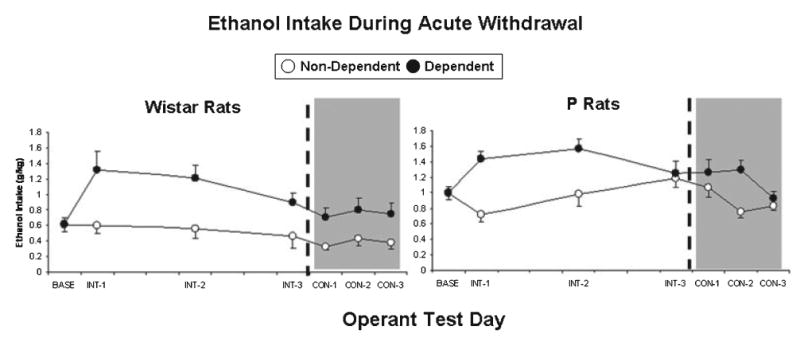
Mean ± SEM ethanol intake (g/kg) by dependent (closed circles) and nondependent (open circles) Wistar rats and P rats during the prevapor baseline period (BASE) and also during 3 intermittent (INT; white background) and 3 consecutive-days (CON; gray background) operant test sessions. All tests occurred 6 hours into acute withdrawal.
Table 1.
Ethanol and Water Lever Presses ± SEM for Nondependent and Dependent P Rats and Wistar Rats During the 5-day Baseline Period That Preceded Vapor Exposure and Also During Withdrawal and Abstinence Tests That Occurred During and Following Vapor Exposure Periods
| Test phase | Test day | P rats | Wistar rats | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nondependent | Dependent | Nondependent | Dependent | ||||||
| Ethanol | Water | Ethanol | Water | Ethanol | Water | Ethanol | Water | ||
| Baseline | 5-day Mean | 52.42 ± 3.69 | 4.46 ± 1.12 | 53.58 ± 3.71 | 6.37 ± 1.08 | 29.33 ± 4.47 | 16.24 ± 8.10 | 29.71 ± 3.33 | 14.33 ± 6.46 |
| Intermittent tests during acute WD | 6-hour Test 1 | 42.30 ± 5.55 | 5.40 ± 0.76 | 81.60 ± 5.66* | 5.00 ± 1.15 | 31.67 ± 5.25 | 19.78 ± 9.01 | 67.78 ± 11.95* | 20.78 ± 6.25 |
| 6-hour Test 2 | 56.90 ±9.18 | 2.90 ± 0.99 | 84.10 ± 8.29 | 4.80 ± 1.02 | 30.00 ± 7.30 | 16.44 ± 5.78 | 61.33 ± 7.30 | 12.33 ± 2.09 | |
| 6-hour Test 3 | 71.80 ± 8.31 | 2.80 ± 0.68 | 71.30 ± 8.07 | 3.90 ± 0.82 | 24.22 ± 7.73 | 14.89 ± 7.14 | 46.22 ± 5.95 | 11.00 ± 5.18 | |
| Consecutive tests during acute WD | 6-hour Test 4 | 64.20 ± 8.59 | 4.00 ± 1.04 | 71.80 ±9.63 * | 5.50 ±1.41 | 17.56 ± 2.60 | 19.44 ±11.30 | 35.56 ± 5.24* | 15.67 ± 5.32 |
| 6-hour Test 5 | 46.00 ± 4.98 | 4.00 ± 1.78 | 73.20 ± 6.87 | 3.90 ± 0.88 | 23.56 ± 4.92 | 14.11 ± 4.86 | 40.56 ± 7.99 | 17.33 ± 8.67 | |
| 6-hour Test 6 | 50.50 ± 4.22 | 4.00 ± 1.15 | 52.90 ± 5.80 | 3.60 ± 1.03 | 21.00 ± 5.55 | 16.11 ± 6.89 | 38.11 ± 6.40 | 12.00 ± 5.35 | |
| Early abstinence | 24 hours | 47.40 ± 5.38 | 2.60 ± 0.72 | 56.20 ± 4.77* | 4.70 ± 1.47 | 22.22 ± 5.58 | 12.00 ± 4.51 | 44.56 ± 5.63* | 10.56 ± 5.30 |
| 48 hours | 50.90 ± 7.01 | 3.20 ± 0.73 | 74.50 ± 6.62 | 4.20 ± 1.37 | 23.11 ± 6.27 | 13.11 ± 4.72 | 36.11 ± 8.08 | 12.22 ± 7.09 | |
| 72 hours | 41.10 ± 3.75 | 4.10 ± 1.23 | 61.60 ± 8.00 | 5.10 ± 1.60 | 18.78 ± 5.76 | 15.22 ± 5.81 | 31.44 ± 7.90 | 9.78 ± 6.12 | |
| Middle abstinence | 7 days | 62.40 ± 4.91 | 3.50 ± 0.95 | 64.80 ± 8.96 | 5.10 ± 1.84 | 17.89 ± 4.66 | 12.11 ± 5.67 | 25.67 ± 4.33 | 10.67 ± 6.59 |
| 8 days | 51.90 ± 5.54 | 2.70 ± 0.86 | 69.20 ± 5.45 | 4.70 ± 1.52 | 18.78 ± 4.96 | 11.00 ± 4.01 | 33.78 ± 7.69 | 14.89 ± 8.03 | |
| 9 days | 50.80 ± 7.02 | 1.60 ± 0.71 | 71.60 ± 8.70 | 6.20 ± 1.71 | 19.00 ± 4.47 | 15.78 ± 4.86 | 24.11 ± 5.89 | 13.56 ± 7.69 | |
| Late abstinence | 14 days | 56.50 ± 7.97 | 3.40 ± 0.71 | 55.10 ± 6.70 | 3.60 ± 1.01 | 20.22 ± 4.57 | 11.11 ± 3.41 | 26.78 ± 4.73 | 9.89 ± 4.43 |
| 15 days | 51.50 ± 6.01 | 2.00 ± 0.77 | 61.90 ± 6.25 | 5.00 ± 2.15 | 19.33 ± 4.42 | 9.33 ± 2.47 | 33.00 ± 7.24 | 10.22 ± 5.33 | |
p < 0.01 significantly higher ethanol responding relative to nondependent controls across test days within a phase and also across rat line (i.e., significant main effect of dependence history).
Table 2.
Ethanol Preference (Ethanol Lever Presses/Total Lever Presses) ± SEM for Nondependent and Dependent P Rats and Wistar Rats During the 5-day Baseline Period That Preceded Vapor Exposure and Also During Withdrawal and Abstinence Tests That Occurred During and Following Vapor Exposure Periods
| Test Phase | Test day | P rats | Wistar rats | ||
|---|---|---|---|---|---|
| Nondependent | Dependent | Nondependent | Dependent | ||
| Baseline | 5-day Mean | 0.91 ± 0.02 | 0.89 ± 0.01 | 0.69 ± 0.08 | 0.73 ± 0.08 |
| Intermittent tests during acute WD | 6-hour Test 1 | 0.87 ± 0.03 | 0.94 ± 0.01 | 0.67 ± 0.09 | 0.75 ± 0.07 |
| 6-hour Test 2 | 0.95 ± 0.02 | 0.95 ± 0.01 | 0.65 ± 0.09 | 0.82 ± 0.03 | |
| 6-hour Test 3 | 0.96 ± 0.01 | 0.95 ± 0.01 | 0.62 ± 0.09 | 0.82 ± 0.06 | |
| Consecutive tests during acute WD | 6-hour Test 4 | 0.93 ± 0.02 | 0.91 ± 0.03 | 0.60 ± 0.08 | 0.72 ± 0.06 |
| 6-hour Test 5 | 0.93 ± 0.03 | 0.95 ± 0.01 | 0.63 ± 0.10 | 0.75 ± 0.10 | |
| 6-hour Test 6 | 0.93 ± 0.02 | 0.94 ± 0.02 | 0.60 ± 0.10 | 0.78 ± 0.09 | |
| Early abstinence | 24 hours | 0.94 ± 0.02 | 0.93 ± 0.02 | 0.66 ± 0.09 | 0.84 ± 0.06 |
| 48 hours | 0.93 ± 0.02 | 0.95 ± 0.01 | 0.61 ± 0.10 | 0.77 ± 0.10 | |
| 72 hours | 0.91 ± 0.03 | 0.92 ± 0.02 | 0.55 ± 0.12 | 0.79 ± 0.09 | |
| Middle abstinence | 7 days | 0.95 ± 0.02 | 0.92 ± 0.03 | 0.61 ± 0.11 | 0.77 ± 0.11 |
| 8 days | 0.94 ± 0.02 | 0.94 ± 0.02 | 0.63 ± 0.10 | 0.76 ± 0.09 | |
| 9 days | 0.95 ± 0.03 | 0.92 ± 0.02 | 0.56 ± 0.10 | 0.70 ± 0.12 | |
| Late abstinence | 14 days | 0.93 ± 0.02 | 0.94 ± 0.02 | 0.63 ± 0.09 | 0.76 ± 0.09 |
| 15 days | 0.96 ± 0.02 | 0.93 ± 0.04 | 0.64 ± 0.08 | 0.79 ± 0.08 | |
Consecutive-Days Tests of Operant Alcohol Responding During Acute Withdrawal
Figure 3 also shows ethanol intake (g/kg) by dependent and nondependent P rats and Wistars during 30-minute operant sessions conducted 6 hours into withdrawal on 3 consecutive test days. In 3 separate 3-way ANOVAs (Line × Vapor treatment × Test day), 6-hour withdrawal ethanol intakes (g/kg) for each of these test days were separately compared to prevapor baseline intakes. There was a significant vapor treatment × test day interaction effect on ethanol intake (g/kg) for the first, F(1,34) = 4.16, p = 0.049, second, F(1,34) = 12.50, p = 0.001, and third, F(1,34) = 4.21, p = 0.48, consecutive-days 6-hour withdrawal tests. Post-hoc analyses indicated that dependent rats consumed more ethanol (g/kg) during the second of these 6-hour withdrawal tests relative to nondependent controls (p < 0.001) and also relative to their own baseline (p = 0.009). There were no significant 3-way interaction effects on ethanol intake (g/kg), indicating that the effects of chronic intermittent vapor exposure on ethanol intake by P rats during consecutive-days testing was similar to the effects of vapor on outbred Wistar rats. Analyses of operant ethanol responses yielded similar results. Relative to P rats, Wistars responded significantly more for water across test days and treatment histories, F(1,34) = 6.21, p = 0.018, and exhibited significantly lower preference for ethanol across test days and treatment histories, F(1,34) = 18.58, p < 0.001. Tables 1 and 2 display ethanol and water lever presses and ethanol preference ratios exhibited by dependent and nondependent P rats and Wistars during consecutive withdrawal tests.
Tests of Operant Alcohol Responding During Early Abstinence
Figure 4 shows ethanol intake (g/kg) by dependent and nondependent P rats and Wistars during 30-minute operant sessions conducted 24, 48, and 72 hours into abstinence from chronic ethanol vapor. In 3 separate 3-way ANOVAs (Line × Vapor treatment × Test day), ethanol intakes (g/kg) from each of these test days were separately compared to prevapor baseline intakes. There was a significant vapor treatment × test day interaction effect on ethanol intake (g/kg) at the 24-hour time point, F(1,34) = 10.00, p = 0.003, 48-hour time point, F(1,34) = 9.56, p = 0.04, and 72-hour time point, F(1,34) = 10.08, p = 0.03, following the termination of chronic intermittent ethanol vapor exposure. Post-hoc analyses indicated that dependent rats consumed more ethanol (g/kg) relative to nondependent controls during each of the 3 early abstinence tests (p < 0.01 in all cases), and also relative to their own baseline during the 48-hour abstinence test (p < 0.05). There were no significant 3-way interaction effects on ethanol intake (g/kg), indicating that the effects of chronic intermittent vapor exposure on ethanol intake by P rats during early abstinence testing was similar to the effects of vapor on outbred Wistar rats. Analyses of operant ethanol responses yielded similar results. Relative to P rats, Wistars responded significantly more for water across test days and treatment histories, F(1,34) = 5.18, p = 0.029, and exhibited significantly lower preference for ethanol across test days and treatment histories, F(1,34) = 15.98, p < 0.001. There was also a significant vapor treatment × test day interaction effect on ethanol preference across time points, F(3,102) = 3.32, p = 0.023. Tables 1 and 2 display ethanol and water lever presses and ethanol preference ratios exhibited by dependent and nondependent P rats and Wistars during early abstinence tests.
Fig. 4.
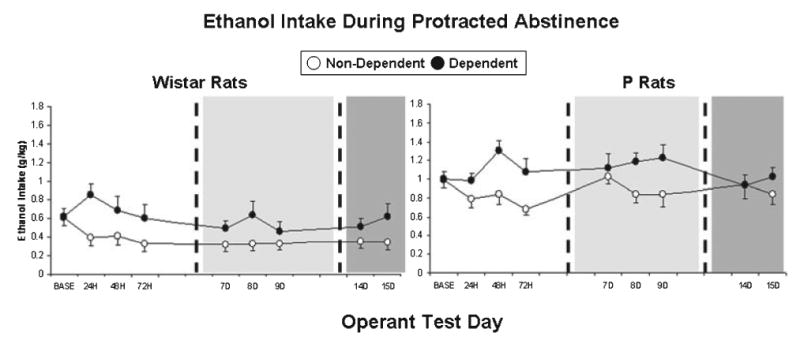
Mean ± SEM ethanol intake (g/kg) by dependent (closed circles) and nondependent (open circles) Wistar rats and P rats during the prevapor baseline period (BASE) and also during three (24, 48, 72 hours) early abstinence (white background), three (7, 8, 9 days) middle-abstinence (light gray background), and two (14, 15 days) protracted-abstinence (dark gray) operant test sessions.
Tests of Operant Alcohol Responding During Middle Abstinence
Figure 4 also shows ethanol intake (g/kg) by dependent and nondependent P rats and Wistars during 30-minute operant sessions conducted 7, 8, and 9 days into abstinence from chronic ethanol vapor. In 3 separate 3-way ANOVAs (Line × Vapor treatment × Test day), ethanol intakes (g/kg) from each of these test days were separately compared to prevapor baseline intakes. There was a significant rat line × test day interaction effect on ethanol intake (g/kg) 7 days into abstinence from ethanol vapor, F(1,34) = 8.17, p = 0.007. Wistar rats consumed less ethanol (g/kg) 7 days into abstinence relative to their own baseline (p = 0.008), but this decrease was not observed in P rats (p < 0.001). There was a significant vapor treatment × test day interaction effect on ethanol intake (g/kg) 8 days into abstinence from ethanol vapor, F(1,34) = 9.97, p = 0.003. Dependent rats consumed more ethanol (g/kg) 8 days into abstinence relative to nondependent controls (p = 0.006). There was a significant rat line × test day interaction effect, F(1,34) = 5.58, p = 0.024, as well as a significant vapor treatment × test day interaction effect, F(1,34) = 4.73, p = 0.037, on ethanol intake (g/kg) 9 days into abstinence from ethanol vapor. Dependent rats consumed more ethanol (g/kg) 9 days into abstinence relative to nondependent controls (p = 0.049); also, Wistar rats consumed less ethanol (g/kg) 9 days into abstinence relative to their own baseline (p = 0.014), but this decrease was not observed in P rats (p < 0.001). There were no significant 3-way interaction effects on ethanol intake (g/kg), indicating that the effects of chronic intermittent vapor exposure on ethanol intake by P rats during middle abstinence testing was similar to the effects of vapor on outbred Wistar rats. Analyses of operant ethanol responses yielded similar results. Relative to P rats, Wistars responded significantly more for water across test days and treatment histories, F(1,34) = 5.59, p = 0.024, and exhibited significantly lower preference for ethanol across test days and treatment histories, F(1,34) = 18.07, p < 0.001. Tables 1 and 2 display ethanol and water lever presses and ethanol preference ratios exhibited by dependent and nondependent P rats and Wistars during middle abstinence tests.
Tests of Operant Alcohol Responding During Protracted Abstinence
Figure 4 also shows ethanol intake (g/kg) by dependent and nondependent P rats and Wistars during 30-minute operant sessions conducted 14 and 15 days into abstinence from chronic ethanol vapor. In 2 separate 3-way ANOVAs (Line × Vapor treatment × Test day), ethanol intakes (g/kg) from each of these test days were separately compared to prevapor baseline intakes. Regardless of vapor treatment history, P rats consumed significantly more ethanol (g/kg) than Wistar rats during day 14 testing, F(1,34) = 37.67, p < 0.001, and day 15 testing, F(1,34) = 31.88, p < 0.001. There was also a significant vapor treatment × test day interaction effect on ethanol intake (g/kg) 15 days into abstinence from ethanol vapor, F(1,34) = 4.30, p = 0.046. Dependent rats consumed more ethanol (g/kg) 15 days into abstinence relative to nondependent controls (p = 0.046). There were no significant 3-way interaction effects on ethanol intake (g/kg), indicating that the effects of chronic intermittent vapor exposure on ethanol intake by P rats during protracted abstinence testing was similar to the effects of vapor on outbred Wistar rats. Tables 1 and 2 display ethanol and water lever presses and ethanol preference ratios exhibited by dependent and nondependent P rats and Wistars during late abstinence tests.
Discussion
The present investigation confirms previous findings (O'Dell et al., 2004; Sommer et al., 2008) that chronic intermittent alcohol vapor exposure produces increases in alcohol drinking in rats that are evident during acute withdrawal. Elevations in drinking occur regardless of whether rats are allowed to drink on an intermittent basis or during consecutive days following the development of dependence, and are confirmed by postoperant blood–alcohol levels (past observations from our lab have confirmed that BALs are zero at the vapor settings and withdrawal test time point used in this study). Furthermore, alcohol-dependent animals consumed more alcohol than nondependent animals up to 15 days following the termination of chronic alcohol vapor. These data are consistent with previous indications that extended periods (≥8 weeks) of chronic intermittent alcohol vapor exposure produce increases in alcohol drinking that last well into the abstinence period (Sommer et al., 2008). Also, P rats consumed more alcohol than Wistar rats throughout the experiment, a result that is consistent with genetic selection of P rats for high alcohol drinking (Lumeng et al., 1977).
The results show that rats selectively bred for high alcohol preference are susceptible to the effects of alcohol dependence on subsequent withdrawal- and abstinence-induced drinking. Although statistical analyses indicate that P rats and Wistars exhibited dependence-induced increases in drinking that were comparable in magnitude (i.e., lack of 3-way interaction effects), there were trends in the data that warrant some discussion. For the duration of the experiment, nondependent P rats exhibited consistently higher alcohol intake than nondependent Wistar rats, and dependent P rats exhibited consistently higher alcohol intake than dependent Wistar rats. Also, for the duration of the experiment, dependent Wistar rats and P rats both exhibited higher alcohol intake than nondependent controls. Relative to nondependent controls, dependence-induced increases in drinking appeared to be larger and more consistent in dependent Wistar rats (during acute withdrawal and abstinence testing), although this may have been an artifact of the descending alcohol intake by nondependent Wistar rats over time. However, relative to prevapor baseline, dependence-induced increases in drinking appeared to be larger and more consistent in P rats (during acute withdrawal and abstinence testing), a result that is impressive considering the already high alcohol intake exhibited by P rats during the baseline period. Together, these results indicate that alcohol-preferring (P) rats are at least as susceptible as outbred Wistar rats to the effects of alcohol dependence on alcohol drinking and related behaviors.
Susceptibility of P rats to the effects of dependence on alcohol drinking-related behaviors is consistent with what is known about dependence-related behaviors in that line of rats. P rats exhibit functional (Gatto et al., 1987) and metabolic tolerance (Lumeng and Li, 1986) following chronic free-choice drinking. Also, following extended periods of chronic free-choice alcohol drinking, P rats exhibit some signs of physical dependence (Waller et al., 1982), such as increased susceptibility to bicuculline-induced seizures (Kampov-Polevoy et al., 2000). P rats exhibit a prolonged alcohol deprivation effect, defined as a transient increase in alcohol intake following long-term access and a subsequent period of abstinence (Rodd-Hendricks et al., 2000, 2001). The vulnerability of P rats to relapse drinking is consistent with human literature that describes a predisposition for the offspring of alcoholics to be affected differently by alcohol and to develop alcoholism (for example, see Cloninger et al., 1981; Pihl et al., 1990; Schuckit, 1986).
It is worth reiterating here that genetic selection for high alcohol preference was not predictive of the magnitude of dependence-induced increases in alcohol drinking in the present study. There is little data in the rat literature to support the notion that genetic background has predictive value for the severity of somatic and motivational disturbances associated with alcohol dependence. Across inbred strains of mice, there appears to be an inverse relationship between alcohol-drinking behavior and somatic alcohol withdrawal severity (Metten and Crabbe, 2005). Specifically, C57BL/6J mice consume far more alcohol than DBA/2J mice and exhibit substantially less intense signs of somatic withdrawal following chronic exposure to high doses of alcohol (Metten et al., 1998), although the relationship between innate alcohol preference in mouse strains and motivational measures of withdrawal is currently not known.
Several limitations to the present experiment should be mentioned. Due to the unavoidable quarantine period for P rats, the P rats but not Wistars were allowed intermittent access to ethanol in the home cage prior to operant training, and this home cage experience with alcohol may have contributed to higher alcohol intake in P rats versus Wistars. It should also be noted that high alcohol intake observed in P rats may have contributed to the lack of 3-way interaction effects on alcohol drinking. That is, the combined effects of genetic selection and dependence on alcohol drinking may produce a ceiling effect for responding in a 30-minute operant session. This issue could be addressed in future studies by extending the length of operant sessions to examine whether dependent P rats maintain responding longer into the session during withdrawal and abstinence.
In summary, the present investigation demonstrates that P rats selectively bred for high alcohol preference exhibit withdrawal- and abstinence-induced elevations in drinking, and that dependence-induced elevations in alcohol drinking last well into abstinence. More comprehensive studies that include alcohol nonpreferring rats and genetically heterogeneous parent rat lines as controls will be needed to more precisely determine whether there is a genetic correlation between innate alcohol preference and the intensity of dependence-related responses and behaviors.
Acknowledgments
This investigation was a collaborative effort between the Scripps Alcohol Research Center and the Indiana University Alcohol Research Center. The authors thank Yanabel Grant for her skilled technical assistance and Mike Arends for his excellent editorial assistance. This is manuscript number 19117 from The Scripps Research Institute. This work was supported by the Pearson Center for Alcoholism and Addiction Research as well as NIAAA grants AA06420, AA08459, AA015512, and AA007611-20.
References
- Bice PJ, Kiefer SW. Taste reactivity in alcohol preferring and non-preferring rats. Alcohol Clin Exp Res. 1990;14:721–727. doi: 10.1111/j.1530-0277.1990.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Murphy JM, Waller MB, McBride WJ, Lumeng L, Li TK. Chronic ethanol tolerance through free-choice drinking in the P line of alcohol-preferring rats. Pharmacol Biochem Behav. 1987;28:111–115. doi: 10.1016/0091-3057(87)90021-9. [DOI] [PubMed] [Google Scholar]
- Hershon HI. Alcohol withdrawal symptoms and drinking behavior. J Stud Alcohol. 1977;38:953–971. doi: 10.15288/jsa.1977.38.953. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–284. [PubMed] [Google Scholar]
- Lankford MF, Roscoe AK, Pennington SN, Myers RD. Drinking of high concentrations of ethanol vs. palatable fluids in alcohol-preferring (P) rats: valid animal model of alcoholism. Alcohol. 1991;8:293–299. doi: 10.1016/0741-8329(91)90417-u. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Waller MB, Hawkins DT. Progress toward a voluntary oral-consumption model of alcoholism. Drug Alcohol Depend. 1979;4:45–60. doi: 10.1016/0376-8716(79)90040-1. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Hawkins DT, Li TK. New strains of rats with alcohol preference and nonpreference. In: Thurman RG, editor. Alcohol and Aldehyde Metabolizing Systems. Vol. 3 Academic Press; New York: 1977. pp. 537–544. [Google Scholar]
- Lumeng L, Li TK. The development of metabolic tolerance in the alcohol-preferring P rats: comparison of forced and free-choice drinking of ethanol. Pharmacol Biochem Behav. 1986;25:1013–1020. doi: 10.1016/0091-3057(86)90079-1. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Musmusculus) strains. Behav Neurosci. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, McBride WJ, Lumeng L, Li TK. Operant responding for oral ethanol in the alcohol-preferring P and alcohol-nonpreferring NP lines of rats. Alcohol. 1989;6:127–131. doi: 10.1016/0741-8329(89)90037-2. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Peterson J, Finn PR. Inherited predisposition to alcoholism: Characteristics of sons of male alcoholics. J Abnorm Psychol. 1990;99:291–301. doi: 10.1037//0021-843x.99.3.291. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Fekete EM, Zhao Y, Funk CK, Zorrilla EP, Koob GF. A novel small molecule antagonist of the corticotropin-releasing factor type 1 receptor (CRF1) is a potent anxiolytic and reduces excessive alcohol intake in dependent male rats. Pharmacol Biochem Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rodd-Hendricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, et al. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Rodd-Hendricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, et al. Alcohol deprivation effect is prolonged in the alcohol preferring P rat after repeated deprivations. Alcohol Clin Exp Res. 2000;24:8–16. [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Genetic aspects of alcoholism. Ann Emerg Med. 1986;15:991–996. doi: 10.1016/s0196-0644(86)80117-2. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science. 1967;157:552–554. doi: 10.1126/science.157.3788.552. [DOI] [PubMed] [Google Scholar]
- Voltaire-Carlsson A, Hiltunen AJ, Koechling UM, Borg S. Effects of long-term abstinence on psychological functioning: a prospective longitudinal analysis comparing alcohol-dependent patients and healthy volunteers. Alcohol. 1996;13:415–421. doi: 10.1016/0741-8329(96)81678-8. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2007;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Lumeng L, Li TK. Induction of dependence on ethanol by free-choice drinking in alcohol-preferring rats. Pharmacol Biochem Behav. 1982;16:501–507. doi: 10.1016/0091-3057(82)90459-2. [DOI] [PubMed] [Google Scholar]


