Abstract
Unraveling the function and evolutionary history of multimodal signaling is a difficult, yet common task of much research in animal communication. Here, I investigated multimodal signal function in the visual and seismic courtship display of the wolf spider Schizocosa stridulans and found that only the seismic courtship signal was important for mating success. First, copulation frequency was assessed in the presence/absence of both visual and seismic courtship signals. The seismic signal was sufficient for successful copulation, whereas the visual signal was neither necessary nor sufficient, suggesting that the signals are not redundant and do not function as backups. Next, female receptivity to video courtship sequences with altered male ornamentation was assessed in the presence of a live male's seismic signal. Female receptivity did not depend on male foreleg ornamentation. Instead, females performed receptivity displays equally to all video stimuli, demonstrating that in the presence of seismic signaling, receptivity is independent of visual signaling—indicating seismic signal dominance. Finally, female responses to isolated seismic cues from crickets and courting males suggest that seismic courtship signals carry both location and identification information. Schizocosa stridulans represents one of the few examples in which a single component likely dominates a multimodal signal.
Keywords: communication, complex signal, female choice, increased detection, intersignal interaction, video playback
Communication via complex signals predominates in many animal displays, and understanding their evolution and function has become a major goal of research focused across disparate taxonomic groups (for reviews, see Partan and Marler 1999, 2005; Candolin 2003; Hebets and Papaj 2005). Complex signals incorporate not only animal displays that combine multiple components within a single signaling modality but also those that combine components or signals across multiple sensory modalities—multimodal signals (Guilford and Dawkins 1991; Partan and Marler 1999; Rowe and Guilford 1999; Narins et al. 2003). The prevalence of multimodal signaling in animal communication is intriguing and hypotheses of multimodal signal function range from those focused only on the information content of signals to those focused on variability in the signaling environment and/or the sensory and processing system of receivers to those focused on the ways in which the signals interact (for reviews, see Candolin 2003; Hebets and Papaj 2005).
Given the diversity of hypotheses relating to multimodal signaling, a reasonable starting point for unraveling function examines receiver responses to isolated unimodal signal components as compared with multimodal composite signals. For example, multimodal signals are often classified as redundant or nonredundant, and signal redundancy can be inferred from equivalent receiver responses to multiple isolated signal components (see Partan and Marler 1999, 2005). Given signal redundancy, more explicit hypotheses of function can then be tested. For example, redundant signals can function to increase the accuracy of a receiver's response or to overcome multiple sensory environments and/or signaling constraints (for overview, see Candolin 2003; Hebets and Papaj 2005). In contrast, nonredundant components are predicted to have different effects when displayed in isolation (Partan and Marler 2005). Nonredundant signals can also function in a variety of content-based and/or efficacy-based ways, or they can interact (see Hebets and Papaj 2005).
Some of the most interesting functions of multimodal signaling involve interactions among nonredundant signals. For example, one signal component can alert a receiver to a second signal or can act as an amplifier, reducing the time to signal detection (see “Increased detection and discrimination” and references therein Hebets and Papaj 2005). In sticklebacks, chemical cues are suggested to alert females to the presence of a mature male before they are in visual proximity (McLennan 2003), and the advertisement calls of the ranid frog Staurois guttatus have recently been shown to direct a receiver's attention to a subsequent visual signal (Grafe and Wanger 2007). In such circumstances, receivers are not likely to respond to the amplifying or alerting signal in isolation. Thus, when attempting to dissect apart the function of such a multimodal signal, responses are compared between the action-causing signal in isolation and the composite signal. If the response to the action-causing signal is equivalent when displayed in isolation as compared with jointly, the multimodal display is considered an example of dominance—a multimodal signal classification suggested to be rare (see Partan and Marler 1999, 2005). Most known nonredundant multimodal signal examples involve modulation, where the response intensity of one isolated signal component is increased or decreased in response to the composite multimodal signal (see Partan and Marler 1999, 2005). Nonetheless, few studies have incorporated experimental designs capable of detecting signal dominance (but see Hebets 2005; Partan et al. 2005).
Unraveling the function and evolutionary history of multimodal signals is unquestionably a difficult and complicated task, requiring both taxonomic groups amenable to experimentation/manipulation and the employment of numerous experimental techniques. As such, the wolf spider genus Schizocosa provides an unmatched opportunity to explore the evolution and function of multimodal signaling. Of the 23 described Nearctic species, there is tremendous variation in the presence/absence and extent of ornamentation on the forelegs of mature males (Stratton 2005). In addition, whereas all species studied use seismic courtship signaling, some ornamented species also produce visual leg-waving signals (for review, see Stratton 2005). A recent phylogenetic analysis suggests that male foreleg ornamentation evolved 5 or 6 times independently and was subsequently lost 2 or 3 times (Stratton 2005). Thus, among closely related species, we find variation in the extent to which males and females rely on visual and/or seismic courtship signals (McClintock and Uetz 1996; Scheffer et al. 1996; Hebets and Uetz 1999, 2000; Stratton 2005; Hebets et al. 2006). In addition, Schizocosa wolf spiders are extremely amenable to techniques ideal for studying multimodal communication such as signal isolation experiments and video playbacks (see Uetz and Roberts 2002).
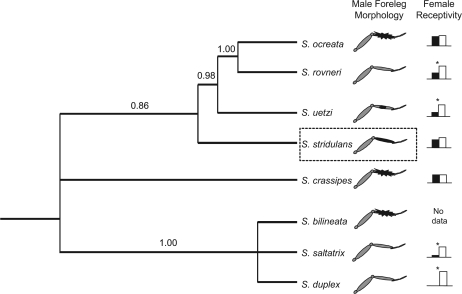
The species of interest, Schizocosa stridulans, belongs to the Schizocosa ocreata clade, within which many of the ornamented Schizocosa species are concentrated (Stratton 2005). Mature male S. stridulans possess dark pigmentation on approximately one half to one-third of their foreleg femora in addition to their entire foreleg patellae and tibiae (Stratton 1991). They also possess short black brushes of hair on their foreleg tibiae (Stratton 2005). Schizocosa ocreata and Schizocosa crassipes are the other brush-legged species in the S. ocreata clade—possessing long, conspicuous brushes of black hair on their foreleg tibiae (Stratton 2005). In prior signal isolation experiments, females of all brush-legged species examined (S. stridulans, S. ocreata, and S. crassipes) did not differ significantly in their receptivity responses to isolated conspecific seismic versus visual courtship signals (Scheffer et al. 1996; Hebets and Uetz 1999, see Figure 1). This is in contrast to less ornamented species (Schizocosa rovneri, Schizocosa uetzi, and Schizocosa duplex), which were significantly more receptive to isolated seismic signals (Scheffer et al. 1996; Hebets and Uetz 1999, see Figure 1). Results of these prior signal isolation experiments suggest that the visual and seismic courtship signals are equivalent in the brush-legged species, indicating signal redundancy. However, further studies on select species have suggested a more complicated story (see below). In addition, female receptivity responses were used as a proxy for female mate choice in the prior studies. Fortunately, recently developed techniques involving artificial environments that alter signal transmission yet allow females and males to interact have enabled a direct test of female mate choice in the presence of unimodal signal components (see Hebets 2005).
Figure 1.
Stylized phylogenetic tree of Schizocosa species based on a Bayesian analysis (general time reversible [GTR] + G model) using data from Hebets and Vink (2007) and sequences of Schizocosa crassipes. Schizocosa ocreata and Schizocosa rovneri are shown as distinct species in this figure; however, this was not supported by the molecular data (see Hebets and Vink 2007). Male foreleg ornamentation is indicated next to each taxon. The graphs next to each foreleg represent the proportion of females that were receptive to isolated courtship signals in prior signal isolation experiments. The y axis is “proportion of females receptive,” and the black bars show female receptivity to visual-only signals, whereas the white bars show female receptivity to seismic-only signals. Asterisk above the graph indicate significant differences in female receptivity to visual-only versus seismic-only stimuli (P < 0.05). Data are compiled from the following sources: (Scheffer et al. 1996; Hebets and Uetz 1999; Uetz and Roberts 2002). The dashed box around Schizocosa stridulans highlights the species used in the present study.
In addition to signal isolation experiments, previous studies have used video playbacks with multiple Schizocosa species to assess female receptivity to visual courtship displays. Most prior studies have used visual-only stimuli in which male foreleg ornamentation was altered while behavior was unchanged (e.g., a single male's courtship sequence was altered into multiple sequences, each only differing in the male's foreleg morphology). Results demonstrated that brush-legged females (S. stridulans, S. ocreata, and S. crassipes) show reduced receptivity when ornamentation is artificially removed from conspecific male forelegs (McClintock and Uetz 1996; Hebets and Uetz 2000). In other words, for these ornamented, multimodal signaling species, female receptivity appears dependent on the visual signal. In addition, in S. ocreata, brush size and symmetry influence female receptivity (McClintock and Uetz 1996; Uetz and Smith 1999) and brush size is condition dependent (Uetz et al. 2002). Results from these studies suggest that in the brush-legged S. ocreata, visual courtship signaling may function to convey information about male quality. However, the playback studies mentioned above did not incorporate the natural multimodal aspect of S. ocreata courtship displays. In contrast to results from the video playback studies, the presence versus absence of brushes did not influence S. ocreata female receptivity when live, seismically signaling males were used (Scheffer et al. 1996). Thus, although the visual signaling component appears important in S. ocreata female mate choice in the absence of a seismic signal, it may not be important when both signals can be perceived by a female. In light of results thus far, authors have suggested that the visual component of S. ocreata’s multimodal courtship display acts as an alerting or amplifying signal prior to the detection of the courting male's seismic signal (Scheffer et al. 1996; Uetz and Roberts 2002 and references therein). Regardless, it is clear that playback experiments assessing receiver responses to more realistic composite signals are crucial for unraveling the true function of multimodal signaling.
Here, in order to first determine signal redundancy/nonredundancy for the multimodal courtship display of S. stridulans, I examined receiver response (i.e., copulation frequency) to isolated and composite courtship signals (visual only, seismic only, visual plus seismic, and no visual or seismic). Next, to determine if the signals interact, I used video playbacks to examine female receptivity to males of varying foreleg morphologies (brushes enlarged, control, and no ornamentation) in the presence of seismic courtship signaling. Finally, in order to explore the information content of seismic courtship signals, I observed female responses to seismic cues from both a prey item (cricket) and a conspecific courting male. Together, the results suggest that the seismic and visual courtship signals of S. stridulans are nonredundant, that the seismic courtship signal is dominant to the visual signal, and that the seismic signal can function to both identify and localize a potential mate.
MATERIALS AND METHODS
Spiders
Schizocosa stridulans is a medium-sized wolf spider (male cephalothorax length [CL] = 3.2 mm, female CL = 3.0 mm, from Stratton 2005) that is closely related to the well-studied sister species S. ocreata and S. rovneri (Stratton 1991, present Figure 1). Schizocosa stridulans is found in mesic upland leaf litter—typically in oak or oak hickory forests ranging from southern Ohio to Mississippi (range map see Stratton 1991). Mature males possess black pigmentation on the tibiae, patellae, and distal third to half of the foreleg femora (Stratton 1991). The foreleg tibiae also have short black hairs characterized as a small brush (Stratton 2005) that are noticeable only under close observation (see Stratton 1991). Male S. stridulans courtship consists of the often simultaneous production of both visual and seismic signals. The visual signal involves a double leg tap in which the 2 forelegs are rapidly tapped on the substrate asynchronously (Stratton 1991, 1997). The seismic courtship signal involves 2 components: “revs” and “idles,” each produced independently. Revs are produced by flexions of the pedipalp (stridulation) and abdomen movements (tremulation) (Elias et al. 2006; see online Supplementary Material). Idles are percussive, produced by the tapping of the forelegs on the substrate followed by flexions of the pedipalp (stridulation) (Elias et al. 2006).
Immature males and females and mature males were collected at night from various sites in northern Mississippi (Lafayette, Marshall, and Panola Counties) in the spring of 1995, 2001, 2003, and 2007. Spiders were housed individually in the laboratory under a 12:12 light:dark light cycle and were provided 2–3 crickets once per week and a constant source of water.
Signal isolation
Using a fully crossed 2 × 2 design with a visual treatment of present versus absent (light/dark) and a seismic treatment of present versus absent (filter paper/granite), I tested the importance of visual and seismic courtship signals for copulation success in S. stridulans. The design was identical to that of Hebets (2005). Briefly, to ablate or reduce the efficacy of the visual signal, trials were run in a completely dark room and were viewed through a Sony DCR-TRV38 MiniDV Handycam with the nightshot option. In contrast, visual present treatments were run under artificial laboratory lighting. To reduce the efficacy of the seismic signal, interacting pairs were placed on a piece of granite rock. Transfer functions of artificially generated seismic vibrations have previously demonstrated that vibrations are highly attenuated (Elias et al. 2004). Furthermore, a rock substrate has previously been shown to inhibit copulation in spider species that rely heavily on seismic signals during courtship (Elias et al. 2004; Hebets 2005). Seismic present treatments were run on filter paper, which does effectively conduct seismic signals (e.g., Scheffer et al. 1996; Hebets and Uetz 1999; Hebets 2005). For further details and discussion of arena and signaling environments, see Hebets (2005).
Mature virgin females ranging in age from 14 to 34 days after maturation were used once in mate choice trials. Females were randomly assigned a signaling treatment (visual+/seismic+, visual+/seismic−, visual−/seismic+, and visual−/seismic−) and were placed in the appropriate arena for a minimum of 2 min prior to the introduction of the male. Female–male pairs were allowed to interact for 1 h during which time the presence/absence of male courtship, presence/absence of copulation, and latency to first courtship and copulation were recorded.
Video playback
A previously published study with S. stridulans demonstrated that in the absence of seismic signals, a female's receptivity is dependent on male foreleg ornamentation (Hebets and Uetz 2000). In the prior study, a S. stridulans male courtship sequence was digitized and manipulated to create 3 test stimuli: 1) a brushes enlarged video: long brushes of black hair were added to the male's forelegs (mimicking the conspicuous brushes of S. ocreata and S. crassipes), 2) a control video: no ornamentation was added or removed, and 3) a no-ornamentation video: all ornamentation was removed from the male's forelegs (for details, see Hebets and Uetz 2000). Using video playback, females were found to be more receptive to the video of males with brushes added as compared with the other 2 video stimuli (Hebets and Uetz 2000). However, female receptivity responses were tested in the absence of a seismic signal. Here, I used the same video sequences and the same individual females as Hebets and Uetz (2000) to test whether female receptivity is dependent on male foreleg ornamentation in the presence of a seismic signal.
The experimental setup was identical to Hebets (2005). Briefly, I altered the video playback arena (Hebets and Uetz 2000) such that each test female resided on the same piece of filter paper as a visually isolated, live courting male (see Hebets 2005 and present Figure 3a). The contiguous substrate enabled the transmission of seismic signals, and thus, each test female could simultaneously watch a video playback and “listen” to a seismic courtship signal. In order to maintain consistency across trials for the seismic stimulus, the same male provided the seismic stimulus across all trials and all females. Using a repeated measures design, each female was tested with each of the 3 video stimuli in the presence of a seismic signal on 3 consecutive days in random order. Previous studies have indicated that female Schizocosa do not show habituation or priming in their response to video stimuli (McClintock and Uetz 1996). Trials lasted 10 min, and females were scored for receptivity (e.g.,Hebets and Uetz 1999, 2000; Hebets 2005). The same 10 females as were used in the video-only experiments (Hebets and Uetz 2000) were used in these video plus seismic experiments. The current study began the day after the last females had completed their visual-only playback trial. For details of video sequences and playback arenas as well as discussions of the validity of video playback with spiders, see Hebets and Uetz (2000), Uetz and Roberts (2002), and Hebets (2005).
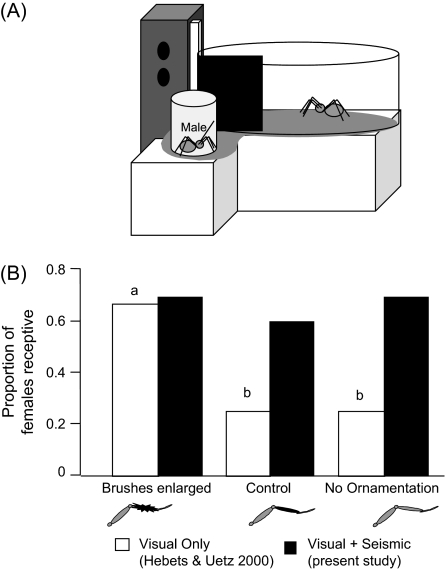
Figure 3.
(A) Representation of the video playback arena where females were exposed to both a video stimulus and the seismic courtship signal from a live courting male (also see Hebets 2005). (B) Female receptivity compared across 3 video stimuli. In contrast to video-only playbacks, in the presence of a seismic courtship signal, female receptivity was not dependent on video stimulus.
A repeated measures Cochran's Q test was used to test the null hypothesis that female receptivity was independent of visual stimuli in the presence of a seismic signal.
Response to seismic signals
In order to determine if females could perceive and discriminate among seismic cues, I assessed female responses to 2 distinct stimuli: 1) normal locomotory cricket vibrations and 2) conspecific male seismic courtship signals. I used an arena measuring 10.16 × 10.16 × 5.08 cm. Four solid white partitions separated the arena into a main compartment bounded on each of 4 sides by side compartments each measuring approximately 2.54 × 5.08 cm. I randomly assigned the seismic stimulus to 1 of the 4 side compartments while the test female sat in the central main compartment. A single piece of filter paper formed the substrate on which both the test subject and the stimulus subjects sat, enabling the transmission of seismic cues. Because the partitions were solid, neither test nor stimulus subject were in visual contact. Due to both the brief timescale within which trials ran and the arena design (e.g., solid barriers), olfactory information was unlikely to play a critical role in female responses.
In order to determine if female age or reproductive status influenced her response to seismic signals, I tested 3 groups of females: 1) penultimate females (spiders were 1 molt away from their final maturation molt), 2) mature virgin females, and 3) mature mated females. All females were tested with both a cricket and a male stimulus in random order. Females were placed in the center of the arena and allowed to acclimate for 1 min before the stimulus individual was introduced. If a female was initially oriented toward the compartment containing the seismic stimulus (i.e., the cricket or male), the female was prodded until she faced a different starting position. Trials did not begin until the cricket started moving or the male started courting. Trials lasted 5 min, during which time females were observed for orientation and approach behaviors. Orientation involved a female positioning herself such that her anterior median eyes were directly facing the side of arena that housed the seismic stimulus.
RESULTS
Signal isolation
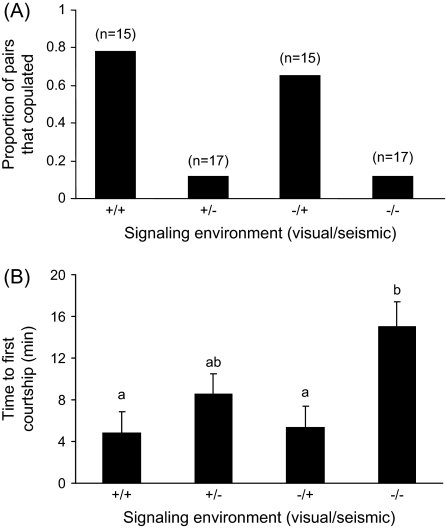
A total of 64 female–male pairs were run through the visual present/absent (light/dark) and seismic present/absent (filter paper/granite) signaling treatments (visual/seismic: +/+: n = 15; +/−: n = 17; −/+: n = 15; −/−: n = 17). Copulation frequency was dependent on treatment. There was no effect of the presence/absence of the visual signal on copulation frequency, but pairs were more likely to copulate in the presence versus absence of a seismic signal, and there was no interaction between the 2 treatments (χ2 = 27.72, P < 0.0001; visual: χ2 = 0.26, P = 0.61; seismic: χ2 = 27.17, P < 0.0001; visual × seismic: χ2 = 0.26, P = 0.61; Figure 2A). Males did not court during all trials (visual/seismic = % courted: +/+ = 93%; +/− = 82%; −/+ = 93%; −/− = 65%), but the presence/absence of male courtship did not depend on signaling environment (χ2 = 6.1, P = 0.11). Nonetheless, only trials in which a male courted were analyzed, and again, copulation frequency was dependent on signaling environment—more pairs copulated in the seismic present versus absent treatments, and there was no effect of the visual environment (χ2 = 23.3, P < 0.0001; visual: χ2 = 0.16, P = 0.69; seismic: χ2 = 22.38, P < 0.0001; visual × seismic: χ2 = 0.64, P = 0.42). Presexual cannibalism occurred in 4 trials (+/+ = 1, +/− = 1, −/+ = 0, −/− = 2).
Figure 2.
(A) Copulation frequencies of female–male pairs under various signaling environments. Pairs were more likely to copulate in the presence versus absence of a seismic signal, whereas the presence/absence of the visual signal did not influence copulation frequency. (B) Males took longer to initiate courtship in the absence of seismic signals, indicating a male reliance on seismic cues. Shared letters indicate nonsignificant differences (P < 0.05).
The time to first courtship (latency to court) was dependent on signaling environment (analysis of variance [ANOVA] F3,48 = 4.27, P = 0.009). A Tukey–Kramer honestly significant difference (HSD) comparison of means (JMP 6.0) revealed that males in the visual absent/seismic absent (−/−) treatment took significantly longer to initiate courtship than males in the visual absent/seismic present (−/+) and visual present/seismic present treatments (+/+) (Figure 2B). However, the time between first courtship and copulation did not depend on the signaling environment (ANOVA F3,22 = 0.1, P = 0.96).
Female age ranged from 14 to 34 days after maturation at the time of mate choice trials, and average female age did not differ among treatments (ANOVA F3,60 = 0.2, P = 0.9). Thirty-seven of the males used in mate choice trials were already mature on collection, and thus, their age is unknown; however, they were all well within the period of time they would be courting in the field. The proportion of already mature males used in mate choice trials did not differ across treatments (visual/seismic—+/+: 67%; +/−: 53%; −/+: 53%; −/−: 59%). The average age of the remaining males of known age also did not vary among treatments (ANOVA F3,23 = 0.5, P = 0.69).
Video playback
This experiment used a video playback design that was more realistic than previous studies to assess female receptivity by allowing visual and seismic signals to interact. Specifically, female receptivity responses to video stimuli were examined in the presence of a seismic signal. It is important to note that the present video playback results involve the same females as were used in the visual-only video playback experiments of Hebets and Uetz (2000). Previously, Hebets and Uetz (2000) used females once and recorded receptivity responses to 1 of 3 video sequences (brushes enlarged, control, and no ornamentation). Immediately after the completion of the Hebets and Uetz (2000) study, the same females were used in the present seismic plus visual playback study. Here, all 10 females engaged in receptivity displays to at least 1 of the 3 video stimuli, and 4 females were receptive to all 3 video sequences. In contrast to the results of Hebets and Uetz (2000), when the video playbacks were paired with a seismic courtship signal, female receptivity did not depend on video stimuli (Cochran's Q test Q = −0.15, P > 0.05; Figure 3B). Among each of the visual stimuli, 60% or more of females engaged in receptivity displays (Figure 3B).
Response to seismic stimuli
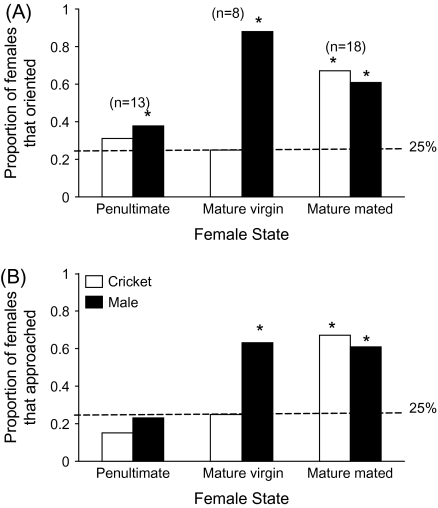
All females were exposed to seismic stimuli from both a live courting male and a moving cricket (penultimate females, n = 13; virgin mature females, n = 8; mated mature females, n = 18). To determine whether or not females could localize seismic cues, I used the null hypothesis that by chance alone a female would have a 0.25 probability of orienting or approaching any one side of the square arena. Thus, using a 1-sided binomial test (JMP 6.0), I asked whether or not a female oriented or approached a stimulus more than expected by chance alone. Penultimate females oriented only to male seismic stimuli more than expected (penultimate females: cricket orient P = 0.42, approach P = 0.87; male orient P = 0.05, approach P = 0.61; Figure 4A). Mature virgin females oriented and approached male seismic stimuli more than expected by chance alone (virgin females: cricket orient P = 0.63, approach P = 0.63; male orient P = 0.0004, approach P = 0.027; Figure 4A). Mature mated females oriented to both cricket and male stimuli more than expected and approached cricket stimuli more than expected by chance alone (mated females: cricket orient P = 0.0002, approach P = 0.019; male orient P = 0.0002, approach P = 0.28; Figure 4A).
Figure 4.
Female orientation and approach responses to isolated seismic cues from mature courting males and moving crickets. Dashed line represents the null expectation that an individual will orient to any one side of the arena 25% of the time by chance alone. * indicates instances where a female's response is significantly higher than expected by chance (P < 0.05). (A) Females oriented to isolated male seismic courtship signals more often than expected by chance, and mature virgin females oriented more often to a male versus a cricket seismic stimulus. (B) Mature virgin and mated females approached isolated seismic courtship signals more often than by chance alone. Mature mated females also approached seismic cricket cues more often than expected by chance.
A nominal logistic model (JMP 6.0) was used to determine whether or not females could discriminate among seismic cues and whether or not their responses were dependent on their reproductive state and developmental stage (i.e., female state). Whether or not a female oriented toward a seismic stimulus was dependent on the stimulus and on an interaction between the female's state and the stimulus, but not on the female's state (degrees of freedom [df] = 5, χ2 = 12.56, P = 0.028; female state: χ2 = 5.5, P = 0.06; seismic stimulus: χ2 = 3.7, P = 0.05; female state × seismic stimulus: χ2 = 5.8, P = 0.05; Figure 4A). Whether or not a female approached a seismic stimulus did not depend on female state or seismic stimulus (df = 5, χ2 = 7.7, P = 0.18; Figure 4B).
A repeated measures Cochran's Q test was used to determine if female orientation responses differed between cricket versus male seismic stimuli. Female orientation was dependent on seismic stimulus only for virgin females (penultimate females n = 7, Q = 0.5, df = 1, P > 0.05; virgin females n = 5, Q = 5, df = 1, P < 0.05; mated females n = 10, Q = 0.14, df = 1, P > 0.05; Figure 4). Virgin females oriented more frequently to the seismic stimulus of a courting male than to a moving cricket.
DISCUSSION
Despite the fact that mature S. stridulans males rapidly tap their conspicuously ornamented forelegs during multimodal courtship displays, their seismic courtship signal appears to dominate the visual component in terms of copulation success and female receptivity. When female–male pairs were allowed to interact through to copulation in manipulated signaling environments, which allowed for the transmission of unimodal courtship signals, copulation frequency was higher in the presence versus absence of the seismic signal. In contrast, the presence/absence of the visual signal had no effect on copulation success. These results suggest that the seismic signal is crucial for successful copulation in S. stridulans and demonstrate that the visual and seismic signals are not redundant, thus allowing us to rule out several hypotheses of complex signal function (e.g., “redundant signal,” “efficacy backup,” “perceptual variability,” see Hebets and Papaj 2005). Furthermore, when displayed and received jointly, the seismic signal appears dominant to the visual signal. A previous video playback study using visual-only stimuli had shown female S. stridulans to be more receptive to more ornamented males (Hebets and Uetz 2000). In direct contrast, the present study demonstrates that when females can also perceive a seismic courtship signal, receptivity responses are independent of male foreleg ornamentation, suggesting that in the presence of seismic courtship signals female mate choice does not rely on the visual signaling component. Finally, female orientation and approach responses to isolated seismic stimuli indicate that females can use seismic courtship signals to identify the direction and likely the identity of a courting male.
Although conspicuous to our eyes, the visual ornamentation and courtship signaling of S. stridulans appears to be neither necessary nor sufficient for successful copulation, as less than 20% of pairs copulated when only the visual signal was present. Nonetheless, even though visual signaling is not necessary for copulation, it may still play an important role in female–male interactions. For example, as suggested for S. ocreata, visual signaling may function to increase the detectability of a male (see Inter-signal interaction hypotheses—Increased detection and discrimination, Hebets and Papaj 2005). In the absence of a seismic signal, we know that the visual signal is capable of attracting a female's attention (Hebets and Uetz 1999, 2000) and females are more responsive to more ornamented males (Hebets and Uetz 1999). Thus, in environments where seismic signals may not travel far, such as the complex leaf litter environment in which S. stridulans lives, a visual signal may attract a female's attention and draw her into a shared signaling surface from a greater distance, whereas the seismic signal ultimately facilitates mate choice and copulation. Although leaf litter transmits seismic courtship signals of spiders more effectively than other substrates (e.g., rock, sand, pine litter [Elias et al. 2004; Hebets et al. 2008]), visual signals may nonetheless travel further than seismic signals in the heterogeneous complex leaf litter habitat of S. stridulans (see Scheffer et al. 1996). In other words, as suggested previously (Hebets and Uetz 2000), the visual courtship signaling of S. stridulans may act as an amplifier or alerting signal—increasing the probability and/or speed of detection of the seismic signal. It is important to note, though, that even in the complex leaf litter habitats of S. stridulans, the visual signal is not sufficient for successful copulation (Hebets EA, unpublished data).
Given the importance of seismic signaling in the multimodal courtship display of S. stridulans, obvious questions arise regarding the information content and function of this signal. To address a part of this question, we asked whether females could obtain location and identity information from seismic courtship signals. It has been well established in other spider species that individuals can determine prey location via seismic cues (Klarner and Barth 1982; Hergenroder and Barth 1983; Bleckmann and Barth 1984). In addition, females of several Cupiennius species have been shown to respond much more strongly to conspecific male seismic signals than heterospecific signals, demonstrating species discrimination based on seismic signals (Barth and Schmitt 1991). It is not surprising then that S. stridulans females are also able to distinguish conspecific male seismic signals from cricket cues and to determine the location of seismic stimuli. For example, mature mated females were the only group that oriented and approached seismic cricket stimuli more often than expected—a result that likely reflects an increased motivation for feeding prior to the production of an egg sac for mated females. More interestingly, mature virgin females, the only females likely to mate (see Norton and Uetz 2005; Persons and Uetz 2005 regarding female remating), were also the only group to approach male seismic signals more frequently than cricket vibrations—a result that likely reflects their motivation to mate. Thus, the different orientation and approach responses observed across female S. stridulans age groups indicate seismic signal discriminability and clear differences in motivation. However, although our data demonstrate that females can likely acquire both location and identification information via male seismic signals, that is not to say that this is the only information these signals convey. Seismic courtship signals may contain additional information about male size or quality and determining this will require detailed studies of the variation observed both across and within individual signaling males.
Seismic cues transmitted through the substrate are the most common means of communication and prey detection in spiders (Barth 1982; Uetz and Stratton 1982), and seismic courtship signaling is thought to be the ancestral state for Schizocosa courtship displays (Stratton 2005). Of the 7 Schizocosa species examined to date, regardless of the degree to which males are ornamented or signal visually, female receptivity responses have never been found to be higher to visual-only signals as compared with seismic-only signals, highlighting the importance of seismic signaling in this group (Figure 1 and Stratton and Uetz 1983; Scheffer et al. 1996; Hebets and Uetz 1999; Uetz and Roberts 2002; Hebets 2005). Thus, it is not necessarily surprising to find seismic signal dominance in the multimodal courtship display of S. stridulans. The dominance of one particular sensory modality is not uncommon across taxonomic groups (for discussion of visual dominance, see Sinnett et al. 2007). For example, vision appears to be the dominant modality in humans. When presented with an auditory and visual stimulus simultaneously, humans often respond only to the visual stimulus (Colavita effect—see Colavita 1974; Koppen and Spence 2007; Sinnett et al. 2007). In addition, as demonstrated by the McGurk effect, our perception of auditory stimuli can be modified by visual stimuli (McGurk and MacDonald 1976), similar to the way that seismic stimuli can modify female S. uetzi responses to visual stimuli (Hebets 2005). Although cases of signal dominance appear rare in the multimodal signaling literature (Partan and Marler 2005), this may simply reflect the difficulty of studies such as that presented here in which receiver responses to isolated versus combined signals are tested.
Interestingly, the results of this study suggest that multimodal signal function varies tremendously even among closely related species (see Figure 1). For example, the first 2 experiments in this study have also been carried out on S. uetzi (Hebets 2005)—a species in which males possess pigmentation on a portion of their foreleg tibiae and have a mostly stationary courtship display consisting of subtle foreleg arches (Stratton 1997). In the signal isolation experiment, the results for S. uetzi were identical to those obtained here—the seismic courtship signal was crucial for copulation success (Hebets 2005). However, results from the video playback studies differed significantly. In the absence of a seismic courtship signal, receptivity displays of S. uetzi were independent of male foreleg ornamentation (Hebets and Uetz 2000). In contrast, S. uetzi females were more receptive to video playbacks of more ornamented males when they could perceive a seismic signal (Hebets 2005). In essence, female receptivity was dependent on visual signaling only in the presence of a seismic signal for S. uetzi, whereas female receptivity was dependent on visual signaling only in the absence of a seismic signal for S. stridulans (Hebets and Uetz 2000, present study). In S. uetzi, the seismic signal appears to alter a female's visual attention (Hebets 2005), whereas in S. stridulans, the seismic signal appears dominant to the visual signal (present study). For both species, the seismic plus visual playback experiments were conducted after the visual-only experiments, and thus, females were necessarily older in the seismic present trials. Recent work on S. ocreata has shown female choosiness to decline with age, and thus, one might argue that females were simply no longer choosy in the seismic plus visual experiment presented here. However, female S. stridulans were run through the seismic present video playbacks immediately after the conclusion of the visual-only playback experiment (see Materials and Methods), and thus, female ages between the 2 experiments were not dramatically different. Furthermore, Norton and Uetz (2005) demonstrate an overall decrease in receptivity response with age (after 3 weeks after maturation), whereas we see an increase in receptivity across all video stimuli (see Figure 3B). Unfortunately, due to differences in experimental design (e.g., females used once in Hebets and Uetz 2000 vs. repeated measures design of present study), it is impossible to directly compare the data. Nonetheless, females of S. stridulans appear much more receptive to visual ornamentation in the presence versus absence of a seismic courtship signal while the opposite was true for S. uetzi.
Studies such as that presented here add to our ever-growing understanding of complex signal function. Ideally, a more complete understanding of current function will lend insights into proximate as well as ultimate factors influencing the evolution of complex signaling. As we acquire the data that enable us to compare and contrast multimodal signal function across closely related taxa such as with the results presented here, we can begin to piece together a picture portraying how past selection has shaped current signal form and function.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://www.beheco.oxfordjournal.org/
FUNDING
National Science Foundation grant to GW Uetz (NSF IBN-9414239) and a National Institute of Health training grant to EAH (2 t32 MH15793).
Supplementary Material
Acknowledgments
As always, I must express my gratitude toward G. Stratton and P. Miller for aid in spider collection, for providing me with food and lodging, and for wonderful conversation and company during my trips to the field. I would also like to thank S. Redella, K. Fowler-Finn, D. Wilgers, A. Nicholas, and R. Willemart for help with spider collections. I am indebted to R. Hoy for laboratory space, insightful comments and suggestions, and inspiration during the signal isolation experiments. K. Cuasay and D. Nee helped with signal isolation trials, and D. Elias and N. VanderSal provided stimulating discussions. The video playback experiment was done in the laboratory of G. Uetz. I would like to thank G. Uetz, M. Persons, K. Delaney, A. Delay, and L. Smith for feedback and critiques on the video playback experiment. The seismic signal localization trials were done in the laboratory of D. Papaj, and I would like to thank him as well as H. Mallory for help during those trials. I am greatly indebted to Cor Vink for constructing the phylogenetic tree for Figure 1 and to K. Fowler-Finn for supplying S. crassipes data. Finally, I would like to thank K. Fowler-Finn, M. Adams, D. Wilgers, A. Rundus, S. Schwartz, R. Santer, and 3 anonymous reviewers for extremely useful comments and criticisms on an earlier version of the manuscript.
References
- Barth FG. Spiders and vibratory signals: sensory reception and behavioral significance. In: Witt PW, Rovner JS, editors. Spider communication: mechanisms and ecological significance. Princeton: Princeton University Press; 1982. pp. 67–120. [Google Scholar]
- Barth FG, Schmitt A. Species recognition and species isolation in wandering spiders (Cupiennius spp., Ctenidae) Behav Ecol Sociobiol. 1991;29:333–339. [Google Scholar]
- Bleckmann H, Barth FG. Sensory ecology of a semi-aquatic spider (Dolomedes triton) II: The release of predatory behavior by water surface waves. Behav Ecol Sociobiol. 1984;14:303–312. [Google Scholar]
- Candolin U. The use of multiple cues in mate choice. Biol Rev. 2003;78:575–595. doi: 10.1017/s1464793103006158. [DOI] [PubMed] [Google Scholar]
- Colavita FB. Human sensory dominance. Percept Psychophys. 1974;16:409–412. [Google Scholar]
- Elias DO, Lee N, Hebets EA, Mason AC. Seismic signal production in a wolf spider: parallel versus serial multicomponent signals. J Exp Biol. 2006;209:1079–1084. doi: 10.1242/jeb.02104. [DOI] [PubMed] [Google Scholar]
- Elias DO, Mason AC, Hoy RR. The effect of substrate on the efficacy of seismic courtship signal transmission in the jumping spider Habronattus dossenus (Araneae: salticidae) J Exp Biol. 2004;207:4105–4110. doi: 10.1242/jeb.01261. [DOI] [PubMed] [Google Scholar]
- Grafe TU, Wanger TC. Multimodal signaling in male and female foot-flagging frogs Staurois guttatus (Ranidae): an alerting function of calling. Ethology. 2007;113:772–781. [Google Scholar]
- Guilford T, Dawkins MS. Receiver psychology and the evolution of animal signals. Anim Behav. 1991;42:1–14. [Google Scholar]
- Hebets EA. Attention-altering interaction in the multimodal courtship display of the wolf spider Schizocosa uetzi. Behav Ecol. 2005;16:75–82. doi: 10.1093/beheco/arn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebets EA, Cuasay K, Rivlin PK. The role of visual ornamentation in female choice of a multimodal male courtship display. Ethology. 2006;112:1062–1070. [Google Scholar]
- Hebets EA, Elias DO, Mason AC, Miller GL, Stratton GE. Substrate dependent signaling success in the wolf spider Schizocosa retrorsa. Anim Behav. 2008;75:605–615. [Google Scholar]
- Hebets EA, Papaj DR. Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol. 2005;57:197–214. [Google Scholar]
- Hebets EA, Uetz GW. Female responses to isolated signals from multimodal male courtship displays in the wolf spider genus Schizocosa (Araneae: Lycosidae) Anim Behav. 1999;57:865–872. doi: 10.1006/anbe.1998.1048. [DOI] [PubMed] [Google Scholar]
- Hebets EA, Uetz GW. Leg ornamentation and the efficacy of courtship display in four species of wolf spider (Araneae: Lycosidae) Behav Ecol Sociobiol. 2000;47:280–286. [Google Scholar]
- Hebets EA, Vink CJ. Experience leads to preference: experienced females prefer brush-legged males in a population of syntopic wolf spiders. Behav Ecol. 2007;18:1010–1020. [Google Scholar]
- Hergenroder R, Barth FG. Vibratory signals and spider behavior: how do the sensory inputs from the eight legs interact in orientation? J Comp Physiol A. 1983;152:361–371. [Google Scholar]
- Klarner D, Barth FG. Vibratory signals and prey capture in orbweaving spiders (Sygiella x-notata, Nephila clavipes; Araneidae) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1982;148:445–455. [Google Scholar]
- Koppen C, Spence C. Seeing the light: exploring the Colavita visual dominance effect. Exp Brain Res. 2007;180:737–754. doi: 10.1007/s00221-007-0894-3. [DOI] [PubMed] [Google Scholar]
- McClintock WJ, Uetz GW. Female choice and pre-existing bias: visual cues during courtship in two Schizocosa wolf spiders (Araneae: Lycosidae) Anim Behav. 1996;52:167–181. [Google Scholar]
- McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264:746–748. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- McLennan DA. The importance of olfactory signals in the gasterosteid mating system: sticklebacks go multimodal. Biol J Linn Soc. 2003;80:555–572. [Google Scholar]
- Narins PM, Hodl W, Grabul DS. Bimodal signal requisite for agonistic behavior in a dart- poison frog, Epipedobates femoralis. Proc Natl Acad Sci USA. 2003;100:577–580. doi: 10.1073/pnas.0237165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S, Uetz GW. Mating frequency in Schizocosa ocreata (Hentz) wolf spiders: evidence for a mating system with female monandry and male polygyny. J Arachnol. 2005;33:16–24. [Google Scholar]
- Partan S, Marler P. Behavior—communication goes multimodal. Science. 1999;283:1272–1273. doi: 10.1126/science.283.5406.1272. [DOI] [PubMed] [Google Scholar]
- Partan S, Yelda S, Price V, Shimizu T. Female pigeons, Columba livia, respond to multisensory audio/video playbacks of male courtship behaviour. Anim Behav. 2005;70:957–966. [Google Scholar]
- Partan SR, Marler P. Issues in the classification of multimodal communication signals. Am Nat. 2005;166:231–245. doi: 10.1086/431246. [DOI] [PubMed] [Google Scholar]
- Persons MH, Uetz GW. Sexual cannibalism and mate choice decisions in wolf spiders: influence of male size and secondary sexual characters. Anim Behav. 2005;69:83–94. [Google Scholar]
- Rowe C, Guilford T. The evolution of multimodal warning displays. Evol Ecol. 1999;13:655–671. [Google Scholar]
- Scheffer SJ, Uetz GW, Stratton GE. Sexual selection, male morphology, and the efficacy of courtship signalling in two wolf spiders (Araneae: Lycosidae) Behav Ecol Sociobiol. 1996;38:17–23. [Google Scholar]
- Sinnett S, Spence C, Soto-Faraco S. Visual dominance and attention: the Colavita effect revisited. Percept Psychophys. 2007;69:673–686. doi: 10.3758/bf03193770. [DOI] [PubMed] [Google Scholar]
- Stratton GE. A new species of wolf spider, Schizocosa stridulans (Araneae, Lycosidae) J Arachnol. 1991;19:29–39. [Google Scholar]
- Stratton GE. A new species of Schizocosa from the southeastern USA (Araneae, Lycosidae) J Arachnol. 1997;25:84–92. [Google Scholar]
- Stratton GE. Evolution of ornamentation and courtship behavior in Schizocosa: insights from a phylogeny based on morphology (Araneae, Lycosidae) J Arachnol. 2005;33:347–376. [Google Scholar]
- Stratton GE, Uetz GW. Communication via substratum-coupled stridulation and reproductive isolation in wolf spiders (Araneae, Lycosidae) Anim Behav. 1983;31:164–172. [Google Scholar]
- Uetz GW, Papke R, Kilinc B. Influence of feeding regime on body size, body condition and a male secondary sexual character in Schizocosa ocreata wolf spiders (Araneae, Lycosidae): condition-dependence in a visual signaling trait. J Arachnol. 2002;30:461–469. [Google Scholar]
- Uetz GW, Roberts JA. Multisensory cues and multimodal communication in spiders: insights from video/audio playback studies. Brain Behav Evol. 2002;59:222–230. doi: 10.1159/000064909. [DOI] [PubMed] [Google Scholar]
- Uetz GW, Smith EI. Asymmetry in a visual signaling character and sexual selection in a wolf spider. Behav Ecol Sociobiol. 1999;45:87–93. [Google Scholar]
- Uetz GW, Stratton GE. Acoustic communication in spiders. In: Witt PN, Rovner JS, editors. Spider communication: mechanisms and ecological significance. Princeton: Princeton University Press; 1982. pp. 123–159. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.