Abstract
Alcohol dependence constitutes a neuroadaptive state critical for understanding alcoholism, and various methods have been utilized to induce alcohol dependence in animals, one of which is alcohol vapor exposure. Alcohol vapor inhalation provides certain advantages over other chronic alcohol exposure procedures that share the ultimate goal of producing alcohol dependence in rats. Chronic alcohol vapor inhalation allows the experimenter to control the dose, duration, and pattern of alcohol exposure. Also, this procedure facilitates testing of somatic and motivational aspects of alcohol dependence. Chronic exposure to alcohol vapor produces increases in alcohol-drinking behavior, increases in anxiety-like behavior, and reward deficits in rats. Alcohol vapor inhalation as a laboratory protocol is flexible, and the parameters of this procedure can be adjusted to accommodate the specific aims of different experiments. This unit describes the options available to investigators using this procedure for dependence induction, when different options are more or less appropriate, and the implications of each.
Keywords: alcohol dependence, ethanol vapor, withdrawal, abstinence, blood-alcohol level, rat, inhalation
Introduction
A variety of experimental approaches have been developed to model alcohol dependence. Alcohol dependence constitutes a neuroadaptive state critical for understanding alcoholism, and various methods have been utilized to induce alcohol dependence in animals, one of which is alcohol vapor exposure. This approach allows for both experimenter control and effective induction of physical dependence in otherwise healthy animals over relatively short periods of time (weeks). The dose, duration, and pattern of exposure (i.e., oscillation of blood-alcohol levels) of alcohol vapors can be precisely controlled by the experimenter. Rats reliably exhibit signs of tolerance and physical dependence following chronic alcohol vapor exposure, which can be measured via a multitude of acute withdrawal– and protracted abstinence–related behaviors. The severity of symptoms associated with alcohol withdrawal increases with each subsequent episode of withdrawal from alcohol vapor exposure. Once animals have been trained to consume alcohol solution prior to vapor exposure, relapse susceptibility can also be measured during acute withdrawal (Roberts et al., 1996) and protracted abstinence (Roberts et al., 2000). Basic Protocol 1, described herein, presents an alcohol vapor inhalation procedure that reliably produces alcohol dependence in rats and allows for the study of a constellation of dependence-related somatic and motivational symptoms. It is worth noting that alcohol vapor inhalation has also been used to produce alcohol dependence in mice, but the procedural parameters and behavioral endpoints associated with this procedure differ across species. The expertise of the authors' laboratory is dependence induction via vapor inhalation in rats, but the following protocol can be used as a general guide for using the same procedure in mice. unit 9.26 also provides an overview of mouse models of alcohol intoxication.
Strategic Planning
Alcohol vapor inhalation systems are available commercially (e.g., La Jolla Alcohol Research Inc.). A typical system includes eight standard rat cages with custom-built lids that have gaskets, air fittings, water bottles, and J-feeders (Fig. 9.29.1). There are two (2) small holes in the lid to string an i.v. connector into the box (if desired). When not in use, these holes are sealed with a white threaded plastic screw with rubber stopper. Cages can hold multiple animals (based on AAALAC regulations); animals that are not cannulated can be normally group-housed, while rats implanted with intracranial cannulae or i.v. catheters can be double-housed with a divider in place to separate the animals. Dividers screw into the lid using two white threaded plastic screws. Lids are affixed to cages with eight metal clasps, and a gasket runs the perimeter of the lid to ensure that air does not enter or escape except through air and vacuum hoses. A thin layer of bedding should cover the floors of cages; excess bedding can adversely affect vapor exposure in animals.
Figure 9.29.1.
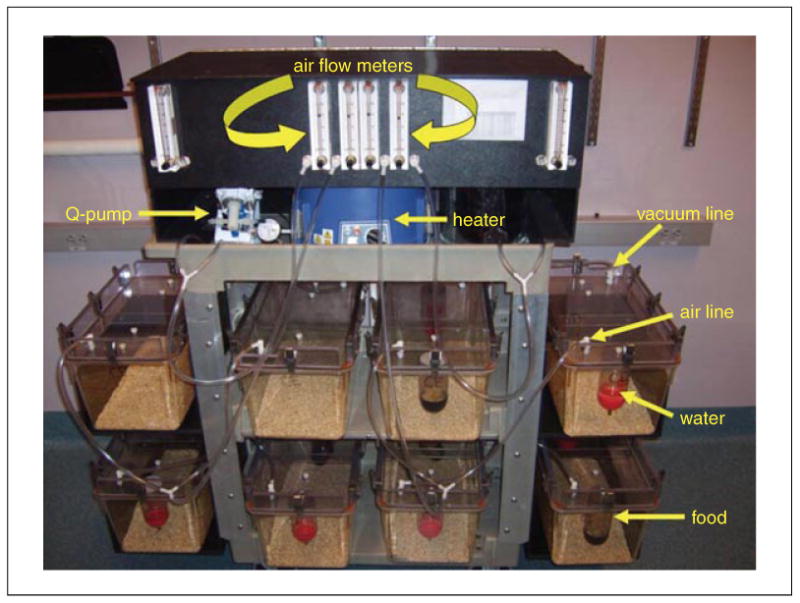
Frontal view of a typical vapor unit (La Jolla Alcohol Research, Inc.) with labeled components. As pictured, the unit is prepared to deliver ethanol vapor to all eight housing chambers. Alternatively, the unit can be used to deliver ethanol vapor to four housing chambers and ambient air to the other four housing chambers.
Other equipment in the alcohol vapor inhalation system (Fig. 9.29. 2) includes a bottle of 95% (v/v) ethanol (alcohol) that sits in an ethanol reservoir, a “Q”-pump (Fluid Metering, Inc.), a spherical flask that sits in a hemispherical EM series electromantle heater (Electrothermal Engineering Ltd.), air flow gauges (Series VF Visi-Float Flowmeter; Dwyer Instruments) that control the influx of ambient air into rat cages, air and vacuum tubes that push pure “house” air from the building into the system and pull ethanol vapor away from the system, as well as tubes between the various components listed above through which ethanol fluid and ethanol vapor travel, and a power strip to plug in the various electrical components of the system.
Figure 9.29.2.
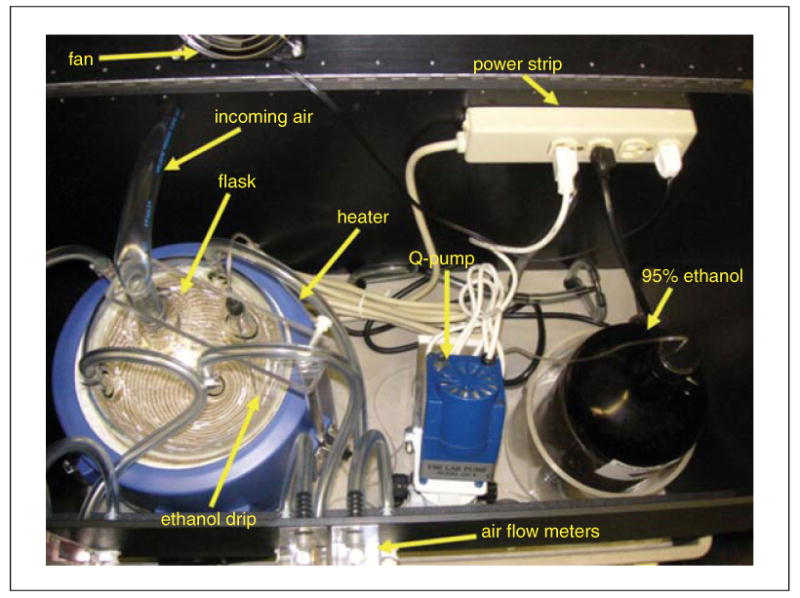
Aerial view of labeled components housed within the upper shelf of the unit. Ethanol is pulled from the 95% ethanol jug by the Q-pump, delivered and dripped into the heated flask, and evaporated there. The vapor is then delivered to housing chambers via plastic tubing and air flow meters.
Dependence Induction via Alcohol Vapor Inhalation
This section describes in detail the pre-exposure preparation of vapor inhalation chambers and animals, optimal exposure procedures for multiple experimental aims, and post-exposure maintenance of vapor inhalation chambers. Vapor inhalation produces alcohol dependence in rats regardless of strain, gender, or age. The age range used for juvenile exposure should be determined by the experimenter, but a reasonable range would be postnatal days 28 to 42. Exposure to alcohol vapor in adult rats typically begins at an age of at least 8 weeks.
Materials
Rats (e.g., Charles River or Harlan; for optimal age of rats see above)
95% (v/v) ethanol
Benzodiazepine
Reconstituted alcohol oxidase/buffer reagent
Alcohol vapor inhalation systems (e.g., La Jolla Alcohol Research Inc.; see Strategic Planning)
Animal scale
Razor blade to cut the tip of the tail (in animals that are not implanted with catheters) or 1-ml syringes to pull blood from i.v. catheters
Centrifuge tubes (heparinized if possible for better separation of blood and plasma)
High-speed centrifuge
Analox AM1 analyzer (Analox Instruments)
Pre-exposure preparation of chambers
1. Assemble the air and vacuum gauges and lines to facilitate linking multiple alcohol systems. Ensure that the main air and vacuum lines and gauges are securely connected to the air and vacuum lines for the vapor inhalation system(s). If multiple systems are linked together, it is important to use copper tubing that can easily connect to the pre-assembled line.
The vapor inhalation system has pre-assembled air pressure gauges for “house” air and vacuum hook-ups. The air pressure gauge controls the amount of air entering the system. The vacuum pressure gauge dilutes the air coming from the chambers to decrease the ethanol concentration of the air entering the building vacuum system.
2. Using the air and vacuum gauges, slowly open the main air line and main vacuum lines that lead into and out of the system. Set the main air line at 3 pounds per square inch (psi; this is a suggested starting point but house air should be set at the lowest level that produces desired air flow rate as denoted by liter per minute gauges) and the main vacuum line at 3 to 5 psi.
3. Set air flow gauges at ∼15 psi (again, this a suggested starting point but these settings can be adjusted; liters per minute on the air flow gauge and the ethanol concentration of air entering chambers are inversely proportional).
Air and vacuum lines must be attached to all eight cage lids to accurately set air flow gauges.
4. Place a one-gallon bottle of 95% (v/v) ethanol in the reservoir and secure the cap.
Do not use 100% (v/v) ethanol, as it is toxic to animals. Ethanol should be replaced frequently and kept in sealed containers to prevent its evaporation.
Never use ethanol that has been diluted with benzonite.
5. Choose appropriate pump settings for animal weight and target blood-alcohol levels (BALs; a general rubric should be provided by the pump manufacturer to guide this process).
6. Set the heater at level 3.
Never force heater past the stop point at level 3.
7. Ensure that pump, heater, and fan are plugged into the power strip.
Never plug the power strip in the vapor chamber unit into another external power strip due to increased risk of power failure.
8. For continuous exposure experiments, plug the power strip directly into an electrical outlet. For intermittent exposure experiments, plug the power strip into a timer (provided with unit) and plug the timer into an electrical outlet.
The timer forces the unit to turn on and off at different times during the day as determined by the experimenter.
9. Fill water and food reservoirs within each of the eight cages and securely attach dividers to cage lids, if desired (if animals are implanted with intracranial cannulae or i.v. catheters).
10. Place animals in cages and secure lids with the eight attached clamps.
Never turn off incoming house air with animals in the chambers.
Follow all AAALAC guidelines for animal numbers in individual chambers (defined by weight). Vapor inhalation systems should be designed and manufactured to AAALAC specifications. For example, three rats that each weigh ≤350 g may be housed together in a single standard chamber, but should be separated if one or all of the animals exceed this body weight.
11. Attach air and vacuum lines to the lids of each of the eight cages.
Pre-exposure preparation of animals
12. Weigh animals before the first exposure to vapor and intermittently thereafter (e.g., twice per week).
Body weight loss is an early indicator of adverse health consequences of overexposure. Also, animals of similar body weights should be placed together in cages so that BALs for the group can be more precisely controlled later.
13. If animals are implanted with i.v. catheters for blood collection, attach catheters to lines and thread lines through the chamber lid after removing the white, threaded plastic screw. Leave enough “play” in the lines to enable animals to move about freely, but make sure the animals are separated by a divider to avoid entanglement and/or chewing of lines.
14. Perform vapor exposure.
The first 24 hr. The protocol for the first 24 hr of vapor exposure will vary based on the following factors: target peak BAL range, target BAL ascent rate, projected total amount of time animals will be exposed to vapor, vapor exposure schedule (i.e., continuous versus intermittent), as well as the gender, age, weight, and genetic background of the animals. Table 9.29.1 illustrates representative vapor settings and resulting BALs in previously alcohol-naïve animals of various strains and body weights. These parameters will be used to determine vapor settings during the initial 24 hr of exposure; however, there are two guidelines that apply to almost all exposure scenarios to ensure the health of the animals:
The first rule is to gradually raise BALs in previously naïve animals; this means a gradual ascent of vapor exposure and BALs over the hours of the first day (i.e., raising ethanol exposure settings over the course of the day) and also over the first few days (i.e., raising ethanol exposure settings from one day to the next).
The second rule is to collect blood samples early and often to monitor BALs. For example, in a rapid ascent or continuous exposure scenario, blood should be collected multiple times across the first 24 hr of exposure and also during each of several subsequent days. Close monitoring of BALs allows for precise and frequent adjustments to ethanol exposure parameters. The major risk early in the vapor inhalation procedure is overexposure (i.e., excessive intoxication). Overexposure criteria will vary as target BAL ranges vary, but the behavioral states described in Table 9.29.2 can be used as a guide for how to identify overexposed rats.
Blood should be collected and BALs measured often during vapor inhalation to ensure intoxication and preserve the health of animals. Blood can be collected into microcentrifuge tubes from animals by making a cut 1 mm from the tip of the tail (the same wound is used for subsequent bleeds), from the retro-orbital cavity, or from intravenous catheters (see above). The time points for blood collection will be determined by the specific vapor exposure protocol, but a general rule is that animals exposed to continuous alcohol vapor must be monitored more closely (and BALs measured more frequently) than animals exposed to intermittent alcohol vapor, at least until it has been determined that BALs are within the target range (e.g., 150 to 225 mg/dl, but see Critical Parameters for more discussion of target BAL range determination). Tail blood samples might be collected from animals multiple times every 2 to 4 hr during the first day of exposure (depending on whether animals are eased into exposure or immediately exposed to high doses), and as little as once every 7 to 10 days once BALs have stabilized in long-term exposed animals. Following each BAL measurement, adjustments should be made to vapor settings as appropriate. In experiments involving behavioral testing or sacrifice/brain removal during withdrawal, BALs should also be measured at corresponding withdrawal time points on nontest days. Blood samples contained in microcentrifuge tubes are centrifuged to separate plasma serum from blood. Plasma serum is then refrigerated until BALs are determined with an alko-analyzer (AM1 series, Analox Instruments Ltd.). The alko-analyzer measures oxygen levels (proportional to ethanol concentration) during the oxidation of ethanol to acetaldehyde and hydrogen peroxide by alcohol oxidase. Reconstituted alcohol oxidase/buffer reagent is necessary to enable the enzymatic breakdown of ethanol by the Analox AM1 analyzer. The shelf-life of alcohol oxidase/buffer reagents is 6 months when unopened and stored at 0° to 5°C, and they can be stored ∼5 days after opening at 0° to 5°C. Fresh aliquots may be frozen for several weeks at −20°C or lower to extend the life of the alcohol oxidase/buffer reagent.
Table 9.29.1.
Representative Vapor System Settings and Resultant Mean BALs Across Hours for Wistar, Long-Evans, and Harlan Sprague-Dawley Ratsa
| Wistar | |||
|---|---|---|---|
| Weight (g) | Time exposed (hr) | Drip rate (drops/pulse) | Average BAL (mg/dl) |
| 200 | 1 | 11/12 | 56.1 |
| 2 | 11/12 | 127.0 | |
| 3 | 11/12 | 241.3 | |
| 4 | 11/12 | 296.9 | |
| 300 | 1 | 11/12 | 24.3 |
| 2 | 11/12 | 53.8 | |
| 3 | 11/12 | 78.2 | |
| 4 | 11/12 | 108.8 | |
| Long Evans | |||
|
| |||
| Weight (g) | Time exposed (hr) | Drip rate (drops/pulse) | Average BAL (mg/dl) |
|
| |||
| 200 | 1 | 11/12 | 43.6 |
| 2 | 11/12 | 85.6 | |
| 3 | 11/12 | 123.0 | |
| 4 | 11/12 | 177.1 | |
| 300 | 1 | 11/12 | 68.4 |
| 2 | 11/12 | 101.2 | |
| 3 | 11/12 | 181.7 | |
| 4 | 11/12 | 211.7 | |
| Sprague-Dawley | |||
|
| |||
| Weight (g) | Time exposed (hr) | Drip rate (drops/pulse) | Average BAL (mg/dl) |
|
| |||
| 200 | 1 | 11/12 | 39.1 |
| 2 | 11/12 | 85.7 | |
| 3 | 11/12 | 134.9 | |
| 4 | 11/12 | 194.1 | |
| 300 | 1 | 11/12 | 28.4 |
| 2 | 11/12 | 70.6 | |
| 3 | 11/12 | 121.6 | |
| 4 | 11/12 | 142.7 | |
All rats weighed between 200 and 300 g and were housed 3 per cage during vapor exposure. All rats were ethanol-naïve prior to vapor exposure. A drip rate of 10/11 in two consecutive bursts is equivalent to ∼ 108 ml ethanol/hr; a drip rate of 11/12 in two consecutive bursts is equivalent to ∼ 120 ml ethanol/hr. BALs ascend rapidly in naïve animals, but tend to ascend more slowly in animals with higher body weights.
Table 9.29.2.
General Guideline for BALs and Associated Behavioral Changes in Rats that can be Visually Observed During Alcohol Vapor Exposurea
| Ascending limb | Descending limb | |||
|---|---|---|---|---|
| BALs (mg%) | Behavior | BALs (mg%) | ||
| 0-200 | Normal mobility, staggered gait | Hyperexcitability, tremors, rigidity, convulsions | 150-0 | |
| 200-300 | Sedation | 300-150 | ||
| 300-400 | Ataxia | 400-300 | ||
| 400-500 | Loss of righting reflex | 500-400 | ||
| >500 | Coma and death | >500 | ||
Most behavioral signs are identical during the ascending (intoxication) and descending (detoxication) limbs of the BAL curve, with the exception of behaviors associated with lower BAL ranges. As BALs approach zero on the descending limb, behavioral signs indicative of alcohol withdrawal appear. Vapor exposure should be closely monitored and frequently adjusted for individual rats that exhibit these behaviors at radically different BAL ranges relative to other experimental animals.
Daily to-do list
The following is an example of a daily protocol for monitoring rats during exposure to ethanol vapor. Other protocols could be adjusted from this template according to specific experimental parameters and institutional procedures (e.g., the level of involvement of vivarium staff with care of vapor-exposed animals).
Early morning
15. Check and record maximum and minimum temperature and humidity in room.
16. Determine and record health of each animal by visibly observing behavior (see Table 9.29.2) and making sure it matches the target BAL range.
17. Check food amounts and water levels, refill as necessary.
18. If bedding is soaked through with water, replace bedding.
19. Ensure air flow meters for each cage are adjusted to correct settings.
20. Refill ethanol jug with 95% (v/v) ethanol if necessary.
21. Ensure that ethanol drip rate matches overnight Q-pump setting (Table 9.29.3).
Table 9.29.3.
Sample Chart Illustrating the Q-Pump Settings for Two Vapor Units and the Corresponding Ethanol Drip Rates and Ethanol Evaporation Rates
| Q-pump setting for vapor unit A | Q-Pump setting for vapor unit B | Ethanol drip ratea (number of drips in 2 consecutive bursts) | Ethanol evaporationb rate (ml/hr) |
|---|---|---|---|
| 1.45 | 1.50 | N/A | 44 |
| 1.65 | 1.70 | N/A | 68 |
| 1.95 | 1.95 | N/A | 76 |
| 2.20 | 2.23 | 9/9 | 84 |
| 2.43 | 2.50 | 10/10 | 100 |
| 2.65 | 2.75 | 11/11 | 108 |
| 2.95 | 3.00 | 11/12 | 120 |
| 3.20 | 3.25 | 12/13 | 132 |
Ethanol drip rates represent the number of ethanol drips that fall into the heated flask to be evaporated during two consecutive drip bursts. Ethanol drip bursts last ∼2 sec and are separated by ∼10-sec intervals.
Ethanol evaporation rates represent the volume of 95% (v/v) ethanol that is evaporated by the vapor unit per hour.
22. Increase flow rate to desired ml/hr drip rate and count drip rate again.
23. Switch locations of rats and adjust air flow meter settings according to health of individual rats.
24. If an individual animal is extremely intoxicated (see Table 9.29.2), remove the animal from the vapor unit, administer subcutaneous injection of 3 ml saline, and leave the animal out of vapor until it is able to right itself. Only then return the animal to the vapor unit.
25. If an animal exhibits severe withdrawal symptoms (e.g., in the form of seizures), treat the animal with a benzodiazepine (e.g., 2 to 16 mg/kg chlordiazepoxide) and/or return to alcohol vapor to relieve symptoms.
Afternoon
26. Determine and record health of each animal by visibly observing behavior (see Table 9.29.2) and making sure it matches the target BAL range.
27. Check food amounts and water levels and refill as necessary.
28. Ensure air flow meters for each cage are adjusted to correct settings.
29. Ensure that ethanol drip rate matches daytime Q-pump setting (Table 9.29.3).
Evening
30. Determine and record health of each animal by visibly observing behavior (see Table 9.29.2) and making sure it matches the target BAL range.
31. Check food amounts and water levels and refill as necessary.
32. Ensure air flow meters for each cage are adjusted to correct settings.
33. Ensure that ethanol drip rate matches daytime Q-pump setting (Table 9.29.3).
34. Adjust ethanol flow to desired overnight drip rate and count drip rate again.
Overnight drip rate should be conservative relative to daytime drip rate as it is likely that animals will not be closely monitored during that time.
35. Switch locations of animals and adjust air flow meter settings according to health of the animals in individual cages. As BAL information becomes available, house animals that are more resistant to the intoxicating effects of ethanol vapor together, and house less resistant animals together.
In this way, air flow rates to individual cages can be adjusted to administer slightly higher or lower doses to rats as needed.
Visual observations of intoxication
Short of constantly measuring BALs, behavioral observation is often sufficient to determine the status of animals during alcohol vapor exposure. Here are some general guidelines:
100 to 200 mg/dl BAL: rat has a staggering gait
200 to 300 mg/dl BAL: rat has trouble staying on its feet or cannot stand at all
300 to 400 mg/dl (or higher) BAL: rat is totally unresponsive.
Many of these symptoms often appear at lower BALs during the first several days of exposure. Majchrowicz (1975; unit 9.28) provides more detailed information on behavioral symptoms to expect from animals during chronic exposure to high alcohol doses. A summary of the behavioral signs associated with ascending (intoxication) and descending BALs (termed “prodromal detoxication” and “dependence signs” by Majchrowicz) is presented in Table 9.29.2. More discussion is also provided in the Critical Parameters section.
Animals that are unresponsive at the end of the exposure often lose a significant amount of body weight, and subsequent exposure rates for those animals should therefore be reduced. Some animals (usually <10% of all animals) may have exceptionally high or low BALs compared to the rest of the group (e.g., due to individual differences in alcohol metabolism capacity). Over- and under-exposure problems can often be resolved by some combination of the following actions: (1) moving cages to different lines, (2) re-pairing animals such that rats with low BALs are housed together and rats with high BALs are housed together, and (3) adjusting air flow gauge settings. Often, one line will deliver more or less ethanol vapor to the attached cage and produce higher or lower BALs in the animals housed in that cage relative to other lines. To minimize weight loss during alcohol vapor exposure, check water bottles often, and provide food in feeders and also on the cage floor when animals are housed in vapor chambers for extended periods of time.
Post-exposure operation of chambers
Turning the vapor inhalation system off
Once vapor exposure is complete in a group of animals and the system is going to be idle for any significant amount of time, the following steps should be followed when shutting the system down.
36. Turn off all electrical functions (heater, pump, and fan) if not on timer (each piece of equipment has an individual on/off switch).
37. Leave air running with air and vacuum hoses attached to cage lids for ∼1 hr to allow hoses and gauges time to completely dry.
Cleaning the vapor inhalation system
The following steps should be followed when cleaning the system.
38. Clean water reservoirs with a mixture of water and 3 to 4 ml of any washroom detergent to avoid mold growth in reservoir.
39. Machine-wash chambers in a dishwasher or tunnel washer.
40. Clean lids by spraying them with a washroom detergent and rinsing and wiping them thoroughly, being careful not to dampen the rubber seal around the edge of the lids.
Chamber lids should not be machine washed because this will compromise the rubber seal around the perimeter of the lids.
Data Analysis for Ethanol Vapor Inhalation Experiments
Typically, withdrawal scores are summated to yield a single datum (0 to 10) for each animal (see Basic Protocol 2). These sum withdrawal scores can be subsequently analyzed using mixed-design (dependence history × time) analyses of variance. These analyses allow for (1) comparison between dependent and nondependent animals (animals are scored for withdrawal by a blind observer but scores for nondependent animals should be negligible), and (2) comparison of withdrawal scores in dependent animals at different time points. Scores related to the specific symptoms and signs associated with alcohol withdrawal are typically presented in a descriptive fashion to illustrate the distribution of somatic disturbances over time.
BALs can be analyzed using mixed-design (dependence history × time) analyses of variance. These analyses allow for examination of changes in BALs of dependent animals over time (from day to day of vapor exposure, and also from hour to hour during vapor exposure or withdrawal). Again, BALs in control animals exposed to ambient air should be negligible throughout the experiment. BAL data from samples collected from dependent animals during alcohol withdrawal can also be correlated with somatic withdrawal scores at the same time points. Presumably, withdrawal scores will rise as BALs progressively decline toward zero.
Sample Protocol for Assessment of Physical Dependence in Rats following Termination of Ethanol Vapor Inhalation
Rats were removed from ethanol vapor inhalation units at 10:00 a.m. Behavioral testing, as described below, occurred at 2-hr intervals: 6, 8, 10, 12, 14, 16, and 24 hr following removal from vapor chambers. Therefore, testing occurred at ∼4:00 p.m., 6:00 p.m., 8:00 p.m., and 10:00 p.m. on the day of removal from vapor chambers and at 12:00 a.m., 2:00 a.m., and 10:00 a.m. on the following day. Tail blood samples were taken at 2-hr intervals: 6, 8, and 10 hr following removal from vapor chambers.
Lift rat by scruff of neck to assess ventromedial dorsal limb flexion (VMD), which is characterized by retraction of limbs toward body when rat is lifted into the air. Score 0 in the case that the behavior appears normal, 1 in the case that it appears moderately abnormal, and 2 in the case that it appears severely abnormal.
Place rat on its back in V-shaped trough and assess for righting reflex (RR). Measure the amount of time (in seconds) the rat requires to right itself such that at least three of the four legs are not visible from above. Maximum time allowed for rat to right itself = 30 sec. Once a rat successfully rights itself in <3 sec, this behavior will not be tested at the later time points.
-
Place rat on the floor of its cage and observe for a period of 2 min. Score each of the following 0, 1, or 2 based on the criteria described in step 1.
Assess abnormal body posture—broad-based gait, immobility, and slow or unsteady forward locomotion.
Assess tail stiffness—presence of rigid tail that extends parallel to the back of the rat or up toward the ceiling.
Assess hyperlocomotion—excessive running, vigorous escape attempts, spontaneous convulsions.
Assess other behaviors indicative of acute alcohol withdrawal in rats—these will include the presence of body/head/tail tremors, wet-dog shakes, chattering teeth, stereotyped repetitive behavior, aimless circling, and restless head turning.
Sum scores from steps 1 and 3 to determine a total withdrawal score ranging between 0 (no withdrawal signs) and 10 (severe withdrawal signs).
Commentary
Background Information
Why vapor inhalation as route of administration for alcohol dependence induction?
Methods of inducing alcohol dependence in animals include not only alcohol vapor inhalation, but also intragastric alcohol infusion (unit 9.28), alcohol-liquid diet, schedule-induced polydipsic alcohol drinking, single-bottle alcohol access, and long-term voluntary alcohol drinking. It is beyond the scope of this unit to thoroughly describe each of these methods of chronic alcohol exposure, but it is worth briefly mentioning the advantages and disadvantages of each.
Intragastric alcohol administration involves tube feeding high doses of alcohol solution in daily fractional doses (3 to 5 times/day) over consecutive days (Majchrowicz, 1975) and allows the experimenter to control the dose, duration, and pattern of alcohol exposure in animals. However, this procedure presents more difficulties in maintaining stable BALs over time, is more labor-intensive, may require surgery (e.g., in self-administration procedures; see Fidler et al., 2006), and animals are more likely to be rendered comatose and/or die of alcohol overdose.
In an alcohol-liquid diet procedure, the liquid diet is typically the sole source of calories available to rats (for example, see Moy et al., 1997), thereby forcing rats to consume the alcohol. Animals develop alcohol dependence via chronic consumption of liquid diet in the absence of significant health risks (Frye et al., 1981), but the major disadvantage of this procedure is individual variability (i.e., the dose, duration, and pattern of alcohol exposure bouts are determined by the animal). Also, animals generally consume fewer calories from alcohol-liquid diet than they otherwise would from solid lab chow, complicating interpretation of the data (E. Zorrilla, pers. comm.).
Single-bottle alcohol access procedures simply involve providing animals with ad libitum access to food and alcohol solution in the absence of any other fluids (Cicero et al., 1971). Rats are forced to consume the alcohol solution for survival; however, this procedure is criticized for the use of long-term water deprivation, it has not reliably produced alcohol dependence in genetically heterogeneous rats (for example, see Carey, 1972), and it has been largely abandoned as a method of dependence induction.
In schedule-induced polydipsia procedures, animals perform “normal” behaviors (e.g., cork-gnawing, wheel running, water or alcohol drinking) in excess during the interim periods between reinforcer deliveries. Schedule-induced alcohol polydipsia is therefore high alcohol drinking produced by the intermittent delivery schedule for another reinforcer, usually small amounts of food in food-deprived animals. This procedure produces somatic symptoms of alcohol dependence (Falk et al., 1972), but has been largely abandoned in recent years due to several methodological concerns, most prominently whether food-deprived animals are drinking alcohol strictly for its caloric value (Freed and Lester, 1970).
Long-term voluntary alcohol drinking provides a useful model for certain aspects of alcohol dependence (Spanagel and Hölter, 1999). Both genetically heterogeneous rats (Sinclair and Senter, 1967; Wolffgramm and Heyne, 1995; Hölter et al., 2000) and P rats (Waller et al., 1982; Lumeng and Li, 1986; Gatto et al., 1987; McKinzie et al., 1998; Kampov-Polevoy et al., 2000) can exhibit specific signs indicative of alcohol dependence following long-term alcohol drinking procedures.
There are several advantages of alcohol vapor inhalation (Rogers et al., 1979) over other methods of chronic forced alcohol administration. Primarily, alcohol vapor inhalation is a non-invasive procedure that allows for precise control of the dose, duration, and pattern of exposure (i.e., minimal oscillation of BALs) as determined by the experimenter, and is not limited by the predisposition of an animal to voluntarily consume alcohol. Secondly, stable BALs can be maintained for long periods of time in the presence of normal body weight regulation and general ingestive behavior. Third, animals are routinely not rendered comatose and they exhibit signs of tolerance and physical dependence upon termination of alcohol vapor exposure. Upon removal from chambers, otherwise healthy animals may be tested for a multitude of acute withdrawal– and protracted abstinence–related behaviors.
Critical Parameters and Troubleshooting
Choosing the appropriate exposure parameters for dependence induction
Table 9.29.4 displays the parameters and endpoints of two published studies that used alcohol vapor inhalation to produce dependence in rats. This table provides a general idea of how one study might differ from the next according to specific experimental aims, or even when the aims of the two studies are quite similar.
Table 9.29.4.
Comparison of Two Studies Published that used Alcohol Vapor Inhalation Proceduresa
| Published study | Roberts et al., 2000b | O'Dell et al., 2004 |
| Exposure pattern | Continuous (24 hr/day) | Intermittent (14 hr/day) |
| BAL target range | 150-200 mg% | 150-200 mg% when vapor on 0 mg% when vapor off |
| Exposure duration before start of testing | 2-4 weeks | 2-4 weeks |
| Rat gender | Male | Male |
| Rat strain | Wistar | Wistar |
| Body weight at start of experiment | 180-200 g | 180-200 g |
| Dependent variable (behavioral endpoint) | Self-administration of alcohol | Self-administration of alcohol and saccharin |
| Time points for behavioral testing | 0 hr withdrawal to 8 weeks abstinence | 2-8 hr withdrawal |
This table displays the parameters and endpoints of two different experiments.
This study utilized a continuous vapor exposure procedure to examine dependence-induced elevations in alcohol-drinking behavior.
Chronic intermittent versus chronic continuous alcohol exposure
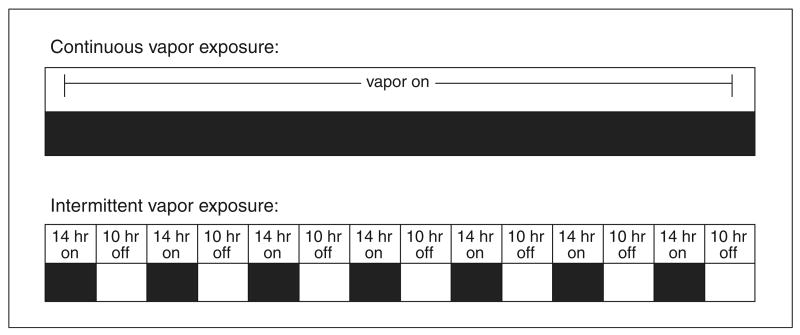
In order to develop alcohol dependence, animals must be chronically exposed to high doses of alcohol vapor, but the pattern of this exposure is left to the discretion of the experimenter according to the experimental objectives of the study. The two patterns of alcohol exposure most often used to induce alcohol dependence in animals are chronic intermittent and chronic continuous alcohol exposure (Fig. 9.29.3). Procedures that utilize intermittent exposure schedules are newer and may provide a superior alternative to continuous exposure schedules by accelerating dependence induction and producing exaggerated relapse behavior (O'Dell et al., 2004).
Figure 9.29.3.
Sample protocol for continuous (upper panel) and chronic intermittent (lower panel) ethanol vapor exposure. In continuous ethanol exposure protocols, ethanol vapor delivery never terminates. In chronic intermittent ethanol exposure protocols, vapor is activated a certain number of hours per day (e.g., 14 hr) and terminated for a certain number of hours per day (e.g., 10 hr).
Chronic intermittent ethanol (CIE) exposure describes procedures in which animals undergo cycles of alcohol exposure and alcohol abstinence over long periods of time. Animals exposed to CIE vapor exhibit elevations in voluntary alcohol consumption relative to chronic continuous alcohol vapor–exposed controls equalized for exposure time (O'Dell et al., 2004). This intermittent procedure typically entails daily cycles of alcohol vapor exposure (12 to 17 hr) and alcohol withdrawal (7 to 12 hr; see Tables 9.29.1 and 9.29.4). CIE procedures accelerate the development of behavioral alterations indicative of alcohol dependence. For example, genetically heterogeneous rats exhibit increases in alcohol self-administration after only 2 weeks of chronic intermittent alcohol vapor exposure (O'Dell et al., 2004), whereas a continuous procedure only produces such elevations in alcohol intake following 4 to 6 weeks of alcohol vapor exposure (Schulteis et al., 1995; Roberts et al., 1999). In animals not previously trained to self-administer alcohol, chronic intermittent alcohol vapor exposure also produces increases in initiation and maintenance of alcohol self-administration (Rimondini et al., 2002) following seven weeks of exposure (Rimondini et al., 2003). A logistical advantage of chronic intermittent alcohol vapor exposure is the facilitation of acute withdrawal testing and within-subjects designs since animals can be tested during daily withdrawal periods as BALs approach zero, and then returned to alcohol vapor chambers for the next exposure period.
Duration of alcohol vapor exposure
The temporal parameters used in studies of alcohol dependence are often determined by the method employed to induce dependence. For example, animals show signs of alcohol dependence in as few as 2 to 3 weeks using CIE vapor inhalation (O'Dell et al., 2004), but this threshold vapor exposure time doubles in chronic continuous vapor procedures (Schulteis et al., 1995; Roberts et al., 1999). The number and length of abstinence periods in intermittent alcohol-exposed groups is usually also determined by the dependence induction method used, the latter of which can range from hours to weeks. Specifically, CIE vapor inhalation procedures include daily abstinence periods of ∼7 to 12 hr (O'Dell et al., 2004; Funk et al., 2007), while chronic continuous vapor inhalation procedures are often used to examine behavior further into abstinence (e.g., Valdez et al., 2002); other dependence-induction procedures often incorporate abstinence periods on the order of days and weeks (e.g., Overstreet et al., 2002). A final consideration is whether to equalize exposure and abstinence times (i.e., 12 hr on/12 hr off CIE; 2 weeks on/2 weeks off continuous vapor). Many of these parameters are determined in part by the dependent variable of interest, such that acute withdrawal-related behaviors lend themselves more to procedures that incorporate daily cycles of alcohol exposure and withdrawal, whereas protracted abstinence–related behaviors require longer periods with and without alcohol availability. Measurable acute withdrawal– and protracted abstinence–related behaviors are described in detail below in the Dependent Variables section.
Choosing the target blood-alcohol level (BAL) range
Target BALs vary across experiments but there are two major concerns when choosing this range. The first major concern is the health of the animals. Obviously, higher BALs present increased risk of health complications and mortality. However, for most studies, BALs can be maintained in a range (150 to 225 mg/dl) that reliably produces alcohol dependence in the absence of these increased risks. The second major determining factor in choosing a target BAL range is the set of desired parameters for subsequent testing. The somatic and motivational aspects of withdrawal are typically measured as BALs approach zero. Higher BALs during alcohol vapor exposure require longer periods of time for alcohol to be eliminated from blood and largely determine when dependence-induced behavioral testing will be conducted.
Confirming achievement of dependence
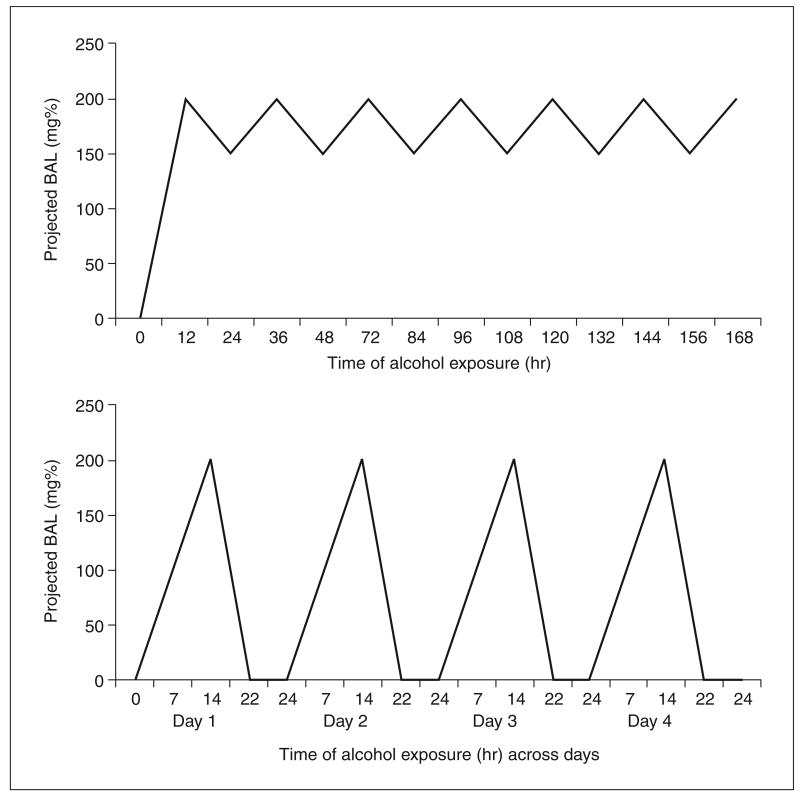
Experiments that employ dependence models can and must be validated by (1) tracking BALs during the induction of alcohol dependence (Fig. 9.29.4) and (2) testing for symptoms of dependence following termination of alcohol exposure.
Figure 9.29.4.
Sample BAL target ranges for continuous (upper panel) and chronic intermittent (lower panel) ethanol vapor exposure protocols. In continuous ethanol exposure protocols BALs are gradually increased to the target range and then oscillated between the upper and lower bounds of that range (e.g., 150 to 200 mg%). In chronic intermittent ethanol exposure protocols, BALs are increased daily to the target level (e.g., 200 mg%) and subsequently allowed to return to zero within the same 24-hr period; this procedure is repeated daily.
Dependent variables measured following chronic alcohol vapor exposure
Somatic aspects of alcohol dependence
Physical aspects of alcohol dependence usually manifest during acute withdrawal from chronic alcohol exposure and dissipate within the first 48 hr without alcohol. These symptoms are similar across species, and in rats include, in order of ascending withdrawal severity: general hyperactivity, tail spasticity/tremors, general spasticity/tremors, head tremors, wet shakes and teeth chattering, and spontaneous tonic-clonic convulsions (Majchrowicz, 1975). The physical symptoms observed during withdrawal are most appropriately regarded as an index of the presence and severity of alcohol dependence (for an example of a withdrawal scoring sheet, see Table 9.29.5), but physical withdrawal symptoms are not effective in motivating subsequent alcohol-seeking behavior (Meisch and Stewart, 1994).
Table 9.29.5.
Rubric Used to Assign Scores During Assessment of Physical Dependence in Vapor-Abstinent Wistar Rats Following Removal from First Week of Ethanol Vapor Inhalation
| Rat withdrawal scores | |||||||
| Coded rat ID: | Actual rat ID: | ||||||
| Body weight: | |||||||
| Time to regain righting reflex (maximum 30 sec) | |||||||
| Time of day | 6 hr | 8 hr | 10 hr | 12 hr | 14 hr | 16 hr | 24 hr |
| Time to regain RR (sec) | |||||||
| Withdrawal behavior ratings (score between 0 and 2) | |||||||
| Time of day | 6 hr | 8 hr | 10 hr | 12 hr | 14 hr | 16 hr | 24 hr |
| VMD | |||||||
| Body posture | |||||||
| Tail stiffness | |||||||
| Hyperlocomotion | |||||||
| Tremor/stereotyped behavior | |||||||
| Total score (0-10) | |||||||
| Blood-alcohol level (mg%) | |||||||
| Time of day | 6 hr | 8 hr | 10 hr | 12 hr | 14 hr | 16 hr | |
| BAL score (mg%) | |||||||
| NOTES:____________________________________________________________________________ | |||||||
It is not difficult to produce physical withdrawal symptoms in rats made dependent on alcohol via vapor inhalation. Continuous and intermittent vapor exposure procedures are both effective in producing these symptoms, although the severity of withdrawal symptoms increases with repeated episodes of withdrawal from alcohol vapor exposure (i.e., intermittent vapor exposure).
Motivational aspects of alcohol dependence
Motivational aspects of alcohol dependence also manifest during acute withdrawal from chronic alcohol exposure, but these symptoms endure throughout protracted abstinence from alcohol. Anxiety is often listed as a symptom in both the acute withdrawal and protracted abstinence syndromes and is rated by alcoholics as an important precipitating factor in relapse to alcohol. Voluntary alcohol consumption has anxiolytic effects in rats (Gallate et al., 2003) and in humans (Hershon, 1977). Likewise, increased anxiety and sensitized anxiety responses to external stressors persist in rats for many weeks following the end of chronic alcohol exposure as measured by the elevated plus-maze (Baldwin et al., 1991; Lal et al., 1991; Rassnick et al., 1993; Gatch et al., 1999; Rasmussen et al., 2001; Valdez et al., 2002, 2003; Cagetti et al., 2004). Another important factor in relapse to alcohol drinking is “craving” (i.e., shifts in reinforcing properties of alcohol) produced by abstinence from alcohol (Voltaire-Carlsson et al., 1996). Although it is beyond the scope of this unit to review these models in detail, it should be noted that there are three behavioral endpoints thought to reflect craving for alcohol in its absence: (1) the alcohol deprivation effect, (2) cue-induced resistance to extinction and cue-induced reinstatement of operant alcohol responding, and (3) protracted elevations in alcohol responding (for review of alcohol craving models, see Koob, 2000). Common to the procedures that produce all of these endpoints is the hypothesis that observed relapse behavior should be excessive in alcohol-dependent animals relative to nonde-pendent animals.
Motivational symptoms associated with alcohol dependence are more difficult to observe reliably than somatic symptoms in animal models. Both chronic continuous and intermittent vapor exposure produce increases in alcohol-drinking behavior in rats, and this effect is augmented by multiple withdrawals in both procedures (Roberts et al., 1996, 2000; O'Dell et al., 2004). However, for experiments that aim to measure alcohol dependence-induced drinking, it is extremely important that BALs be maintained within a target range between 125 and 250 mg%. BALs below this range are not sufficient to produce motivational aspects of alcohol dependence, whereas BALs above this range produce health complications and/or kindle withdrawal symptoms that inhibit observation and interpretation of behavior thought to reflect those aspects of dependence. When animals are exposed to extended periods of chronic alcohol vapor (e.g., >8 weeks), BALs should be maintained on the lower end of this range to ensure the health of subjects.
Potential problems and other considerations
More often than not, health problems in animals exposed to chronic alcohol vapor are more accurately symptoms of dependence that manifest regardless of the route of alcohol administration used, and these complications usually fall into two categories. Excessive intoxication due to overexposure can be easily resolved by lowering vapor settings to produce lower BALs in animals; animals that are extremely intoxicated should be removed from vapor chambers, given soft food, and rehydrated with saline injections until they recover motor coordination and the ability to feed and drink normally. Extreme physical disturbances and seizures associated with alcohol withdrawal are literally a defining characteristic of alcohol dependence. However, certain steps should be taken in cases in which the survival of the animal is threatened. Excessive physical withdrawal should be treated acutely by administration of benzodiazepines. Animals enduring severe physical withdrawal can also be immediately re-exposed to alcohol vapor to elevate BALs until these symptoms subside; animals can then be gradually (over a period of days) weaned off of alcohol vapor to avoid unwanted seizure activity. When these disturbances are accompanied by hypothermia (animal is cold to the touch), animal cages should be placed on heating pads until animals have recovered the ability to thermoregulate (presumably several hours following initial intervention).
Another issue to consider occurs when alcohol vapor–exposed animals do not gain body weight at the same rate as nondependent controls. This phenomenon is particularly characteristic of the early phases (first week or less) of vapor exposure before animals develop functional tolerance to alcohol. This effect of vapor on body weight gain can be minimized in intermittent procedures by coordinating the alcohol exposure phase with the inactive (light) phase of the light/dark cycle in rats, since animals are far less active during that period and consume much more food and water during the active (dark) phase of this cycle. Complications in consummatory data produced by body weight differences between groups can usually be resolved by expressing alcohol intake in milliliter or gram ethanol per kilogram body weight. Experimenters should also be mindful of potential differences in alcohol metabolism between different strains and lines of animals, animals of different body ages and body weights (e.g., juvenile versus adult vapor exposure), and animals with different exposure histories (e.g., total duration of exposure, continuous versus intermittent exposure). Parameters used during initiation and maintenance of alcohol vapor exposure will be largely determined by these factors (see Tables 9.29.1 and 9.29.4).
Almost all problems encountered during chronic alcohol vapor exposure are the products of human error. Therefore, it is extremely important that alcohol vapor inhalation systems and animals housed therein be monitored closely and frequently for the duration of the exposure period. Animals should never be placed in the vapor system for extended periods of time if adequate monitoring is not going to be possible at least several times per day for the duration of the exposure. It is also helpful for multiple people to be knowledgeable in operation of the vapor systems such that mistakes by one person can be corrected by another.
“Always” and “never” lists
The following are lists of things to always and never do when operating ethanol vapor inhalation chambers:
Always
make sure all connectors/hoses/clamps are tightly secured.
open air gauges slowly.
check amount of ethanol remaining (consider adding a “fill to” line in the ethanol jug).
maintain “house” air at lowest possible setting that produces desired air flow (∼15 psi).
have air flow gauges on front of system set at a minimum of 10 psi.
allow spherical flask to cool for 15 to 20 min by running air only.
Never
leave heater on when not in use.
set heater higher than level 3.
have system in operation without air running.
leave animals in chamber without air running.
operate system without connecting all cages (even if some cages are empty).
Anticipated Results
Alcohol self-administration behavior
A common goal of chronic alcohol vapor exposure in rats is to examine the increased prevalence of behaviors reflective of the motivational aspects of alcohol dependence. One of the major dependent variables included in this domain is alcohol self-administration behavior (i.e., susceptibility to relapse). A detailed description of these animal models is beyond the scope of this unit, but behavioral endpoints indicative of this pathology are defined by increased alcohol-seeking and alcohol-drinking behaviors by alcohol-dependent animals.
Parameters of studies examining alcohol self-administration/relapse behavior vary widely and at least in part determine, and are determined by, vapor parameters employed in a particular experiment. These parameters include, but are not limited to, duration of access (continuous versus limited access) and self-administration procedure (two-bottle choice versus operant). An important consideration in studies of dependence-induced drinking (produced by vapor and otherwise) is to analyze alcohol consumption in terms of intake per body weight (e.g., gram ethanol/kilogram body weight) since dependent animals typically weigh slightly less than nondependent animals following long-term exposure to high doses of alcohol. Other self-administration paradigms of interest include progressive ratio testing to determine shifts in the reinforcement value of alcohol in dependent animals, and reinstatement testing to examine whether dependent animals exhibit heightened sensitivity to reinstatement of alcohol-seeking behavior elicited by alcohol-paired cues.
Affective disturbances
Other animal models assess emotionality (e.g., anxiety-like behavior and reward reduction) by taking advantage of the natural ecology of rodent behavior. Animal models of anxiety-like behavior can be used to examine the effects of alcohol dependence as well as the combined effects of alcohol dependence and external stressors/alcohol-conditioned cues on anxiety-like behavior (Kliethermes, 2005). Generally, these tests aim to measure behaviors indicative of anxiety-like states. The procedures most commonly used to assess anxiety-like behavior in alcohol-dependent animals are the elevated plus-maze, light-dark box, open field test, and social interaction test (unit 8.3). The conflict test (i.e., the Geller-Seifter conflict test or Vogel's conflict test; unit 8.3) has also been used to assess tolerance to the anxiolytic effects of alcohol. In general, alcohol-dependent rats exhibit increases in anxiety-like behavior early in alcohol withdrawal and well into protracted abstinence from alcohol (Kliethermes, 2005).
Reward deficits can be defined as a state marked by the decreased ability of an organism to perceive the reinforcing properties of stimuli. Rodent models of reward deficits following chronic exposure to high alcohol doses include intracranial self-stimulation (ICSS) and consumption of natural reinforcers (e.g., sucrose). For example, rats exhibit increases in ICSS current thresholds (Schulteis et al., 1995) and decreases in operant responding for natural reinforcers (Slawecki, 2006) following termination of chronic alcohol vapor exposure, results thought to reflect decreased function of brain reward systems.
Time Considerations
The typical amount of time required to produce alcohol dependence in animals, as defined by the manifestation of withdrawal symptoms upon termination of vapor exposure, is ∼4 weeks. However, this duration is highly variable and largely determined by the many vapor exposure parameters chosen by the experimenter. Typically, vapor exposure continues throughout the course of behavioral testing and therefore, the final duration of cumulative vapor exposure will be determined by the length of that testing period and the experimental design in general.
Acknowledgments
The authors thank Mike Arends for his excellent editorial assistance. This is manuscript number 19073 from The Scripps Research Institute. Supported by the Pearson Center for Alcoholism and Addiction Research and NIAAA grants AA06420, AA08459, and AA12602.
Footnotes
Note: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
Disclaimer: Maury Cole has a commercial interest in La Jolla Alcohol Research, Inc. and is also an employee of The Scripps Research Institute. The other authors have no apparent conflicts of interest.
Literature Cited
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology. 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Baicy KJ, Olsen RW. Topiramate attenuates withdrawal signs after chronic intermittent ethanol in rats. Neuroreport. 2004;15:207–210. doi: 10.1097/00001756-200401190-00040. [DOI] [PubMed] [Google Scholar]
- Carey RJ. A decrease in ethanol preference in rats resulting from forced ethanol drinking under fluid deprivation. Physiol Behav. 1972;8:373–375. doi: 10.1016/0031-9384(72)90385-x. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Snider SR, Perez VJ, Swanson LW. Physical dependence on and tolerance to alcohol in the rat. Physiol Behav. 1971;6:191–198. doi: 10.1016/0031-9384(71)90088-6. [DOI] [PubMed] [Google Scholar]
- Falk JL, Samson HH, Winger G. Behavioral maintenance of high concentrations of blood ethanol and physical dependence in the rat. Science. 1972;177:811–813. doi: 10.1126/science.177.4051.811. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Clews TW, Cunningham CL. Reestablishing an intragastric ethanol self-infusion model in rats. Alcohol Clin Exp Res. 2006;30:414–428. doi: 10.1111/j.1530-0277.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- Freed EX, Lester D. Schedule-induced consumption of alcohol: Calories or chemotherapy? Physiol Behav. 1970;5:555–560. doi: 10.1016/0031-9384(70)90080-6. [DOI] [PubMed] [Google Scholar]
- Frye GD, Chapin RE, Vogel RA, Mailman RB, Kilts CD, Mueller RA, Breese GR. Effects of acute and chronic 1,3-butanediol treatment on central nervous system function: A comparison with ethanol. J Pharmacol Exp Ther. 1981;216:306–314. [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. CRF1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiat. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallate JE, Morley KC, Ambermoon P, McGregor IS. The consequences of beer consumption in rats: Acute anxiolytic and ataxic effects and withdrawal-induced anxiety. Psychopharmacology. 2003;166:51–60. doi: 10.1007/s00213-002-1291-z. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Wallis CJ, Lal H. Effects of NMDA antagonists on ethanol-withdrawal induced “anxiety” in the elevated plus maze. Alcohol. 1999;19:207–211. doi: 10.1016/s0741-8329(99)00045-2. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Murphy JM, Waller MB, McBride WJ, Lumeng L, Li TK. Chronic ethanol tolerance through free-choice drinking in the P line of alcohol-preferring rats. Pharmacol Biochem Behav. 1987;28:111–115. doi: 10.1016/0091-3057(87)90021-9. [DOI] [PubMed] [Google Scholar]
- Hershon HI. Alcohol withdrawal symptoms and drinking behavior. J Stud Alcohol. 1977;38:953–971. doi: 10.15288/jsa.1977.38.953. [DOI] [PubMed] [Google Scholar]
- Hölter SM, Linthorst ACE, Reul JMHM, Spanagel R. Withdrawal symptoms in a long-term model of voluntary alcohol drinking in Wistar rats. Pharmacol Biochem Behav. 2000;66:143–151. doi: 10.1016/s0091-3057(00)00196-9. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: Changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–284. [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Koob GF. Animal models of craving for ethanol. Addiction. 2000;95:S73–S81. doi: 10.1080/09652140050111663. [DOI] [PubMed] [Google Scholar]
- Lal H, Prather PL, Rezazadeh SM. Anxiogenic behavior in rats during acute and protracted ethanol withdrawal: Reversal by buspirone. Alcohol. 1991;8:467–471. doi: 10.1016/s0741-8329(91)90153-n. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Li TK. The development of metabolic tolerance in the alcohol-preferring P rats: Comparison of forced and free-choice drinking of ethanol. Pharmacol Biochem Behav. 1986;25:1013–1020. doi: 10.1016/0091-3057(86)90079-1. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li TK. The alcohol deprivation effect in the alcohol-preferring P rat under free-access and operant access conditions. Alcohol Clin Exp Res. 1998;22:1170–1176. [PubMed] [Google Scholar]
- Meisch RA, Stewart RB. Ethanol as a reinforcer: A review of laboratory studies of non-human primates. Behav Pharmacol. 1994;5:425–440. [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Criswell HE, Breese GR. Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology. 1997;131:354–360. doi: 10.1007/s002130050303. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25:999–1005. [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdale reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: Behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol. 2003;64:445–449. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Koob GF. Operant self-administration of sweetened versus unsweetened ethanol: Effects on blood alcohol levels. Alcohol Clin Exp Res. 1999;23:1151–1157. [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: Animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: Advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Increased preference for ethanol in rats following alcohol deprivation. Psychon Sci. 1967;8:11. [Google Scholar]
- Slawecki CJ. Two-choice reaction time performance in Sprague-Dawley rats exposed to alcohol during adolescence or adulthood. Behav Pharmacol. 2006;17:605–614. doi: 10.1097/01.fbp.0000236272.10418.62. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Hölter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: An animal model of alcoholism? Alcohol Alcoholism. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute withdrawal and protracted abstinence: Regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Voltaire-Carlsson A, Hiltunen AJ, Koechling UM, Borg S. Effects of long-term abstinence on psychological functioning: a prospective longitudinal analysis comparing alcohol-dependent patients and healthy volunteers. Alcohol. 1996;13:415–421. doi: 10.1016/0741-8329(96)81678-8. [DOI] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Lumeng L, Li TK. Induction of dependence on ethanol by free-choice drinking in alcohol-preferring rats. Pharmacol Biochem Behav. 1982;16:501–507. doi: 10.1016/0091-3057(82)90459-2. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. From controlled drug intake to loss of control: The irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]