Abstract
T cell-mediated immunity to microbes and to cancer can be enhanced by the activation of dendritic cells (DCs) via Toll-like receptors (TLRs). In this study, we evaluated the safety and feasibility of topical imiquimod, a TLR7 agonist, in a series of vaccinations (26) proteins,(27) and DNA, (28, 29) as well as in vaccines using recombinant Listeria(30) or DCs.(31) In humans, it was shown that topical imiquimod treatment may enhance the immunogenicity of a melanoma peptide vaccine when given with systemic FLT3 ligand. (32) In addition, injection of immature DCs into imiquimod pretreated skin lead to DC activation in situ and enhanced migratory capacity to draining lymph nodes in cancer patients. (33)
In this study, we test the safety and feasibility of imiquimod in a vaccine against the cancer/testis antigen NY-ESO-1, and evaluate the immunogenicity of the combination. NY-ESO-1 is detectable in approximately 30% of metastatic melanomas. (34-36) It is against the cancer/testis antigen NY-ESO-1 in patients with malignant melanoma. Recombinant, full-length NY-ESO-1 protein was administered intradermally into imiquimod pre-conditioned sites followed by additional topical applications of imiquimod. The regimen was very well-tolerated with only mild and transient local reactions and constitutional symptoms. Secondarily, we examined the systemic immune response induced by the imiquimod/NY-ESO-1 combination, and show that it elicited both humoral and cellular responses in a significant fraction of patients. Skin biopsies were assessed for imiquimod's in situ immunomodulatory effects. Compared with untreated skin, topical imiquimod induced dermal mononuclear cell infiltrates in all patients composed primarily of T cells, monocytes, macrophages, myeloid DCs and natural killer (NK) cells, and to a lesser extent plasmacytoid DCs. DC activation was evident. This study demonstrates the feasibility and excellent safety profile of a topically applied TLR7 agonist utilized as a vaccine adjuvant in cancer patients. Imiquimod's adjuvant effects require further evaluation and likely need optimization of parameters such as formulation, dose and timing relative to antigen exposure for maximal immunogenicity.
Keywords: Toll-like receptor, TLR7 agonist, imiquimod, cancer vaccine
INTRODUCTION
TLRs are a family of highly conserved transmembrane receptors on cells of the immune system that function to alert the host to the presence of specific molecular patterns found in microbes.(1, 2) TLR ligands or agonists control the activation of antigen-presenting cells (APCs) such as DCs by triggering their maturation program, which involves up-regulation of HLA and co-stimulatory molecules and secretion of proinflammatory cytokines such as TNFα, IL-6, IL-12 and IFNα.(3) In addition to activating the innate immune response, stimulation of TLRs on APCs can direct adaptive immunity, including the induction of a T helper 1 (Th1) cell response, which is believed necessary for anti-tumor immunity.(4-6) Thus, incorporating TLR agonists into vaccines could be a very effective way to boost vaccine activity.(7)
At least ten members of the TLR family have been identified so far in humans. Two of these, TLR7 and 8, are closely related molecules that induce antiviral immune responses through the recognition of single-stranded viral RNA in the endosomal compartment (8-11). Synthetic ligands belonging to the imidazoquinoline family have been identified that also trigger TLR7 signaling and immune activation (4, 12). Ligation of TLR7 on human myeloid and plasmacytoid DCs by these compounds induces DC maturation and the secretion of important inflammatory mediators such as IL-12 (by myeloid DCs) and IFNα (by plasmacytoid DCs).(13, 14) Imidazoquinolines have also been shown to induce cytokine production when applied to human condylomata acuminata, (15) to induce Langerhans cell and plasmacytoid DC migration in mouse skin, (16-18) and to lead to accumulation of plasmacytoid and myeloid DCs in treated lesions of patients with various skin diseases (18, 19). Vaccination studies in animal models have indicated that imidazoquinolines can boost the magnitude and quality of antigen-specific T cell responses, particularly when either conjugated to an antigen (20) or administered mixed with antigen in a water-oil emulsion.(21)
Imiquimod (Aldara™) is one of the better characterized imidazoquinolines and is the only one currently approved for clinical use, although only as a topical ointment. It has been demonstrated to possess anti-viral and anti-tumor properties, and is approved for the treatment of anogenital warts caused by human papillomavirus as well as for basal cell carcinoma and actinic keratosis.(22-25) In animal models, imiquimod given either topically or systemically has demonstrated adjuvant activity in vaccines using antigenic peptides, expressed by a variety of cancers but not in adult somatic tissues, making it an attractive target for immunotherapies.(37, 38) We show that topical imiquimod used as a vaccine adjuvant is well tolerated, and that the vaccine induces measurable NY-ESO-1-specific antibody and CD4+ T cell responses. Immunohistochemistry studies indicate that imiquimod induces dermal inflammatory infiltrates that are rich in APCs and T cells, which suggests a mechanism for the immunogenicity of this combination.
MATERIALS AND METHODS
Study design, patients and treatment schedule
This was a pilot, single arm study in nine patients. The primary objective was to evaluate the safety of the vaccine combination; the secondary objective was to determine the frequency of induced T and B cell immunity. Additional exploratory endpoints included the characterization of the vaccine-induced immunity and the immunostimulatory effect of imiquimod in treated skin. Patients with histologically confirmed, resected malignant melanoma (AJCC stages (39) IIB, IIC and III) were eligible. Additional eligibility criteria included age ≥ 18 years, ECOG performance status(40) ≤ 2 and adequate organ and marrow function. Patients were excluded for any of the following: anti-cancer therapy within four weeks, prior NY-ESO-1 vaccination, immunodeficiency or use of immunosuppressive medications, autoimmune diseases other than vitiligo, intercurrent illnesses, pregnancy or lactation, or inflammatory skin disorders. Tumor NY-ESO-1 expression was not required for study entry, but was offered optionally as part of enrollment into the NYU Interdisciplinary Melanoma Cooperative Group (IMCG) database. Immunohistochemistry for NY-ESO-1 expression was performed as previously described in (36). The clinical trial (NCT00142454, clinicaltrials.gov) was approved by the NYU School of Medicine Institutional Review Board and written informed consent was obtained from all patients. Nine patients were screened, all were found eligible and enrolled. All completed four vaccinations and two follow-up visits.
250 mg of 5% imiquimod cream (Aldara™, 3M) was self-applied topically by patients to a 4 x 5 cm outlined area of healthy extremity skin overnight on days 1−5 of each cycle. Application and removal times were recorded in treatment diaries. 100 μg of recombinant human NY-ESO-1 protein (in 4 M urea and 50 mM glycine, provided by the Ludwig Institute for Cancer Research) was injected intradermally into the imiquimod-treated site on day 3. Cycles were repeated every 3 weeks for a total of 4 injections. Imiquimod was omitted on day 5 of the last cycle to avoid biopsy site irritation.
Blood samples and peptides
Blood was drawn at baseline (day 1/cycle 1), on days 1 and 8 of each treatment cycle and 3 and 6 weeks following the fourth vaccine. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by Ficoll centrifugation and frozen in aliquots in 90% pooled human serum / 10% dimethyl sulfoxide (DMSO). Supernatant plasma from each time point was also frozen. A library of 17 overlapping peptides (20- to 22-mers, 10 amino acid overlap) spanning the entire NY-ESO-1 sequence was obtained from NeoMPS via the Ludwig Institute.(41) The peptide sequences (starting from the NY-ESO-1 N-terminus) are: MQAEGRGTGGSTGDADGPGG, STGDADGPGGPGIPDGPGGN, PGIPDGPGGNAGGPGEAGAT, AGGPGEAGATGGRGPRGAGA, GGRGPRGAGAARASGPGGGA, ARASGPGGGAPRGPHGGAAS, PRGPHGGAASGLNGCCRCGA, GLNGCCRCGARGPESRLLEF, RGPESRLLEFYLAMPFATPM, YLAMPFATPMEAELARRSLA, EAELARRSLAQDAPPLPVPG, QDAPPLPVPGVLLKEFTVSG, PGVLLKEFTVSGNILTIRLTAADHR, NILTIRLTAADHRQLQLSIS, AADHRQLQLSISSCLQQLSLLM, SCLQQLSLLMWITQCFLPVF, WITQCFLPVFLAQPPSGQRR. Each lyophilized peptide was resuspended in DMSO at a concentration of 2 mg/ml and stored frozen. A stock solution of 17 pooled overlapping peptides, 100 μg/ml each, was used for immune monitoring assays at a final concentration of 1 μg/ml each.
IFNγ ELISPOT assay
PBMCs were thawed and cultured overnight in R-10 medium (RPMI-1640 with 10% heat-inactivated human AB serum, 10 mM HEPES and 20 μg/ml gentamicin), and plated the following day at 300,000 per well in 96-well PVDF filter plates (Millipore) coated with anti-IFNγ monoclonal antibody (Mabtech) in the presence or absence of pooled NY-ESO-1 peptides (1 μg/ml each), or in the presence of 1 μg/ml Staphylococcus enterotoxin A (SEA, Sigma). Plates were cultured overnight, then washed and developed with 2 μg/ml biotinylated anti-IFNγ antibody (Mabtech), avidinhorseradish peroxidase complex and AEC substrate (Vector Labs). Spots were counted with a CTL ImmunoSpot analyzer (Cellular Technology), and average values for triplicate wells multiplied by 3.33 to determine the number of spot-forming cells (SFC) per million PBMCs.
In vitro T cell pre-sensitization (IVS)
In vitro pre-sensitizations were performed as described.(41) PBMCs were thawed and cultured overnight in I-10 medium (Iscove's Modified Dulbecco's Medium with 10% heat-inactivated human AB serum, 1mM HEPES, 0.1 mM MEM non-essential amino acids and 2 mM GlutaMAX), then separated into CD4+, CD8+, and CD4−CD8− (APC) fractions using MACS MS columns (Miltenyi). Each fraction was then washed and resuspended in I-10 medium containing 10 U/ml IL-2 and 10 ng/ml IL-7. APCs were irradiated (3,000 rads) and then co-cultured with CD4+ or CD8+ cells (500,000 to 1 million cells per well) and pooled NY-ESO-1 peptides (1 μg/ml each) in a 96-well round bottom plate for 7 days, replenishing medium and cytokines every 2 to 3 days.
Intracellular cytokine staining (ICS)
All reagents were obtained from BD Biosciences. For initial screening, 1 wk IVS cultures were harvested, washed and re-plated in I-10 medium in duplicate wells of a 96-well V-bottom plate. A pool of all 17 NY-ESO-1 peptides (1 μg/ml each) was added to one of the wells, the other well left unstimulated. Unstained control and a SEA-stimulated control well were also included. For epitope mapping, IVS cultures were plated into 18 wells, 17 of which contained one of the NY-ESO-1 overlapping peptides, with the final well unstimulated. For all ICS cultures, plates were incubated for 1 hr at 37°C, after which Brefeldin A (GolgiPlug) was added to each well and the cultures incubated another 5 hr. Cells were then stained for CD4 and CD8, fixed and permeabilized with BD cytofix/cytoperm solution, then washed with 1X BD perm/wash buffer and stained for CD3 and IFNγ. Cells were analyzed on a BD LSR II flow cytometer using FACSDiva software and compensated using BD Comp Beads. Data were analyzed using FlowJo software (TreeStar).
Measurement of antibody responses
Patient plasma samples were analyzed by ELISA for seroreactivity against various recombinant protein antigens (NY-ESO-1, LAGE-1, MAGE-3, SSX2, p53, Melan-A, Tyrosinase), as well as against pools of overlapping peptides (10 μg/ml each) covering the NY-ESO-1 or MAGE-3 sequences. Plasma was serially diluted from 1/100 to 1/100,000 and added to low- volume 96-well plates (Corning, NY) coated with 1μg/ml antigen and blocked with PBS containing 5% non-fat milk. After incubation, plates were washed with PBS containing 0.2% Tween-20 and rinsed with PBS. Plasma IgG (total or subclasses) bound to antigens was detected with alkaline phosphatase conjugated specific monoclonal antibodies (Southern Biotech, Birmingham, AL). Following addition of ATTOPHOS substrate (Fisher Scientific, Waltham, MA), absorbance was measured using a fluorescence reader Cytofluor Series 4000 (PerSeptive Biosystems, Framingham, MA). A reciprocal titer was calculated for each plasma sample as the maximal dilution still significantly reacting to a specific antigen. This value was extrapolated by determining the intersection of a linear trend regression with a cutoff value. The cutoff was defined as 10x the average of OD values from the first 4 dilutions of a negative control pool made of 5 healthy donor sera. In each assay, sera of patients with known presence or absence of specific reactivity were used as controls. Titers >100 were considered significantly reactive, and specificity determined by comparing reactivity to control antigens and to pooled NY-ESO-1 peptides. Epitope mapping was performed using individual NY-ESO-1 peptides. For these assays, plasma samples were chosen at the peak of antibody response. Sera of three patients with known spontaneous NY-ESO-1 immunity were chosen as positive control, serum from one patient with known seronegativity for NY-ESO-1 served as a negative control.
DTH measurement and skin biopsies
Forty-eight hours after the final vaccination patients were evaluated for DTH, defined as induration ≥ 5 mm. 4 mm punch biopsies were then obtained from imiquimod treated skin, from the vaccine site within the imiquimod area, and from untreated skin. Formalin fixed, paraffin embedded tissue sections were stained with hematoxylin and eosin or analyzed by immunohistochemistry with antibodies to CD1a and DC-LAMP (Immunotech), CD3, CD4, CD8 and CD20 (Ventana), CD25, CD11c, CD83 and Langerin (Novo Castra), CD57 (Cell Marque), CD68 (Dako), and CD123 (BD Biosciences). Slides were developed using biotinylated secondary antibodies, avidinhorseradish peroxidase conjugate, DAB substrate enhanced with copper sulfate, and hematoxylin counterstain. Positive and negative controls were included with the study sections, which were evaluated by three investigators including a pathologist blinded to the biopsied site. Positive cells were counted manually per 10 consecutive high-power fields (400x) in each of 3 layers: epidermis, papillary dermis and reticular dermis, and the sum of all 30 HPF reported.
Statistical analysis
Adverse events were graded by CTC criteria (version 3.0) and summarized. Positive B cell immune responses were defined as a titer >100 as determined by reactivity to overlapping NY-ESO-1 peptides and rNY-ESO-1 protein. Positive T cell responses were defined as a 3-fold or greater increase over pre-vaccination levels that was also 3 times greater than a parallel unstimulated control, with a minimum value of 10 SFC per million PBMCs for ELISPOT assays or 0.1% IFNγ+ cells for ICS. ELISPOT, ICS and ELISA data were summarized using descriptive statistics. The exact test (McNamar's) was used to compare the presence or absence of inflammatory infiltrates in biopsied skin by H&E stain. The Wilcoxon signed-rank test was used to compare immunohistochemistry results.
RESULTS
Patient characteristics and clinical data
The patient population consisted of 7 women and 2 men, 38 to 76 years of age (mean age of 54 years, Table I). All patients were of White/Caucasian/Non-Hispanic background. All had resected malignant melanoma (3 patients AJCC stage II, 6 patients AJCC stage III) and were disease free (NED) at the time of enrollment. All patients previously declined adjuvant α-IFN, a standard option after surgical resection for high-risk primary or regional disease. NY-ESO-1 expression in the resected tumor was not required for study entry, since we have previously shown that NY-ESO-1 expression is more prevalent in metastatic disease compared to primary tumors (36), suggestive of evolution with disease progression or recurrence. This observation provides a rationale for immunotherapeutic interventions even in patients with NY-ESO-1 negative tumors. Consistent with the reported frequencies, in our subset of patients tested, only one of three tumors expressed the antigen (Table I).
Table I.
Patient demographics, tumor stage and NY-ESO-1 expression, HLA typing results, immunological and clinical summary
| Pt ID | Age | Sex | AJCC Stage | Prior therapy | HLA | Tumor NY-ESO-1 expression | Latest follow up (days) | Current disease status | NY-ESO-1 antibody | NY-ESO-1 CD4+ T resonses |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | F | IIIB | Surgery | A1,A1,B57,B8,DR13,DR14,DQ5,DQ6,DP2,DP6 | N/A | 785 | NED | + | + |
| 2 | 76 | M | IIIB | Surgery | A11,A24,B18,B51,Cw1,Cw5,DR3,DR11,DQ2,DQ3,DP4,DP6 | − | 856 | R (827 d), RDF | − | + |
| 3 | 38 | M | IIIC | Surgery | A1,A33,B51,B56,C8,C8,DR4,DR7,DQ2,DQ3,DP2,DP5 | + (<5% of tumor cells) | 581 | R (456 d), LFU | + | − |
| 4 | 50 | F | IIB | Surgery | A3,A11,B44,B61,C2,C4,DR13,DR13,DQ6,DQ6DP4,DP34 | N/A | 709 | NED | + | + |
| 5 | 48 | F | IIIC | Surgery, Radiation | A24,A32,B39,B44,C7,C16,DR7,DR12,DQ3,DQ3,DP4,DP6 | N/A | 869 | NED | − | − |
| 6 | 62 | F | IIIB | Surgery | A11,A25,B13,B39,C6,C12,DR1,DR7,DQ2,DQ5,DP4,DP4 | N/A | 791 | R (123 d), RDF | + | + |
| 7 | 48 | F | IIB | Surgery | A1,A11,B18,B51,C5,C16,DR3,DR11,DQ2,DQ3,DP2,DP4 | − | 855 | NED | − | + |
| 8 | 51 | F | IIIA | Surgery | A1,A24,B13,B35,Cw4,Cw6,DR4,DR7,DQ2,DQ3,DP4, DP4 | N/A | 744 | NED | − | N/A |
| 9 | 56 | F | IIB | Surgery | A29,A68,B38,B51,DR1,DR7,DQ2,DQ5,DP2,DP2 | N/A | 764 | NED | − | + |
Pt ID patient identification number, NED no evidence of disease, R recurrence (at × days), RDF rendered disease-free following recurrence, LFU lost to follow up, + positive, − negative
All patients remained NED at completion of the study at 4 months. The clinical median follow-up time is 773 days (range of 581 to 869 days) from the start of investigational therapy. 3 of the 9 patients recurred (median time to recurrence of 468 days), 6 patients remain disease free at present (Table I).
Safety of NY-ESO-1 protein/imiquimod
NY-ESO-1/imiquimod was well-tolerated, and all patients completed the study. Treatment-related adverse events (AEs) were mild and transient. Local reactions at the site of imiquimod application or vaccine injection were seen in 8 of 9 patients (89%, Fig. 1). Four of nine patients (44%) reported fatigue and two of nine patients (22%) experienced flu-like symptoms. All AEs were Grade 1 (CTC criteria version 3.0) and were likely related to the immunomodulatory effects of imiquimod and vaccination. A hemoglobin decrease of greater than 1 g/dl was observed in 3 patients over the course of the trial, possibly related to phlebotomy.
Figure 1.
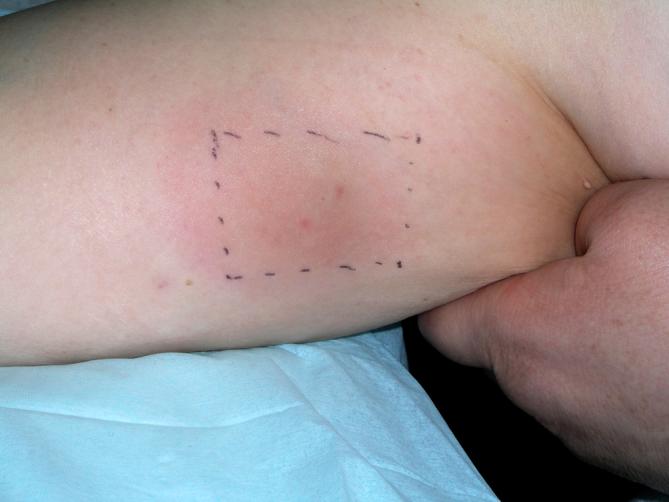
Typical local erythematous skin reaction in the area of imiquimod application (representative patient).
Humoral immune responses to NY-ESO-1
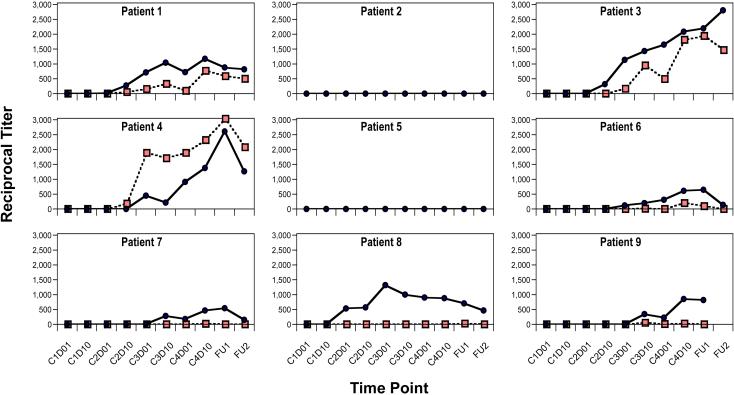
No patient had evidence of humoral immunity to NY-ESO-1 prior to vaccination. Antibody responses to recombinant NY-ESO-1 protein were induced in 7 of 9 patients post-vaccination (Fig. 2, blue circles). However, a majority of these patients also showed comparable seroreactivity to recombinant protein control antigens (not shown), indicating that the vaccine may have induced responses to immunogenic contaminants from the bacterial expression system. To unequivocally demonstrate NY-ESO-1 specificity, sera from the 7 protein-reactive patients were tested against a pool of NY-ESO-1 overlapping peptides, which confirmed NY-ESO-1-specificity in 4 of 7 protein-reactive patients (Fig. 2, red squares).
Figure 2.
NY-ESO-1 antibody responses. Extrapolated reciprocal titers (y axis) for each patient at each vaccination time point (x axis) as measured by ELISA using NY-ESO-1 recombinant protein (blue circles) and NY-ESO-1 peptide pool (red squares). For responders, the mean maximum titer was 1:1,400 using NY-ESO-1 protein, 1:1,500 using NY-ESO-1 peptides. Time points indicated by vaccination cycle (C) and day (D), or by follow-up visit (FU1, FU2).
T cell responses to NY-ESO-1
PBMCs for each time point were tested for the presence of NY-ESO-1-specific T cells by IFNγ ELISPOT assay using a pool of NY-ESO-1 overlapping peptides,(41) which has been shown to be able to detect both CD4+ and CD8+ T cell responses previously.(42) By ELISPOT, no response was identified for any of the study subjects (data not shown). DTH responses to intradermally injected NY-ESO-1 protein were also not detected in any of the subjects.
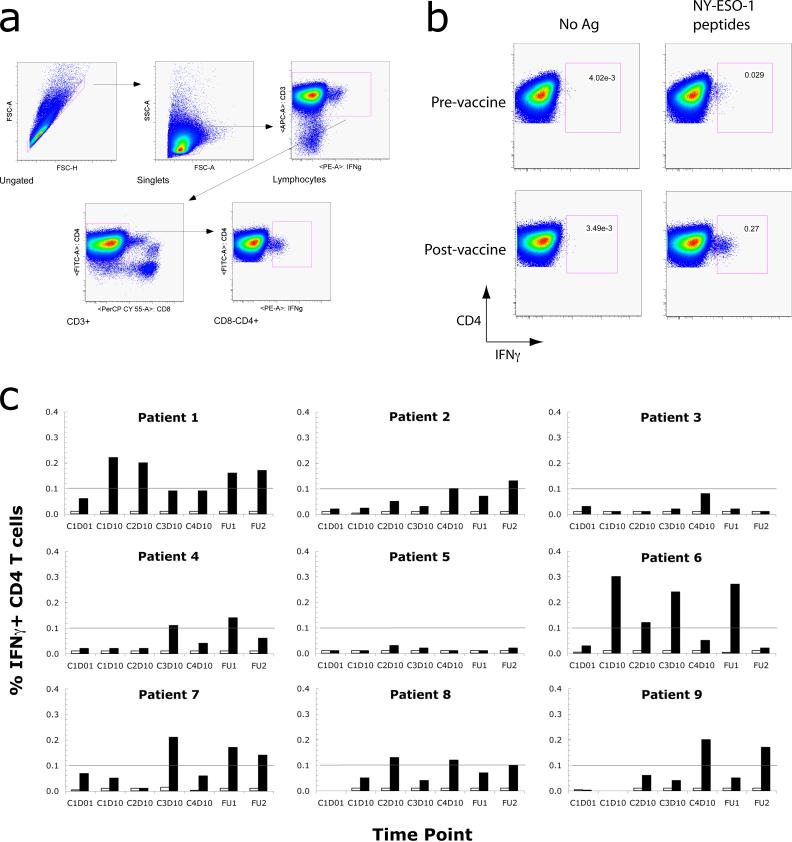
To determine if the vaccine had in fact elicited T cell responses that were not detectable by these screening methods, we analyzed PBMC samples following a 1-week in vitro pre-sensitization (IVS) (41) with the same pool of NY-ESO-1 overlapping peptides. Pre-sensitized T cells were re-stimulated using the NY-ESO-1 peptide pool, then stained for the presence of intracellular IFNγ and analyzed by flow cytometry (Fig. 3, a and b). Following the IVS, CD4+ T cell responses to NY-ESO-1 were detected at 2 or more post-vaccine time points in 7 of the 9 study subjects (Fig 3c). None of the subjects had a detectable response prior to vaccination, although the pre-vaccine sample for one subject (Patient 8) was not evaluable as the cryopreserved cells were not viable after thawing. Of the 4 subjects who had developed NY-ESO-1 antibodies, 3 of these had CD4+ T responses as well (see Table 1). No CD8+ T cell responses were detected following IVS for any of the study subjects, neither pre- nor post-vaccination (data not shown). While this pilot study was not designed to determine an association between immune responses and clinical outcome, survival data in Table I do not indicate a clear correlation between induced immune responses and disease recurrence.
Figure 3.
Intracellular cytokine staining. Following a 1 wk IVS with pooled NY-ESO-1 overlapping peptides, cells were re-stimulated with antigen and stained for intracellular IFNγ. (a) Gating for flow cytometry. Cell aggregates were excluded by gating for singlets (FSC-height vs. FSC-area), and CD4+ T cells were selected by sequentially gating for lymphocytes, CD3+ cells, and CD8− CD4+ cells. This gating strategy removes most autofluorescent dead or dying cells and minimizes non-specific background. (b) Quantification of IFNγ-secreting NY-ESO-1-specific CD4+ T cells. Representative pre- (upper) and post- (lower) vaccine samples for Patient 6 are shown. Plots on the right show T cells re-stimulated with pooled NY-ESO-1 overlapping peptides. Plots on the left show parallel pre-sensitized T cell controls that did not receive a second stimulation. For all plots, CD4 staining is shown on the y axis and IFNγ staining is shown on the x axis. (c) Summary of results for all patients. Filled bars show the percentage of CD4+ T cells secreting IFNγ following re-stimulation with the NY-ESO-1 overlapping peptide pool. Open bars represent the values for parallel controls that did not receive a second stimulation. The threshold for detection for this assay, defined as 0.1% of CD4+ T cells, is indicated by a horizontal line in each graph. Time points are indicated by vaccination cycle (C1, C2, C3 or C4) and day (D01 or D10), or as follow-up visits (FU1, 1-month follow-up; FU2, 2-month follow-up).
Epitope mapping of cellular and humoral immune responses
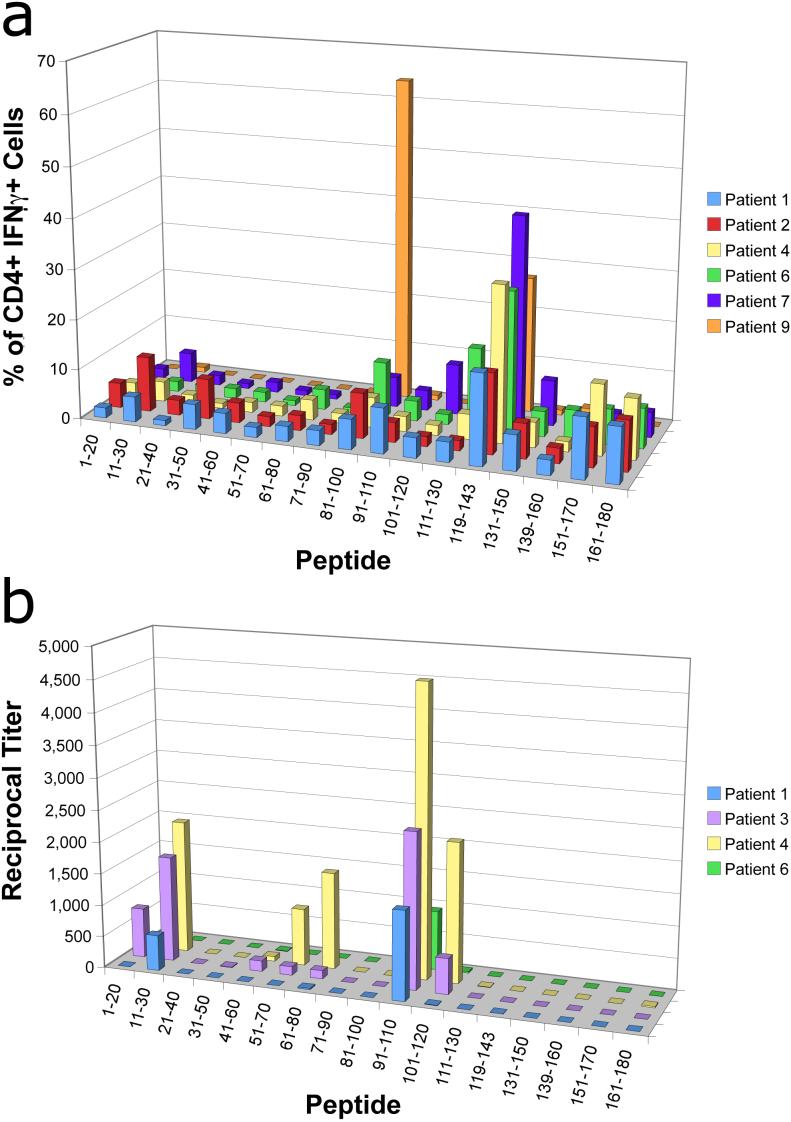
Mapping of the epitope reactivity of vaccine-induced T cells using individual rather than pooled peptides showed the induction of CD4+ T cells to several peptide epitopes, in particular the AA 119−143 epitope, which was mapped in all T cell responders (Fig. 4a). In addition, one patient exhibited a particularly strong response to the AA 81−100 epitope. This reactivity, however, did not correlate with a specific HLA class II subtype (Table I). The reactivity to both these epitopes corresponds with the results of previous studies of both vaccinated patients as well as patients with naturally occurring anti-NY-ESO-1 T cells (38, 42), www.cancerimmunity.org/peptidedatabase/tumorspecific.htm).
Figure 4.
Epitope mapping of cellular and humoral reactivities to NY-ESO-1 (a) Mapped CD4+ T cell responses in patients with reactivity to pooled NY-ESO-1 peptides. The percentage of IFNγ secreting CD4+ T cells responding to a particular AA sequence is shown by patient. (b) Mapping of B cell responses for subjects reactive with pooled NY-ESO-1 peptides. The reciprocal antibody titer for antibody to individual NY-ESO-1 peptide sequences is shown by patient.
Epitope mapping of humoral responses was similarly performed (Fig. 4b). Reactivity was observed toward the N-terminal region of NY-ESO-1 protein (AA 1−30 and AA 51−80), and especially to the AA 91−110 peptide epitope, which was observed in all antibody responders. As with the T cell epitope mapping studies, antibody responses to each of these epitopes have been previously described in vaccinated patients and in patients with spontaneous humoral immunity to NY-ESO-1 (38, 42).
Evaluation of skin biopsies
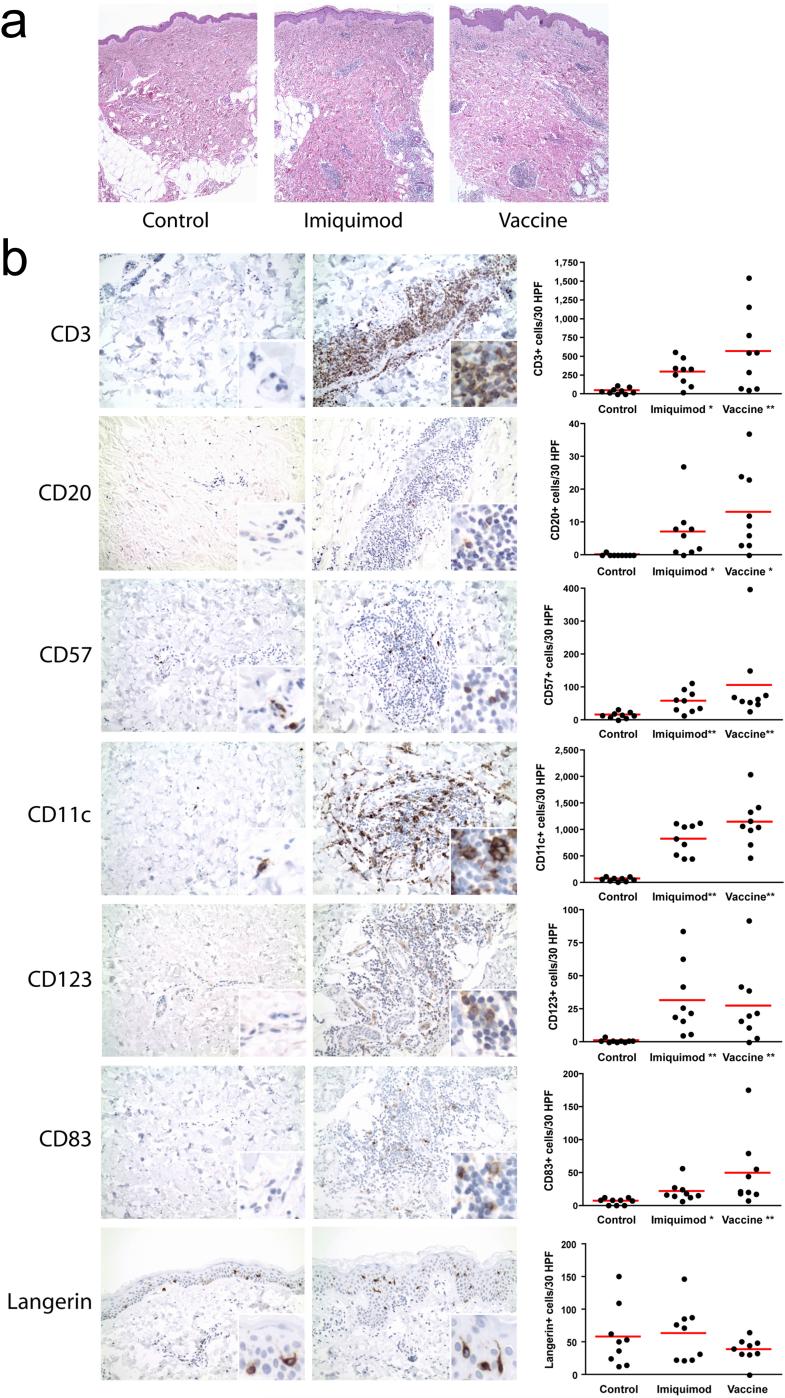
Skin sections showed mild to moderate perivascular and periadnexal mononuclear cell infiltrates in the papillary and reticular dermis in imiquimod treated skin and at imiquimod-treated vaccine injection sites of all patients, but not in untreated skin (p<0.01, Fig. 5a). Imiquimod treatment was associated with no other obvious changes except for occasional epidermal mitoses and apoptotic cells. Immunohistochemistry of the dermal infiltrates revealed that they were composed mostly of T lymphocytes (CD3+, Fig. 5b, CD4:CD8 ratio approximately 3:1, data not shown), CD11c+ and CD68+ cells (monocytes, macrophages and myeloid dendritic cells, Fig. 5b and data not shown) and NK cells (CD57+, Fig. 5b). Smaller numbers of CD123+ cells (plasmacytoid dendritic cells) and occasional B lymphocytes (CD20+) were also identified (Fig. 5b). A minority of the cells in the infiltrate were CD25+ (activated monocytes and T cells, T regulatory cells, data not shown). Occasional cells also expressed CD83 and DC-LAMP, markers of dendritic cell activation. All of the above immune cell types were significantly more frequent in imiquimod treated skin compared to untreated control skin of the same patients (p<0.05) (Fig. 5b). No statistically significant quantitative difference in immune cells was observed between imiquimod treated skin and skin receiving imiquimod plus NY-ESO-1 (Fig. 5b). Interestingly, examination of the epidermis for Langerhans cell markers CD1a and Langerin indicated no statistically significant difference in the number of Langerhans cells in treated and untreated skin (Fig. 5b and data not shown).
Figure 5.
(a) Representative H&E stained sections of control skin (left), imiquimod treated skin (center) and vaccine site (right), 40x magnification. (b) Representative immunohistochemistry sections for each of the tested markers (at 200x magnification, untreated skin (left), vaccine/imiquimod site (middle)) and graphs (right) showing cell counts for all individual patients (* p<0.05, ** p<0.01 compared to untreated control skin).
DISCUSSION
Here we report on the safety and feasibility of the topical TLR7 agonist imiquimod when used to condition vaccination sites for intradermal injection with recombinant cancer/testis antigen NY-ESO-1 in patients with malignant melanoma. The NY-ESO-1 protein / imiquimod vaccine regimen was well tolerated – only mild and transient side effects were observed, and all patients completed the study.
The vaccine combination successfully elicited both humoral and cellular immune responses in a significant fraction of patients. NY-ESO-1-specific antibody responses were detected in 4 of 9 patients (44%). However, antibody titers were significantly lower than those described in a previous study using intramuscular injection of NY-ESO-1 protein with the saponin-based adjuvant ISCOMATRIX (43), as well as in a more recent study by our group using Montanide ISA-51 adjuvant and TLR9 agonist CpG 7909 administered subcutaneously with recombinant NY-ESO-1 (42). In both these studies, all seronegative patients receiving NY-ESO-1 developed antibodies, and average maximum titers were 10-fold or more higher than what we observed here, suggesting that a protective carrier formulation providing a controlled release or depot effect may be required for optimal antibody responses. In the current study, imiquimod / NY-ESO-1 did, however, induce antibodies in a higher percentage (44%) of patients than those in the control group of the ISCOMATRIX study who received NY-ESO-1 protein alone without any adjuvant (25% of patients).(43) Epitope mapping of the antibody responses using individual peptides confirmed that the immunogenic regions of the protein were similar to those found in patients with spontaneous NY-ESO-1 immunity, indicating that the vaccine is capable of inducing B cell responses against epitopes naturally presented by NY-ESO-1 positive tumors. We also have previously shown that these vaccine-induced NY-ESO-1 antibodies facilitated cross-presentation (42).
In this study, NY-ESO-1 / imiquimod vaccination induced IFNγ-secreting NY-ESO-1-specific CD4+ T cells in 6 of 8 (75%) evaluable patients. These responses were clearly evident by intracellular cytokine staining following a one week in vitro presensitization with NY-ESO-1 overlapping peptides, but were not detectable in “ex vivo” ELISPOT assays (i.e., without pre-sensitization). In contrast, CD8+ T cell responses were not detectable using either method.
In the above mentioned ISCOMATRIX study, both CD4+ and CD8+ T cell responses to NY-ESO-1 were detectable in a subset of patients vaccinated with the ISCOMATRIX adjuvant, although a more prolonged in vitro pre-sensitization was used and ex vivo assays were not attempted, making a comparison with our study difficult.(43) However, in our recent CpG / Montanide / NY-ESO-1 protein vaccine study almost all patients developed CD4+ T cell responses and approximately half had detectable CD8+ T cell responses as measured by the same vitro pre-sensitization assay described here. In addition, ex vivo assays showed CD4+ T cell responses in 67% of subjects and CD8+ T cell responses in 39%. (42) These results indicate that CpG plus Montanide is a more potent adjuvant formulation than imiquimod alone, and is capable of cross-priming CD8+ T cell responses. Of note, CpG was administered mixed with the antigen/Montanide emulsion, whereas imiquimod was applied topically before and after antigen injection.
A possible explanation for the absence of CD8+ T cell immunity observed in our study, as well as for the weaker CD4+ T cell and antibody responses, may be the timing used for the application of imiquimod. In recent experiments in mice it was observed that when TLR agonists were provided prior to antigen encounter, cross presentation by APCs was impaired and cross priming inhibited due to premature APC maturation.(44) Thus it is possible that the use of TLR agonists may be most effective at the time of, or immediately following, the delivery of antigen. The use of TLR activation stimuli may also require additional adjuvants or emulsifiers for optimal effect. In recent studies in non-human primates, the use of imidazoquinolines or CpG was most effective when these compounds were either covalently coupled to antigen or mixed in a water-oil emulsion,(20, 21) and Montanide emulsification in our above-mentioned study might have contributed significantly to the vaccine's strong immunogenicity (42). In addition, it may prove that other imidazoquinolines such as resiquimod, which activates both TLR7 and TLR8, are more potent stimulators of immunity than imiquimod. (45) We are currently addressing these issues in a follow-up clinical study.
One question raised by this study is whether similar results could have been obtained by vaccinating with NY-ESO-1 protein alone. Although we did not include an antigen-alone control arm, previous studies have confirmed that NY-ESO-1 or MAGE-3 proteins given alone are poorly immunogenic (43, 46, 47), and that addition of an immunological adjuvant is essential for the induction of persistent immunological memory (48). The above-mentioned NY-ESO-1 protein / ISCOMATRIX study included an NY-ESO-1 protein-alone control group of 16 patients, most of whom had resected melanoma. NY-ESO-1 protein was given intramuscularly at 4 week intervals at the same dose as in our study (100 mcg). However, the frequency of induction of NY-ESO-1 specific antibodies was less than we observed for NY-ESO-1 / imiquimod (25% compared to 44%), and the induction of CD4+ T cells reactive to NY-ESO-1 overlapping peptides was 0% compared to 75%. As in our study, CD8+ T cells reactive to NY-ESO-1 were not induced in the absence of ISCOMATRIX. These data suggest that topical imiquimod can enhance the induction of antibody and CD4+ T cell responses, but not CD8+ T cell responses.
To address the potential the mechanism of action of imiquimod in inducing vaccine responses, we show that topical application of imiquimod is associated with dermal mononuclear cell infiltrates that are composed largely of T cells, antigen-presenting cells (monocytes, macrophages, myeloid DCs and plasmacytoid DCs) and NK cells. Activated DCs (CD83+ and DC-LAMP+ cells) were also evident. Only rare B cells were seen. Although we were unable to further characterize the observed CD123+ cells by double-staining, we expect the majority of them to be plasmacytoid DCs, as we morphologically excluded basophils (which also express CD123). Our findings are in accordance with previous observations of recruitment and activation of myeloid and plasmacytoid DCs in the skin of patients whose skin tumors were treated with imiquimod,(18, 19) as well as observations of accumulation of plasmacytoid DC-like cells in the skin of mice after imiquimod treatment,(17) and of “monocyte-macrophage-dendrocyte” dermal infiltrates in healthy skin chronically exposed to imiquimod.(49) These observations suggest that the recruitment and activation of APCs at the vaccination site by imiquimod may lead to improved uptake and presentation of the injected protein antigen, promoting immunization.
In contrast to other studies, we did not observe a net loss of epidermal Langerhans cells following imiquimod application.(16, 17, 49) These studies reported a decrease in CD1a+ Langerhans cells in the epidermis, suggestive of migration to draining lymph nodes, after imiquimod treatment for up to 10 days in mice and following chronic exposure over months in humans. The observed difference with our results may be due to a dose effect, as studies using the imidazoquinoline resiquimod in healthy volunteers demonstrated a decrease of CD1a+ cells only in the highest dose group.(50) Furthermore, recent analyses have failed to detect TLR7 in Langerhans cells freshly purified from human skin, at least at the mRNA level (51).
In summary, this study demonstrates the feasibility and excellent safety profile of a topically applied TLR agonist as adjuvant for a protein vaccine in cancer patients. Although no conclusions about imiquimod's additive effect can be drawn in the absence of a control arm, we show that the imiquimod/NY-ESO-1 combination elicits both humoral and CD4+ T cell responses in a significant fraction of patients. In light of these findings, further studies evaluating imiquimod's relative effect on the immunogenicity of this combination, including experiments evaluating the dose and timing of its application, are warranted.
ACKNOWLEDGMENTS
The authors wish to thank Drs Gerd Ritter, Herman Yee, Michelle Lowes and Bruce Strober for their support and guidance, Dr. Thomas Del Corral for preparing the vaccines, Dr Iman Osman and the NYU IMCG for access to tissue specimens, Dr. Vandana Mukhi for statistical support, Juliet Escalon, Sean Lemoine and Nadege Gilles for data management. We also wish to thank all patients enrolled in this trial.
Supported by the Ludwig Institute for Cancer Research, the Cancer Vaccine Collaborative and the Cancer Research Institute. S.A. supported in part by an ASCO Career Development Award and NIH 5P30CA016087. Y.S. supported in part by NIH 5P30CA016087. N.B. supported in part by NIH 5R01AIO61684, the Emerald Foundation, CHAVI, the Gates Foundation and is a recipient of the Burroughs Wellcome Fund Clinical Scientist Award and a Doris Duke Distinguished Clinical Scientist Award.
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Disclosures: Sylvia Adams, Nina Bhardwaj, Erika Ritter and Sacha Gnjatic have research funding to disclose (Ludwig Institute for Cancer Research); Sacha Gnjatic, Erika Ritter, Achim Jungbluth, Linda Pan, Ralph Venhaus, Eric Hoffman and Lloyd Old have employment relationships to disclose (Ludwig Institute for Cancer Research).
REFERENCES
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Kabelitz D, Medzhitov R. Innate immunity--cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr Opin Immunol. 2007;19:1–3. doi: 10.1016/j.coi.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, Roederer M, Seder RA, Koup RA. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol. 2003;171:4320–4328. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 5.Pasare C, Medzhitov R. Toll-like receptors and acquired immunity. Semin Immunol. 2004;16:23–26. doi: 10.1016/j.smim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 7.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27:49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 9.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 10.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101(18):6835–6836. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diebold SS, Massacrier C, Akira S, Paturel C, Morel Y, Reis e Sousa C. Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur J Immunol. 2006;36:3256–3267. doi: 10.1002/eji.200636617. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 13.Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, Noelle RJ, Miller RL, Kedl RM, Fitzgerald-Bocarsly P, Tomai MA, Vasilakos JP. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 15.Tyring SK, Arany I, Stanley MA, Tomai MA, Miller RL, Smith MH, McDermott DJ, Slade HB. A randomized, controlled, molecular study of condylomata acuminata clearance during treatment with imiquimod. Journal of Infectious Diseases. 1998;178:551–555. doi: 10.1086/517472. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Wang B, Shivji GM, Toto P, Amerio P, Tomai MA, Miller RL, Sauder DN. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J Invest Dermatol. 2000;114:135–141. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 17.Palamara F, Meindl S, Holcmann M, Luhrs P, Stingl G, Sibilia M. Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol. 2004;173:3051–3061. doi: 10.4049/jimmunol.173.5.3051. [DOI] [PubMed] [Google Scholar]
- 18.Urosevic M, Dummer R, Conrad C, Beyeler M, Laine E, Burg G, Gilliet M. Disease-independent skin recruitment and activation of plasmacytoid predendritic cells following imiquimod treatment. J Natl Cancer Inst. 2005;97:1143–1153. doi: 10.1093/jnci/dji207. [DOI] [PubMed] [Google Scholar]
- 19.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beutner KR, Spruance SL, Hougham AJ, Fox TL, Owens ML, Douglas JM., Jr. Treatment of genital warts with an immune-response modifier (imiquimod). J Am Acad Dermatol. 1998;38:230–239. doi: 10.1016/s0190-9622(98)70243-9. [DOI] [PubMed] [Google Scholar]
- 23.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. 2002;27:571–577. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 24.Schulze HJ, Cribier B, Requena L, Reifenberger J, Ferrandiz C, Garcia Diez A, Tebbs V, McRae S. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from a randomized vehicle-controlled phase III study in Europe. Br J Dermatol. 2005;152:939–947. doi: 10.1111/j.1365-2133.2005.06486.x. [DOI] [PubMed] [Google Scholar]
- 25.Lebwohl M, Dinehart S, Whiting D, Lee PK, Tawfik N, Jorizzo J, Lee JH, Fox TL. Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J Am Acad Dermatol. 2004;50:714–721. doi: 10.1016/j.jaad.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Rechtsteiner G, Warger T, Osterloh P, Schild H, Radsak MP. Cutting edge: priming of CTL by transcutaneous peptide immunization with imiquimod. J Immunol. 2005;174:2476–2480. doi: 10.4049/jimmunol.174.5.2476. [DOI] [PubMed] [Google Scholar]
- 27.Johnston D, Bystryn JC. Topical imiquimod is a potent adjuvant to a weakly-immunogenic protein prototype vaccine. Vaccine. 2006;24:1958–1965. doi: 10.1016/j.vaccine.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 28.Smorlesi A, Papalini F, Orlando F, Donnini A, Re F, Provinciali M. Imiquimod and S-27609 as adjuvants of DNA vaccination in a transgenic murine model of HER2/neu-positive mammary carcinoma. Gene Ther. 2005;12:1324–1332. doi: 10.1038/sj.gt.3302559. [DOI] [PubMed] [Google Scholar]
- 29.Zuber AK, A Brave, Engstrom G, Zuber B, Ljungberg K, Fredriksson M, Benthin R, Isaguliants MG, Sandstrom E, Hinkula J, Wahren B. Topical delivery of imiquimod to a mouse model as a novel adjuvant for human immunodeficiency virus (HIV) DNA. Vaccine. 2004;22:1791–1798. doi: 10.1016/j.vaccine.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 30.Craft N, Bruhn KW, Nguyen BD, Prins R, Lin JW, Liau LM, Miller JF. The TLR7 agonist imiquimod enhances the anti-melanoma effects of a recombinant Listeria monocytogenes vaccine. J Immunol. 2005;175:1983–1990. doi: 10.4049/jimmunol.175.3.1983. [DOI] [PubMed] [Google Scholar]
- 31.Prins RM, Craft N, Bruhn KW, Khan-Farooqi H, Koya RC, Stripecke R, Miller JF, Liau LM. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176:157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 32.Shackleton M, Davis ID, Hopkins W, Jackson H, Dimopoulos N, Tai T, Chen Q, Parente P, Jefford M, Masterman KA, Caron D, Chen W, Maraskovsky E, Cebon J. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun. 2004;4:9–19. [PubMed] [Google Scholar]
- 33.Nair S, McLaughlin C, Weizer A, Su Z, Boczkowski D, Dannull J, Vieweg J, Gilboa E. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J Immunol. 2003;171:6275–6282. doi: 10.4049/jimmunol.171.11.6275. [DOI] [PubMed] [Google Scholar]
- 34.Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, Cebon J. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12:764–771. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
- 35.Bolli M, Schultz-Thater E, Zajac P, Guller U, Feder C, Sanguedolce F, Carafa V, Terracciano L, Hudolin T, Spagnoli GC, Tornillo L. NY-ESO-1/LAGE-1 coexpression with MAGE-A cancer/testis antigens: a tissue microarray study. Int J Cancer. 2005;115:960–966. doi: 10.1002/ijc.20953. [DOI] [PubMed] [Google Scholar]
- 36.Velazquez EF, Jungbluth AA, Yancovitz M, Gnjatic S, Adams S, O'Neill D, Zavilevich K, Albukh T, Christos P, Mazumdar M, Pavlick A, Polsky D, Shapiro R, Berman R, Spira J, Busam K, Osman I, Bhardwaj N. Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)--correlation with prognostic factors. Cancer Immun. 2007;7:11–17. [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 38.Gnjatic S, Nishikawa H, Jungbluth AA, Gure AO, Ritter G, Jager E, Knuth A, Chen YT, Old LJ. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 39.Greene FL. AJCC Cancer Staging Manual. 6th edition Springer Verlag; 2002. pp. 209–217. [Google Scholar]
- 40.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 41.Ayyoub M, Souleimanian NE, Godefroy E, Scotto L, Hesdorffer CS, Old LJ, Valmori D. A phenotype based approach for the immune monitoring of NY-ESO-1-specific CD4+ T cell responses in cancer patients. Clin Immunol. 2006;118:188–194. doi: 10.1016/j.clim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O'Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci U S A. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, Scott AM, Maraskovsky E, McArthur G, MacGregor D, Sturrock S, Tai TY, Green S, Cuthbertson A, Maher D, Miloradovic L, Mitchell SV, Ritter G, Jungbluth AA, Chen YT, Gnjatic S, Hoffman EW, Old LJ, Cebon JS. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci U S A. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, de Koning-Ward TF, Belz GT, Carbone FR, Crabb BS, Heath WR, Villadangos JA. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 45.Thomsen LL, Topley P, Daly MG, Brett SJ, Tite JP. Imiquimod and resiquimod in a mouse model: adjuvants for DNA vaccination by particle-mediated immunotherapeutic delivery. Vaccine. 2004;22:1799–1809. doi: 10.1016/j.vaccine.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 46.Kruit WH, van Ojik HH, Brichard VG, Escudier B, Dorval T, Dreno B, Patel P, van Baren N, Avril MF, Piperno S, Khammari A, Stas M, Ritter G, Lethe B, Godelaine D, Brasseur F, Zhang Y, van der Bruggen P, Boon T, Eggermont AM, Marchand M. Phase 1/2 study of subcutaneous and intradermal immunization with a recombinant MAGE-3 protein in patients with detectable metastatic melanoma. Int J Cancer. 2005;117:596–604. doi: 10.1002/ijc.21264. [DOI] [PubMed] [Google Scholar]
- 47.Atanackovic D, Altorki NK, Stockert E, Williamson B, Jungbluth AA, Ritter E, Santiago D, Ferrara CA, Matsuo M, Selvakumar A, Dupont B, Chen YT, Hoffman EW, Ritter G, Old LJ, Gnjatic S. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol. 2004;172:3289–3296. doi: 10.4049/jimmunol.172.5.3289. [DOI] [PubMed] [Google Scholar]
- 48.Atanackovic D, Altorki NK, Cao Y, Ritter E, Ferrara CA, Ritter G, Hoffman EW, Bokemeyer C, Old LJ, Gnjatic S. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci U S A. 2008;105:1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hermanns-Le T, Paquet P, Nikkels AF, Pierard-Franchimont C, Pierard GE. Prolonged imiquimod treatment and graft-versus-host reaction: histological mimicry in the skin infiltration pattern of the monocyte-macrophage-dendrocyte lineage. Dermatology. 2003;206:361–365. doi: 10.1159/000069958. [DOI] [PubMed] [Google Scholar]
- 50.Sauder DN, Smith MH, Senta-McMillian T, Soria I, Meng TC. Randomized, single-blind, placebo-controlled study of topical application of the immune response modulator resiquimod in healthy adults. Antimicrob Agents Chemother. 2003;47:3846–3852. doi: 10.1128/AAC.47.12.3846-3852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flacher V, Bouschbacher M, Verronese E, Massacrier C, Sisirak V, Berthier-Vergnes O, de Saint-Vis B, Caux C, Dezutter-Dambuyant C, Lebecque S, Valladeau J. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]