Abstract
Background and Objective
Platelet-derived growth factor-BB is a potent mediator of tooth-supporting periodontal tissue repair and regeneration. A limitation of the effects of topical platelet-derived growth factor-BB application is its short half-life in vivo. Gene therapy has shown strong promise for the long-term delivery of platelet-derived growth factor in both skin ulcer healing and periodontal tissue engineering. However, little is known regarding the extended effects of platelet-derived growth factor-B on cell signaling via gene delivery, especially at the level of phosphorylation of intracellular kinases. This study sought to evaluate the effect of gene transfer by Ad-PDGF-B on human gingival fibroblasts (HGFs) and the subsequent regulation of genes and cell-surface proteins associated with cellular signaling.
Material and Methods
HGFs from human subjects were treated by adenoviral PDGF-B, PDGF-1308 (a dominant negative mutant of PDGF) and recombinant human platelet-derived growth factor-BB, and then incubated in serum-free conditions for various time points and harvested at 1, 6, 12, 24, 48, 72 and 96 h. Exogenous PDGF-B was measured by RT-PCR and Western blot. Cell proliferation was evaluated by [methyl-3H]thymidine incorporation assay. We used proteomic arrays to explore phosphorylation patterns of 23 different intracellular kinases after PDGF-B gene transfer. The expression of α and β PDGFR and Akt were measured by Western blot analysis.
Results
Sustained in vitro expression of PDGF-B in HGFs by Ad-PDGF-B transduction was seen at both the mRNA and protein levels. Compared to rhPDGF-BB and Ad-PDGF-1308, Ad-PDGF-B maintained cell growth in serum-free conditions, with robust increases in DNA synthesis. Gene delivery of PDGF-B also prolonged downregulation of the growth arrest specific gene (gas) PDGFαR. Of the 23 intracellular kinases that we tested in proteomic arrays, Akt revealed the most notable long-term cell signaling effect as a result of the over-expression of Ad-PDGF-B, compared with pulse recombinant human platelet-derived growth factor BB. Prolonged Akt phosphorylation was induced by treatment with Ad-PDGF-B, for at least up to 96 h.
Conclusion
These findings further demonstrate that gene delivery of PDGF-B displays sustained signal transduction effects in human gingival fibroblasts that are higher than those conveyed by treatment with recombinant human platelet-derived growth factor-BB protein. These data on platelet-derived growth factor gene delivery contribute to an improved understanding of these pathways that are likely to play a role in the control of clinical outcomes of periodontal regenerative therapy.
Keywords: platelet-derived growth factor, wound repair, tissue engineering, periodontal regeneration
Platelet-derived growth factor, a member of a multifunctional polypeptide family, is composed of A, B, C and D polypeptide chains that form homodimeric or heterodimeric molecules (1). Two receptor isoforms with tyrosine kinase activity have been identified: platelet-derived growth factor-α receptor and platelet-derived growth factor-β receptor (1). Platelet-derived growth factor binding to receptors activates a variety of intracellular signal molecules, such as phosphatidylinositol-3-kinase and Akt/PKB, and subsequently exerts its biological effects on cell migration, proliferation, extracellular matrix synthesis and anti-apoptosis (2–7).
Platelet-derived growth factor-BB, which binds both α and β platelet-derived growth factor receptors, promotes wound healing in both hard and soft tissues, such as skin and periodontium. Platelet-derived growth factor-α and -β receptors are induced in human gingival fibroblasts and tooth-supporting periodontal ligament cells during periodontal tissue repair (8,9). In addition, platelet-derived growth factor initiates tooth-supporting periodontal ligament cell chemotaxis (10), mitogenesis (11), matrix synthesis (12,13) and attachment to tooth dentinal surfaces (14). In an experimental model of periodontitis in macaques, platelet-derived growth factor-BB was shown to promote new attachment and new bone formation in vivo (15). Furthermore, it has been recently shown that the application of platelet-derived growth factor-BB in an osteoconductive scaffold promotes periodontal regeneration in humans (16).
A limitation of the effects of topical platelet-derived growth factor application is its short half-life in vivo (17–19). Gene delivery is a candidate approach to overcome this problem. Delivery of platelet-derived growth factor by gene transfer has been shown to stimulate the mitogenesis and proliferation of gingival fibroblasts, periodontal ligament and tooth-lining cells (cementoblasts) more strongly than the continuous administration of platelet-derived growth factor protein in vitro (20,21). In terms of gene expression duration, in vitro studies have shown that adenovirus-encoded platelet-derived growth factor-A delivered to cells derived from periodontal tissues (gingival and periodontal ligament fibroblasts, cement-blasts and osteoblasts) elicits elevated and prolonged expression of the PDGF-A gene for at least 1 wk (20,22). Furthermore, transfer of the PDGF-A gene using adenovirus resulted in sustained tyrosine kinase phosphorylation and downregulation of the platelet-derived growth factor-α receptor for at least 96 h in dermal fibroblasts (22). Our previous work has also shown that in vivo, PDGF-B gene transfer promotes the regeneration of tooth- supporting structures when adenovirus-encoded PDGF-B is applied to periodontal osseous defects (23). Another study examined the effect of gene transfer by adenovirus encoding PDGF-A and PDGF-B on a human gingival fibroblast repopulation in an ex vivo wound-healing model and found that gene expression was maintained for at least 10 d and that PDGF-B gene transfer stimulated an increase of more than four-fold in cell repopulation above that of PDGF-A and controls (24). Although adenoviral delivery of PDGF-B demonstrates a promising future in periodontal regeneration, little is known about its long-term effect on signal transduction in cells isolated from the periodontium. Human gingival fibroblasts are a possible cell target for periodontal tissue regeneration because of several advantages, such as easy accessibility, richness in cell resource and higher proliferation potential. In our previous work, we have already shown that human gingival fibroblasts can respond to adenoviral-encoded platelet-derived growth factor-A (21) and can be used to stimulate periodontal soft tissue repair ex vivo (24). The present study sought to evaluate the effect of gene transfer of PDGF-B by adenovirus on human gingival fibroblasts and the subsequent regulation of genes and cell-surface proteins associated with cellular signaling.
Material and methods
Adenovirus construction
The construction of the adenovirus encoding PDGF-1308 (the dominant negative mutant of platelet-derived growth factor-A) has been previously described (25). The recombinant adenoviruses encoding human PDGF-B (Ad-PDGF-B) and luciferase (Ad-Luc) are driven by a cytomegalovirus promoter and were gifts from Tissue Repair Company (26), San Diego, CA, USA.
Cell culture and gene transduction
Primary human gingival fibroblasts used for this study were kindly gifted to us by Dr Martha Somerman (University of Washington, Seattle, WA, USA). Human gingival fibroblasts were isolated separately from gingival tissue biopsy samples from three healthy human patients. Low passage cells (passages 4–7) were cultured and maintained in Dulbecco’s modified Eagle’s minimal essential medium supplemented with 10% fetal bovine serum, 100 U/mL of penicillin/streptomycin and 2 mM glutamine, then plated in 60 × 15-mm dishes. Confluent cultures were brought to a stage of quiescence by rinsing the monolayers with phosphate-buffered saline and reducing the serum concentration to 0.1% for 24 h prior to adenovirus transduction or treatment with recombinant human platelet-derived growth factor-BB (a kind gift of BioMimetic Therapeutics Inc., Franklin, TN, USA). For all experiments the cells were treated either with recombinant adenoviruses (Ad-PDGF-B, Ad-PDGF-1308, Ad-Luc) for 2 h at a multiplicity of infection of 200 or with 20 ng/mL of recombinant human platelet-derived growth factor-BB. Cells were incubated for various periods of time and harvested at 1, 6, 12, 24, 48, 72 and 96 h.
[Methyl-3H]thymidine incorporation assay
Human gingival fibroblast cells were seeded in 12-well plates at a concentration of 2 × 104 cells per well. After different treatments (recombinant human platelet-derived growth factor-BB, Ad-PDGF-B, Ad-PDGF-1308 or no treatment), cells were cultured with serum-free Dulbecco’s modified Eagle’s minimal essential medium. Two days later, 2 × 105 counts per minute of [methyl-3H]thymidine was added to each well. On day 7, the medium was removed and each well was washed twice with cold phosphate-buffered saline. The DNA in each well was precipitated with 5% cold trichloro-acetic acid at 4°C for 2 h, solubilized with 1% sodium dodecyl sulfate solution at 55°C for 2 h and then the radioactivity of [methyl-3H]thymidine in the solution was counted using a scintillation counter (Wallac 1410; Perkin-Elmer, Waltham, MA, USA).
Western blot analysis
Whole-cell lysates were obtained from transduced human gingival fibroblast cells by washing cell monolayers twice with ice-cold phosphate-buffered saline, adding 250 µL of ice-cold cell lysis buffer (Bio-Rad, Alameda, CA, USA) directly to each dish and harvesting using cell scrapers. The total protein concentration was determined using the Bio-Rad Protein Assay with measurement of the absorbance at 595 nm. After heat denaturation (95°C, 5 min), 10 µg of total protein from each sample was added to 5× sample buffer (0.06 m Tris, pH 6.8, 2% sodium dodecyl sulfate, 0.3 m β-mercaptoethanol, 0.1% bromophenol blue, 10% glycerol) and the samples were electrophoresed (80 V, 2 h) through 10% acrylamide gels in Tris-glycine-sodium dodecyl sulfate running buffer. Protein samples were then transferred to immunoblot poly(vinylidene difluoride) membranes (Bio-Rad) in transfer buffer, pH 8.3 (25 mM Tris, 192 mM glycine, 20% methanol) for 90 min at 400 mA in a 4°C cold room. The membrane was then incubated in TTBS (100 mM Tris, 0.9% NaCl, 0.5% Tween 20) containing 5% nonfat dry milk for 1 h to block nonspecific binding. The blocking solution was discarded and the membrane was incubated overnight in fresh blocking solution containing diluted primary antibodies. The membranes were then washed with TTBS for 15 min, three times, then incubated in fresh blocking solution containing a 1:3000 dilution of anti-rabbit horseradish peroxidase (Amersham Corp. Piscataway, NJ, USA) for 1 h. The membranes were washed as described above and prepared for chemiluminescent detection by immersing the blot in a 1:1 mixture of reagents from an ECL Detection Kit (Amersham Corp.), then exposed to Biomax MS film (Kodak, Rochester, NY, USA) for 30–120 s before development. Densitometric scanning (Bio-Rad Molecular Imager ChemiDoc XRS System; Bio-Rad) was performed by normalizing platelet-derived growth factor receptor expression to glyceraldehyde-3-phosphate dehydrogenase for each of the treated primary cell cultures from three different human biopsies to ascertain receptor expression patterns following gene or protein delivery. Antibodies used in the present study were: rabbit anti-human platelet-derived growth factor-α receptor polyclonal antibody and rabbit anti-human platelet-derived growth factor-b receptor polyclonal antibody (Upstate Cell Signaling, Charlottesville, VA, USA), rabbit polyclonal anti-phospho-Akt (Ser473) , rabbit polyclonal anti-Akt, rabbit polyclonal anti-GAPDH (all from Cell Signaling Technology, Danvers, MA, USA). In order to detect the secreted platelet-derived growth factor-BB, cell culture medium was collected on day 4, followed by dialysis in Amicon Ultra spin columns (Millipore, Billerica, MA, USA) and lyophilization. Then, the samples were reconstituted in H2O and loaded for western blot analysis. Rabbit polyclonal anti-platelet-derived growth factor-B (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used to detect platelet-derived growth factor-B expression.
Reverse transcription-polymerase chain reaction
Total RNA was extracted from human gingival fibroblast cells using the Qiagen RNeasy Mini kit (Qiagen, Valencia, CA, USA). Approximately 1 µg of RNA was reverse transcribed in a 20-µL reverse transcription reaction using a Retroscript kit (Ambion, Austin, TX, USA). cDNA was generated using random primers. Ten microliters of the cDNA was used as a template for polymerase chain reaction amplification to detect PDGF-B as well as the housekeeping gene, GAPDH.
Proteomic array for phosphoproteins
In order to assess the relative level of phosphorylation in a given sample, a human Phospho-MAPK array kit (R&D Systems, Minneaplolis, MN, USA) was utilized to measure simultaneously the phosphorylation levels of 23 different mitogen-activated protein kinases (MAPKs) and other serine/threonine kinases. Each array contains duplicate validated control and capture antibodies for specific kinases. The MAPK array was performed according to the manufacturer’s protocol. Briefly, human gingival fibroblast cells that had undergone the different experimental treatments were lysed using the lysis buffer in the kit. Array membranes were blocked at room temperature for 1 h and were then incubated with 800 µg of total cell lysate over-night at 4°C. The next day, after washing three times, array membranes were incubated with a phospho-site specific biotinylated antibody cocktail for 2 h at room temperature. The membranes were washed a further three times, developed using the ECL Detection Kit (Amersham), then exposed to Biomax MS film (Kodak).
Results
Adenoviral gene delivery of PDGF-B results in sustained expression of platelet-derived growth factor-BB in human gingival fibroblast cells
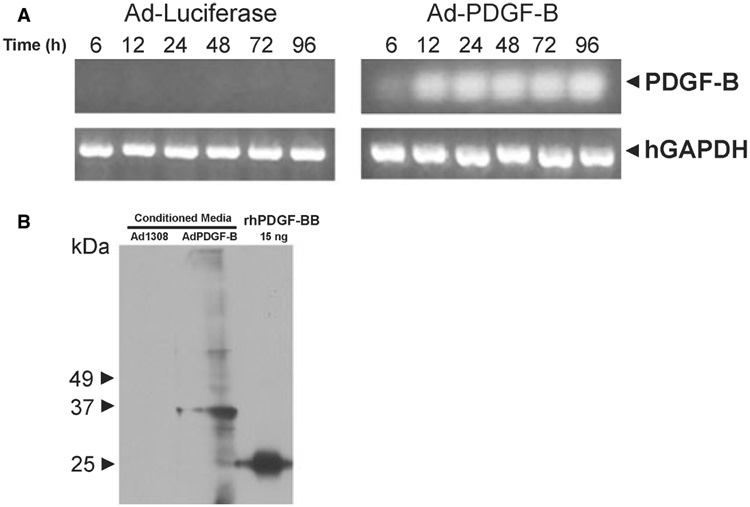
We first assessed the expression of exogenous PDGF-B in primary human gingival fibroblasts by adenoviral gene delivery. In our previous study, using green fluorescent protein reporter protein, we showed that adenoviral gene delivery has a high transduction efficiency in fibroblasts (> 95% of dermal fibroblasts were transduced) (22). Similarly, in the present study we detected the transcription of exogenous PDGF-B 6 h after exposure to Ad-PDGF-B, and transcription was sustained for at least 96 h (Fig. 1A). This effect was not observed after transfection of either the Ad-Luc control (Fig. 1A) or Ad-PDGF-1308 (data not shown). Furthermore, exogenously expressed PDGF-B was translated and secreted into cell culture medium as a dimer, which was detected by western blot analysis 4 d after treatment with Ad-PDGF-B but not after treatment with Ad-PDGF-1308 (Fig. 1B). Our Ad-PDGF-B vector encodes a 160 amino acid platelet-derived growth factor-B isoform with a molecular weight of 18.1 kDa; therefore, in non-reducing conditions, this results in a dimer of about 36.2 kDa. For the recombinant human platelet-derived growth factor-BB control, the dimer of the 109 amino acid isoform displayed a molecular weight of 25 kDa.
Fig. 1.
Adenoviral gene delivery of PDGF-B results in the sustained expression of platelet-derived growth factor-B in human gingival fibroblast cells. (A) Human gingival fibroblast cells were cultured to confluence and were then transferred to serum-free medium containing Ad-PDGF-B (multiplicity of infection = 200), Ad-Luc, Ad-PDGF-1308, or platelet-derived growth factor-BB protein (20 ng/mL). The cells were then allowed to incubate for 2–3 h before removal and replacement with serum-free medium. The cells were then harvested for RNA extraction at 6, 12, 24, 48, 72 and 96 h post-infection. Approximately 1.0 µg of total RNA was then used to generate cDNA with oligo dT primers. The resulting cDNA was used as a template for polymerase chain reaction amplification using human gene-specific primers for PDGF-B or GAPDH. The results show that only the treatment with Ad-PDGF-B caused a measurable increase in PDGF-B gene expression. The results obtained following treatment with Ad-Luc, Ad-PDGF-1308 or platelet-derived growth factor-BB protein showed no detectable expression of the PDGF-B gene (data not shown). (B) Approximately 2 × 105 cells were seeded in 100-cm dishes. After transient infection with Ad-PDGF-1308 or Ad-PDGF-B, the cells were cultured in serum-free medium. Conditioned media were collected at 96 h. After dialysis and lyophilization, samples were reconstituted in H2O, loaded for non-reduced sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and detected using anti-PDGF-BB. Our Ad-PDGF-B vector encodes a 160 amino acid platelet-derived growth factor-B isoform with a molecular weight of 18.1 kDa; therefore, a dimer in the non-reduced SDS gel was about 36.2 kDa. For the recombinant human platelet-derived growth factor-BB control, the dimer of the 109 amino acid isoform displayed a molecular weight of 25 kDa. hGAPDH, human glyceraldehyde-3-phosphate dehydrogenase; PDGF-B, platelet-derived growth factor-B; rhPDGF-BB, recombinant human platelet-derived growth factor-BB.
Adenoviral gene delivery of PDGF-B maintains human gingival fibroblast proliferation
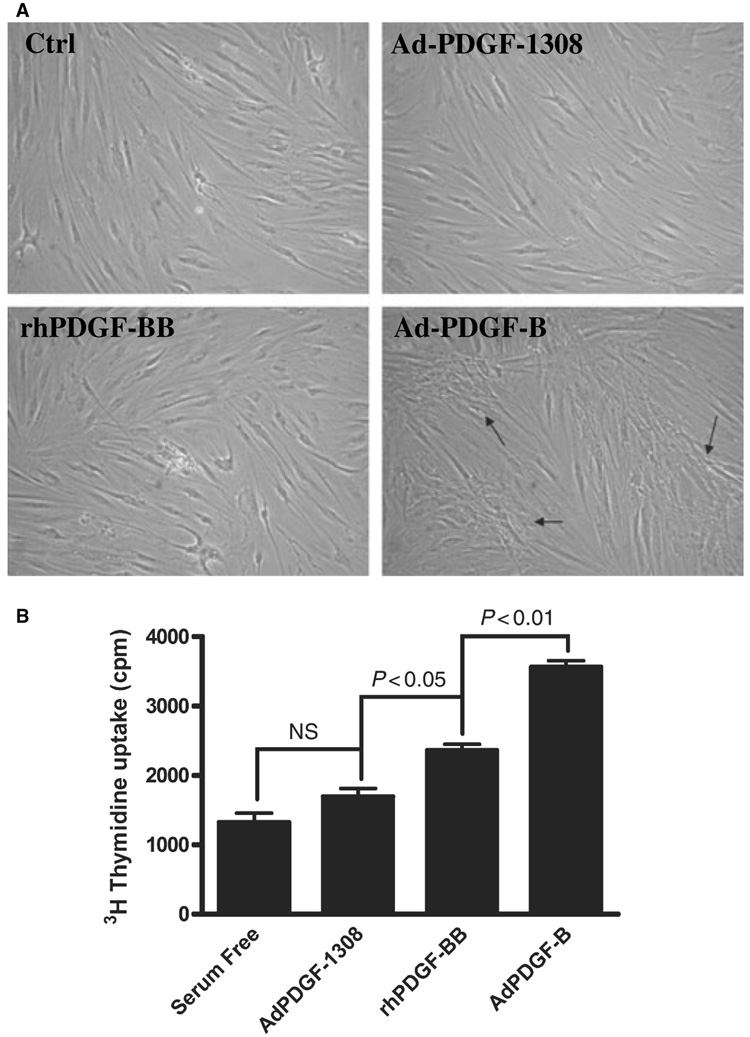
Next, we sought to determine whether the exogenous expression of PDGF-B was biologically active. After treatment with Ad-PDGF-B, human gingival fibroblasts tended to form greater cell polarization distinct from that observed following treatment with recombinant human platelet-derived growth factor-BB or with the Ad-PDGF-1308 control (Fig. 2A). Compared to treatment with Ad-PDGF-1308 or transient recombinant human platelet-derived growth factor-BB, Ad-PDGF-B administration maintained the proliferation of human gingival fibroblasts in serum-free conditions and stimulated robust DNA synthesis, as shown by the [methyl-3H]thymidine-incorporation assay (Fig 2B).
Fig. 2.
(A) Cells were treated as described in the legend to Fig. 1 and photographed at 72 h. Compared to treatment with control (serum-free), Ad-PDGF-1308, or recombinant human platelet-derived growth factor-BB, human gingival fibroblasts transduced with Ad-PDGF-B resulted in colony-like growth, as indicated by the arrows (20× magnification). (B) Human gingival fibroblast cells were seeded in 12-well plates at a concentration of 2 × 104 cells per well. After different treatments (recombinant human platelet-derived growth factor-BB, Ad-PDGF-B, or Ad-PDGF-1308), the cells were cultured with serum-free Dulbecco’s modified Eagle’s minimal essential medium without medium change during the experiment. Two days later, 2 × 105 counts per minute of [methyl-3H]thymidine was added to each well. On day 7, the medium was removed and each well was washed twice with cold phosphate-buffered saline. The DNA in each well was precipitated with 5% cold trichloroacetic acid at 4°C for 2 h, solubilized with 1% sodium dodecyl sulfate solution at 55°C for 2 h, then the radioactivity of [methyl-3H]thymidine in the solution was counted using a scintillation counter. Ad-PDGF-B stimulated robust DNA synthesis when compared with the other treatments. NS, no significant difference; (n = 6 samples).
Gene delivery of Ad-PDGF-B prolonged downregulation of the growth arrest-related gene PDGFαR and of PDGFβR
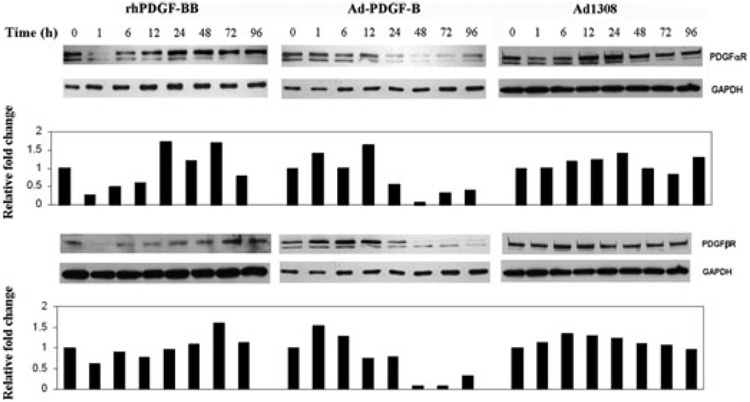
In the condition of serum deprivation, fibroblasts remain in the state of growth arrest (G0), which is associated with increased expression of the gas gene PDGFαR as well as of PDGFβR. We examined the impact of Ad-PDGF-B transduction on the levels of platelet-derived growth factor-α receptor and platelet-derived growth factor-β receptor. The steady-state levels of platelet-derived growth factor-α receptor and platelet-derived growth factor-β receptor for the Ad-PDGF-1308-treated human gingival fibroblasts remained at similar levels over the 96-h observation period. Treatment of human gingival fibroblasts with recombinant human platelet-derived growth factor-BB protein elicited a reduction in the levels of platelet-derived growth factor-α receptor and platelet-derived growth factor-β receptor within 1 h and these levels remained low for 12 h before returning to normal baseline levels by 24 h (Fig 3). By contrast, human gingival fibroblast cells treated with Ad-PDGF-B (multiplicity of infection = 200) showed a delayed, but prolonged and greater, reduction in both platelet-derived growth factor-α and -β receptor protein levels starting at about 24 h, with the levels remaining reduced for at least 96 h (Fig. 3).
Fig. 3.
Gene delivery of Ad-PDGF-B prolongs the downregulation of the growth arrest-related gene (gas)PDGFαR as well as PDGFβR. Western blot was used to analyze platelet-derived growth factor-α receptor and platelet-derived growth factor-β expression levels in human gingival fibroblasts in response to treatment with either recombinant human platelet-derived growth factor-BB (20 ng/mL), or Ad-PDGF-B. Human gingival fibroblasts from three individuals were tested, with representative data shown here. Human gingival fibroblasts were exposed to Ad-PDGF-1308 (multiplicity of infection = 200), Ad-PDGF-B (multiplicity of infection = 200) or 20 ng/mL of recombinant human platelet-derived growth factor-BB in serum-free medium for 2 h. Then, the medium was removed and human gingival fibroblasts were maintained in serum-free medium for 1, 6, 12, 24, 48, 72 and 96 h. Cells were lysed and 30 µg of total protein from cell lysates was subjected to western blotting using antibodies to platelet-derived growth factor-α receptor and platelet-derived growth factor-β receptor. The blots were then stripped and reprobed with anti-GAPDH to determine the relative loading of protein in each lane. Densitometric scanning was performed by normalizing platelet-derived growth factor receptor expression to glyceraldehyde-3-phosphate dehydrogenase for the treated primary cell cultures from three different human biopsies. The graphs demonstrate the cumulative results from three individuals with the blots showing the typical result. The graphs show that treatment with Ad-PDGF-B resulted in sustained downregulation of platelet-derived growth factor-a receptor and platelet-derived growth factor-β receptor for up to 96 h. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PDGFαR, platelet-derived growth factor-α receptor; PDGFβR, platelet-derived growth factor-β receptor; rhPDGF-BB, recombinant human platelet-derived growth factor-BB.
Proteomic array to determine phosphorylation of intracellular proteins
In these experiments our goal was to identify the effects on signal transduction elicited by Ad-PDGF-B gene delivery. After recombinant human platelet-derived growth factor-BB binds to tyrosine receptor kinases, it triggers a cascade of downstream signal transduction pathways, in which kinase activation plays a central role. Because we observed (by western blotting) prolonged downregulation of platelet-derived growth factor-α and -β receptor protein levels following treatment with Ad-PDGF-B, we wanted to determine whether adenoviral delivery would also extend intracellular signaling events. Therefore, we examined the phosphorylation state of numerous kinases by using a human phospho-MAPK array that contains 21 different specific intracellular kinase antibodies (Table 1). This array allowed the simultaneous relative quantification of the phosphorylation state of MAPKs in three major families (ERK1/2, JNK1–3 and p38), and also detected another nine important intracellular tyrosine/serine kinases, including Akt, GSK-3 and p70 S6 kinase, in the same cell lysate (Table 1). With this array we focused on phophorylation patterns at early stage (1 h) and late-stage (72 h and 96 h) time-points. Akt1, Akt2 and Akt3 were the most sensitive intracellular signal markers to platelet-derived growth factor treatment of all the 21 kinases that were tested. Our results showed that Ad-PDGF-B treatment revealed a similar, but delayed, phosphorylation pattern as that of recombinant human platelet-derived growth factor-BB: significant Akt phosphorylation was seen by 1 h after stimulation of human gingival fibroblasts by recombinant human platelet-derived growth factor-BB, but the effect disappeared by 72 h; exposure to Ad-PDGF-B resulted in Akt phosphorylation at the late stage, which can be seen from arrays at both 72 and 96 h (Fig. 4). Unstimulated human gingival fibroblast lysates demonstrated very low basal levels of phosphorylation in all the tested kinases under serum-deprivation conditions, and this was also found for the Ad-PDGF-1308 control.
Table 1.
Human phosphoproteins simultaneously measured the phosphorylation levels of 21 different mitogen-activated protein kinases (MAPKs) and other serine/threonine kinases following PDGF-B gene therapy
| MAPK family | ERK1, ERK2 |
| JNK1, JNK2, JNK3, JNK pan | |
| p38α, p38β, p38γ, p38δ | |
| Others | Akt1, Akt2, Akt3, Akt pan, RSK1, RSK2, MSK2, GSK-3α/β, GSK-3β, HSP27, p70 S6 kinase |
Fig. 4.
Proteomic array to identify intracellular phosphorylation proteins that mediate either immediate or long-term effects of protein or gene delivery. The human phospho-MAPK array kit was utilized to measure simultaneously the phosphorylation levels of target kinases. Human gingival fibroblast cells treated with 20 ng/mL of recombinant human platelet-derived growth factor-BB, Ad-PDGF-B (multiplicity of infection = 200), Ad-PDGF-1308 (multiplicity of infection = 200) or serum-free medium were harvested at 1, 72 and 96 h post-treatment. After blocking, 800 µg of human gingival fibroblast cell lysates were incubated with membranes at 4°C overnight. Each membrane contained duplicates, validated controls and capture antibodies for specific kinases. After washing, a phospho-site-specific biotinylated antibody cocktail was added the following day. Each array was identically treated with detection reagent and film. Treatment with Ad-PDGF-B resulted in the phosphorylation of Akt in human gingival fibroblast cells at the late stage, which is similar to the immediate response to recombinant human platelet-derived growth factor-BB protein. 1, Akt1; 2, Akt2; 3, Akt3; 4, pan-Akt; Ctrl, control.
Sustained phosphorylation of Akt in adenoviral gene delivery
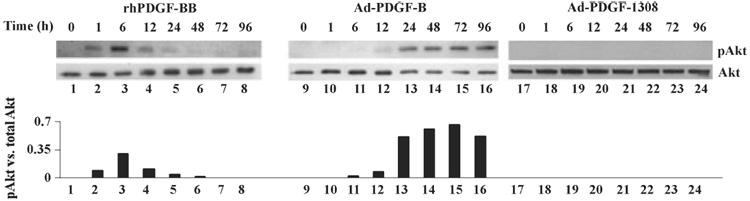
Based on our findings from the proteomics array, Akt was further evaluated as a key intracellular molecule that mediates the biological function of adenoviral PDGF gene delivery. These experiments were designed to explore in further detail the time curve of Akt activation during Ad-PDGF-B transduction, compared with exposure to recombinant human platelet-derived growth factor-BB. Our results showed that in cells exposed to recombinant human platelet-derived growth factor-BB, phosphorylation of Akt, which peaked at 1–6 h, was sustained only for 12–24 h. In human gingival fibroblasts treated with Ad-PDGF-B, the phosphorylation of Akt was initially seen as early as 6 h post-transduction and remained elevated for at least 96 h, to a greater relative extent than platelet-derived growth factor-BB (Fig. 5). Following Ad-PDGF-1308 delivery, human gingival fibroblasts did not show any Akt phosphorylation. We also examined the phosphorylation of Erk1/2 in different treatments. Ad-PDGF-B transduction elicited modest increases of Erk1/2 phosphorylation at the later time-points of 48–96 h, a result that was consistent with our proteomic array data (data not shown).
Fig. 5.
Sustained phosphorylation of Akt in adenoviral gene delivery. Western blotting was used to examine the time curve of Akt phosphorylation after gene delivery. Human gingival fibroblasts from three patients were tested and representative data are shown here. Human gingival fibroblast cells were exposed to Ad-PDGF-1308 (multiplicity of infection = 200), Ad-PDGF-B (multiplicity of infection = 200) or 20 ng/mL of recombinant human platelet-derived growth factor-BB in serum-free medium for 2 h. Then, cell lysates at 1, 6, 12, 24, 48, 72 and 96 h were subjected to western blotting using antibodies to phosphor-Akt and pan-Akt. The graphs show the relative ratio of phosphorylated Akt levels normalized to total Akt (analyzed by densitometric scanning). Recombinant human platelet-derived growth factor-BB induced transient Akt phosphorylation, which lasted about 24 h. However, with Ad-PDGF-B transduction, the phosphorylation of Akt was first seen as early as 6 h post-infection, and remained elevated for at least 96 h to a greater relative extent.
Discussion
A major goal of this study was to gain knowledge of the molecular mechanisms by which adenoviral gene delivery of PDGF-B mediates periodontal tissue regeneration. Our previous work showed that tooth-supporting structures could be re-engineered by Ad-PDGF-B. By using human gingival fibroblast cells, we also demonstrated that platelet-derived growth factor gene transfer has a potential use in periodontal soft tissue-engineering applications (24). Although the use of Ad-PDGF-B has enabled several advancements to be made in the promotion of periodontal regeneration, little is known about its effects in signal transduction from cells isolated from the periodontium. This study examined some of the cellular signaling events within human gingival fibroblasts resulting from adenoviral gene delivery of PDGF-B compared with direct protein delivery. This information aids in an improved understanding of these pathways that are likely to be critical in controlling the clinical outcomes of periodontal regenerative therapies.
Adenoviral vectors are known to be an effective tool for delivering genes to a broad range of cell types and tissues (27). Also, adenovirus has a high transduction efficiency and does not integrate into the host genome of the transduced cells (28). Compared with the short half-life of recombinant platelet-derived growth factor protein, our previous work showed that adenoviral gene delivery to rat periodontal sites induced marker gene expression, which was maintained at a high level at least until day 4 and rapidly decreased to 20% by day 14 (23). Therefore, we believe that by establishing the changes in signaling molecules in the first 96 h after treatment with Ad-PDGF-B we can understand its extended effects in more detail. We first examined the transduction efficiency of PDGF by Ad-PDGF-B gene transfer to human gingival fibroblasts using reverse transcription-polymerase chain reaction and observed a high level of PDGF gene expression for at least 96 h, and evident as early as 6 h post-infection (Fig 1). This correlates well with previous work by our group and by others, which consistently showed a sustained production of platelet-derived growth factors delivered by adenovirus (21,24,29) within other cells from periodontia. In view of the fact that some of the platelet-derived growth factor-BB protein may adhere to the polymer surface because of strong negative charges, we would expect the yield of platelet-derived growth factor-BB protein from the Ad-PDGF-B-transduced human gingival fibroblasts to be in the order of ~40 ng of protein per 106 cells. This was effective to maintain cell proliferation in serum-free medium (Fig. 2B). Also it would provide an efficacious dose of the growth factor in vivo for periodontal wound healing applications and could abrogate the need for continuous delivery compared with the topical ‘pulse’ administration of plate-let-derived growth factor-BB protein that typically results in the transient (< 24 h) release of platelet-derived growth factor-BB in vivo (16).
Platelet-derived growth factor is a soluble protein that stimulates cell proliferation and migration through membrane receptors of the receptor tyrosine kinase family. Platelet-derived growth factor-A only binds to platelet-derived growth factor-α receptor, whereas platelet-derived growth factor-B can bind to both αand βreceptors. Platelet-derived growth factor binding to the receptors also activates a variety of intracellular signaling molecules (3) and the platelet-derived growth factor α and –βreceptors activate many overlapping signaling pathways. The activated receptors phosphorylate a large number of substrates (more than 20), including themselves, which initiates a complex network of signaling cascades. Activation of either the α or the βreceptor can stimulate cell migration, although platelet-derived growth factor-B is generally a stronger inducer than platelet-derived growth factor-A (30–32). In addition to their traditional role as receptor tyrosine kinases, platelet-derived growth factor-α receptor and platelet-derived growth factor-β receptor have been associated with cell growth arrest (17). Lih et al. showed that platelet-derived growth factor-α receptor was highly expressed during the cell growth arrest stage induced by serum deprivation (33). Vaziri et al. reported that serum deprivation induced growth arrest in G0 and is associated with increased expression of platelet-derived growth factor-β receptor (34). We previously found that Ad-PDGF-A gene transfer resulted in sustained activation and subsequent downregulation of the platelet-derived growth factor-α receptor, a direct result of continued signaling by ligand binding (22). Vaziri et al. also found that the adenovirus continuously produced platelet-derived growth factor-AA dimers and that prolonged platelet-derived growth factor expression activated and downregulated platelet-derived growth factor-α receptor for an extended period of time. Carmen et al. reported that treatment with recombinant human platelet-derived growth factor-BB induced the downregulation of plate-let- derived growth factor receptor in mouse hepatic stellate cells (35). In the present study we measured a similar downregulation of both α and β platelet-derived growth factor receptors by Ad-PDGF-B gene transfer within human gingival fibroblast cells. The kinetics of platelet-derived growth factor-α and platelet-derived growth factor-β receptor protein expression in human gingival fibroblast cells were nearly identical: downregulated after 6 h post-transduction and maintained at very low levels for at least 96 h (Fig 3).
Protein phosphorylation is a fundamental mechanism for the regulation of many aspects of cellular functions. One of the important roles of protein phosphorylation is in signal transduction pathways, which are regulated at many levels, from the activation of cell-surface receptors to the stimulation of transcription factors. Detection of the phosphorylation state of intracellular proteins would provide valuable information on the activation state of human gingival fibroblasts following the long-term delivery of platelet-derived growth factor. In the present study, by using a human phospho-MAPK array, we simultaneously assessed the phosphorylation of 23 different intracellular kinases, including three major members of the MAPK family (ERK1/2, JNK1-3 and p38), Akt, GSK-3 and p70 S6 kinase. Our results from the protein array (Fig. 4), which were confirmed by western blotting, showed that Akt activation may play an important role by mediating the effect of long-term platelet-derived growth factor delivery in human gingival fibroblasts. In our experiment, adenovirus was delivered to human gingival fibroblasts by transient transfection (only 2 h), which explains in part why the phosphorylation levels of Akt at later time-points were relatively low. Akt (also known as protein kinase B), functions as a downstream target of phosphatidylinositol-3-kinase and plays a critical role in controlling cell survival and apoptosis (36,37). Akt is inactive in serum-starved conditions, but is activated by various growth factors or stimuli, including platelet-derived growth factor (25). It is a general survival signal mediator, which inhibits apoptosis by phosphorylating or inactivating several downstream targets, such as nuclear factor-κB, Bad, fork-head transcription factors and caspase-9 (38). Thus, the sustained activation of Akt after adenoviral platelet-derived growth factor-B delivery in human gingival fibroblasts helps us to understand, in more detail, a mechanism by which PDGF gene therapy supports tissue repair (24). This is also supported by the work of Mangi et al., which showed that mesenchymal stem cells transduced with Akt1 can repair infarcted myocardium, probably via promoting cell survival after cell transplantation (39). Recently, Akt was also found to regulate the growth arrest-related genes PDGFαR and PDGFβR. Activation of Akt inhibits the expression of PDGFαR and PDGFβR via activating mTOR. This suppression probably occurs as a result of decreased transcription of PDGFR and/or reduced mRNA half-life (40,41). Therefore, the sustained decrease of the platelet-derived growth factor-α and -β receptors (Fig. 3) could be partly caused by the prolonged activation of Akt.
In conclusion, this study demonstrated a high efficiency of PDGF-B gene transfer into human gingival fibroblasts using adenovirus, with expression of the PDGF gene detected up to 96 h post-infection. This has promise for clinical applications as direct application of recombinant human platelet-derived growth factor-BB for periodontal wound healing applications has proven effective yet typically displays a much shorter half-life. Our data also shows that specific intracellular signaling pathways (i.e., those involving platelet-derived growth factor receptors α and β, and Akt) mediate unique responses to growth factors via gene delivery within human gingival fibroblasts. These data on PDGF gene delivery contribute to an improved understanding of these pathways that are likely to play a role in the control of clinical outcomes of periodontal regenerative therapy.
Acknowledgements
This study was supported by NIH/NIDCR Grants DE13397, DE15384 and the AO Foundation Switzerland.
References
- 1.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 4.Heldin P, Laurent TC, Heldin CH. Effect of growth factors on hyaluronan synthesis in cultured human fibroblasts. Biochem J. 1989;258:919–922. doi: 10.1042/bj2580919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan DR, Chao FC, Stiles CD, Antoniades HN, Scher CD. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979;53:1043–1052. [PubMed] [Google Scholar]
- 6.Rosenkranz S, Kazlauskas A. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors. 1999;16:201–216. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- 7.Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982;92:584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green RJ, Usui ML, Hart CE, Ammons WF, Narayanan AS. Immunolocalization of platelet-derived growth factor A and B chains and PDGF-alpha and beta receptors in human gingival wounds. J Periodontal Res. 1997;32:209–214. doi: 10.1111/j.1600-0765.1997.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 9.Parkar MH, Kuru L, Giouzeli M, Olsen I. Expression of growth-factor receptors in normal and regenerating human periodontal cells. Arch Oral Biol. 2001;46:275–284. doi: 10.1016/s0003-9969(00)00099-6. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura F, Terranova VP. Comparative study of the chemotactic responses of periodontal ligament cells and gingival fibroblasts to polypeptide growth factors. J Dent Res. 1996;75:986–992. doi: 10.1177/00220345960750041401. [DOI] [PubMed] [Google Scholar]
- 11.Oates TW, Rouse CA, Cochran DL. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J Periodontol. 1993;64:142–148. doi: 10.1902/jop.1993.64.2.142. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda N, Lin WL, Kumar NM, Cho MI, Genco RJ. Mitogenic, chemotactic, and synthetic responses of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. J Periodontol. 1992;63:515–525. doi: 10.1902/jop.1992.63.6.515. [DOI] [PubMed] [Google Scholar]
- 13.Haase HR, Clarkson RW, Waters MJ, Bartold PM. Growth factor modulation of mitogenic responses and proteoglycan synthesis by human periodontal fibroblasts. J Cell Physiol. 1998;174:353–361. doi: 10.1002/(SICI)1097-4652(199803)174:3<353::AID-JCP9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Zaman KU, Sugaya T, Kato H. Effect of recombinant human platelet-derived growth factor-BB and bone morphogenetic protein-2 application to demineralized dentin on early periodontal ligament cell response. J Periodontal Res. 1999;34:244–250. doi: 10.1111/j.1600-0765.1999.tb02250.x. [DOI] [PubMed] [Google Scholar]
- 15.Giannobile WV, Hernandez RA, Finkelman RD, et al. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J Periodontal Res. 1996;31:301–312. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 16.Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 17.Bowen-Pope DF, Malpass TW, Foster DM, Ross R. Platelet-derived growth factor in vivo: levels, activity, and rate of clearance. Blood. 1984;64:458–469. [PubMed] [Google Scholar]
- 18.Park JB, Matsuura M, Han KY, et al. Periodontal regeneration in class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor. J Periodontol. 1995;66:462–477. doi: 10.1902/jop.1995.66.6.462. [DOI] [PubMed] [Google Scholar]
- 19.Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Control Release. 2006;112:103–110. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannobile WV, Lee CS, Tomala MP, Tejeda KM, Zhu Z. Platelet-derived growth factor (PDGF) gene delivery for application in periodontal tissue engineering. J Periodontol. 2001;72:815–823. doi: 10.1902/jop.2001.72.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Z, Lee CS, Tejeda KM, Giannobile WV. Gene transfer and expression of platelet-derived growth factors modulate periodontal cellular activity. J Dent Res. 2001;80:892–897. doi: 10.1177/00220345010800030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen QP, Giannobile WV. Adenoviral gene transfer of PDGF downregulates gas gene product PDGFa and prolongs ERK and Akt/PKB activation. Am J Physiol Cell Physiol. 2002;282:C538–C544. doi: 10.1152/ajpcell.00419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9:519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anusaksathien O, Webb SA, Jin QM, Giannobile WV. Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng. 2003;9:745–756. doi: 10.1089/107632703768247421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franke TF, Yang SI, Chan TO, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 26.Doukas J, Chandler LA, Gonzalez AM, et al. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum Gene Ther. 2001;12:783–798. doi: 10.1089/104303401750148720. [DOI] [PubMed] [Google Scholar]
- 27.Hitt MM, Addison CL, Graham FL. Human adenovirus vectors for gene transfer into mammalian cells. Adv Pharmacol. 1997;40:137–206. doi: 10.1016/s1054-3589(08)60140-4. [DOI] [PubMed] [Google Scholar]
- 28.Bromberg JS, Debruyne LA, Qin L. Interactions between the immune system and gene therapy vectors: bidirectional regulation of response and expression. Adv Immunol. 1998;69:353–409. [PubMed] [Google Scholar]
- 29.Tyrone JW, Mogford JE, Chandler LA, et al. Collagen-embedded platelet-derived growth factor DNA plasmid promotes wound healing in a dermal ulcer model. J Surg Res. 2000;93:230–236. doi: 10.1006/jsre.2000.5912. [DOI] [PubMed] [Google Scholar]
- 30.Bornfeldt KE, Raines EW, Graves LM, Skinner MP, Krebs EG, Ross R. Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Ann NY Acad Sci. 1995;766:416–430. doi: 10.1111/j.1749-6632.1995.tb26691.x. [DOI] [PubMed] [Google Scholar]
- 31.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Moon A, Kim HR. Both platelet-derived growth factor receptor (PDGFR)-alpha and PDGFR-beta promote murine fibroblast cell migration. Biochem Biophys Res Commun. 2001;282:697–700. doi: 10.1006/bbrc.2001.4622. [DOI] [PubMed] [Google Scholar]
- 33.Lih CJ, Cohen SN, Wang C, Lin-Chao S. The platelet-derived growth factor alpha-receptor is encoded by a growth-arrest-specific (gas) gene. Proc Natl Acad Sci U S A. 1996;93:4617–4622. doi: 10.1073/pnas.93.10.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaziri C, Faller DV. Repression of plate-let-derived growth factor beta-receptor expression by mitogenic growth factors and transforming oncogenes in murine 3T3 fibroblasts. Mol Cell Biol. 1995;15:1244–1253. doi: 10.1128/mcb.15.3.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechuga CG, Hernandez-Nazara ZH, Hernandez E, et al. PI3K is involved in PDGF-beta receptor upregulation post-PDGF-BB treatment in mouse HSC. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1051–G1061. doi: 10.1152/ajpgi.00058.2005. [DOI] [PubMed] [Google Scholar]
- 36.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 37.Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 38.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 39.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Cicchetti G, Onda H, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through down-regulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Bajraszewski N, Wu E, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–738. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]