Abstract
Neuroendocrinology of the aging (male) gonadal and (male and female) somatotropic axes will be reviewed. A companion chapter (see J.E. Hall) discusses reproductive hormonal changes in aging women. Both the gonadal and growth-hormone/insulin-like growth factor (GH/IGF-I) axes function as ensembles. The ensembles comprise tripartite interactions among the brain (hypothalamus), anterior pituitary gland (gonadotrope and somatotrope cells) and target organs (testis, liver, muscle, fat and brain). Compelling evidence indicates that combined hypothalamic and gonadal adaptations operate in the reproductive axis of older men, and multiple hypothalamic adaptations prevail in the GH axis of elderly men and women. Evolving investigative methods allow more precise parsing of the particular mechanisms that subserve such age-related changes, and suggest novel interventional strategies to evaluate the physiological impact of the dynamic alterations discerned in aging individuals.
Keywords: human, brain, anterior pituitary, gonadotropin, somatotropin, androgen, men, growth hormone, luteinizing hormone, IGF-I
2. Introduction
Aging is accompanied by incremental adaptations in behavior, motivation, cognition, physical activity, body composition, energy expenditure and the endocrine milieu. This review will highlight prominent age-associated adaptations in gonadotropin (luteinizing hormone, LH), testosterone (Te), and somatotropin (GH) secretion in the human.
3. The Male Gonadal Axis
3.1 Overview of Te deficiency in aging
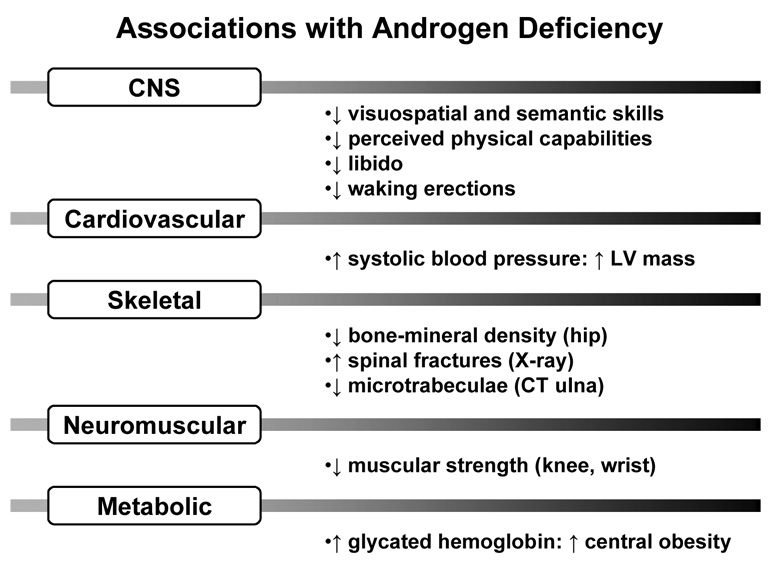
Albeit recognized biochemically nearly 55 yr ago, Te depletion in aging is an increasingly pertinent clinical issue (Liu et al. 2004; Liu et al. 2005a; Liverman and Blazer 2004). The current medical focus is motivated by reported epidemiological linkages between hypoandrogenemia and muscle weakness, sarcopenia, osteopenia, diminished physical stamina, erectile dysfunction, systolic hypertension, carotid artery-wall thickness, abdominal visceral-fat mass, insulin resistance, low HDL concentrations, postprandial somnolence, impaired quality of life, depressive mood, diminished working memory, and decreased executive-cognitive function (Kenny et al. 2001; Moffat et al. 2002; Schroeder et al. 2004; Wang et al. 1996): Figure 1. Among such postulated relationships, low Te availability correlates with reduced grip strength, decreased lean-body mass and increased visceral adiposity in metaanalyses (Isidori et al. 2005). The same pathophysiological features are significantly reversed by Te supplementation (Liu et al. 2004; Liu et al. 2005a; Liverman and Blazer 2004).
Figure 1.
Epidemiological associations reported with androgen deficiency. Unpublished summary. CNS = central nervous system, LV = left ventricle, CT = computed tomography
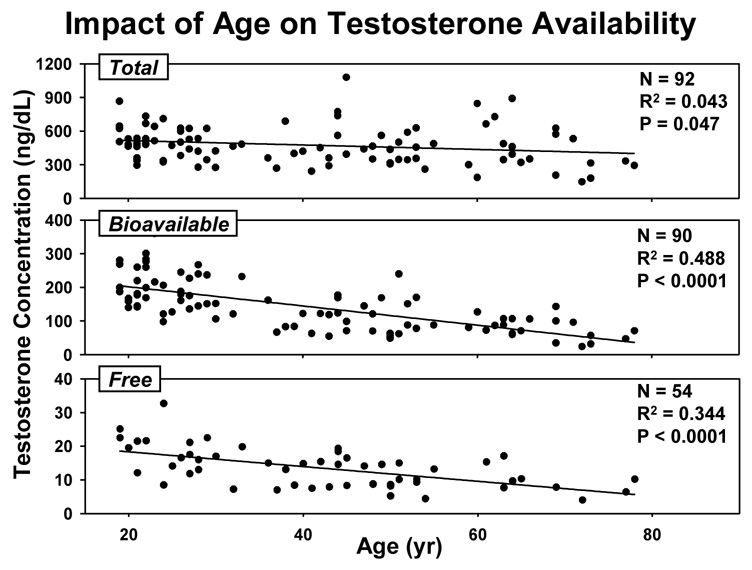
Impoverished Te production in older men has been documented by (i) direct sampling of the spermatic vein, (ii) metaanalysis of cross-sectional, and (iii) longitudinal investigations in healthy cohorts. A 15-yr prospective analysis in New Mexico reported that total Te concentrations fall by approximately 110 ng/dL per decade in men after the age of 60 yr; the Massachusetts Male Aging Cohort study forecast a 0.8–1.3% annual decrement in bioavailable (nonSHBG-bound) Te concentrations; and, the Baltimore Longitudinal Study of Aging predicted a yearly decline of 4.9 pmol Te/nmol SHBG (Harman et al. 2001; Liu et al. 2005a; Liu et al. 2004; Morley et al. 1997). In the last study, the age-related prevalence of hypogonadal serum Te/SHBG ratios according to young-adult normative criteria exceeded 20%, 30% and 50% at ages 60, 70 and 80 yr, respectively. In addition, medications, comorbidity and intercurrent illness in aging populations exacerbate androgen deficiency (Gray et al. 1991). Cross-sectional data collected in healthy men in Olmsted County, MN, verify age-associated decrements in Te concentrations measured by tandem mass spectrometry: Figure 2.
Figure 2.
Impact of age in cross-sectional analyses of total, bioavailable and free Te concentrations in healthy men in Olmsted County, MN. To convert ng/dL to international units (nmol/L), multiply by 0.0347. Unpublished.
3.2 Need for mechanistic understanding of gonadal axis as a whole
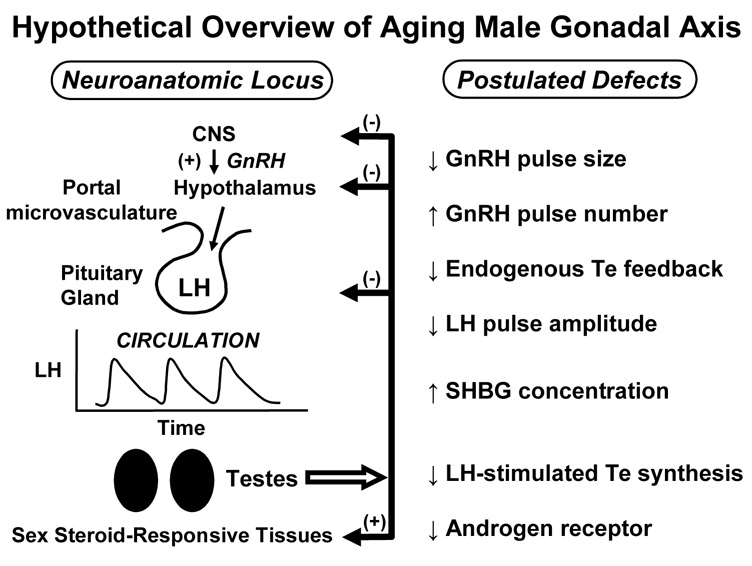
As pointed out by the U.S.A. Institute of Medicine (Liverman and Blazer 2004), the primary mechanisms mediating gradual Te depletion in older men are unknown Available data point to presumptive multisite impairment; viz., reduced hypothalamic GnRH outflow, decreased testicular responsiveness to hCG/LH, and attenuated androgenic negative feedback (Keenan et al. 2006; Keenan and Veldhuis 2004): Figure 3. Which of these proposed mechanisms is primary is not known. Because of strong physiological connectivity among GnRH, LH and Te, definitive mechanistic assessment necessitates ensemble-level analyses of feedback (inhibitory) and feedforward (stimulatory) dose-response pathways interlinking these signals. The analytical formalism for this approach has been validated by analyses of hypothalamo-pituitary, jugular and spermatic-vein hormone release in 3 mammalian species (Keenan et al. 2006; Keenan et al. 2004). Clinical implications of determining definitive loci of impaired regulation are suggested in Table 1.
Figure 3.
Principal mechanistic alterations in aging male gonadal axis deduced to date. Unpublished précis. CNS = central nervous system, GnRH = gonadotropin-releasing hormone, Te = testosterone, SHBG = sex-hormone binding globulin, LH = luteinizing hormone
Table 1.
Translational implications of understanding the physiology of aging
| Age impairs the efficacy of LH drive of testicular | ||
| steroidogenesis | ||
| • | rescue Leydig cells via COX2 inhibitors, cytokine antagonists, adenosine analogs, selective estrogen-receptor modulators, or oxygen scavengers | |
| • | reconstitute Leydig cell by bone-marrow mesenchymal pluripotent stem cell | |
| Age decreases GnRH secretion | ||
| • | develop GnRH-neuronal secretagogues | |
| • | explore GnRH disinhibitory agents | |
| Age reduces sex-steroid feedback via CNS | ||
| androgen receptors | ||
| • | evaluate therapeutic action of selective androgen- and estrogen-receptor modulators | |
| • | assess generality of reduced brain steroid action | |
LH = luteinizing hormone
COX2 = cyclooxygenase type 2; GnRH = gonadotropin-releasing hormone; CNS = central nervous system
(i) Aging attenuates testis responses to LH
Administration of the LH surrogate, hCG, in pharmacological amounts fails to stimulate maximal young-adult testosterone concentrations in many older men (Liu et al. 2005a). However, pharmacological hCG stimulation paradigms are difficult to interpret because: (i) baseline LH concentrations differ in young and older men; (ii) the half-life of hCG (24 hr) is much longer than that of LH (1 hr), resulting in nearly continuous stimulation of Leydig cells; (iii) hCG unlike LH rapidly downregulates Leydig-cell steroidogenesis even in young men (iv) the dose of hCG used clinically is multifold greater than the bioactivity contained in a normal pulse of LH (Liu et al. 2005a). Therefore, clinical hCG stimulation protocols, albeit utilized for 3 decades, are inadequate to evaluate the physiological basis for declining Te secretion in aging.
A recent approach to quantify Leydig-cell Te secretion entails infusing fixed pulses of recombinant human (rh) LH after suppressing LH secretion overnight with a potent GnRH-receptor antagonist, ganirelix. In young men, 7 consecutive i.v. pulses of rhLH normalized ganirelix-suppressed LH and Te concentrations, thus validating the model. The stimulation regimen elevated unbound Te concentrations by 50% less in older than young men (Veldhuis et al. 2005g). Extending the ganirelix clamp and rhLH pulses for a full 2 days also failed to reconstitute young-adult bioavailable (nonSHBG-bound) and free (equilibrium-dialyzed) Te concentrations in aging individuals (Liu et al. 2005b). However, these studies used a single fixed LH dose. What remains unknown is how aging alters the LH→Te dose-response function in healthy men. In corollary, given that LH/hCG can rapidly downregulate (desensitize) testicular responses, the query emerges, Do gradually rising LH concentrations in older men contribute to gonadal downregulation? If so, higher LH concentrations due to lower Te production in aging individuals could create a vicious cycle leading to progressive androgen deficiency. These hypotheses will be important to test given the implications underscored in Table 1.
(ii) Age diminishes hypothalamic GnRH secretion
The nearly 3 decade-long intuition that hypothalamic outflow of GnRH is reduced in older men has been difficult to establish or refute (Winters and Troen 1982): Figure 3. The hypothesis is congruent with laboratory observations in the male rodent, which include: (i) attenuated postcastration, anesthesia and restraint stress-induced LH release with preserved acute responses to exogenous GnRH (Liu et al. 2005a); (ii) diminished LH pulse amplitude in vivo and GnRH release by hypothalamic tissue in vitro (Veldhuis et al. 2007b); (iii) fewer GnRH neuronal synapses (Witkin 1987); and (iv) restoration of sexual activity in the impotent aged male rat by implanting fetal hypothalamic tissue (Huang et al. 1987). Most clinical observations are consistent with an hypothesis of reduced GnRH secretion: Table 2. However, a few studies have inferred that older men have blunted gonadotrope responses to exogenous GnRH, increased LH pulse size and/or decreased in vitro LH bioactivity (Liu et al. 2005a; Veldhuis et al. 2007b). Discrepancies motivated the recent development of more compelling methods to appraise how age affects hypothalamic GnRH secretion. Among these, an ensemble-based analysis strongly predicts a significant (> 30%) fall in brain GnRH output to the pituitary in healthy older men (Keenan et al. 2006).
Table 2.
Indirect evidence for impaired GnRH outflow in older men
|
(iii) Age impairs testosterone-mediated negative feedback on brain GnRH outflow and/or pituitary LH secretion
How age affects Te-dependent negative feedback has been controversial. Three studies described excessive suppression of LH concentrations in older men by short-term (4-day) i.v. infusion of Te and longer-term (11-day and 15-month) transscrotal administration of Te or transdermal delivery of 5 alpha-dihydroTe (DHT) (Liu et al. 2005a). Two other investigations reported less suppression of LH concentrations in older than young subjects following i.m. injections of Te. Five experimental paradigms suggest impaired feedback regulation of LH production by endogenous Te concentrations in elderly men. Three other noninvasive model-based analyses indicate that aging restricts negative feedback on GnRH/LH secretion by any given Te concentration. In contrast, only one study has assessed whether estrogen-dependent negative feedback changes in aging men (Veldhuis and Iranmanesh 2005).
Histochemical studies 2 decades ago described reduced androgen-receptor (AR) expression in the brain, pituitary gland, prostate and penis of the aged rat and in genital fibroblasts of the older human (Haji et al. 1980; Roth and Heiss 1982): Figure 3. However, the generality and functional implications of these findings remain unclear. Although AR depletion in the hypothalamo-pituitary unit could decrease the feedback efficacy of Te, whether significant AR depletion occurs in critical neuroregulatory regions associated with GnRH secretion is uncertain. Moreover, how age affects local conversion of Te to E2 or 5α-dihydrotestosterone (DHT) in the human hypothalamus and pituitary gland is unknown. A limiting problem in addressing these issues has been that traditional studies based on administering Te, DHT, E2, 5α-reductase inhibitors or aromatase blockers cannot discriminate between CNS and pituitary sites of AR- and ER-mediated feedback.
The translational implications of understanding Te and E2 feedback control in aging men are important: Table 1. Interventions may be envisioned that would enhance androgen or estrogen action selectively in the CNS or pituitary. A broader ensuant question would be whether age limits other effects of Te and E2 on the brain, and whether CNS-selective androgens and estrogens would mitigate the consequences of impaired Te action.
3.3 Implications to elderly patients
Insidious androgen deprivation may adversely impact libido and sexual potency, psychological well being, mood and cognition, exercise tolerance, muscle strength, skeletal mineral content and fracture risk, intraabdominal adiposity, insulin sensitivity, and lipid and carbohydrate metabolism (Liu et al. 2004): Figure 1. Metaanalyses of Te supplementation studies in older men indicate that lean-body mass, grip strength and visceral adiposity specifically respond to short-term androgen repletion (Isidori et al. 2005; Liu et al. 2004). Nonetheless, because the 2004 Institute of Medicine report underscored major uncertainties about therapeutic indications, safety, dosing and benefits of Te treatment in older men, a recommended immediate research focus is to clarify the primary mechanistic bases for age-related Te depletion (Liverman and Blazer 2004). Further experimental and technical advances will be necessary to achieve this fundamental goal.
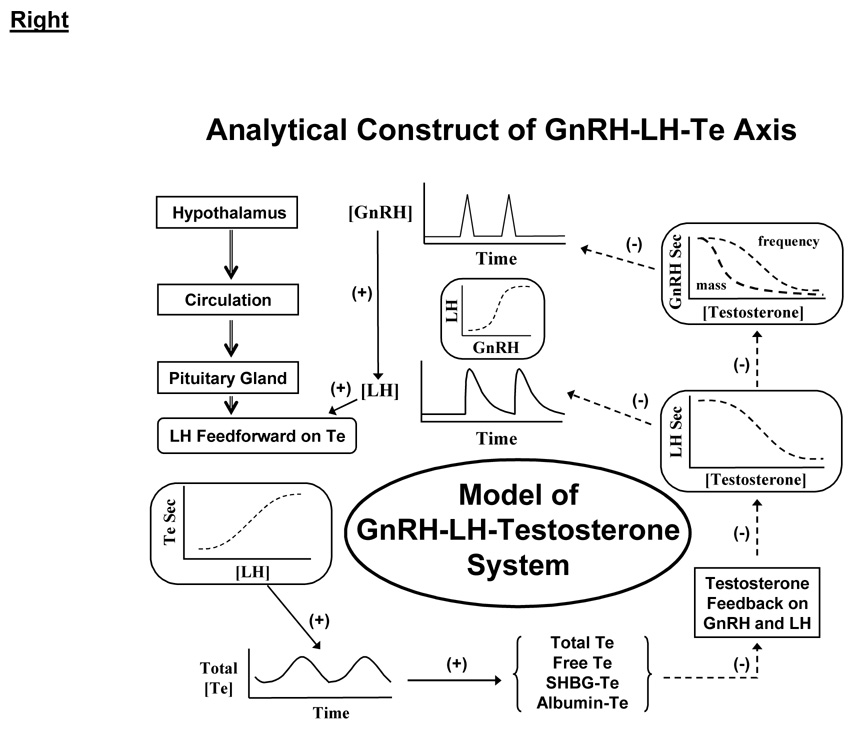
3.4 Concept of hypothalamo-pituitary-Leydig cell feedback system
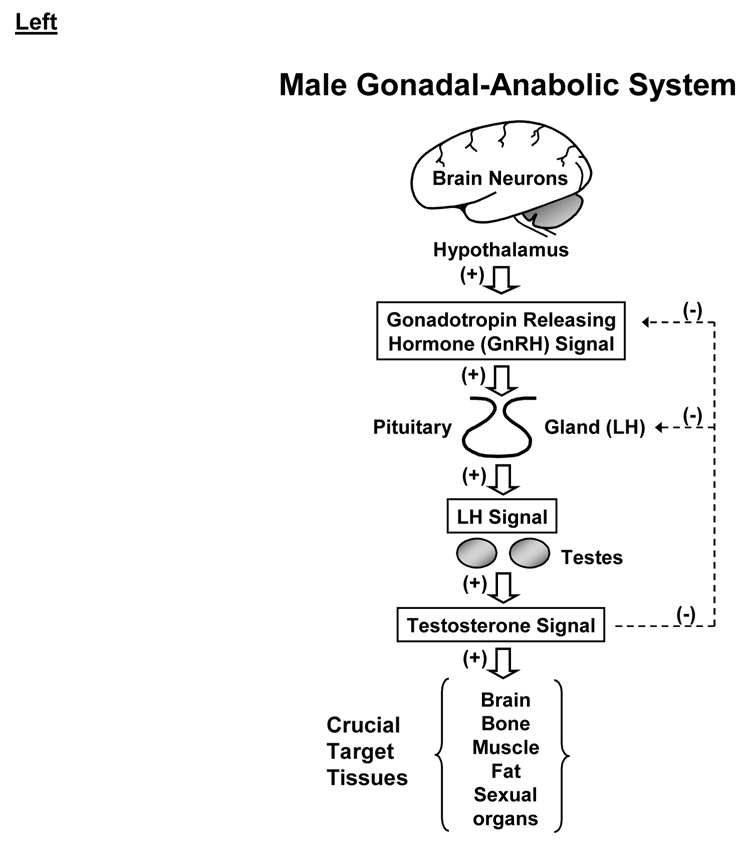
Physiological regulation of the male gonadal axis proceeds via repeated incremental signaling adjustments among GnRH, LH and Te (Keenan et al. 2001; Keenan et al. 2004; Liu et al. 2005a): Figure 4A. According to this thesis, none of GnRH, LH or Te acts alone or may be viewed alone. Analytical predictions based upon this ensemble perspective include impairment of all 3 of GnRH→LH feedforward, LH→Te feedforward and Te→GnRH/LH feedback in older men (Keenan et al. 2006; Keenan and Veldhuis 2004; Liu et al. 2005b; Veldhuis et al. 2004b; Veldhuis et al. 2005d). In such models, signaling connections among GnRH, LH and Te are represented by unknown biomathematical dose-response functions (Keenan et al. 2004; Keenan and Veldhuis 2001): Figure 4B. The resultant interlinked construct has utility both analytically and predictively. Analytical estimates disclose that healthy older men exhibit a tetralogy of low-amplitude, high-frequency and irregular LH pulses and hypoandrogenemia (Keenan et al. 2006; Keenan and Veldhuis 2004). Predictive simulations further reveal that combined deficits in LH action on the testis, hypothalamic GnRH outflow and Te-enforced negative feedback on GnRH/LH could explain this clinical tetrad (Keenan and Veldhuis 2001). In particular, intensive and extended blood-sampling paradigms and newer analytical methods forecast 35% smaller and 50% more frequent LH pulses in older than young men, as corroborated using 4 mathematically distinct pulse-analysis methods, 3 independent LH assays and 5 cohorts of volunteers (Liu et al. 2005a). Given that insufficiently intensive blood-sampling regimens censor LH pulse number and inflate estimated LH pulse size, inadequate sampling regimens probably explain many earlier disparities in the literature.
Figure 4.
Male anabolic axis from a clinical perspective (left) and analytical vantage (right). CNS, GnRH, Te, SHBG and LH are defined in Figure 3.
Viewed mechanistically, smaller incremental LH pulses in older men could denote: (i) less hypothalamic GnRH secretion per burst; (ii) greater hypothalamo-pituitary feedback inhibition by sex steroids; and/or (iii) impaired gonadotrope responses to GnRH. The third notion was excluded recently by demonstrating that age potentiates LH secretion stimulated by submaximal doses of exogenous GnRH (Veldhuis et al. 2005d). This outcome contrasts with some earlier studies using injected GnRH to stimulate LH acutely in young and older men (Liu et al. 2005a). The difference might be explained by our use of a five-fold strategy of: (a) deconvolution analysis of LH secretion; (b) high-specificity robotics-automated LH assay; (c) separate-morning randomly ordered single-bolus i.v. doses of GnRH spanning a 1000-fold range; (d) nonlinear (four-parameter logistic) regression analyses to quantify gonadotrope sensitivity (slope term), GnRH potency (ED50) and GnRH efficacy (maximal LH secretory response); and (e) age comparisons in the eugonadal and Te-depleted state (Veldhuis et al. 2005d). The outcomes indicated that age: (i) does not alter GnRH efficacy; and (ii) increases gonadotrope sensitivity and GnRH potency but only in a low Te milieu. The inference that age and Te availability jointly govern GnRH dose-responsiveness could explain prior discrepancies, which failed to control for both Te concentrations and GnRH dose. In corroboration, infusion of GnRH pulses (100 ng/kg) i.v. every 90 min for 14 days in older and young men sustains age-equivalent LH pulse amplitude and frequency (Mulligan et al. 1999). Therefore, smaller endogenous LH pulses in older men could be explained logically by reduced hypothalamic GnRH secretion to the pituitary gland, rather than by impaired GnRH action on gonadotrope cells. This important inference directs focus to factors that govern neuronal GnRH secretion in the aging male.
Available Te feedback data in healthy young men establish that Te and nonaromatizable androgens (e.g., 5 alpha-DHT or fluoxymesterone) suppress LH pulse frequency; and (ii) ketoconazole (which depletes Te) and flutamide (which inhibits androgen-receptor binding) accelerate LH pulse frequency (Veldhuis et al. 2001). Other studies indicate that frequent pulses of GnRH decrease LH peak increments. This means that the acceleration of LH pulse frequency due to low Te availability in aging might accentuate the reduction in LH pulse increments (Mulligan et al. 1999; Veldhuis et al. 1992). According to expected dose-response relationships, small LH pulses would further attenuate Leydig-cell Te secretion (Keenan and Veldhuis 2004).
4. Age-Associated Mechanisms of Hyposomatotropism
4.1 Overview
Growth hormone (GH) and IGF-I concentrations decline exponentially with age beginning in young adulthood, thus resulting in progressive biochemical hyposomatotropism (Veldhuis et al. 2006). Whereas the causes of hyposomatotropism in aged individuals are not established, two powerful co-predictors of relative GH deficiency are age, sex-steroid depletion and increased abdominal visceral fat (Erickson et al. 2005; Iranmanesh et al. 1998; Veldhuis et al. 2005a; Veldhuis et al. 2005b; Weltman et al. 1994). Epidemiological data correlate hyposomatotropism with osteopenia, sarcopenia, intraabdominal adiposity, insulin resistance, hyperlipidemia, atherosclerotic risk and diminished quality of life. From a mechanistic perspective, the fall in systemic GH and IGF-I availability is due principally to decreased GH secretion. In particular, aging does not alter the elimination kinetics of GH, reduce hypoglycemia-stimulated GH release, or impair hepatic IGF-I production in response to exogenous GH (Arvat et al. 1998; Lissett and Shalet 2003).
4.2 Role of estrogen depletion in aging-related hyposomatotropism
Estrogen is the primary sex-steroid agonist of GH secretion in both women and men. (Veldhuis et al. 2006; Veldhuis et al. 2005a). Estrogens are synthesized from androgen substrates via the aromatase enzyme expressed in the brain, pituitary gland, ovary, fat, muscle, liver, kidney, and other tissues. Actions of E2 may be mediated in principle via ER-alpha, ER-beta, and membrane receptors; modified by prominent target cell-specific factors; and diversified by the negative impact of age and body fat and feedback by GH and IGF-I (Veldhuis et al. 2006). What remains unclear is how age, estrogen and visceral adiposity jointly regulate the secretion of GH.
The proximate cause of diminished GH concentrations in aging individuals is small GH pulses, as quantified by less GH secreted per burst (defined as µg GH released per unit distribution volume per pulse). Age does not affect GH pulse frequency, GH half-life or basal (constitutive) GH release (Gentili et al. 2002; Iranmanesh et al. 1998; Shah et al. 1999). Impoverished pulsatile GH secretion has directed investigative focus to the core mechanisms that drive high-amplitude GH pulses (Veldhuis et al. 2005a). Basic laboratory studies have helped in the dissection of regulatory mechanisms. Nonetheless, the species specificity of sex-steroid actions makes inferences gained in the rat, mouse, pig and sheep illustrative of, rather than definitive to, the human (Veldhuis et al. 2006). For example, estrogens repress and nonaromatizable androgens increase pulsatile GH secretion in the rat, but exert opposite effects in the human. Accurate clinical studies require specific high-sensitivity GH assays, frequent blood sampling, recombinant peptides, validated analytical methods and model-assisted formulations (Veldhuis et al. 2005a; Veldhuis et al. 2006).
GH production doubles in the E2-enriched preovulatory phase of the menstrual cycle, and is higher in pre- than postmenopausal women or comparably aged men (Weltman et al. 1994). Acute and long-term estrogen administration to girls with Turner’s syndrome, postmenopausal women, male-to-female transsexual patients and men with prostatic cancer elevates GH concentrations by 1.8 to 3.3-fold (Veldhuis et al. 2005a). The route is not critical to driving GH, since each of i.v., i.m., oral, intravaginal, intranasal and higher-dose transdermal E2 stimulates GH secretion. However, oral estrogens result in momentarily higher hepatic concentrations of E2 than transdermal estrogen due to the brief estrogen pulse and the absorptive pathway. Indeed, enterically delivered E2 tends to suppress hepatic IGF-I production more and fat oxidation less, while elevating hepatic fibrinogen, angiotensinogen and IGFBP-1 concentrations more, than transdermally conveyed E2. However, equivalent serum E2 concentrations derived from oral and transdermal administration amplify GH secretion comparably in postmenopausal women (Friend et al. 1996).
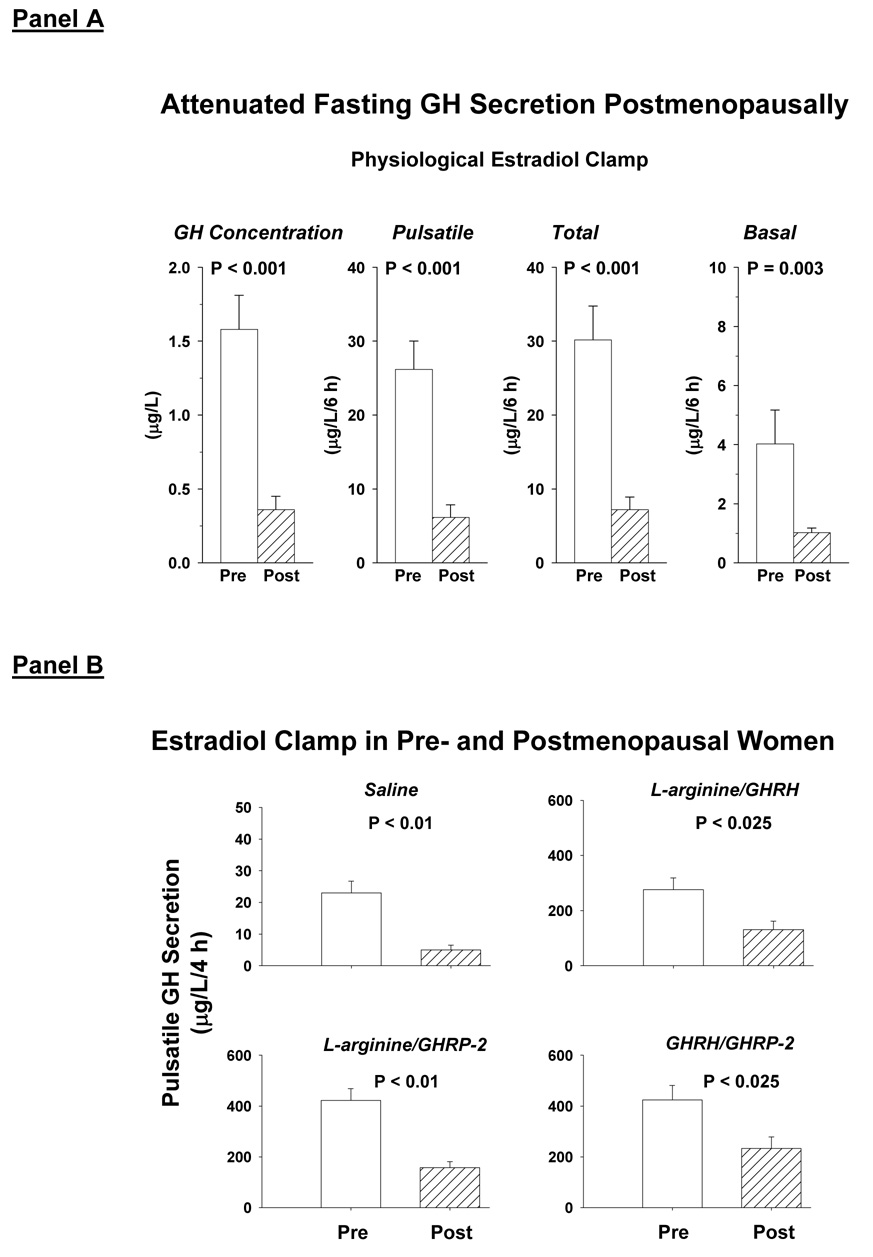
A recent clinical model employs a transdermal estrogen clamp imposed by gonadotropin downregulation and E2 addback to mimic steady-state physiology (Erickson et al. 2004; Erickson et al. 2005). One may thereby dissect age-dependent mechanisms of GH secretion at experimentally defined E2 concentrations in young and older women. During controlled transdermal estrogen repletion, age contributed > 75% of the variability in pulsatile GH secretion both in the fasting state and after secretagogue infusion (Erickson et al. 2004; Erickson et al. 2005): Figure 5. Conversely, despite comparable estrogen depletion, premenopausal women maintained 2-fold higher GH and IGF-I concentrations than postmenopausal individuals (Veldhuis et al. 2005b). The attenuating effect of age may be altered by estrogen-independent factors like concomitant visceral adiposity, inflammatory cytokines, regulatory peptides, catecholamines, thyroxine, cortisol and free fatty acids (Veldhuis et al. 2006). Distinguishing E2-dependent and E2-independent mechanisms of impaired GH and IGF-I production constitutes a major step toward discerning new steroidal and nonsteroidal strategies to maintain GH and IGF-I availability in aging women. Nonsteroidal interventions would have particular relevance in elderly women with elevated risk for neoplastic, cerebro- and cardiovascular, thrombophlebitic or cholestatic disease.
Figure 5.
Fifty percent reduction in fasting GH concentrations and secretion rates (Panel A) and peptide-driven GH pulses (Panel B) in postmenopausal compared with premenopausal women evaluated during a 3-week E2 clamp emulating the late follicular phase in the young adult. Reprinted with permission from (Erickson et al. 2004).
4.3 Concept of ensemble-peptide control
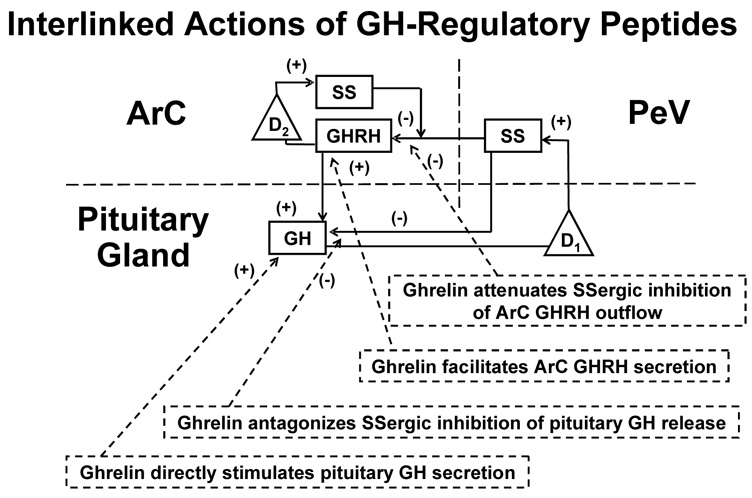
The amount of GH secreted in pulses is significant for several basic reasons, viz.: (a) aging primarily reduces pulsatile GH production; (b) pulsatile (rather than basal time-invariant) GH secretion constitutes the majority (88–94%) of total daily GH output; and (c) the size of GH pulses is determined by a well articulated ensemble of key peptides; viz., growth-hormone releasing hormone (GHRH) and peptide (ghrelin, GHRP) feedforward and somatostatin (SS)-mediated feedback by GH and IGF-I (Gentili et al. 2002; Shah et al. 1999; Veldhuis et al. 2006; Veldhuis et al. 2007a; Veldhuis et al. 2005b). Figure 6 schematizes key reciprocal connections among the foregoing primary peptidic signals. The reciprocal, time-delayed nature of network interactions necessitates novel analytical methods to reconstruct and quantify ensemble control objectively. To this end, several simplified biomathematical constructs embody consensus linkages among GHRH, SS and ghrelin (Farhy et al. 2007; Farhy and Veldhuis 2005). At present, such models permit simulation but not quantitation, which remains necessary to assist in the interpretation of experimental outcomes.
Figure 6.
Ensemble construct of core linkages among ghrelin (GHRP), GHRH, SS and GH feedback. D1 and D2 are feedback delays. GHRH = GH-releasing hormone, GHRP = GH-releasing peptide; SS = somatostatin; ArC = arcuate nucleus; PeV = periventricular nucleus; +, stimulatory; −, inhibitory. Adapted from (Farhy and Veldhuis 2005).
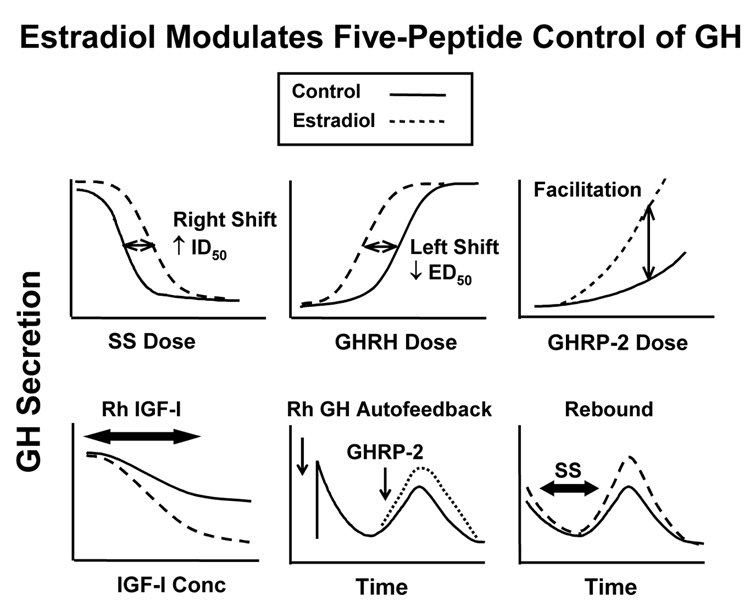
Mechanistic clinical experiments document that age reduces the GH response to individual peptidic secretagogues (Veldhuis et al. 2005a). GH stimuli unaffected by aging are insulin-induced hypoglycemia and combined GHRH, GHRP and L-arginine injection [the last, to limit SS outflow] (Arvat et al. 1998; Veldhuis et al. 2006). Recent clinical investigations demonstrate that short-term E2 supplementation significantly, but not fully, overcomes attenuated secretagogue actions in postmenopausal women. In particular, estrogen repletion: (i) doubles the stimulatory potency of exogenous GHRH; (ii) halves the inhibitory potency of infused SS-14; (iii) potentiates submaximal feedforward by synthetic GHRP and native ghrelin; (iv) mutes negative feedback by injected GH; and (v) paradoxically accentuates feedback by recombinant human (rh) IGF-I: Figure 7. Thus, E2 supplementation in elderly women primarily controls the physiological potency rather than pharmacological efficacy of GHRH, SS, ghrelin, GH and IGF-I.
Figure 7.
Summary of experimentally inferred actions of E2 on regulated GH secretion. Unpublished compilation. GHRH, GHRP and SS are defined in the legend of Figure 6. The terms ID50 and ED50 denote inhibitory and stimulatory potencies, respectively.
A pivotal mechanism of amplifying GH secretion is synergy between ghrelin and GHRH. Transgenic knockdown of the ghrelin-receptor gene in tyrosine hydoxylase-expressing neurons decreases GH and IGF-I concentrations and somatic growth by 30% in the female animal (Shuto et al. 2002). The mechanisms involve a reduction in the density of hypothalamic GHRH-expressing neurons and in GH pulse size. The data indicate that ghrelin normally is trophic to GHRH neurons and probably amplifies the amount of GHRH secreted. Missense mutations of the human ghrelin-receptor gene decrease cell-surface expression and constitutive signaling, and are associated with short stature (Pantel et al. 2006). One would predict that such patients have small GH pulses of normal frequency, according to a model in which brain ghrelin receptors mediate amplification of GHRH outflow (Farhy et al. 2007; Farhy and Veldhuis 2005; Veldhuis et al. 2006; Veldhuis et al. 2007a).
4.4 Significance of GH outflow to target tissues
GH governs anabolism by inducing in situ IGF-I production and modifying IGFBP availability in local target tissues, stimulating lipolysis, prechondrocyte proliferation and growth of erythroid precursors, and driving hepatic and renal secretion of IGF-I and certain IGFBP’s (Veldhuis et al. 2006). The trophic contribution of blood-borne IGF-I (somatomedin hypothesis) to peripheral tissues is not exclusive, since marked depletion of systemic IGF-I concentrations achieved transgenically in mice does not restrict somatic growth after birth, but impairs carbohydrate balance and reduces bone density in later adulthood (Sjogren et al. 2001; Yakar et al. 2001).
Sex steroids regulate the effects of GH, IGF-I and IGFBP’s in target tissues. For example, GH induces and E2 potentiates brain IGF-I production, turnover and signaling, thereby putatively enhancing neurite outgrowth, synaptogenesis, neuronal differentiation and memory (Veldhuis et al. 2006). Estrogen stimulates expression of the GH-receptor gene in human osteoblastic cells and (via a female pattern of GH secretion) upregulates the LDL-receptor gene in liver cells (Slootweg et al. 1997). Conversely, E2 blocks GH-induced IGF-I synthesis in liver by activating an autoinhibitory pathway, SOCS [suppressor of cytokine synthesis]. In the liver, > 850 genes are male-pattern selective and > 750 genes female-selective due to strong dependence on gender-defined GH pulse patterns. Certain of these gene transcripts are uniquely affected by the aging process. No analogous data exist for estrogen-, GH- and IGF-I-targeted genes in the hypothalamus and pituitary gland.
4.5 Adjuvant interventions to replete GH
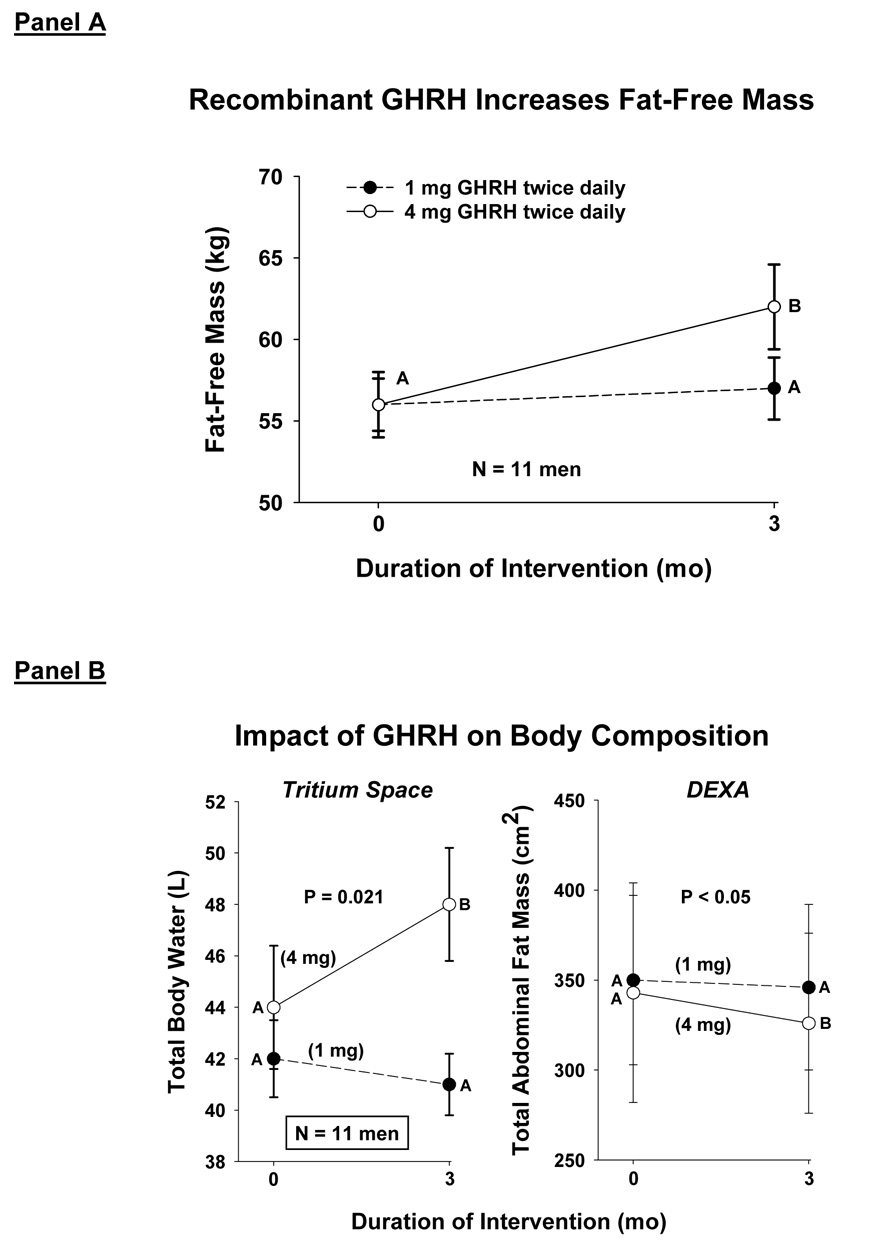
The capability of GHRH supplementation to stimulate pulsatile GH production in older adults was evaluated recently by twice-daily s.c. injections of 1 or 4 mg of recombinant human GHRH-1,44-amide for 3 mo (Veldhuis et al. 2004c; Veldhuis et al. 2005f). Detailed body-compositional and functional assessments in men revealed a decline in abdominal visceral fat (AVF), an increase in lean-body mass, and a decrease in stair-climbing and walking times: Figure 8. Concomitantly, GH and IGF-I concentrations rose by 1.8–2.1-fold. These outcomes are consistent with (but not proof of) the hypothesis that GHRH deficiency exists in the elderly and that amelioration of GHRH deficiency may have salutary effects.
Figure 8.
Administration of recombinant human GHRH for 3 mo twice daily increases fat-free (lean-body) mass (Panel A) and total body water (tritium spaces), and decreases total abdominal fat mass (Panel B).
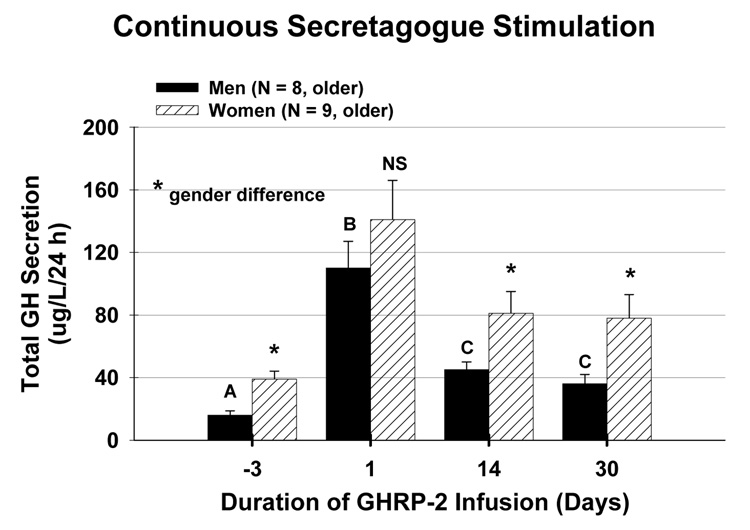
The implications of putatively impaired ghrelin drive in older adults were explored recently in a 1-mo trial of continuous s.c. GHRP-2 infusion. GHRP-2 is a synthetic hexapeptide analog of ghrelin. During the second and fourth wk of the intervention, GHRP-2 infusion stimulated 24-h GH secretion by 1.45-fold and elevated IGF-I concentrations by 1.65-fold (Bowers et al. 2004): Figure 9. In absolute terms, responses were greater in women than men. What is not known is whether favorable body-compositional, biochemical and functional performance changes can be induced by such regimens. Preliminary investigations by Nagaya et al. reported trophic effects of ghrelin in cachectic patients with congestive heart failure (Nagaya et al. 2004). Beneficial effects included increases in cardiac ejection fraction, peak oxygen consumption, skeletal muscle strength and Karnofsky performance score. A major unresolved issue is whether augmented GH and IGF-I availability in frail elderly individuals would be safe and beneficial.
Figure 9.
Continuous GHRP-2 (a ghrelin analog) infusion for 30 days drives 24-h GH secretion in older men and women (Bowers et al. 2004).
The consequences of diminished production of GH and IGF-I in aging adults might in theory be overcome by supplementation with recombinant GH. However, there are no safety data that justify long-term GH administration in healthy older individuals. In fact, high doses of GH in patients with critical illness increase mortality and in GH-deficient adults elicit acromegalic features.
4.6 Feedback inhibition of GH secretion by IGF-I
Pulsatile GH secretion rates are reduced in postmenopausal women and older men, despite 50% lower IGF-I concentrations. In contrast, a 32% reduction in IGF-I concentrations stimulates GH secretion by 1.8-fold in young adults (Veldhuis et al. 2006). Lower GH output in the face of lower IGF-I availability (and thus lesser feedback) in older adults implies that hypothalamo-pituitary drive to GH secretion is impaired.
4.7 Role of abdominal visceral fat (AVF)
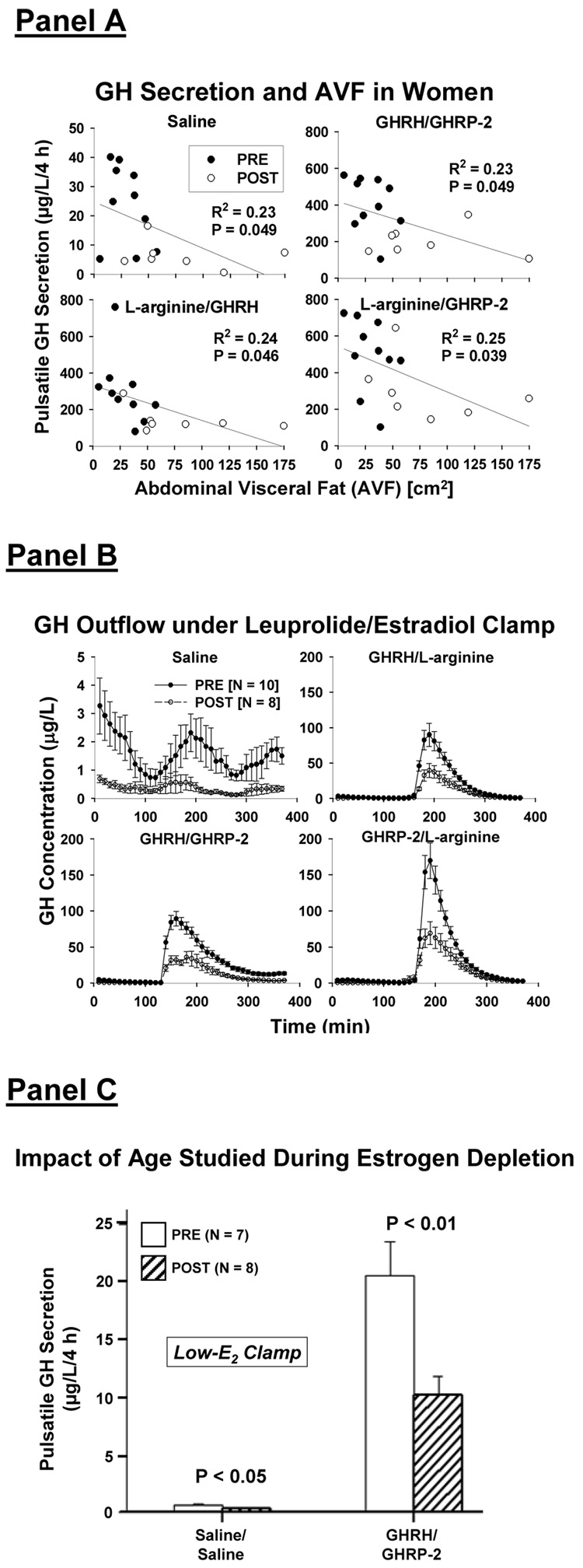
There is a strong negative impact of computed tomography-estimated AVF on fasting and dual secretagogue-stimulated GH secretion in an estrogen-enriched milieu: Figure 10A. In particular, AVF explains 23–25% (and age the remainder) of the variability in pulsatile GH secretion in older and young women studied after leuprolide injection and transdermal E2 addback (E2 clamp) (Erickson et al. 2004; Erickson et al. 2005; Veldhuis et al. 2007a; Veldhuis et al. 2005b). During E2 repletion, postmenopausal individuals had: (i) 50% lower mean concentrations of GH and IGF-I and pulsatile GH secretion; and (ii) 50% smaller GH responses to combined injections of: (a) L-arginine/GHRH; (b) L-arginine/GHRP-2; and (c) especially GHRH/GHRP-2: Figure 10B. Combining a peptide with L-arginine was used to presumptively limit hypothalamic SS outflow (Veldhuis et al. 2006), thereby unmasking maximal agonistic effects of GHRH and GHRP-2.
Figure 10.
Panel A. Regression of pulsatile GH secretion on abdominal visceral fat mass (AVF) estimated by computed tomography (CT) in pre- and postmenopausal (PRE and POST) women exposed to gonadal downregulation and E2 repletion (Erickson et al. 2004). Panel B. Unstimulated (saline/saline) and maximal paired secretagogue-induced GH secretion during an E2 clamp in 8 POST and 10 PRE women. Note differing y-axis scales. Data are the mean ± SEM. Adapted from (Erickson et al. 2004). Panel C. Reduced baseline (saline/saline) and GHRH/GHRP-2-stimulated GH secretion in POST compared with PRE women administered leuprolide and placebo to enforce a low-E2 clamp (Veldhuis et al. 2005b).
A model of leuprolide injections to downregulate the gonadal axis with placebo (rather than E2) addback yields a low-estrogen clamp, which allows one to unmask E2-independent effects of age on GH secretion (Veldhuis et al. 2005b). Figure 10C shows that postmenopausal women achieve 56% lower fasting and 50% lower GHRH/GHRP-2-stimulated pulsatile GH secretion than young subjects during equivalent E2 deprivation. According to an ensemble model (Farhy et al. 2007; Farhy and Veldhuis 2005; Veldhuis et al. 2007a), these outcomes are consistent with postulated excessive outflow (secretion and action) of SS and/or deficient efficacy of GHRH and GHRP in aging women.
4.8 Analytical insights
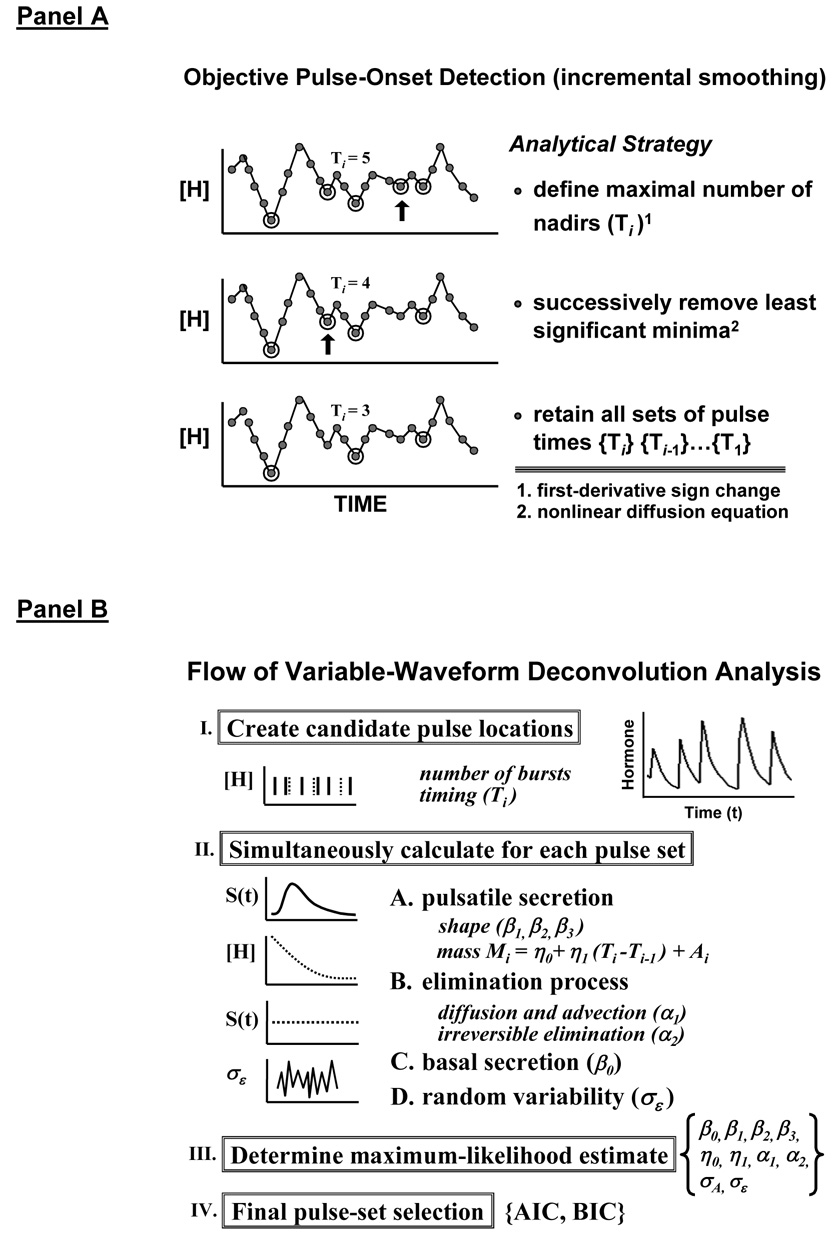
To quantify pituitary hormone secretion validly requires a composite analytical model of basal hormone release, variably shaped secretory bursts, biexponential elimination kinetics and random effects (Keenan et al. 2003; Keenan et al. 2004). The mathematical formalism is termed maximum-likelihood estimation (MLE) deconvolution, conditioned statistically on candidate sets of pulse-onset times objectively estimated a priori (Keenan et al. 2003): Figure 11A. The construct was validated empirically by frequent (5 min) and extended (4–12 h) sampling of hormone release into hypothalamo-pituitary portal and jugular-venous blood in the conscious sheep and horse (Keenan et al. 2001; Keenan et al. 2006; Keenan et al. 2004). The statistical framework was verified by direct mathematical proof (Keenan et al. 2005). The parameters estimated by the deconvolution procedure include secretory-burst number (frequency), size (mass) and shape, elimination kinetics and random effects, as schematized in Figure 11B. In complementation, Bayesian estimates of parameter distributions can be made on single hormone profiles: Table 3. Application of such methods has demonstrated that administration of E2 compared with placebo augments the amount (mass) of GH released per burst by 2-fold, and accelerates initial release of GH within each bursts by 1.8-fold (Veldhuis et al. 2005b). Rapid release of GH denotes prompt exocytosis of somatotrope stores, strongly suggesting withdrawal of hypothalamic SSergic restraint.
Figure 11.
New variable-waveform deconvolution method comprising incremental smoothing to create candidate sets of pulse times, Ti (Panel A), and nonlinear estimation of all secretion and elimination parameters simultaneously (Panel B). The algorithm was validated experimentally and verified mathematically (Keenan et al. 2003; Keenan et al. 2005; Keenan et al. 2004). AIC and BIC refer to the Akaike and Bayesian information criteria, respectively. Greek symbols denote parameters of secretion, elimination and random effects. Unpublished line drawings.
Table 3.
Precision of Bayesian parametric estimates on individual LH profiles
| Young Man |
Postmenopausal Woman |
|
|---|---|---|
| Frequency (#/24 h) | 12 ± 1.7 (14) | 27 ± 2.5 (9.3) |
| Mass/Burst (IU/L) | 9 ± 3 (33) | 36 ± 7 (19) |
| Pulsatile Sec (IU/L/24 h) | 90 ± 25 (28) | 560 ± 90 (16) |
| Half-life (min) | 47 ± 20 (42) | 119 ± 8 (6.7) |
Data are the mean ± SD of 100 realizations from two individual 10-min LH time series.
Parentheses give the CV (SD/mean × 100%).
Adapted from analyses given in (Keenan et al. 2005).
4.9 Ensemble model-based predictions
Computer-assisted models can be used to formalize reversible, time-delayed, dose-responsive interactions among the key components that regulate neuroendocrine networks (Farhy et al. 2007; Farhy and Veldhuis 2005; Keenan et al. 2001; Meier et al. 2005; Veldhuis et al. 2007a). Ensemble formulations allow one to: (a) examine clinical intuition based upon objective multipeptide linkages; (b) test integrative implications of observed outcomes; and (c) explore regulatory hypotheses in followup experiments. The GH model in Figure 6 incorporates the published capabilities of ghrelin/GHRP to: (i) stimulate pituitary GH secretion directly by 2 to 4-fold; (ii) induce hypothalamic GHRH release; and (iii) oppose the actions of SS in the arcuate nucleus and on pituitary gland (Farhy et al. 2007; Farhy and Veldhuis 2005). Suitable model-based simulations of complex ensembles should enhance insights into network control.
5. Actions of Te on the Aging Male GH Axis
5.1 Supplementation with Te and/or GH improves body composition in older men
Single and combined administration of transdermal Te (5 mg daily) and s.c. GH (6.25 µg/kg/day) for 1 mo in older men increases muscle IGF-I gene expression, lean body mass, measures of physical performance and GH, IGF-I and osteocalcin concentrations (Brill et al. 2002). Continuous s.c. infusion of GHRP-2 (a synthetic analog of ghrelin) for 30 days elevates GH, IGF-I and IGFBP-3 concentrations into the middle-age range in older adults (Bowers et al. 2004). And, s.c. administration of GHRH twice-daily for 3 mo improves performance in a 30-meter walk and 4-flight stair climb, decreases abdominal visceral adiposity and increases lean-body mass in aging men (Veldhuis et al. 2004c): Figure 8.
How the safety profiles of these 3 regimens differ is not yet known.
5.2 Mechanistic bases for impaired GH secretion in older men
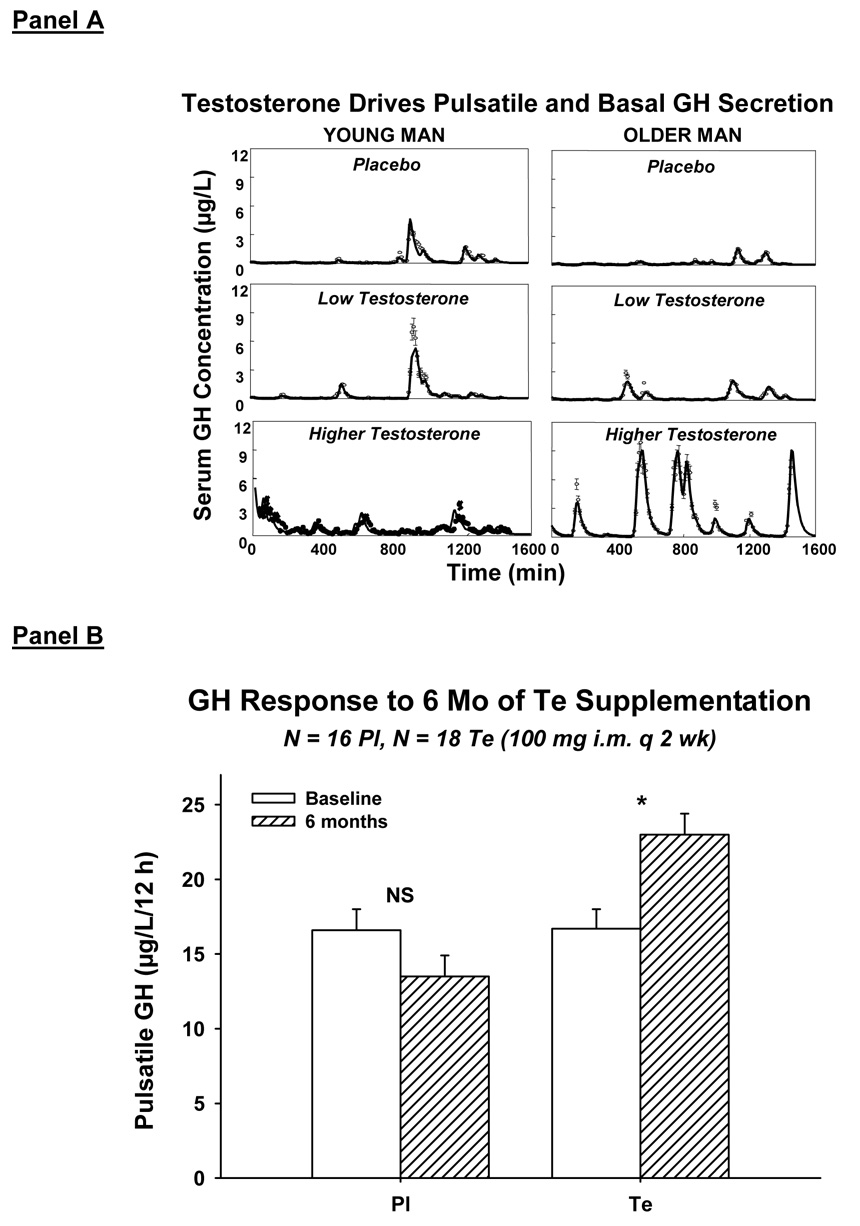
Clinical studies establish that GH, IGF-I and Te concentrations begin to decline in early adulthood (Iranmanesh et al. 1998; Liu et al. 2005a; Veldhuis et al. 2007b; Weltman et al. 1994). Understanding the genesis of hyposomatotropism and hypoandrogenemia in aging is important, because both states correlate with intraabdominal adiposity, insulin resistance, dyslipidemia, cardiovascular mortality, sarcopenia, osteopenia, cognitive loss and diminished quality of life. In addition, sex-steroid depletion and visceral obesity are jointly correlated, and strongly predict GH and IGF-I deficiency (Gentili et al. 2002; Liu et al. 2005a; Shah et al. 1999; Veldhuis et al. 2005a; Veldhuis et al. 2006; Veldhuis et al. 2007b; Weltman et al. 1994). The relevance of relative Te deprivation in aging men is indicated by the fact that supplementation with Te for 2 wk or 6 mo stimulates GH production in older and hypogonadal individuals, but not in Te-replete young men (Gentili et al. 2002; Muniyappa et al. 2007): Figure 12. A prevailing view is that systemic aromatization of Te to estradiol (E2) mediates androgenic drive of GH secretion in young adults. However, this inference may be too narrow, inasmuch as Te but not E2 elevates IGF-I concentrations and blunts negative feedback by IGF-I (Brill et al. 2002; Gentili et al. 2002). Conversely, E2 but not Te lowers IGF-I concentrations and abbreviates GH secretory bursts (Erickson et al. 2004; Erickson et al. 2005; Shah et al. 1999). Thus, the question arises whether certain products of Te metabolism modulate GH secretion in a manner distinct from that of E2.
Figure 12.
Panel A. Illustrative 24-h GH concentration profiles in a young (23 yr-old) and older (68 yr-old) man given placebo, low Te and higher Te parenterally for 2 wk in randomly assigned order at least 6 wk apart (Gentili et al. 2002). Panel B. Six months of near-physiological testosterone supplementation compared with placebo (Pl) increases overnight pulsatile GH secretion in older men (Muniyappa et al. 2007).
A central principle is that aging selectively reduces, whereas Te supplementation augments, pulsatile GH production by regulating the amount (mass) of GH secreted in each burst (Gentili et al. 2002; Iranmanesh et al. 1998; Muniyappa et al. 2007). In contradistinction, neither age nor Te availability determines GH pulse frequency, pharmacologically stimulated GH secretion, GH elimination kinetics or the hepatic action of GH to stimulate IGF-I production (Arvat et al. 1998; Gentili et al. 2002; Lissett and Shalet 2003; Muniyappa et al. 2007; Veldhuis et al. 2006). Therefore, a major investigative issue becomes, How do age and Te availability together control GH secretory-burst size? Clinical studies are needed to address this query, because estrogens suppress and nonaromatizable androgens augment GH pulse size in the rat, but exert opposite effects in the human. However, the size (mass) of GH secretory bursts is determined by species-independent peptidyl signals, which include GHRH, somatostatin (SS), ghrelin/GHRP, GH and IGF-I (Veldhuis et al. 2006): Figure 6. The 5 peptides interact by way of time-delayed, reciprocal, concentration-dependent, nonlinear dose-response interfaces, which are not observed directly (Farhy et al. 2007; Farhy and Veldhuis 2005). This ensemble can be simulated by simplified biomathematical constructs of key feedback and feedforward connections that are both necessary and sufficient to confer self-renewing, high-amplitude GH secretory bursts (Farhy et al. 2007; Farhy and Veldhuis 2005). What remains lacking are analytical capabilities to estimate unobserved interactions.
5.3 Investigative strategies
A necessary consequence of multisignal control is that a change in any one pathway alters output of all connecting loci. For example, suppose that Te (like E2) potentiates a submaximal GHRH stimulus (Veldhuis et al. 2003). This outcome could signify that Te: (a) upregulates somatotrope GHRH receptors; (b) augments GHRH-releasable GH stores; (c) attenuates SSergic inhibition; (d) reduces negative feedback by GH or IGF-I; and/or (e) enhances stimulation by ghrelin, which synergizes with GHRH (Veldhuis et al. 2005a). To determine which mechanisms actually pertains requires not only accounting for all ensemble interactions correctly (Figure 6), but also constructing relevant peptide-infusion paradigms to unmask endogenous signaling.
5.4 Insights from new analytical tools
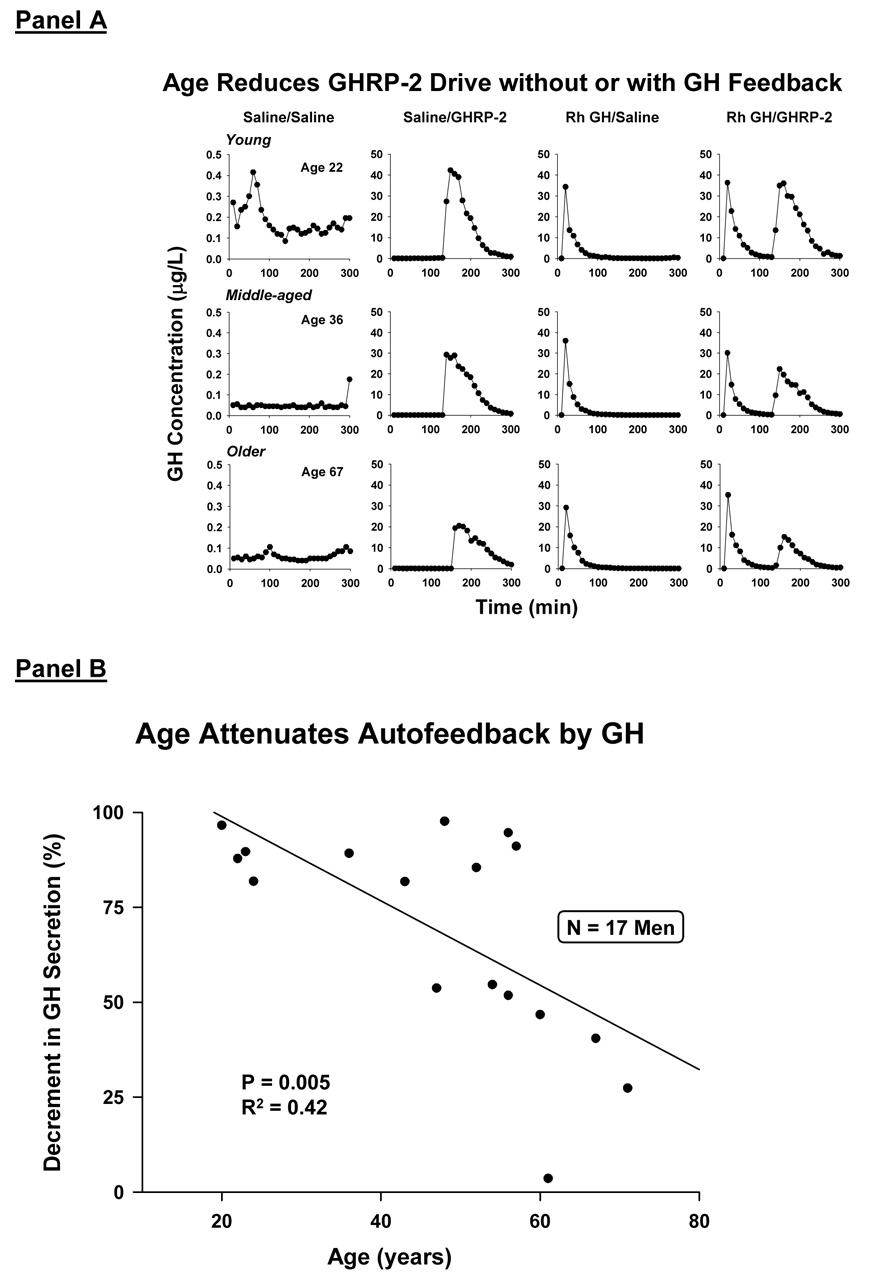
The ensemble schema in Figure 6 incorporates recently discovered ghrelin (Farhy and Veldhuis 2005), which is both an appetitive stimulus and a potent activator of the GHRP receptor. Ghrelin and its cognate receptor are expressed primarily in the hypothalamus, pituitary gland, stomach and vasculature (Kojima et al. 1999). Analyses of GH secretory bursts induced by a synthetic ghrelin analog, GHRP-2, has revealed that this class of peptides augments the mass of GH secreted per burst, and stimulates rapid exocytosis of GH stores, possibly by repressing SS outflow (Veldhuis et al.2005e). Increasing age in men reduces GHRP-2 efficacy in the presence or absence of imposed negative feedback by GH (Veldhuis et al. 2005c): Figure 13A. Age also attenuates percentage feedback inhibition by a pulse of GH, raising the possibility of decreased brain GH-receptor signaling (Veldhuis et al. 2005c): Figure 13B. Testosterone does not augment GHRP efficacy, but blunts feedback inhibition by both GH and IGF-I (Veldhuis et al. 2005e; Veldhuis et al. 2005c; Veldhuis et al. 2004a).
Figure 13.
Panel A. Reduced unstimulated (baseline) GH secretion and GHRP-2-stimulated GH secretion in older men in the absence and presence of exogenous GH feedback. Data are GH concentration profiles in one young (age 22 y), middle-aged (age 36 y) and older (age 67 y) man [rows, top-to-bottom], each studied on 4 different mornings fasting [columns, left-to-right]. The 4 sessions comprised consecutive iv infusion of saline/saline, saline/GHRP-2, rh GH/saline or rh GH/GHRP-2. Data reflect sampling every 10 min for 5 h beginning at 0800 h clock time (zero min on x axis). Panel B. Age reduces fractional (%) inhibition of GH secretion by a fixed pulse of recombinant human (rh) GH compared with saline.
5.5 Influence of Te on the actions of GH and IGF-I
Sex steroids regulate tissue-specific effects of GH and IGF-I (Pons and Torres-Aleman 1993; Yu et al. 2003). Te potentiates selected anabolic actions of GH and IGF-I (Gibney et al. 2005), whereas E2 can both antagonize and potentiate GH/IGF-I signaling depending upon the target tissue. Positive interactions suggest that combined GH and Te deficiencies would be especially detrimental to bone, muscle, brain and metabolic regulation in aging individuals.
5.6 Clinical implications
Androgen-deprivation therapy in men with prostatic cancer reduces concentrations of both Te and GH. Significant sequelae in such patients include osteopenia, sarcopenia, visceral adiposity, insulin resistance, decreased physical stamina, hip and vertebral fractures and reduced quality of life (Chen et al. 2002). Nonetheless, no data establish the safety of GH or IGF-I administration in the setting of prior neoplasia. The concern reflects epidemiological linkages between GH/IGF-I availability and risk of colonic polyposis, breast-cancer growth and prostatic cancer.
6. Summary
Gradual age-related depletion of anabolic hormones (Te and GH/IGF-I) putatively contributes to frailty, visceral adiposity, insulin resistance, osteopenia, sarcopenia and (possibly) impaired cognitive function. New methodologies and laboratory discoveries have permitted important insights into the multipathway mechanisms that mediate relative hypogonadism and hyposomatotropism in older adults.
Acknowledgments
We thank Kay Nevinger for support of manuscript preparation; Ashley Bryant for data analysis and graphics; the Mayo Immunochemical Laboratory for assay assistance; and the Mayo research nursing staff for implementing the protocol. Supported in part via the Clinical-Translational Research-Center Grant MO1 RR00585 to the Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD) and R01 NIA AG019695 and AG29362 from the National Institutes of Health (Bethesda, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arvat E, Ceda GP, Di Vito L, Ramunni J, Gianotti L, Ghigo E. Age-related variations in the neuroendocrine control, more than impaired receptor sensitivity, cause the reduction in the GH-releasing activity of GHRP's in human aging. Pituitary. 1998;1:51–58. doi: 10.1023/a:1009970909015. [DOI] [PubMed] [Google Scholar]
- Bowers CY, Granda R, Mohan S, Kuipers J, Baylink D, Veldhuis JD. Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. Journal of Clinical Endocrinology Metabolism. 2004;89:2290–2300. doi: 10.1210/jc.2003-031799. [DOI] [PubMed] [Google Scholar]
- Brill KT, Weltman AL, Gentili A, Patrie JT, Fryburg DA, Hanks JB, Urban RJ, Veldhuis JD. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. Journal of Clinical Endocrinology Metabolism. 2002;87:5649–5657. doi: 10.1210/jc.2002-020098. [DOI] [PubMed] [Google Scholar]
- Chen Z, Maricic M, Nguyen P, Ahmann FR, Bruhn R, Dalkin BL. Low bone density and high percentage of body fat among men who were treated with androgen deprivation therapy for prostate carcinoma. Cancer. 2002;95:2136–2144. doi: 10.1002/cncr.10967. [DOI] [PubMed] [Google Scholar]
- Dufau ML, Veldhuis JD. Pathophysiological relationships between the biological and immunological activities of luteinizing hormone. 1987;1:153–176. doi: 10.1016/s0950-351x(87)80057-5. [DOI] [PubMed] [Google Scholar]
- Erickson D, Keenan DM, Farhy LS, Mielke K, Bowers CY, Veldhuis JD. Determinants of dual secretagogue drive of burst-like GH secretion in premenopausal women studied under a selective estradiol clamp. Journal of Clinical Endocrinology Metabolism. 2005;90:1741–1751. doi: 10.1210/jc.2004-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson D, Keenan DM, Mielke K, Bradford K, Bowers CY, Miles JM, Veldhuis JD. Dual secretagogue drive of burst-like growth hormone secretion in postmenopausal compared with premenopausal women studied under an experimental estradiol clamp. Journal of Clinical Endocrinology Metabolism. 2004;89:4746–4754. doi: 10.1210/jc.2004-0424. [DOI] [PubMed] [Google Scholar]
- Farhy LS, Bowers CY, Veldhuis JD. Model-projected mechanistic bases for sex differences in growth-hormone regulation in humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1577–R1593. doi: 10.1152/ajpregu.00584.2006. [DOI] [PubMed] [Google Scholar]
- Farhy LS, Veldhuis JD. Deterministic construct of amplifying actions of ghrelin on pulsatile GH secretion. Am J Physiol Regul Integr Comp. 2005;288:R1649–R1663. doi: 10.1152/ajpregu.00451.2004. [DOI] [PubMed] [Google Scholar]
- Friend KE, Hartman ML, Pezzoli SS, Clasey JL, Thorner MO. Both oral and transdermal estrogen increase growth hormone release in postmenopausal women -- a clinical research center study. Journal of Clinical Endocrinology Metabolism. 1996;81:2250–2256. doi: 10.1210/jcem.81.6.8964860. [DOI] [PubMed] [Google Scholar]
- Gentili A, Mulligan T, Godschalk M, Clore J, Patrie J, Iranmanesh A, Veldhuis JD. Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. Journal of Clinical Endocrinology Metabolism. 2002;87:825–834. doi: 10.1210/jcem.87.2.8222. [DOI] [PubMed] [Google Scholar]
- Gibney J, Wolthers T, Johannsson G, Umpleby AM, Ho KK. Growth hormone and testosterone interact positively to enhance protein and energy metabolism in hypopituitary men. Am J Physiol Endocrinol Metab. 2005;289:266–271. doi: 10.1152/ajpendo.00483.2004. [DOI] [PubMed] [Google Scholar]
- Gray A, Berlin JA, McKinlay JB, Longcope C. An examination of research design effects on the association of testosterone and male aging: results of a meta-analysis. J Clin Epidemiol. 1991;44:671–684. doi: 10.1016/0895-4356(91)90028-8. [DOI] [PubMed] [Google Scholar]
- Haji M, Kato KI, Nawata H, Ibayashi H. Age-related changes in the concentrations of cytosol receptors for sex steroid hormones in the hypothalamus and pituitary gland of the rat. Brain Res. 1980;204:373–386. doi: 10.1016/0006-8993(81)90596-5. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. Journal of Clinical Endocrinology Metabolism. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Huang HH, Kissane JQ, Hawrylewicz EJ. Restoration of sexual function and fertility by fetal hypothalamic transplant in impotent aged male rats. Neurobiol Aging. 1987;8:465–472. doi: 10.1016/0197-4580(87)90042-x. [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, South S, Liem AY, Clemmons D, Thorner MO, Weltman A, Veldhuis JD. Unequal impact of age, percentage body fat, and serum testosterone concentrations on the somatotrophic, IGF-I, and IGF-binding protein responses to a three-day intravenous growth hormone-releasing hormone pulsatile infusion in men. Eur J Endocrinol. 1998;139:59–71. doi: 10.1530/eje.0.1390059. [DOI] [PubMed] [Google Scholar]
- Isidori AM, Giannetta E, Gianfrilli D, Greco EA, Bonifacio V, Aversa A, Isidori A, Fabbri A, Lenzi A. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63:381–394. doi: 10.1111/j.1365-2265.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Alexander SL, Irvine CHG, Clarke IJ, Canny BJ, Scott CJ, Tilbrook AJ, Turner AI, Veldhuis JD. Reconstruction of in vivo time-evolving neuroendocrine dose-response properties unveils admixed deterministic and stochastic elements. Proc Natl Acad Sci USA. 2004;101:6740–6745. doi: 10.1073/pnas.0300619101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DM, Chattopadhyay S, Veldhuis JD. Composite model of time-varying appearance and disappearance of neurohormone pulse signals in blood. J Theor Biol. 2005;236:242–255. doi: 10.1016/j.jtbi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA. 2001;98:4028–4033. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD. Physiological control of pituitary hormone secretory-burst mass, frequency and waveform: a statistical formulation and analysis. Am J Physiol. 2003;285:R664–R673. doi: 10.1152/ajpregu.00195.2003. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, Veldhuis JD. An Ensemble Model of the Male Gonadal Axis: illustrative application in aging men. Endocrinology. 2006;147:2817–2828. doi: 10.1210/en.2005-1356. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD. Disruption of the hypothalamic luteinizing-hormone pulsing mechanism in aging men. Am J Physiol. 2001;281:R1917–R1924. doi: 10.1152/ajpregu.2001.281.6.R1917. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD. Divergent gonadotropin-gonadal dose-responsive coupling in healthy young and aging men. Am J Physiol. 2004;286:R381–R389. doi: 10.1152/ajpregu.00376.2003. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol.A Biol.Sci.Med.Sci. 2001;56:M266–M272. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Lissett CA, Shalet SM. The insulin-like growth factor-I generation test: peripheral responsiveness to growth hormone is not decreased with ageing. Clin Endocrinol (Oxf) 2003;58:238–245. doi: 10.1046/j.1365-2265.2003.01703.x. [DOI] [PubMed] [Google Scholar]
- Liu PY, Iranmanesh A, Nehra AX, Keenan DM, Veldhuis JD. Mechanisms of hypoandrogenemia in healthy aging men. 2005a;34:935–955. doi: 10.1016/j.ecl.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Liu PY, Swerdloff RS, Veldhuis JD. The rationale, efficacy and safety of androgen therapy in older men: future research and current practice recommendations. Journal of Clinical Endocrinology Metabolism. 2004;89:4789–4796. doi: 10.1210/jc.2004-0807. [DOI] [PubMed] [Google Scholar]
- Liu PY, Takahashi PY, Roebuck PD, Iranmanesh A, Veldhuis JD. Aging in healthy men impairs recombinant human LH-stimulated testosterone secretion monitored under a two-day intravenous pulsatile LH clamp. Journal of Clinical Endocrinology Metabolism. 2005b;90:5544–5550. doi: 10.1210/jc.2005-0909. [DOI] [PubMed] [Google Scholar]
- Liverman CT, Blazer DG. Testosterone and aging: clinical research directions. Institute of Medicine. The National Academies Press; 2004. [PubMed] [Google Scholar]
- Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54:1649–1656. doi: 10.2337/diabetes.54.6.1649. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. Journal of Clinical Endocrinology Metabolism. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metab: Clin Exp. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig-cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol. 1999;141:257–266. doi: 10.1530/eje.0.1410257. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Sorkin JD, Veldhuis JD, Harman SM, Munzer T, Bhasin S, Blackman MR. Long-term testosterone supplementation augments overnight growth hormone secretion in healthy older men. Am J Physiol Endo Metab. 2007;293:E769–E775. doi: 10.1152/ajpendo.00709.2006. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–3679. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- Pantel J, Legendre M, Cabrol S, Hilal L, Hajaji Y, Morisset S, Nivot S, Vie-Luton MP, Grouselle D, de Kerdanet M, Kadiri A, Epelbaum J, Le Bouc Y, Amselem S. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest. 2006;116:760–768. doi: 10.1172/JCI25303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM, Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proc Natl Acad Sci USA. 1996;93:14100–14105. doi: 10.1073/pnas.93.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S, Torres-Aleman I. Estradiol modulates insulin-like growth factor I receptors and binding proteins in neurons from the hypothalamus. J Neuroendocrinol. 1993;5:267–271. doi: 10.1111/j.1365-2826.1993.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Roth GS, Heiss GI. Changes in the mechanisms of hormone and neurotransmitter action during aging: current status of the role of receptor and post-receptor alterations. Mech Ageing Dev. 1982;20:175–194. doi: 10.1016/0047-6374(82)90086-0. [DOI] [PubMed] [Google Scholar]
- Schroeder ET, Zheng L, Ong MD, Martinez C, Flores C, Stewart Y, Azen C, Sattler FR. Effects of androgen therapy on adipose tissue and metabolism in older men. Journal of Clinical Endocrinology Metabolism. 2004;89:4863–4872. doi: 10.1210/jc.2004-0784. [DOI] [PubMed] [Google Scholar]
- Shah N, Evans WS, Veldhuis JD. Actions of estrogen on the pulsatile, nyctohemeral, and entropic modes of growth hormone secretion. Am J Physiol. 1999;276:R1351–R1358. doi: 10.1152/ajpregu.1999.276.5.R1351. [DOI] [PubMed] [Google Scholar]
- Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, Tamura H, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest. 2002;109:1429–1436. doi: 10.1172/JCI13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren K, Wallenius K, Liu JL, Bohlooly Y, Pacini G, Svensson L, Tornell J, Isaksson OG, Ahren B, Jansson JO, Ohlsson C. Liver-derived IGF-I is of importance for normal carbohydrate and lipid metabolism. Diabetes. 2001;50:1539–1545. doi: 10.2337/diabetes.50.7.1539. [DOI] [PubMed] [Google Scholar]
- Slootweg MC, Swolin D, Netelenbos JC, Isaksson OG, Ohlsson C. Estrogen enhances growth hormone receptor expression and growth hormone action in rat osteosarcoma cells and human osteoblast-like cells. J Endocrinol. 1997;155:159–164. doi: 10.1677/joe.0.1550159. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Cosma M, Erickson D, Paulo R, Mielke K, Farhy LS, Bowers CY. Tripartite control of growth hormone secretion in women during controlled estradiol repletion. Journal of Clinical Endocrinology Metabolism. 2007a;92:2336–2345. doi: 10.1210/jc.2007-0043. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Erickson D, Iranmanesh A, Miles JM, Bowers CY. Sex-steroid control of the aging somatotropic axis. 2005a;34:877–893. doi: 10.1016/j.ecl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Erickson D, Mielke K, Farhy LS, Keenan DM, Bowers CY. Distinctive inhibitory mechanisms of age and relative visceral adiposity on GH secretion in pre- and postmenopausal women studied under a hypogonadal clamp. Journal of Clinical Endocrinology Metabolism. 2005b;90:6006–6013. doi: 10.1210/jc.2005-0854. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Evans WS, Bowers CY. Estradiol supplementation enhances submaximal feedforward drive of growth hormone (GH) secretion by recombinant human GH-releasing hormone-1,44-amide in a putatively somatostatin-withdrawn milieu. Journal of Clinical Endocrinology Metabolism. 2003;88:5484–5489. doi: 10.1210/jc.2003-030410. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Evans WS, Iranmanesh A, Weltman AL, Bowers CY. Short-term testosterone supplementation relieves growth hormone autonegative feedback in men. Journal of Clinical Endocrinology Metabolism. 2004a;89:1285–1290. doi: 10.1210/jc.2003-031017. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A. Short-term aromatase-enzyme blockade unmasks impaired feedback adaptations in luteinizing hormone and testosterone secretion in older men. Journal of Clinical Endocrinology Metabolism. 2005;90:211–218. doi: 10.1210/jc.2004-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Bowers CY. Joint mechanisms of impaired GH pulse renewal in aging men. Journal of Clinical Endocrinology Metabolism. 2005c;90:4177–4183. doi: 10.1210/jc.2005-0336. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Godschalk M, Mulligan T. Older men manifest multifold synchrony disruption of reproductive neurohormone outflow. Journal of Clinical Endocrinology Metabolism. 2000;85:1477–1486. doi: 10.1210/jcem.85.4.6546. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Keenan DM. Erosion of endogenous testosterone-driven negative feedback on pulsatile LH secretion in healthy aging men. Journal of Clinical Endocrinology Metabolism. 2004b;89:5753–5761. doi: 10.1210/jc.2004-0399. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Mulligan T. Age and testosterone feedback jointly control the dose-dependent actions of gonadotropin-releasing hormone in healthy men. Journal of Clinical Endocrinology Metabolism. 2005d;90:302–309. doi: 10.1210/jc.2004-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Iranmanesh A, Takahashi PY, Nehra AX. The ensemble male hypothalamo-pituitary-gonadal axis. 2007b;12:185–203. [Google Scholar]
- Veldhuis JD, Keenan DM, Mielke K, Miles JM, Bowers CY. Testosterone supplementation in healthy older men drives GH and IGF-I secretion without potentiating peptidyl secretagogue efficacy. Eur J Endocrinol. 2005e;153:577–586. doi: 10.1530/eje.1.02001. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Patrie J, Frick K, Weltman JY, Weltman AL. Administration of recombinant human GHRH-1,44-amide for three months reduces abdominal visceral fat mass and increases physical-performance measures in postmenopausal women. Eur J Endocrinol. 2005f;153:669–677. doi: 10.1530/eje.1.02019. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Patrie JT, Frick K, Weltman JY, Weltman A. Sustained GH and IGF-I responses to prolonged high-dose twice-daily GHRH stimulation in middle-aged and older men. Journal of Clinical Endocrinology Metabolism. 2004c;89:6325–6330. doi: 10.1210/jc.2004-0430. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev. 2006;27:101–140. doi: 10.1210/er.2005-0006. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Urban RJ, Lizarralde G, Johnson ML, Iranmanesh A. Attenuation of luteinizing hormone secretory burst amplitude is a proximate basis for the hypoandrogenism of healthy aging in men. Journal of Clinical Endocrinology Metabolism. 1992;75:52–58. doi: 10.1210/jcem.75.3.1517359. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Veldhuis NJ, Keenan DM, Iranmanesh A. Age diminishes the testicular steroidogenic response to repeated intravenous pulses of recombinant human LH during acute GnRH-receptor blockade in healthy men. Am J Physiol. 2005g;288:E775–E781. doi: 10.1152/ajpendo.00410.2004. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Zwart A, Mulligan T, Iranmanesh A. Muting of androgen negative feedback unveils impoverished gonadotropin-releasing hormone/luteinizing hormone secretory reactivity in healthy older men. Journal of Clinical Endocrinology Metabolism. 2001;86:529–535. doi: 10.1210/jcem.86.2.7200. [DOI] [PubMed] [Google Scholar]
- Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V, Steiner B, Hull L, Callegari C, Swerdloff RS. Testosterone replacement therapy improves mood in hypogonadal men - a clinical research center study. Journal of Clinical Endocrinology Metabolism. 1996;81:3578–3583. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- Weltman A, Weltman JY, Hartman ML, Abbott RD, Rogol AD, Evans WS, Veldhuis JD. Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: effects of gender. Journal of Clinical Endocrinology Metabolism. 1994;78:543–548. doi: 10.1210/jcem.78.3.8126124. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Troen P. Episodic luteinizing hormone (LH) secretion and the response of LH and follicle-stimulating hormone to LH-releasing hormone in aged men: evidence for coexistent primary testicular insufficiency and an impairment in gonadotropin secretion. Journal of Clinical Endocrinology Metabolism. 1982;55:560–565. doi: 10.1210/jcem-55-3-560. [DOI] [PubMed] [Google Scholar]
- Witkin JW. Aging changes in synaptology of luteinizing hormone-releasing hormone neurons in male rat preoptic area. Neurosci. 1987;22:1003–1013. doi: 10.1016/0306-4522(87)92976-9. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Fernandez AM, Wu Y, Schally AV, Frystyk J, Chernausek SD, Mejia W, Le Roith D. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes. 2001;50:1110–1118. doi: 10.2337/diabetes.50.5.1110. [DOI] [PubMed] [Google Scholar]
- Yu H, Shu XO, Li BD, Dai Q, Gao YT, Jin F, Zheng W. Joint effect of insulin-like growth factors and sex steroids on breast cancer risk. Cancer Epidemiology, Biomarkers and Prevention. 2003;12:1067–1073. [PubMed] [Google Scholar]