Abstract
Sperm competition occurs when 2 or more males copulate with a particular female during the same reproductive cycle, and their sperm compete to fertilize the female's available eggs. One strategy that male voles use to assess the risk and intensity of sperm competition involves responding to the presence of scent marks of conspecific males found near a sexually receptive female. Previously, we have shown that if a male vole copulated with a female while he was in the presence of the odors of another male he increased his sperm investment relative to his investment if another male's odors were not present. The aim of the present study was to test the hypothesis that males assess differences in the relative quality of competing males and adjust their sperm investment accordingly. We did so by allowing males to copulate when they were exposed to the scent mark of a 24-h food-deprived male (low-quality male) or the scent mark of a male that was not food deprived (high-quality male). The data indicate that male meadow voles did not increase their sperm investment during copulation when exposed to the scent mark of a food-deprived male but did so when they were exposed to the scent mark of a male that was not food deprived. The results support the hypothesis that male voles are able to adjust sperm investment when they encounter the scent marks of males that differ in quality.
Keywords: chemical signals, copulatory behavior, food deprivation, scent marking, sperm competition, voles
Sperm competition occurs when 2 or more males copulate with a particular female during the same reproductive cycle, and their sperm compete to fertilize the female's available eggs (Smith 1984; Birkhead and Møller 1998; Birkhead 2000; Simmons 2001). There are more than 95% of mammalian species that show some degree of promiscuity (Kleiman 1977), and sperm competition has been found to be prevalent in mammals (Ginsberg and Huck 1989; Gomendio et al. 1998). The frequent occurrence of sperm competition may have forced males to develop different strategies to reduce the risk of displacement of their own sperm by competing males and to displace or overcome the sperm of competing males (Huck et al. 1985). One strategy for overcoming the sperm of other males is by adjusting the amount of sperm allocated to the ejaculate (Parker et al. 1996; Williams et al. 2005). Males may increase their sperm investment in response to the risk of sperm competition (Parker et al. 1996) as shown by the bush cricket, Kawanaphila nartee (Simmons and Kvarnemo 1997), the house cricket and the decorated cricket, Acheta domesticus and Gryllodes supplicans (Gage and Barnard 1996), the white butterfly, Pieris rapae (Wedell and Cook 1999), the bitterling, Rhodeus sericeus (Candolin and Reynolds 2002; Smith et al. 2003), the black goby and sneaker males of the grass goby, Gobius niger and Zosterisessor ophiocephalus (Pilastro et al. 2002), territorial gobies (Scaggiante et al. 2005), parental bluegill sunfish, Lepomis macrochirus (Neff et al. 2003), Norway rats, Rattus norvegicus (Pound and Gage 2004), and meadow voles, Microtus pennsylvanicus (delBarco-Trillo and Ferkin 2004, 2006a). Alternatively, males may not adjust sperm investment as the risk of sperm competition increases as described in a species of cricket, Gryllus texensis (Schaus and Sakaluk 2001), and the quacking frog, Crinia georgiana (Byrne 2004). Finally, male house mice, Mus musculus domesticus may reduce their sperm investment if the risk of sperm competition increases (Ramm and Stockley 2007).
During the breeding season, male meadow voles occupy large home ranges that encompass the territories of one or more females. Females inhabit mutually exclusive territories (Madison 1980). Male and female meadow voles are promiscuous, and most interactions between opposite-sex conspecifics are limited to mating attempts (Madison 1980; Boonstra et al. 1993). Despite the high frequency of encounters between males and females, encounters between same-sex conspecifics, particularly between males, are less frequent (Madison 1980). Male–male agonism is not common (Ferkin and Seamon 1987) and when it occurs males do not establish dominance hierarchies (Ferkin 2007). Thus, male voles do not directly restrict other males from having access to sexually receptive female voles, and therefore, the incidence of sperm competition is likely to be high (Dewsbury 1981; Boonstra et al. 1993; Berteaux et al. 1999). Consequently, male voles are likely to have developed physiological, morphological, and/or behavioral strategies to confront the normal occurrence of sperm competition (Dewsbury 1981; Boonstra et al. 1993).
One strategy that male voles use to allocate sperm during copulation is to assess the risk and intensity of sperm competition by the presence of scent marks of conspecific males found near a sexually receptive female, which may be a good estimate of the number of males that will copulate with that female (Salo and Dewsbury 1995). Our recent work has supported and expanded this hypothesis by showing that if a male meadow vole is paired with a female vole and both are exposed to the odor of a male conspecific, the copulating male will increase his sperm investment by over 116% (delBarco-Trillo and Ferkin 2004). A male vole's sperm investment, however, does not rise as high if he is exposed to the scent marks of several males (delBarco-Trillo and Ferkin 2006a), suggesting that male voles are able to assess differences in the number of potential mates near a receptive female. Interestingly, the male did not alter his sexual behavior (delBarco-Trillo and Ferkin 2004, 2006a, 2006b, 2006c, 2007) as has been shown in other animals (Stockley and Preston 2004). Given that male meadow voles adjust their sperm investment during mating when exposed to the scent marks of other males, it begs the question as to whether they adjust their sperm investment based on the information contained in the scent marks of competing males. For example, do males adjust their sperm investment if they encounter the scent marks of males that differ in some feature of their quality?
The aim of the present experiment was to determine whether males assess differences in the relative quality of competing males and adjust their sperm investment accordingly. We selected males that were not food deprived and males that were food deprived as odor donors to represent differences in their relative quality and resultant risk of sperm competition. Recent work has reported that food-deprived male voles may be of “lower quality” relative to males that were not food deprived (Pierce and Ferkin 2005). First, food-deprived males produced odors that were less attractive to sexually receptive females than those of males that were not food deprived. Next, food-deprived males spent less time than males that were not food deprived investigating the odors of receptive females. Lastly, food-deprived males engaged in coitus fewer times than males that were not food deprived when paired with a sexually receptive female conspecific (Pierce and Ferkin 2005; Pierce et al. 2005). Thus, males that are food deprived may produce odors or scent marks that are associated with a decreased risk of sperm competition, whereas odors or scent marks from males that were not food deprived may represent a higher risk of sperm competition. If so, a prediction of the hypothesis is that a copulating male will increase his sperm investment if he encounters the scent mark of a male conspecific that was not food deprived for 24 h but will not increase his sperm investment if he encounters the scent mark of a male that was food deprived for 24 h. Such a finding would suggest that males are able to adjust their sperm investment when they encounter males that represent different risks of sperm competition.
METHODS
Animals
The meadow voles used in this study were offspring of field-caught animals, all of which were born and raised at The University of Memphis in a room that was controlled for temperature and on a 14:10 h light:dark cycle to simulate day length during breeding season. Meadow voles are weaned at 19 days of age and kept with littermates until they are 34 days old. They are then housed singly in clear polycarbonate cages (27 × 16.5 × 12.5 cm). Cages contain hardwood shaving as bedding and cotton for nesting material. Food and water are provided ad libitum (except for odor donors in the food-deprived condition, as explained below).
Treatment groups
In all, 36 male and 36 female meadow voles were used in this study, with 12 different males and 12 different females used in each sperm competition treatment group. This resulted in 36 pairs of voles being used in the experiment. Adult male meadow voles copulated with sexually receptive females in one of 3 groups that only differed in the type of scent mark the copulating male was exposed to during the trial. In one group (n = 12 male–female pairs), we paired a female and a male vole that mated in the presence of no scent marks from a conspecific male; this group represented the control condition (CONTROL). In the control condition, water was used instead of a scent mark. In the second group (n = 12 male–female pairs), we paired a male and female in the presence of the scent mark of a male that was food deprived (FD-M) for 24 h. As mentioned earlier, this group represents the scent marks of males considered to be of lower quality relative to the copulating male. In the third group (n = 12 male–female pairs), we paired a female and male vole in the presence of the scent mark of a male that was not food deprived for 24 h; this male scent donor had continuous access to food (1M). This group is similar to that described in delBarco-Trillo and Ferkin (2004, 2006a), in that it represents the scent marks of males considered to be of similar quality to the copulating male.
Testing procedure
We used control (fresh water) and fresh male scent marks for each male–female pairing using methods detailed elsewhere (Ferkin et al. 1999; Pierce et al. 2005). Briefly, in the control condition, fresh distilled water was placed on a sterile cotton applicator and rubbed for 5 s on the center portion of a clean glass microscope slide (7.5 × 2.5 cm). In the food-deprived (FD-M) and non–food-deprived conditions (1M), the anogenital area of the male scent donor was rubbed against the center portion of a clean glass slide for 5 s. The resulting scent marks from the male donors and the water marks were roughly the same size, approximately 1.2 × 0.3 cm (l × w). We used a single slide for each pairing. A different male's scent mark was used in each trial, and each donor was only used once (n = 12 FD-M donors and n = 12 1M donors). None of the male scent donors were familiar or related to the copulating male. However, all male scent donors and copulating males were similar in age (between 6 and 9 months old), weight (within 8 g), and sexual experience (having previously sired a litter).
Immediately after the scent mark slide was prepared, we placed a female vole into the testing cage (37 × 21 × 15 cm). The female voles were injected with 0.05 mg of estradiol 60 h prior to pairing to increase the chance that the females would be receptive and mate (delBarco-Trillo and Ferkin 2004). Five minutes after the female was placed in the cage, we placed a glass slide containing a scent mark of a male donor or the control into the cage. The slide was suspended 2 cm above the substrate by a clean metal clip and hook. Five minutes after the slide was placed into the cage, we placed the subject male into the cage. We allowed these males to mate until sexual satiety, which is 30 min without any intromission (Gray and Dewsbury 1975; delBarco-Trillo and Ferkin 2004).
We recorded copulatory behavior of voles using methods similar to those detailed elsewhere (delBarco-Trillo and Ferkin 2004). Briefly, copulatory behavior of voles was recorded using a video camcorder connected to a VCR recorder. We later scored the tapes to determine the total number of ejaculations, the latency to first ejaculation, and the mean ejaculation interval. The latency to first ejaculation was the amount of time (seconds) from the start of the trial to the first ejaculation. The mean ejaculation interval was the average amount of time (seconds) between each ejaculation. The methods for scoring these 2 variables are similar but not exactly the same as was seen in an earlier article examining copulatory behavior in meadow voles (delBarco-Trillo and Ferkin 2007). The scorers of the videotapes were blind to the treatment group of the voles.
Immediately after the male reached sexual satiety, he was removed from the cage and returned to his home cage, the glass slide was discarded, and the female was removed from the cage and euthanized using an overdose of isoflurane vapors. The female reproductive tract was removed, opened, and all the semen diluted in 25 ml of distilled water as detailed in delBarco-Trillo and Ferkin (2004, 2006a). The solution was gently homogenized. Four sperm counts were conducted using an improved Neubauer hemocytometer. The average of the 4 sperm counts was used to estimate the total number of sperm ejaculated by the male or his sperm investment (delBarco-Trillo and Ferkin 2004, 2006a). The sperm counter was blind to the treatment group being tested.
Statistical analyses
The experimental design of this study is more similar to that of delBarco-Trillo and Ferkin (2006a) than it is to the earlier delBarco-Trillo and Ferkin (2004) in that we do not use a “within-animal” design in the current study. This was due to the difficulty of obtaining 3 successful trials with the same male. Generally, not using a within-animal design may be a problem in this type of study if there is much unexplained variation among males (Pound and Gage 2004). However, previous work has shown that much of the variation in sperm investment of male voles is explained by male body size (delBarco-Trillo and Ferkin 2004) and therefore may be controlled by incorporating male body size in the statistical analyses as a covariate.
It has been previously reported that sperm investment is significantly correlated with male body weight (delBarco-Trillo and Ferkin 2004). Therefore, we used an analysis of covariance (ANCOVA) to control for the effect of male body weight on sperm investment (delBarco-Trillo and Ferkin 2006a). The grouping variable was treatment group (CONTROL, 1M, and FD-M), and the covariate was male body weight. Before running the ANCOVA, we tested whether the assumption of homogeneity of regression was met using a Kolmogorov–Smirnov test. Levene's homogeneity of variance test was used to test the assumption of homoscedasticity. We used ANCOVA, the covariate being male body weight, with a Fisher's least significant difference adjustment for the pairwise comparisons (delBarco-Trillo and Ferkin 2006a). Statistical analyses were performed using SPSS 16 for Windows. Differences were considered significant at P < 0.05. We also used one-way analysis of variance (ANOVA) to determine whether males in the different treatment groups had different numbers of ejaculations, latencies to first ejaculation, and mean ejaculation intervals. The independent variable was treatment group (CONTROL, 1M, and FD-M). The dependent variable was the number of ejaculations, latency to first ejaculation, or the mean ejaculation interval.
RESULTS
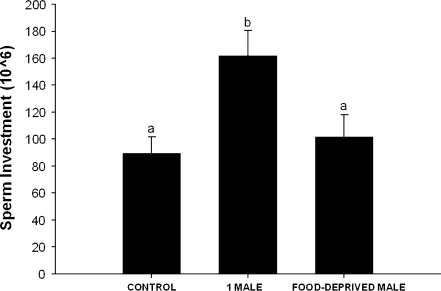
We found significant differences in sperm investment between the 3 groups (ANCOVA: F2,32 = 6.213, P = 0.005; Figure 1). Sperm investment was lowest in the CONTROL group, which was statistically similar to the FD-M group (F1,32 = 0.028, P = 0.868). The highest sperm investment was in the 1M group (Figure 1). A significant difference was found between the CONTROL and 1M groups, with the 1M males having a significantly higher sperm investment (F1,32 = 9.79, P = 0.005). There was also a significant difference between the FD-M and 1M groups, with the 1M males again investing more sperm (F1,32 = 5.827, P = 0.025). Although we controlled for body size of males, a subsequent analysis revealed that it did not affect sperm investment in male voles. The ANOVA results also showed a difference between the 3 groups F2,33 = 5.984, P = 0.006. The Tukey post hocs also showed a similar result; there was a significant difference between the CONTROL and the 1M groups and also between the 1M group and the FD-M group (both comparisons, P < 0.05).
Figure 1.
The mean + standard error of the mean sperm investment of copulating males exposed to a clean glass slide (control), a glass slide containing the scent mark of an unrelated, unfamiliar male conspecific (1M), and a glass slide containing the scent mark of an unrelated, unfamiliar male conspecific that was food deprived for 24 h (FD-M). Histograms capped with different letters are significantly different at P < 0.05.
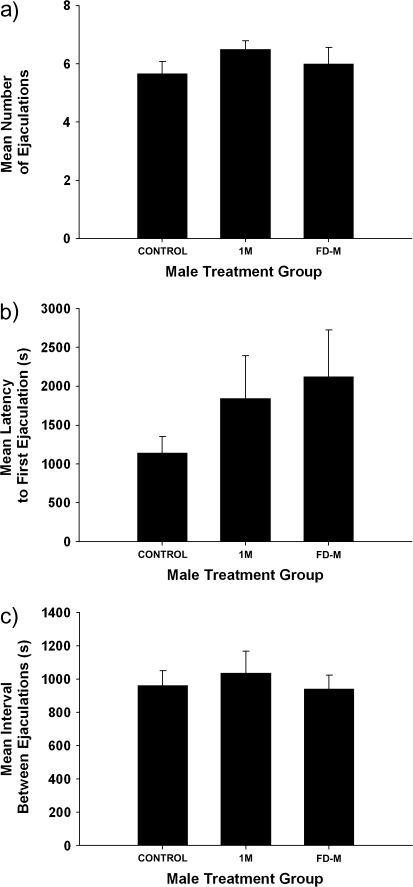
We found that different risks of sperm competition did not affect aspects of the copulatory behavior of male voles. There was not a significant difference among the 3 different treatment groups in the number of ejaculations (6.03 ± 0.36 ejaculations; F2,33 = 0.771, P = 0.471; Figure 2a), latency to first ejaculation (1704.7 ± 453.1 s; F2,33 = 1.095, P = 0.347; Figure 2b), and mean ejaculation interval (979.6 ± 100.9 s; F2,33 = 0.238, P = 0.790; Figure 2c). Typically, male and female voles completed their mating bouts within 40 min–3.5 h of being paired.
Figure 2.
The mean + standard error of the mean number of (a) ejaculations by males, (b) latency (seconds) to first ejaculation, and (c) mean interval (seconds) between ejaculations by males exposed to a clean glass slide (control), a glass slide containing the scent mark of an unrelated, unfamiliar male conspecific (1M), and a glass slide containing the scent mark of an unrelated, unfamiliar male conspecific that was food deprived for 24 h (FD-M). There were no significant differences between the groups of males.
DISCUSSION
Differences in male quality were established by selecting male voles that were not food deprived or that were food deprived for 24 h prior to testing. Previous work has shown that food-deprived male voles may be of “lower quality” relative to males that were not food deprived. Briefly, male voles that were food deprived for 24 h produced odors that were less attractive to females, spent less time investigating the odors of receptive females, and were less likely to copulate than males that were not food deprived (Pierce et al. 2005). Our results show that males are able to adjust their sperm investment when they encounter the scent marks of males that were not food deprived for 24 h but do not increase their sperm investment during copulation when they are exposed to the scent mark of a male that was food deprived for 24 h. Indeed, sperm investment was similar in the presence of the scent mark of a food-deprived male and in the absence of any scent marks from male conspecifics. These findings suggest that food-deprived males may represent a reduced risk of sperm competition relative to males that were not food deprived. Our results are consistent with those of previous studies showing that sperm investment of a copulating male mammal will increase if he encounters the scent marks of a conspecific male of similar relative quality, which represents a stronger risk of sperm competition (delBarco-Trillo and Ferkin 2004, 2006a; Pound and Gage 2004). Males also increase their sperm investment when the risk of sperm competition is high as seen in the white butterfly (Wedell and Cook 1999), the house cricket and the decorated cricket (Gage and Barnard 1996), and the black goby and sneaker males of the grass goby (Pilastro et al. 2002). More importantly, our study extends the hypothesis that male mammals can assess the risk and intensity of sperm competition (delBarco-Trillo and Ferkin 2004, 2006a; Pound and Gage 2004) by showing that male mammals can assess the relative quality of nearby males and use the information found in their scent marks to adjust their own sperm investment.
Our present findings and those from previous studies demonstrate that male voles can allocate different amounts of sperm when they encounter males that represent different relative risks of sperm competition (this study, delBarco-Trillo and Ferkin 2004, 2006a). The ability to adjust sperm investment depending on both the relative risk of sperm competition and the intensity of sperm competition may be a strategy employed by males to use sperm prudently (Parker 1970; Dewsbury 1982; Dewsbury and Sawrey 1984; Parker et al. 1996). If there are multiple competitors, then the likelihood of siring the offspring of a particular female will decrease. The ability to adjust sperm investment may be an advantage to individuals in species characterized by a promiscuous mating system (Birkhead 2000), a social system where male mammals visit the territories of females that likely contain the scent marks of males that are able to represent different relative risks of sperm competition (Madison 1980; Boonstra et al. 1993; Ferkin and Pierce 2007), a high incidence of sperm competition (Dewsbury and Sawrey 1984; Gomendio et al. 1998; Berteaux et al. 1999), and an environment containing variable food availability (Getz et al. 2001). It is worth mentioning that multiple mating may occur in other species of voles, including those species that have mating systems characterized by either polygyny or monogamy (Wolff and Dunlap 2002; Klemme et al. 2006). It would be interesting to know if males in these species make similar sperm allocation adjustments when they encounter the scent marks of conspecific males.
Male meadow voles did not adjust aspects of their copulatory behavior when they were exposed to males that represent different risks of sperm competition. This result is interesting because males in many other species do adjust copulatory behavior according to risk of sperm competition. Much evidence suggests that when faced with a high risk of sperm competition males alter their copulatory behavior in such a way as to increase the likelihood that they will fertilize the female's eggs (Stockley and Preston 2004). In rats, it has been found that increasing the intromission length leads to more vaginal stimulation of the female (Adler and Toner 1986). It may also cause a reduction in female receptivity, which may reduce the future risk of a male competitor mating with that particular female (Hardy and DeBold 1972; Stockley and Preston 2004). Roof rats, Rattus rattus, and montane voles, Microtus montanus, have been found to decrease the latency to copulate when there is a perceived risk of sperm competition (Shapiro and Dewsbury 1986; Estep 1988). In contrast, our results showed that for male meadow voles the number of ejaculations, the latency to first ejaculation, and the mean ejaculation interval did not differ significantly across treatment conditions. Similar results have also been reported in other experiments on meadow voles, showing that males exposed to different risks and intensities of sperm competition do not alter their copulatory behavior (delBarco-Trillo and Ferkin 2004, 2006a, 2007). For male meadow voles, it appears that the number of ejaculations and other aspects of copulatory behavior in a mating bout may be somewhat fixed. The lack of change in the copulatory behavior of male voles in the face of different risks of sperm competition may provide males with benefits that outweigh the costs. Male and female meadow voles are promiscuous and can mate with multiple partners during a breeding event (Boonstra et al. 1993; Berteaux et al. 1999). To increase the likelihood of reproductive success, males must provide females, which are induced ovulators (Milligan 1982), with sufficient vaginal stimulation during coitus to ensure she ovulates and he must provide sufficient sperm to increase his chances of getting the female pregnant (Gray and Dewsbury 1975; Seabloom 1985; Bakker and Baum 2000). If there are too few intromissions and ejaculations, the female may not ovulate and become pregnant. If the numbers of intromissions and subsequent ejaculations are sufficient to allow a female to become pregnant, males may not need to increase the number of ejaculations they have with a particular female, especially if by doing so, he reduces the likelihood that he can impregnate additional females. As seems to be the case for meadow voles, a better strategy than modifying the number of ejaculations that males have during a copulatory bout with a female may be to adjust the number of sperm per ejaculation. This adjustment of sperm investment, especially during the first ejaculations, may account for the uncertainty of whether a male meadow vole will be able to complete a full mating bout with a given female (delBarco-Trillo and Ferkin 2006a, 2006c, 2007).
FUNDING
National Science Foundation (grant IOB 04553); National Institutes of Health (grant HDO 49525 to M.H.F.).
Supplementary Material
Acknowledgments
We thank Dr Jeremy Field and the 2 anonymous reviewers for their comments. This research adhered to the Animal Behavior Society Guidelines for the Use of Animals in Research. All procedures involving voles were approved by the Institutional Animal Care and Use Committee of The University of Memphis.
References
- Adler NT, Toner JP., Jr The effects of copulatory behavior on sperm transport and fertility in rats. Ann N Y Acad Sci. 1986;474:21–32. doi: 10.1111/j.1749-6632.1986.tb27995.x. [DOI] [PubMed] [Google Scholar]
- Bakker J, Baum MJ. Neuroendocrine regulation of GnRH release in induced ovulators. Front Neuroendocrinol. 2000;21:220–262. doi: 10.1006/frne.2000.0198. [DOI] [PubMed] [Google Scholar]
- Berteaux D, Bety J, Rengifo E, Bergeron J. Multiple paternity in meadow voles (Microtus pennsylvanicus): investigating the role of the female. Behav Ecol Sociobiol. 1999;45:283–291. [Google Scholar]
- Birkhead TR. Promiscuity: an evolutionary history of sperm competition. Cambridge: Harvard University Press; 2000. [Google Scholar]
- Birkhead TR, Møller AP. Sperm competition and sexual selection. San Diego (CA): Academic Press; 1998. [Google Scholar]
- Boonstra R, Xia X, Pavone L. Mating system of the meadow vole, Microtus pennsylvanicus. Behav Ecol. 1993;4:83–89. [Google Scholar]
- Byrne PG. Male sperm expenditure under sperm competition risk and intensity in quacking frogs. Behav Ecol. 2004;15:857–863. [Google Scholar]
- Candolin U, Reynolds JD. Adjustments of ejaculation rates in response to risk of sperm competition in a fish, the bitterling (Rhodeus sericeus) Proc R Soc Lond B Biol Sci. 2002;269:1549–1553. doi: 10.1098/rspb.2002.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- delBarco-Trillo J, Ferkin MH. Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature. 2004;431:446–449. doi: 10.1038/nature02845. [DOI] [PubMed] [Google Scholar]
- delBarco-Trillo J, Ferkin MH. Male meadow voles respond differently to risk and intensity of sperm competition. Behav Ecol. 2006a;17:581–585. [Google Scholar]
- delBarco-Trillo J, Ferkin MH. Similarities between female meadow voles mating during post-partum oestrus and raising two concurrent litters and females raising only one litter. Reprod Fertil Dev. 2006b;18:751–756. doi: 10.1071/rd06004. [DOI] [PubMed] [Google Scholar]
- delBarco-Trillo J, Ferkin MH. Female meadow voles, Microtus pennsylvanicus, cause their mates to ejaculate outside their reproductive tract. Behaviour. 2006c;143:1425–1437. [Google Scholar]
- delBarco-Trillo J, Ferkin MH. Risk of sperm competition does not influence copulatory behavior in the promiscuous meadow vole (Microtus pennsylvanicus) J Ethol. 2007;25:139–145. [Google Scholar]
- Dewsbury DA. An exercise in the prediction of monogamy in the field from laboratory data on 42 species of muroid rodents. Biologist. 1981;63:138–162. [Google Scholar]
- Dewsbury DA. Ejaculate cost and male choice. Am Nat. 1982;119:601–610. [Google Scholar]
- Dewsbury DA, Sawrey DK. Male capacity as related to sperm production, pregnancy initiation, and sperm competition in deer mice (Peromyscus manicualtus) Behav Ecol Sociobiol. 1984;16:37–47. [Google Scholar]
- Estep DQ. Copulations by other males shorten the post-ejaculatory intervals of pairs of roof rats, Rattus rattus. Anim Behav. 1988;36:299–300. [Google Scholar]
- Ferkin MH. Effects of previous interactions and sex on over-marking in meadow voles. Behaviour. 2007;144:1297–1313. [Google Scholar]
- Ferkin MH, Pierce AP. Perspectives on over-marking: is it good to be on top? J Ethol. 2007;25:107–116. [Google Scholar]
- Ferkin MH, Seamon JO. Odor preference and social behavior in meadow voles, Microtus pennsylvanicus: seasonal differences. Can J Zool. 1987;65:2931–2937. [Google Scholar]
- Ferkin MH, Dunsavage J, Johnston RE. What kind of information do meadow voles, Microtus pennsylvanicus, use to distinguish between the top and bottom scent of an over-mark? J Comp Psychol. 1999;113:43–51. [Google Scholar]
- Gage AR, Barnard CJ. Male crickets increase sperm number in relation to competition and female size. Behav Ecol Sociobiol. 1996;38:349–353. [Google Scholar]
- Getz LL, Hofmann JE, McGuire B, Dolan TW., III Twenty-five years of population fluctuations of Microtus ochrogaster and M. pennsylvanicus in three habitats in east-central Illinois. J Mammal. 2001;82:22–34. [Google Scholar]
- Ginsberg JR, Huck UW. Sperm competition in mammals. Trends Ecol Evol. 1989;4:74–79. doi: 10.1016/0169-5347(89)90152-3. [DOI] [PubMed] [Google Scholar]
- Gomendio M, Harcourt AH, Roldan ERS. Sperm competition in mammals. In: Birkhead TR, Møller AP, editors. Sperm competition and sexual selection. London: Academic Press; 1998. pp. 667–751. [Google Scholar]
- Gray GD, Dewsbury DA. A quantitative description of the copulatory behaviour of meadow voles (Microtus pennsylvanicus) Anim Behav. 1975;23:261–267. [Google Scholar]
- Hardy DF, DeBold JF. Effects of coital stimulation upon behavior of the female rat. J Comp Physiol Psychol. 1972;78:400–408. doi: 10.1037/h0032536. [DOI] [PubMed] [Google Scholar]
- Huck UW, Quinn RP, Lisk RD. Determinants of mating success in the golden hamster (Mesocricetus auratus) IV. Sperm competition. Behav Ecol Sociobiol. 1985;17:239–252. [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Klemme I, Eccard JA, Ylönen H. Do female bank voles (Clethrionomys glareolus) mate multiply to improve on previous mates? Behav Ecol Sociobiol. 2006;60:415–421. [Google Scholar]
- Madison DM. An integrated view of the social biology of Microtus pennsylvanicus. Biologist. 1980;62:20–33. [Google Scholar]
- Milligan SR. Induced ovulation in mammals. In: Finn CA, editor. Oxford reviews of reproductive biology. Vol. 4. London: Clarendon Press; 1982. pp. 1–46. [Google Scholar]
- Neff BD, Peng F, Gross MR. Sperm investment and alternative mating tactics in bluegill sunfish (Lepomis macrochirus) Behav Ecol. 2003;14:634–641. [Google Scholar]
- Parker GA. Sperm competition and its evolutionary consequences in insects. Biol Rev. 1970;45:525–567. [Google Scholar]
- Parker GA, Ball MA, Stockley P, Gage MJG. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc R Soc Lond B Biol Sci. 1996;263:1291–1297. [Google Scholar]
- Pierce AA, Ferkin MH. Re-feeding and restoration of odor attractivity, odor preferences, and sexual receptivity in food-deprived female meadow voles. Physiol Behav. 2005;84:553–561. doi: 10.1016/j.physbeh.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Pierce AA, Ferkin MH, Williams TK. Food-deprivation-induced changes in sexual behavior of meadow voles, Microtus pennsylvanicus. Anim Behav. 2005;70:339–348. [Google Scholar]
- Pilastro A, Scaggiante M, Rasotto M. Individual adjustment of sperm expenditure accords with sperm competition theory. Proc Natl Acad Sci USA. 2002;99:9913–9915. doi: 10.1073/pnas.152133499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound N, Gage MJG. Prudent sperm allocation in Norway rats, Rattus norvegicus: a mammalian model of adaptive ejaculate adjustment. Anim Behav. 2004;68:819–823. [Google Scholar]
- Ramm SA, Stockley P. Ejaculate allocation under varying sperm competition risk in the house mouse, Mus musculus domesticus. Behav Ecol. 2007;18:491–495. [Google Scholar]
- Salo AL, Dewsbury DA. Three experiments on mate choice in meadow voles (Microtus pennsylvanicus) J Comp Psychol. 1995;109:42–46. doi: 10.1037/0735-7036.109.1.42. [DOI] [PubMed] [Google Scholar]
- Scaggiante M, Raostto MB, Romualdi C, Pilastro A. Territorial male gobies respond aggressively to sneakers but do not adjust their sperm expenditure. Behav Ecol. 2005;16:1001–1007. [Google Scholar]
- Schaus JM, Sakaluk SK. Ejaculate expenditures of male crickets in response to varying risk and intensity of sperm competition: not all species play games. Behav Ecol. 2001;12:740–745. [Google Scholar]
- Seabloom RW. Endocrinology. In: Tamarin RH, editor. Biology of New World Microtus. Special Publication No.8. Lawrence (KS): the American Society of Mammalogists; 1985. pp. 685–724. [Google Scholar]
- Shapiro LE, Dewsbury DA. Male dominance, female choice and male copulatory behavior in two species of voles (Microtus ochrogaster and Microtus montanus) Behav Ecol Sociobiol. 1986;18:267–274. [Google Scholar]
- Simmons LW. Sperm competition and its evolutionary consequences in the insects. Princeton: Princeton University Press; 2001. [Google Scholar]
- Simmons LW, Kvarnemo C. Ejaculate expenditure by male bushcrickets decreases with sperm competition intensity. Proc R Soc Lond B Biol Sci. 1997;264:1203–1208. [Google Scholar]
- Smith C, Reichard M, Jurajda P. Assessment of sperm competition by European bitterling, Rhodeus sericeus. Behav Ecol Sociobiol. 2003;53:206–213. [Google Scholar]
- Smith RL. Sperm competition and the evolution of animal mating systems. Orlando (FL): Academic Press; 1984. [Google Scholar]
- Stockley P, Preston BT. Sperm competition and diversity in rodent copulatory behaviour. J Evol Biol. 2004;17:1048–1057. doi: 10.1111/j.1420-9101.2004.00742.x. [DOI] [PubMed] [Google Scholar]
- Wedell N, Cook PA. Butterflies tailor their ejaculate in response to sperm competition risk and intensity. Proc R Soc Lond B Biol Sci. 1999;266:1033–1039. [Google Scholar]
- Williams PD, Day T, Cameron E. The evolution of sperm-allocation strategies and the degree of sperm competition. Evolution. 2005;59:492–499. [PubMed] [Google Scholar]
- Wolff JO, Dunlap AS. Multi-male mating, probability of conception, and litter size in the prairie voles (Microtus ochrogaster) Behav Processes. 2002;58:105–110. doi: 10.1016/s0376-6357(02)00022-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.