Abstract
This review summarizes recent advances in electrochemical biosensors based on carbon nanotubes (CNTs) and carbon nanofibers (CNFs) with an emphasis on applications of CNTs. CNTs and CNFs have unique electric, electrocatalytic and mechanical properties, which make them efficient materials for developing electrochemical biosensors.
We discuss functionalizing CNTs for biosensors. We review electrochemical biosensors based on CNTs and their various applications (e.g., measurement of small biological molecules and environmental pollutants, detection of DNA, and immunosensing of disease biomarkers). Moreover, we outline the development of electrochemical biosensors based on CNFs and their applications. Finally, we discuss some future applications of CNTs.
Keywords: Biosensing, Carbon nanofiber, Carbon nanotube, Disease biomarker, DNA detection, Electrochemical biosensor, Environmental pollutant, Functionalization, Immunosensing, Small biological molecule
1. Introduction
Since the early 1990s, the science and technology of carbon nanotubes (CNTs) has been one of the fastest growing areas of research in chemistry, physics, materials, and life technologies [1–4]. The important properties and possible potential applications of CNTs have been extensively reviewed in recent years [3,5–9]. Of the applications, the development of CNT-based biosensors is one of most interesting areas, which has increased tremendously in recent decades. CNTs are attractive because of their unique properties (e.g., high electrical conductivity, great chemical stability and extremely high mechanical strength [3–6].
CNTs can be divided into two major groups – single-walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs) [10]. SWCNTs comprise a single graphite sheet rolled with a tube diameter of 1–2 nm, whereas MWCNTs are concentric, closed graphite tubules with diameters of 2–50 nm and an interlayer distance approximately 0.34 nm. Typical SWCNTs have an open-ended nanostraw or a capped nanohorn tubular structure. Because of their highly oriented architectures, these novel nanostructures possess unique electronic, chemical, thermal, and mechanical properties that differ from those of bulk graphite and diamond [3–6]. SWCNTs and MWCNTs are prepared with different approaches (e.g., chemical-vapor deposition (CVD)), which have been summarized [7,11]. In this article, we discuss electrochemical biosensors based on MWCNTs and SWCNTs.
Carbon nanofibers (CNFs) are cylindrical nanostructure with grapheme layers arranged as stacked cones, cups or plates [12]. They have lengths of the order of µm, while their diameter varies between some tens of nm to several hundred nm. Due to their specific properties, CNFs have also been used for biosensor development [13].
This article reviews recent progress in the development of electrochemical biosensors based on CNTs and CNFs with the emphasis on CNT-based electrochemical biosensors and their applications (e.g., enzyme sensors, DNA sensors, and immunosensors). In particular, we review layer-by-layer (LBL) assembly of enzyme nanocomposites on CNT-based and enzyme-based biosensors for detecting organophosphate pesticides. We briefly cover recent advances in developing CNF-based electrochemical biosensors. Finally, we discuss the outlook for future biosensor development.
2. Electrochemical biosensors based on carbon nanotubes
Recent studies have demonstrated that CNTs are promising materials for electrochemical biosensors and show great potential for use in the next generation of biosensors [4–6]. For example, CNTs can enhance the electroactivity of biomelecules and promote the electron-transfer reaction of proteins due to their electrocatalytic capabilities [14,15]. Moreover, CNTs are robust and have mechanical strength. These properties make CNTs efficient materials for use in a wide range of electrochemical biosensors ranging from amperometric enzyme electrodes to DNA hybridization biosensors and immunosensors.
2.1. Attachment of biomolecules
For biological applications, the lack of solubility of CNTs in aqueous media has been a major technical barrier. Great efforts have been devoted to finding cost-effective approaches to functionalize CNTs for attachment of biomolecules, such as proteins (e.g., enzymes, antibodies), DNA and aptamers, as summarized in review articles [6,11,16,17].
Some methods (i.e. chemical, electrochemical, thermal, and plasma oxidation) have been developed for treatment of the CNTs. With these treatments, the surface of CNTs can be generated with functional groups (e.g., carboxyl), which make the CNTs more hydrophilic and lay the foundation for further derivitization on its surface. This is a critical step for using CNTs in biosensor developments. For CNT-based biosensors, biomolecules are generally immobilized on CNTs by two approaches: non-covalent attachment and covalent binding [6,10,15].
For non-covalent attachment, physical adsorption and entrapment are generally used. However, either stability or durability of biosensors based on this approach may be problematic.
For covalent binding, some cross-linker agents (e.g., 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC)/ N-hydroxysuccinimide (NHS)) or affinity binding (e.g., biotin and avidin interaction) are usually used. The activity of the immobilized biomolecules may be affected due to steric hindrance by covalent binding.
In general, to achieve maximum attachment of biomelecules and keep the activity of the biomelecules on the CNTs, the choice of methods for treating CNTs and the method for attaching biomolecules are very critical for the performance of the biosensors.
According to the orientation of CNTs on the electrode surface, two types are generally used for immobilizing CNTs on electrodes: (i) non-aligned; and, (ii) aligned.
In the non-aligned approach, CNTs are randomly immobilized on the electrode surface by physical adsorption (e.g., casting or spin casting) and composite entrapment using polymer [18,19] and sol-gel [20].
There have been several approaches to achieving density-controlled, aligned CNTs [8,21–23] (e.g., CNT arrays by self-assembling functionalized CNTs (e.g., -COOH) [21], using pyrolyzing CVD of relevant catalysts and carbon materials followed by transferring CNTs onto a substrate support [22] or direct growth using CVD or plasma-enhanced CVD, offering a controllable size and a given location for the catalyst spots that allow the growth of a given numbers of CNTs [23,24]).
Both approaches are widely used for biosensor developments. The adsorption of CNTs on an electrode surface is a simple approach, while aligned CNTs on the electrode surface make electron transfer easier due to the high conductance of the edge plane of CNTs. Moreover, aligned CNTs can be prepared in parallel for fabricating CNT arrays on a substrate [24].
2.2. Enzyme-protein-based biosensors
Mostelectroactive sites of enzymes are embedded inside surrounding peptides. To fabricate effective protein/enzyme-based biosensors, recent research on CNTs-based biosensors has focused on two issues:
improving the electron-transfer reactions between protein/enzyme and electrodes; and,
enhancing the electrochemical reactivity of enzymatic products.
Due to their unique electric, electrocatalytic, and mechanical properties, CNTs are the excellent candidates for development of enzyme/protein-based biosensors [4–6].
2.2.1. Direct electrochemistry
Recently, direct electrochemical communication between redox-active biomacromolecules and conventional electrode substrates mediated by CNTs has received great attention because of their potential in the study of the structure–function relationship of these biomolecules and in biosensor design.
Wang et al. reported the direct electrochemistry of cytochrome c at electrochemically-activated SWCNT-modified electrodes [15]. Rusling et al. reported covalently attaching enzymes onto the ends of vertically-oriented SWCNT-forest arrays [25]. Quasi-reversible FeIII/FeII voltammetry was used to examine iron-heme enzymes myoglobin and horseradish peroxidase (HRP) coupled to the carboxylated ends of the nanotube forests by amide linkages. Observation suggested that the “trees” in the nanotube forest behaved similarly electrically to a metal, conducting electrons from the external circuit to the enzymes [25]. Gooding and co-authors also observed direct charge transfer between redox-active enzymes and the surface of aligned CNTs [21]. Their study revealed that the rate of electron transfer remained the same regardless of the lengths of the tubes. These findings enabled electroactive molecules to be located several hundred nm of a macroscopic substrate electrode with no loss in performance.
Willner et al. demonstrated that SWCNTs could plug into the active site of GOx, making feasible direct electron transfer from the redox center to electrodes through CNTs [14].
These studies demonstrated that direct electron-transfer reactions of redox-active biomacromolecules can be much improved through the use of CNTs, which enhanced the sensitivity of given biosensors and offered an opportunity to develop reagentless biosensors.
2.2.2. Enzyme-CNT biosensors
Most reported CNT-based biosensors are enzyme-modified electrodes for detecting different biomolecules (e.g., glucose [9]) and environment pollutants (e.g., organophosphate pesticides [26]). These types of sensor take advantage of enzymatic reaction-generated electroactive products, which can be sensitively detected on CNT-modified electrodes. Here, the CNT on the electrode surface functions as both an electrode for attaching biomelecules and a transducer for enhancing electrochemical response. Moreover, the CNTs can promote the electron-transfer reaction between the enzyme and the electrode, and can be used to develop reagentless enzyme biosensor.
Wang and co-workers employed CNT/Nafion-based electrodes for immobilizing glucose-oxidase (GOx) enzyme for sensitive detection of glucose [18]. This CNT/Nafion composite was prepared by dispersing solubilized CNTs in Nafion solution onto an electrode surface. The CNT-based biosensor offers substantially greater signals, especially at low potential, reflecting the electrocatalytic activity of CNTs. Such lowpotential operation of the CNT-based biosensor results in a wide linear range and a fast response time.
Several hundred enzymatic reactions of NAD+/NADH-dependent dehydrogenases have NADH as a cofactor. There is a large overpotential encountered for NADH oxidation at ordinary electrodes. CNTs have thus been examined as a new electrode material to reduce the overpotential [27]. It is found that the CNT offers remarkably decreased overvoltage for NADH oxidation as well as reducing the surface-fouling effects of the electrodes. These characteristics indicate great promise for CNTs in developing highly-sensitive, low-potential, stable, amperometric biosensors based on dehydrogenase enzymes [27].
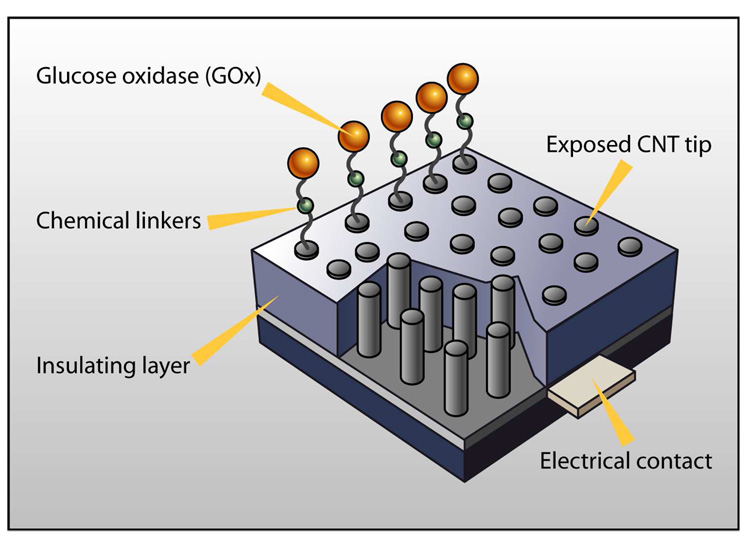
Lin and coworkers have demonstrated a novel glucose biosensor using CNT-nanoelectrode ensembles (Fig. 1) [28]. The CNT array was fabricated by growing CNTs directly on the patterned catalysts [24]. The density of the CNT array can be adjusted by changing the density of the catalysts.
Figure 1.
Glucose sensor based on carbon-nanotube (CNT) nanoelectrode ensembles.
The GOx molecules were attached to the open-ended tips of the CNTs by forming amide linkages between their amine residues and carboxylic-acid groups on the CNT tips via carbodiimide chemistry. For this CNT-array sensor, each CNT electrode acts as nanoelectrode. Because the distance between two nanoelectrodes is much larger than the diameter of each nanoelectrode, there is negligible overlap of diffusion layers between nanoelectrodes, so this kind of CNT-array biosensor has faster response time, lower background current, and higher signal/noise ratio than bulk-CNT-modified electrodes. Furthermore, glucose detection at a lower operating potential (−0.2 V) is significantly less influenced by the interferants, indicating high selectivity towards glucose. Such development of interference-free transducers will significantly simplify the design and the fabrication of biosensors. Biosensors based on low-site-density, aligned CNTs are also suitable for highly-selective detection of glucose in a variety of biological fluids (e.g., saliva, sweat, urine, and serum) [28].
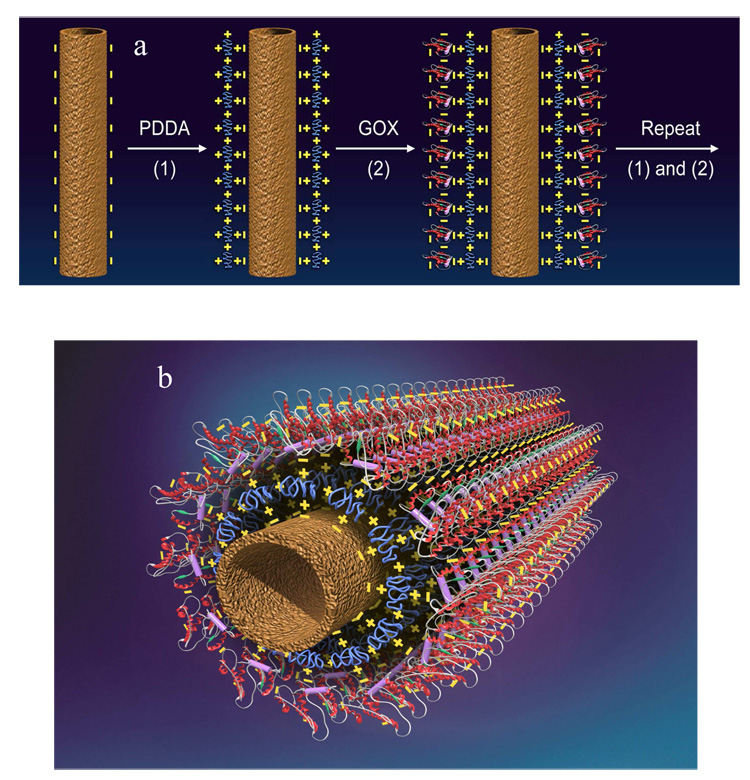
Recently, some enzyme/CNT biosensors using the LBL approach to immobilizing enzymes on CNTs were developed by Lin and co-workers [26,29,30]. Fig. 2 illustrates the procedure for attaching enzymes to CNTs using the LBL technique. A cationic polymer, poly(diallyldimethylammonium (PDDA), is used as a building block for this approach.
Figure 2.
(A) Procedure of assembling enzymes on carbon nanotube (CNT) by layer-by-layer (LBL) technique. (B) 3-D image of PDDA/enzyme/PDDA/CNT nanocomposite. (Reproduced from [29], with permission from ASP).
Two systems were developed for constructing enzyme-modified electrodes by this technique:
For the sensor of a binary enzyme system, the two enzymes are generally coupled because the enzymatic product of the first enzymatic reaction is the substrate of the second enzymatic reaction on the electrode surface. Enzyme-modified electrodes (PDDA/[enzyme/PDDA]n/MWCNT/glassy carbon (GC)) fabricated by the LBL technique have been used for different applications (e.g., glucose biosensor and choline biosensor).
A glucose biosensor was fabricated by immobilizing a single GOx enzyme on the CNT-modified electrodes with LBL technique [31]. This sensor has excellent electrocatalytic activity toward H2O2 with a great decrease in the overpotential, which makes it possible to detect glucose at very low potential. The results indicated that the polyelectrolyte-protein multilayer does not retard the electrocatalytic properties of CNTs. Furthermore, an amperometric choline biosensor was also demonstrated, based on an LBL assembly of a bi-enzyme of choline oxidase (ChO) and horseradish peroxidase (HRP) onto a CNT/GC electrode [30]. In this sensor design, choline oxidation was detected at CNT-modified electrodes through two coupled enzymatic reactions. The measurement of Faradic responses resulting from enzymatic reactions has been realized at low potential with good sensitivity because of the electrocatalytic effect of CNTs [30].
Enzyme/CNT-based biosensors have been used to detect not only small biomolecules but also environmental pollutants (e.g., organophosphate (OP) pesticides [32,33]). The mechanism of this biosensor for detecting OPs is based on inhibiting enzyme activity by these toxic chemicals. The cholinesterase (ChE) activity in this system is monitored by measuring the oxidation or reduction current of the enzymatic products and the extent of ChE activity is inversely correlated with the amount of chemical inhibitor present in the system. More inhibitors will result in less enzyme activity, so the sensor can be used for quantitation of chemicals that are known to inhibit blood, saliva or tissue ChE activity. OPs can be detected with one enzyme system or binary enzymes systems. The amperometric biosensors for OPs and nerve agents have been reported based on the self-assembly of acetylcholinesterase (AChE) on a CNT electrode using the LBL approach [26]. The electrocatalytic activity of CNT leads to a greatly improved electrochemical detection of the enzymatically generated thiocholine product, including a low oxidation overpotential (+150 mV), higher sensitivity, and stability. The biosensor was used to measure as low as 0.4 pM paraoxon with a 6-min inhibition time under optimal conditions [26].
With different immobilization approaches for one or two enzyme system, various enzyme/CNT based biosensors have been developed for detection of OPs [33]. Ju and coworkers reported a sensitive, fast, cheap flow injection biosensor for an organophosphorus insecticide based on immobilizing acetylcholinesterase (AChE) by binding covalently to a cross-linked chitosan-MWCNT composite [34]. The sensor shows signal in a wide linear range and fast response. Both bio-compatibility of chitosan and inherent conductive properties of MWCNTs favored detection of the insecticide with good stability and reproducibility [34].
Lin reported on a disposable CNT-based biosensor for OPs using a binary AChE and ChO/CNT-modified screen-printed carbon electrode [35]. The biosensors provided high sensitivity, large linear range, and low limits of detection (LODs) for the analysis of OP compounds. Such characteristics may be attributed to the catalytic activity of in promoting the redox reaction of hydrogen peroxide produced during AChE/ChO enzymatic reactions with their substrate, as well as the large surface area of the CNT materials.
Very recently, Deo and co-workers reported an amperometric biosensor for OP pesticides based on a CNT-modified transducer and an organophosphorus hydrolase (OPH) biocatalyst [32]. A bilayer approach with the OPH layer at the top of the CNT film was used to prepare the CNT/OPH biosensor. The CNT layer leads to a greatly improved anodic detection of the enzymatically-generated p-nitrophenol product, including higher sensitivity and stability. Under optimal conditions, the biosensor was used to measure levels as low as 0.15 mM paraoxon and 0.8 mM methyl parathion with sensitivities of 25 nA/mLM and 6 nA/mLM, respectively [32].
2.3. DNA/CNT biosensors
Interest in the detection of DNA has grown rapidly because of its importance in diagnosis and treatment of genetic disease, drug discovery, and anti-bioterrorism efforts [36]. The combination of the unique electric, thermal, chemical, mechanical, and 3-D spatial properties of CNTs with DNA hybridization (based on the Watson-Crick base-pair principle) offers the possibility of constructing DNA biosensors with simplicity, high sensitivity, and multiplexing.
For CNT-based electrochemical DNA sensors, the large surface area and electrocatalytic properties of CNTs can be used for sensitively detecting intrinsic electroactive residues of target DNA (e.g., guanine [37]) or enzymatic products from enzyme labels [38] or electroactive labels (e.g., ferrocene [39]). The mechanical and 3-D spatial properties of CNTs make them excellent carriers to load a large amount of enzymes or other electroactive species for labeling and amplifying the electrochemical signals. In addition, DNA adsorption onto the CNTs will lead to change in the electronic properties of the CNTs, which can be employed for constructing CNT-based field effect-transistors (FETs). These functionalized FETs, coupled with advanced sensor-array techniques, can provide a new direction in CNT-based bioassays. Some details of CNT-based FETs can be found in recent review articles [40].
Erdem reported an electrochemical DNA biosensor based on pencil graphite electrodes (PGEs) modified with MWCNTs, using direct electrochemical detection of the guanine signal [37]. The new DNA biosensor has shown some important advantages such as being inexpensive, sensitive, selective, and able to generate reproducible results using a simple, direct electrochemical protocol [37].
Wang and co-workers developed an ultrasensitive DNA biosensor based on a “dual amplification route” by using CNTs as both recognition sites and transducers, namely as carriers for numerous enzyme tags and as transducers for accumulating the product of the enzymatic reaction [38]. He and co-workers recently developed a novel electrochemical DNA biosensor by immobilizing single-strand chains onto aligned CNTs and detecting the oxidation signal from ferrocene-labeled complementary DNA [41].
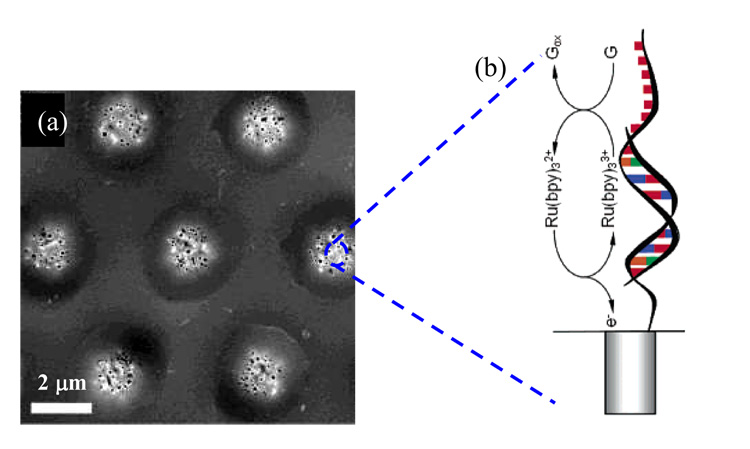
Li developed a microarray-based, ultrasensitive DNA sensor using aligned CNTs [23]. The mechanism of the sensor for DNA detection was demonstrated in Figure 3. The intrinsic guanine residues are used for electrochemical detection and a mediator, Ru(bpy)32+, was used to expedite guanine oxidation and enhance the electrochemical signals [23].
Figure 3.
(a) SEM image of carbon-nanotube (CNT) electrode array. (b) DNA sensor based on mediator-induced guanine oxidation. (Reproduced from [23], with permission from ACS).
2.4. CNT-based immunosensors
Proteins are essential components of organisms and are involved in a wide range of biological functions. There are increasing demands for ultra-sensitive protein detection because many important protein biomarkers are present at ultra-low levels, especially during the early stages of disease [42]. Electrochemical immunosensors based on CNTs have been extensively explored for sensitive detection of proteins in recent years [16]. The specific recognition interaction between antibody (or aptamer) and antigen are used for immunosensor. There are different detection strategies for CNT-based electrochemical immuosensors:
label-free immunosensor; and,
immunosensors that use labels and/or mediators.
Due to their large surface area, excellent conductivity, great toughness, and mechanical flexibility, CNTs can function as transduction materials in which both enzymatic products and electroactive tags of the immunoassay or electroactive part of analyte proteins with label-free can be sensitively detected at CNT-modified electrodes with amperometric and voltammetric techniques [43,44]. CNTs can also act as carriers and labels of immunoassay to load a large amount of enzymes or other electroactive species for amplifying electrochemical signals. CNTs have been used to develop FETs for detection of proteins. This approach is label-free and has been used for detecting a wide range of proteins. There are some details on CNT-based FETs in recent review articles [16,45,46].
CNT-based electrochemical immunosensors have been developed for detecting proteins and cancer biomarkers [47]. Rusling reported amperometric enzyme-linked immunoassays of proteins based on a vertically-aligned array of SWCNT forests [48–50]. A prototype amperometric immunosensor was evaluated based on the adsorption of antibodies onto perpendicularly-oriented assemblies of SWCNT forests [48]. The biosensor platform is also being developed to accommodate a flow-through sensor design with direct electron-transfer detection of the enzyme label on a CNT matrix.
Traditional electrochemical immunosensors were based on mediated electron transfer. However, efficient direct electron transfer will offer some advantages, such as simplicity, for a reagentless immunoassay. Wang reported CNTs as carriers to load a large amount of enzymes for amplified detection of proteins. Here, CNTs not only act as carriers but also concentrate enzymatic products on the electrode surface because the enzymatic products are absorbed onto the surface of the CNTs [38].
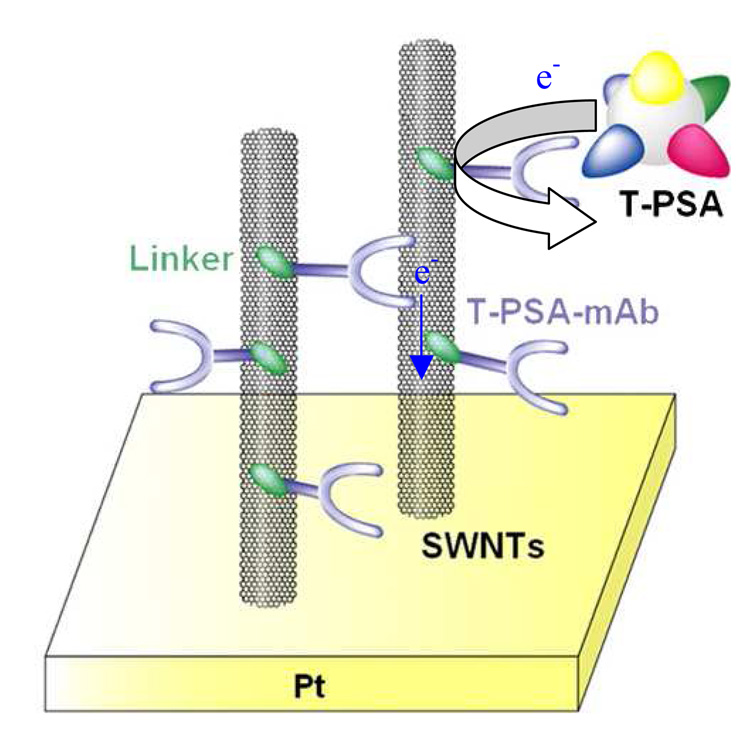
Okuno et al fabricated microelectrode arrays modified with SWCNTs for label-free immunosensing of total prostate-specific antigen (T-PSA) using differential pulse voltammetry (DPV) [51]. Fig. 4 shows the principle of this label-free immunosensor for detecting PSA. The monoclonal anti-T-PSA was covalently linked to the CNTs. It was found that the current signals, derived from the oxidation of tyrosine (Tyr), and tryptophan (Trp) residues in the PSA, increased with the interaction between T-PSA and T-PSA-mAb covalently immobilized on SWCNTs. The LOD for T-PSA was determined as 0.25 ng/mL.
Figure 4.
A label-free electrochemical immunosensor based on oxidation of tyrosine on carbon nanotubes (CNTs). (Reproduced from [51], with permission from Elsevier).
The performance of the label-free electrochemical immunosensor seems promising for further clinical applications [51]. Viswanathan et al developed a CNT-based immunosensor for sensitive detection of cholera toxin (CT), in which monoclonal antibody against the B subunit of CT is linked to poly(3,4-ethylenedioxythiophene) coated on a Nafion-supported MWCNT caste film on a glassy-carbon electrode, and potassium ferrocyanide-encapsulated and ganglioside (GM1)-functionalized liposomes act as highly specific recognition labels for the amplified detection of CT [43]. CT was detected on CNT using square-wave voltammetric measurement of the released potassium ferrocyanide molecules from the bound liposomes. The sandwich assay provided the amplification route for the detection of the CT present in ultratrace levels [43]. Rusling developed an electrochemical immunosensor combining SWCNT forest platforms with multi-label secondary antibody-nanotube bioconjugates for highly sensitive detection of PSA [49]. Greatly amplified sensitivity was attained by using bioconjugates featuring HRP labels and secondary antibodies (Ab2) linked to a CNT at high HRP/Ab2 ratio. This approach provided an LOD of 4 pg/mL (100 amol/mL), for PSA in 10 µL of undiluted calf serum.
3. Carbon nanofiber-based biosensors
The use of CNF for biosensor development has grown in recent years because of their physical and chemical properties (e.g., conductivity, surface area, inherent and induced chemical functionalities, and biocompatibility). Recent activity on CNF-based biosensors has focused on functionalization of the CNFs for biosensing DNA and proteins.
3.1. Functionalization of carbon nanofibers
The functionalization of CNFs is critical for utilizing their specific properties for biosensor development. Some approaches to biochemical functionalization of synthetic CNFs have been studied, including physical adsorption and covalent bonding. Electrochemical techniques are often used to produce functional groups on the surface of CNFs for further modifications.
Lee reported the use of reactions to generate amino groups by electrochemical oxidation of nitro groups on specific nanostructures for covalently linking DNA to only nanostructures of vertically-aligned CNFs (VACNFs) [52]. DNA hybridization shows that the method works well and DNA-modified nanoscale structures have excellent biological selectivity.
Baker reported two different strategies (photochemical reaction and electrochemical reaction) for covalently modifying CNFs with biological molecules, such as DNA. Both methods yield DNA-modified CNFs exhibiting excellent specificity and reversibility in binding to DNA-probe molecules in solution having complementary vs non-complementary sequences [53].
McKnight introduced site-specific biochemical functionalization along the height of vertically-aligned CNF arrays by a photoresist-blocking method, which can be used for site-specific physical, chemical, and electrochemical functionalization of nanofiber arrays both spatially across regions of the device as well as along the length of the vertical nanofibers [54].
Fletcher developed two attachment schemes making use of a class of heterocyclic aromatic dye compounds for specific adsorption onto VACNFs and covalently coupling biomolecules through cross-linking to carboxylic-acid sites on the sidewalls of the CNFs [55]. The adsorption and covalent coupling properties were consistent with the physical structure and the chemical characteristics of the VACNFs.
These investigations have extended the application of functionalized CNFs for biosensor development.
3.2. Biosensors based on carbon nanofibers
Potentially, CNF-based biosensors have extensive applications (e.g., glucose biosensors).
Vamvakaki reported a glucose biosensor based on highly-activated CNFs with direct enzyme immobilization [56]. This is a highly efficient method for developing very sensitive, stable, reproducible electrochemical biosensors.
Wu and co-workers reported a CNF-based amperometric glucose sensor [57], which showed excellent catalytic activity of soluble CNF. It was obtained with a simple nitricacid treatment, with electro-reduction of dissolved oxygen at a low operating potential. The CNF membrane showed good stability and provided fast response to dissolved oxygen. The use of a low operating potential (−0.3 V) and a Nafion membrane also produced good selectivity toward glucose detection.
Li and co-workers studied the effect of CNF microstructure on the electrochemical sensing of hydrogen peroxide [58]. The investigation of three types of CNFs included platelet-like CNFs (PCNFs), fish-bone-like CNFs (FCNFs), and tube-shaped CNFs (TCNFs). It revealed significant diversity of electrocatalytic activity of these CNFs toward the oxidation of hydrogen peroxide, which could have resulted from differences of the CNFs in morphology, texture, and crystalline structure.
Arvinte and co-workers reported the use of CNFs to design electrochemical biosensors based on the electro-catalytic activity of CNFs toward NADH [59]. The direct electrochemistry of NADH was studied at a CNF-modified carbon electrode and a decrease of the oxidation potential of NADH by more than 300 mV was observed compared with a bare glassy-carbon electrode.
These results confirmed that CNFs provide a promising material for assembling electrochemical sensors and biosensors.
The excellent conductivity and strong mechanical strength of CNFs have been utilized to design a biocompatible architecture for attaching and cytosensing K562 cells on an electrode [60]. K562 cells are the human immortalized myelogenous leukemia line. The electrochemical impedance spectroscopic and cyclic voltammetric measurement of the K562 cells modifying the CNF-nanocomposite film revealed that the impedance of electronic transduction was related to the amount of the adhered cells, thus producing a highly sensitive impedance sensor for K562 cells.
4. Conclusion and outlook
This review has highlighted recent progress in the development of electrochemical biosensors based on CNTs and CNFs and their typical applications (e.g., enzyme-based biosensors, and DNA and protein-based bioassays). CNTs and CNFs not only improve the sensitivity of given methods but also diversify research in electrochemical-biosensor development. CNTs and CNFs have great potential to create the next generation of electrochemical biosensors due to their unique structural, electrical and mechanical properties.
The highly-ordered CNT array combined with multiple biorecognition holds the promise of developing multiplexed electrochemical biosensors. It will be very useful for high-throughput diagnosis and screening.
Due to their small size and robustness, CNTs may be a good candidate material for fabricating nanoscale biosensors for in-body biosensing and for making remote-controlled electrochemical biosensors for environmental monitoring.
In general, electrochemical biosensors based on CNTs and CNFs show great promise for future applications in heath-care testing, disease diagnostics and environmental monitoring.
Acknowledgements
This work was supported by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke (Award # NS058161-01) and partially by CDC/NIOSH Grant R01 OH008173-01. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the US Federal Government. This research was performed at the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the US Department of Energy’s Office of Biological and Environmental Research. Pacific Northwest National Laboratory is operated by Battelle for DOE under Contract DE-AC05-76RL01830.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tasis D, Tagmatarchis N, Bianco A, Prato M. Chem. Rev. 2006;106:1105. doi: 10.1021/cr050569o. [DOI] [PubMed] [Google Scholar]
- 2.Baxendale M. J. Mater. Sci.: Mater. Electron. 2003;14:657. [Google Scholar]
- 3.Yang WR, Thordarson P, Gooding JJ, Ringer SP, Braet F. Nanotechnology. 2007;18:412001. [Google Scholar]
- 4.Pagona G, Tagmatarchis N. Current Med. Chem. 2006;13:1789. doi: 10.2174/092986706777452524. [DOI] [PubMed] [Google Scholar]
- 5.Kim SN, Rusling JF, Papadimitrakopoulos F. Adv. Mater. 2007;19:3214. doi: 10.1002/adma.200700665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yun YH, Dong ZY, Shanov V, Heineman WR, Halsall HB, Bhattacharya A, Conforti L, Narayan RK, Ball WS, Schulz MJ. Nano Today. 2007;2:30. [Google Scholar]
- 7.Sinha N, Ma JZ, Yeow JTW. J. Nanosci. Nanotechnol. 2006;6:573. doi: 10.1166/jnn.2006.121. [DOI] [PubMed] [Google Scholar]
- 8.Lin YH, Yantasee W. J. Wang, Front. Biosci. 2005;10:492. doi: 10.2741/1545. [DOI] [PubMed] [Google Scholar]
- 9.Wang J. Electroanalysis (NY) 2005;17:7. [Google Scholar]
- 10.Ajayan PM. Chem. Rev. 1999;99:1787. doi: 10.1021/cr970102g. [DOI] [PubMed] [Google Scholar]
- 11.Merkoci A, Pumera M, Llopis X, Perez B, del Valle M, Alegret S. Trends Anal. Chem. 2005;24:826. [Google Scholar]
- 12.Rodriguez NM. J. Mater. Res. 1993;8:3233. [Google Scholar]
- 13.Vamvakaki V, Fouskaki M, Chaniotakis N. Anal. Lett. 2007;40:2271. [Google Scholar]
- 14.Patolsky F, Weizmann Y, Willner I. Angew. Chem. Int. Ed. Engl. 2004;4:2113. doi: 10.1002/anie.200353275. [DOI] [PubMed] [Google Scholar]
- 15.Wang JX, Li MX, Shi ZJ, Li NQ, Gu ZN. Anal. Chem. 2002;74:1993. doi: 10.1021/ac010978u. [DOI] [PubMed] [Google Scholar]
- 16.Veetil JV, Ye KM. Biotechnol. Progr. 2007;23:517. doi: 10.1021/bp0602395. [DOI] [PubMed] [Google Scholar]
- 17.Souza AG, Fagan SB. Quim. Nova. 2007;30:1695. [Google Scholar]
- 18.Wang J, Musameh M, Lin YH. J. Am. Chem. Soc. 2003;125:2408. doi: 10.1021/ja028951v. [DOI] [PubMed] [Google Scholar]
- 19.Rege K, Raravikar NR, Kim DY, Schadler LS, Ajayan PM, Dordick JS. Nano Lett. 2003;3:829. [Google Scholar]
- 20.Gavalas VG, Andrews R, Bhattacharyya D, Bachas LG. Nano Lett. 2001;1:719. [Google Scholar]
- 21.Gooding JJ, Wibowo R, Liu JQ, Yang WR, Losic D, Orbons S, Mearns FJ, Shapter JG, Hibbert DB. J. Am. Chem. Soc. 2003;125:9006. doi: 10.1021/ja035722f. [DOI] [PubMed] [Google Scholar]
- 22.Gao M, Dai LM, Wallace GG. Electroanalysis (NY) 2003;15:1089. [Google Scholar]
- 23.Li J, Ng HT, Cassell A, Fan W, Chen H, Ye Q, Koehne J, Han J, Meyyappan M. Nano Lett. 2003;3:597. doi: 10.1088/0957-4484/14/12/001. [DOI] [PubMed] [Google Scholar]
- 24.Tu Y, Lin YH, Ren ZF. Nano Lett. 2003;3:107. [Google Scholar]
- 25.Yu X, Chattopadhyay D, Galeska I, Papadimitrakopoulos F, Rusling JF. Electrochem. Commun. 2003;5:408. [Google Scholar]
- 26.Liu GD, Lin YH. Anal. Chem. 2006;78:835. doi: 10.1021/ac051559q. [DOI] [PubMed] [Google Scholar]
- 27.Musameh M, Wang J, Merkoci A, Lin YH. Electrochem. Commun. 2002;4:743. [Google Scholar]
- 28.Lin YH, Lu F, Tu Y, Ren ZF. Nano Lett. 2004;4:191. [Google Scholar]
- 29.Liu GD, Lin YH. J. Nanosci. Nanotechnol. 2006;6:948. doi: 10.1166/jnn.2006.133. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Liu GD, Lin YH. Analyst (Cambridge, UK) 2006;131:477. doi: 10.1039/b516038c. [DOI] [PubMed] [Google Scholar]
- 31.Liu GD, Lin YH. Electrochem. Commun. 2006;8:251. [Google Scholar]
- 32.Deo RP, Wang J, Block I, Mulchandani A, Joshi KA, Trojanowicz M, Scholz F, Chen W, Lin YH. Anal. Chim. Acta. 2005;530:185. [Google Scholar]
- 33.Liu NY, Cai XP, Lei Y, Zhang Q, Chan-Park MB, Li CM, Chen W, Mulchandani A. Electroanalysis (NY) 2007;19:616. [Google Scholar]
- 34.Kandimalla VB, Ju HX. Chem.-Euro. J. 2006;12:1074. doi: 10.1002/chem.200500178. [DOI] [PubMed] [Google Scholar]
- 35.Lin YH, Lu F, Wang J. Electroanalysis (NY) 2004;16:145. [Google Scholar]
- 36.Sassolas A, Leca-Bouvier DL, Blim LJ. Chem. Rev. 2008;108:109. doi: 10.1021/cr0684467. [DOI] [PubMed] [Google Scholar]
- 37.Erdem A, Papakonstantinou P, Murphy H. Anal. Chem. 2006;78:6656. doi: 10.1021/ac060202z. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Liu GD, Jan MR. J. Am Chem. Soc. 2004;126:3010. doi: 10.1021/ja031723w. [DOI] [PubMed] [Google Scholar]
- 39.Seiwert B, Karst U. Anal. Bioanal. Chem. 2008;390:181. doi: 10.1007/s00216-007-1639-7. [DOI] [PubMed] [Google Scholar]
- 40.Allen BL, Kichambare PD, Star A. Adv. Mater. 2007;19:1439. [Google Scholar]
- 41.He PG, Dai LM. Chem. Commun. 2004:348. doi: 10.1039/b313030b. [DOI] [PubMed] [Google Scholar]
- 42.Zhang HQ, Zhao Q, Li XF, Le XC. Analyst (Cambridge, UK) 2007;132:724. doi: 10.1039/b704256f. [DOI] [PubMed] [Google Scholar]
- 43.Viswanathan S, Wu LC, Huang MR, Ho JAA. Anal. Chem. 2006;78:1115. doi: 10.1021/ac051435d. [DOI] [PubMed] [Google Scholar]
- 44.Yun Y, Bange A, Heineman WR, Halsall HB, Shanov VN, Dong ZY, Pixley S, Behbehani M, Jazieh A, Tu Y, Wong DKY, Bhattacharya A, Schulz MJ. Sens. Actuators, Part B. 2007;123:177. doi: 10.1166/jnn.2007.408. [DOI] [PubMed] [Google Scholar]
- 45.Katz HE. Electroanalysis (NY) 2004;16:1837. [Google Scholar]
- 46.Wanekaya AK, Chen W, Myung NV, Mulchandani A. Electroanalysis (NY) 2006;18:533. [Google Scholar]
- 47.Ou CF, Yuan R, Chai YQ, Tang MY, Chai R, He XL. Anal. Chim Acta. 2007;603:205. doi: 10.1016/j.aca.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 48.Yu X, Kim SN, Papadimitrakopoulos F, Rusling JF. Mol. Biosys. 2005;1:70. doi: 10.1039/b502124c. [DOI] [PubMed] [Google Scholar]
- 49.Yu X, Munge B, Patel V, Jensen G, Bhirde A, Gong JD, Kim SN, Gillespie J, Gutkind JS, Papadimitrakopoulos F, Rusling JF. J. Am. Chem. Soc. 2006;128:11199. doi: 10.1021/ja062117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Connor M, Kim SN, Killard AJ, Forster RJ, Smyth MR, Papadimitrakopoulos F, Rusling JF. Analyst (Cambridge, UK) 2004;129:1176. doi: 10.1039/b412805b. [DOI] [PubMed] [Google Scholar]
- 51.Okuno J, Maehashi K, Kerman K, Takamura Y, Matsumoto K, Tamiya E. Biosens. Bioelectron. 2007;2:2377. doi: 10.1016/j.bios.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 52.Lee CS, Baker SE, Marcus MS, Yang WS, Eriksson MA, Hamers RJ. Nano Lett. 2004;4:1713. [Google Scholar]
- 53.Baker SE, Tse KY, Hindin E, Nichols BM, Clare TL, Hamers RJ. Chem. Mater. 2005;17:4971. [Google Scholar]
- 54.McKnight TE, Peeraphatdit C, Jones SW, Fowlkes JD, Fletcher BL, Klein KL, Melechko AV, Doktycz MJ, Simpson ML. Chem. Mater. 2006;18:3203. [Google Scholar]
- 55.Fletcher BL, McKnight TE, Melechko AV, Simpson ML, Doktycz MJ. Nanotechnol. 2006;17:2032. [Google Scholar]
- 56.Vamvakaki V, Tsagaraki K, Chaniotakis N. Anal. Chem. 2006;78:5538. doi: 10.1021/ac060551t. [DOI] [PubMed] [Google Scholar]
- 57.Wu L, Zhang XJ, Ju HX. Biosens. Bioelectron. 2007;23:479. doi: 10.1016/j.bios.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Li ZZ, Cui XL, Zheng JS, Wang QF, Lin YH. Anal. Chim. Acta. 2007;597:238. doi: 10.1016/j.aca.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 59.Arvinte A, Valentini F, Radoi A, Arduini F, Tamburri E, Rotariu L, Palleschi G, Bala C. Electroanalysis (NY) 2007;19:1455. [Google Scholar]
- 60.Hao C, Ding L, Zhang XJ, Ju HX. Anal. Chem. 2007;79:4442. doi: 10.1021/ac062344z. [DOI] [PubMed] [Google Scholar]
- 61.Singh S, Nalwa HS. J. Nanosci. Nanotechnol. 2007;7:3048. doi: 10.1166/jnn.2007.922. [DOI] [PubMed] [Google Scholar]