Abstract
Actin filaments that serve as ‘rails’ for the myosin-based transport of membrane organelles [1-4] continuously turn over by concurrent growth and shortening at the opposite ends [5]. While it is known that dynamics of actin filaments is essential for many of the actin cytoskeleton functions, the role of such dynamics in the myosin-mediated organelle transport was never studied before. Here we addressed the role of turnover of actin filaments in the myosin-based transport of membrane organelles by treating cells with the drugs that suppress actin filament dynamics and found that such a suppression significantly inhibited organelle transport along the actin filaments without inhibiting their intracellular distribution or the activity of the myosin motors. We conclude that dynamics of actin filaments is essential for myosin-based transport of membrane organelles and suggest a previously unknown role of actin filament dynamics in providing the ‘rails’ for continuous organelle movement resulting in the increased distances traveled by membrane organelles along the actin filaments.
To address the role of dynamics of actin filaments in myosin-based transport of membrane organelles we used Xenopus melanophores as an experimental system [6]. The major function of melanophores is fast redistribution of pigment granules, which aggregate at the cell center or redisperse uniformly throughout the cytoplasm [6]. While pigment aggregation is exclusively microtubule-dependent, dispersion combines kinesin-driven transport of granules along the radial microtubules and myosin-driven transport along the randomly arranged actin filaments [7-9]. Treatment of melanophores with microtubule-depolymerizing drugs followed by application of pigment dispersion stimuli provides an established experimental system for studying the actin component of the organelle transport [7].
To test the hypothesis that dynamics of actin filaments is important for actin-mediated organelle transport, we examined pigment aggregation and dispersion in melanophores treated with the actin-stabilizing drug jasplakinolide [10]. To reduce the possibility of non-specific effects of the drug treatment, jasplakinolide was taken at a low concentration (1 μM) [11] and applied for a short period of time (5 min). We found that while aggregation of pigment granules (a solely microtubule-dependent process) occurred with normal kinetics, the rate of pigment dispersion (a process that combines microtubule-based and actin-based transport) was significantly inhibited in jasplakinolide-treated cells (Fig. 1A, and Supplemental Videos 1-4), suggesting that stabilization of actin filaments by jasplakinolide specifically affects the actin filament-based component of pigment transport.
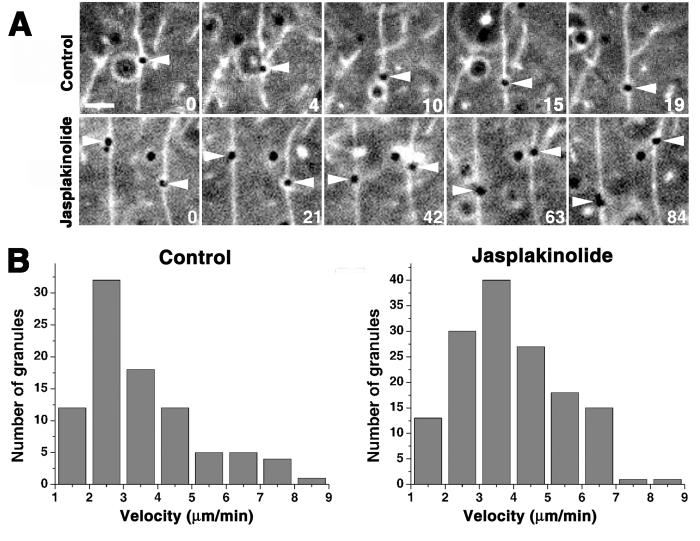
Figure 1. Treatment of melanophores with actin filament-stabilizing drugs inhibits transport of pigment granules.
A, Phase contrast images of melanophores treated with melatonin to induce pigment aggregation (top row), or with melanocyte-stimulating hormone to induce pigment dispersion (bottom row) in the absence (left panel) or in the presence (right panel) of jasplakinolide (1 μM); pairs of images in each panel show the same cells before or 10 min after hormone treatment; while pigment aggregation occurred with a normal kinetics, the rate of pigment dispersion was significantly inhibited in jasplakinolide-treated cells; numbers indicate time in min; bar, 20 μm. B, Quantification of actin-dependent movement of pigment granules measured in cells with disrupted cytoplasmic microtubules; plots show changes with time of averaged squared distances traveled by pigment granules from a starting point in control cells (black squares), cells treated with jasplakinolilde (1 μM) for 5 min (red circles), cells injected with phalloidin solution (100 μM; purple triangles), buffer-injected cells (blue diamonds), GFP-overexpressing cells (green triangles), or cells overexpressing dominant-negative myosin Va construct (MST-GFP; dark blue triangles); treatment of cells with jasplakinolide or microinjection with phalloidin solution reduces the movement of pigment granules to the levels seen in melanophores overexpressing dominant-negative myosin Va. C, Decomposition of the trajectories of pigment granule movement in cells with disrupted microtubules into linear (blue) and random diffusion-like displacements (green) using multiscale trend analysis in control (two top trajectories) and jasplakinolide-treated (two bottom trajectories) cells; the lengths of linear segments of the trajectories that likely correspond to actin-based runs are significantly shorter in jasplakinolide-treated cells. See also Supplemental Videos 1-6.
To study the effects of jasplakinolide specifically on the actin-based transport in the absence of the microtubule component, we examined the effects of actin filament stabilizing drugs on the movement of pigment granules in melanophores treated with a microtubule depolymerizing drug nocodazole [7]. Consistent with previous observations [7], pigment granules continuously moved in nocodazole-treated cells in the presence of pigment dispersion stimuli, as seen in a light microscope. Shortly after the application of jasplakinolide to such cells (5 min), visible movement of pigment granules dramatically decreased (Supplemental Videos 5 and 6), suggesting that jasplakinolide treatment inhibited granule movement along the actin filaments. To quantify this effect, we plotted averaged squared distances traveled by individual pigment granules over time (Fig. 1B) and used the 2-dimensional diffusion model to quantitatively characterize pigment granule movement by calculating the diffusion coefficients (defined as the slope of the curve of the averaged square distance over time, approximated to a straight line)[12] for granule movement under different conditions. Jasplakinolide treatment resulted in a decrease of the diffusion coefficient value by about 8-fold compared to control (to 0.48 × 10−3μm2/s, compared to 4.03 × 10−3 μm2/s in control). A significant inhibition was also observed if instead of jasplakinolide treatment cells were injected with phalloidin, an actin filament-stabilizing drug chemically distinct from jasplakinolide [13]. Phalloidin injection resulted in the diffusion coefficient value dropping to 0.91 × 10−3 μm2/s compared to the 3.79 × 10−3 μm2/s seen in control mock-injected cells (Fig. 1B). Thus, the effect of actin filament-stabilizing drugs on pigment transport is indeed related to the inhibition of dynamics of actin filaments and not to the specific chemical properties of jasplakinolide.
It was previously shown that inhibition of intracellular myosin Va by overexpression of a dominant-negative truncated construct of myosin Va results in a complete inhibition of actin-based pigment granule transport [9]. To compare the levels of this inhibition with those seen in jasplakinolide, we measured actin-based pigment granule movement in cells transfected with the dominant negative myosin construct and treated with nocodazole to disrupt microtubules and found that in such cells actin-based transport was reduced to the levels similar to jasplakinolide treatment (Fig. 1B, MST-GFP; 0.39 × 10−3 μm2/s; Fig. 1B). Therefore treatment of cells with actin-stabilizing drug jasplakinolide indeed caused a substantial decrease in the transport of pigment granules along the actin filaments, suggesting that dynamics actin filaments is required for such transport.
While the diffusion coefficient value is an accepted parameter to describe the overall displacement of pigment granules over time [14], it does not allow measurements of the specific velocity and length of linear particle excursions that correspond to the transport events along the actin filaments. Rather, it encompasses all types of pigment granule behavior, including pauses between the runs and random displacement of pigment granules in a motor-independent way. This limitation makes it difficult to evaluate the effect of stabilization of actin filaments specifically on the myosin-driven actin-based transport component rather than the overall displacement of the pigment in the cell. To specifically evaluate this effect we performed a detailed analysis of pigment granule trajectories by decomposing pigment granule movement into linear excursions along actin filaments and random, diffusion-like movement using the Multiscale Trend Analysis (MTA) [15] (Fig. 1C). We found that, consistent with the results of visual observations and diffusion coefficient measurements, jasplakinolide treatment substantially (∼3-fold) reduced the lengths of linear actin runs and the amount of time that pigment granules spend moving along the actin filaments (Table 1). The velocity of actin-based movement was also reduced to some extent. Thus, actin-dependent movement of pigment granules was indeed inhibited in cells with stabilized actin filaments.
Table 1.
Parameters of actin-based motion of pigment granules and lysosomes.
| Pigment granules | Lysosomes | |||
|---|---|---|---|---|
| Control | JSP | Control | JSP | |
| Number of examined runs |
767 | 504 | 559 | 532 |
| Duration of runs (min) | 0.21 ± 0.01 | 0.10 ± 0.01 | 0.14 ± 0.01 | 0.10 ± 0.01 |
| Length of runs (μm) | 0.41 ± 0.02 | 0.13 ± 0.01 | 0.34 ± 0.01 | 0.21 ± 0.01 |
| Velocity or runs (μm/min) |
2.05 ± 0.04 | 1.40 ± 0.04 | 2.60 ± 0.05 | 2.40 ± 0.05 |
| Time spent on actin (%) |
66 ± 0.5 | 24 ± 0.5 | 68 ± 0.8 | 42 ± 0.7 |
Several types of control experiments were performed to validate this result. First, to confirm that our jasplakinolide treatment indeed stabilized actin filaments in melanophores, we studied their dynamics in the absence and presence of jasplakinolide in cells microinjected with rhodamine-labeled actin, using fluorescence recovery after photobleaching (FRAP). The recovery of actin fluorescence after bleaching of a small zone in the cytoplasm was much slower in jasplakinolide-treated than in control cells, as evidenced from the quantification of actin fluorescence in the bleached zone (Fig. 2 and Supplemental Videos 7 and 8). We conclude that, as expected, jasplakinolide treatment indeed resulted in the inhibition of actin filament dynamics.
Figure 2. Jasplakinolide treatment stabilizes actin filaments, but does not significantly change their organization, or the levels of actin polymer in the cytoplasm.
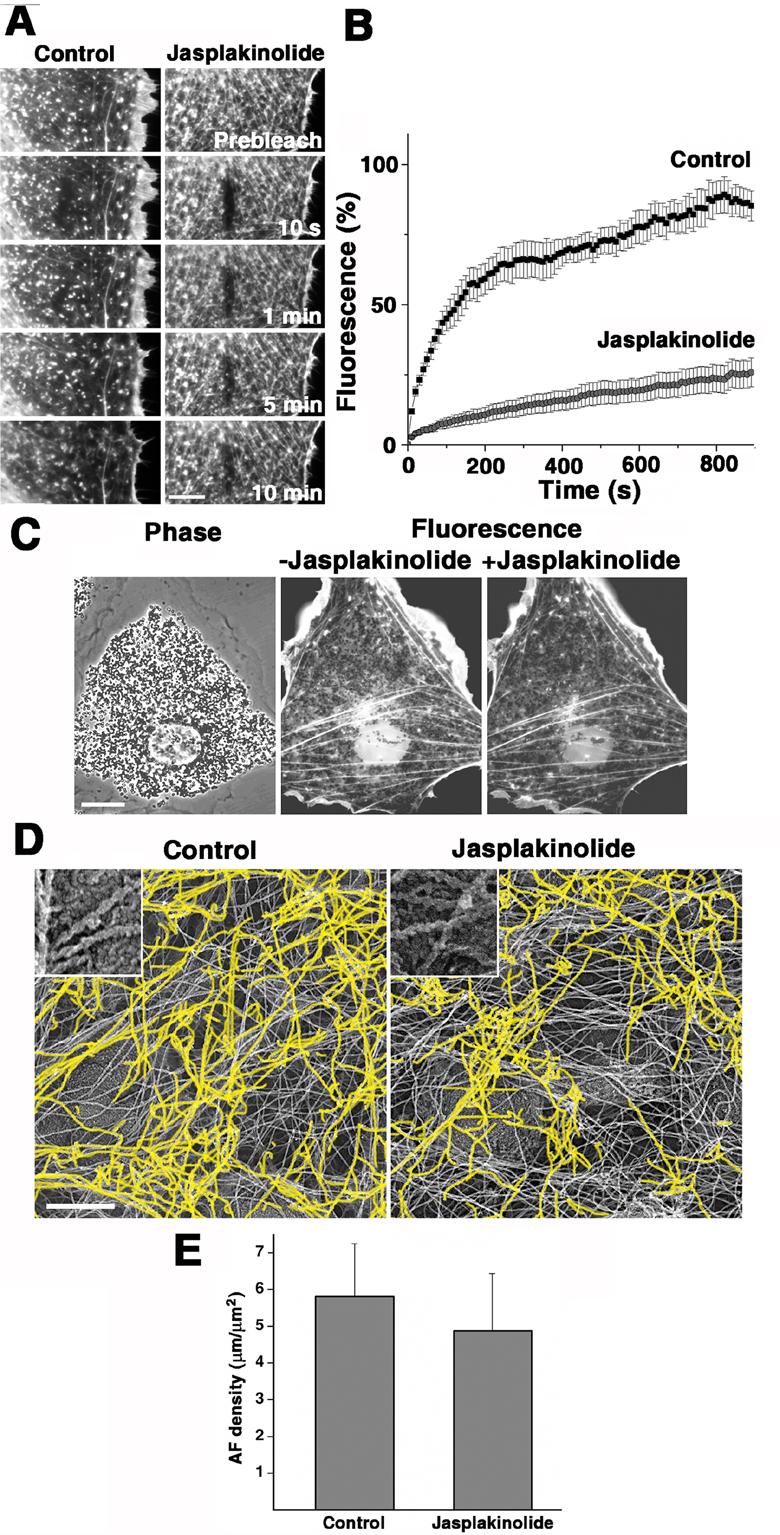
A and B, FRAP analysis of the turnover rates of actin filaments in control and jasplakinolide-treated cells. A, Sets of successive images of the bleached zones in control non-treated (left) or jasplakinolide-treated (right) cells; bar, 10 μm. B, Quantification of fluorescence recovery in the bleached zones located at approximately equal distances from the cell center and cell margin in the areas of the cytoplasm capable for supporting actin-based transport; black squares, control cells; gray circles, jasplakinolide-treated cells; error bars represent SEM for measurements in ten different cells; recovery of actin fluorescence is significantly faster in control than in jasplakinolide-treated cells; numbers shown on panel A indicate time after the photobleaching. C, Distribution of actin fluorescence in the same cell before (middle) or 5 min after (right) application of jasplakinolide; for the labeling of actin filaments, the cell was injected with rhodamine-actin 2 hours prior to acquisition of images; left, phase contrast image that shows distribution of pigment granules in the same cell; jasplakinolide treatment does not significantly change the distribution of actin fluorescence; bar, 20 μm. D, Electron micrographs of platinum replicas of cytoplasmic regions located at approximately equal distances from the cell center and cell margin in control non-treated (left) and a jasplakinolide-treated (right) melanophore; characteristic rope-like appearance of the actin filaments (inserts), which allowed their identification in electron micrographs, is explained by their decoration with the S1 subfragment of myosin during the preparation of samples for electron microscopy [7]; the S1-decorated actin filaments are highlighted in yellow; jasplakinolide treatment did not significantly change actin filament distribution; bar, 0.5 μm. E, Quantification of the actin filament density by measuring length of actin filaments in electron micrographs of control non-treated and jasplakinolide-treated cells; error bars represent SEM for measurements in ten different cells; jasplakinolide treatment does not significantly the density of actin filaments. See also Supplemental Videos 7-8.
Second, we examined the possibility that inhibition of pigment granule transport upon jasplakinolide treatment could be explained by the reorganization of actin cytoskeleton caused by the drug. To address this, we examined the organization of actin filaments before and after jasplakinolide treatment by light microscopy imaging of live cells microinjected with rhodamine-labeled actin and by electron microscopy of platinum replicas of regions of fixed cells treated with myosin subfragment 1 (S1) to decorate actin filaments. Live imaging of the same cells before and 5 min after the application of the drug indicated that jasplakinolide treatment did not cause significant changes in the distribution of actin fluorescence (Fig. 2C). Electron microscopy of platinum replicas demonstrated that the overall distribution of actin filaments (Fig. 2D) as well as their local density (determined as the total length of actin filaments per area in 10 different fields of view, Fig. 2E) were similar in jasplakinolide-treated and control untreated melanophores. Therefore brief exposure of melanophores to the low concentrations of jasplakinolide used in our experiments did not lead to significant changes in the organization of actin filaments in the treated cells, suggesting that the inhibition of actin-based transport of pigment granules observed in jasplakinolide-treated cells could not be explained by changes in actin filament organization.
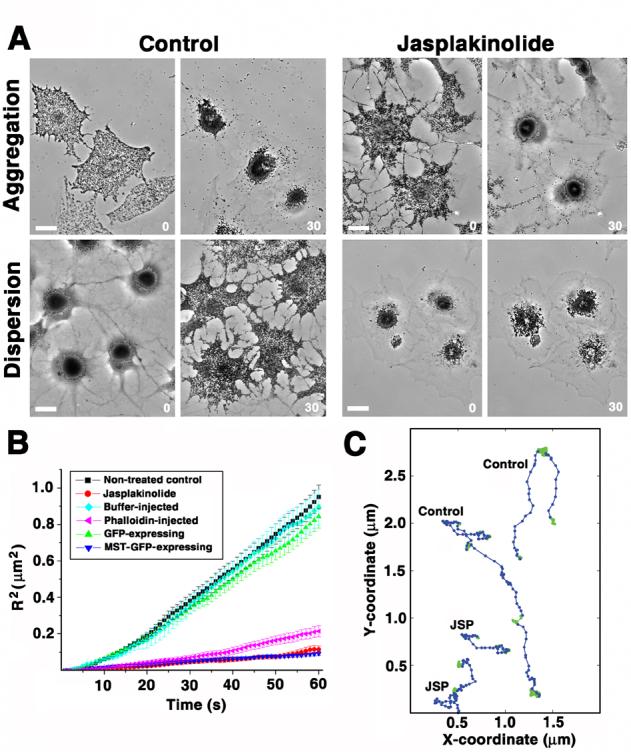
Finally, we tested the possibility that jasplakinolide suppressed transport of pigment granules along actin filaments by inhibiting the activity of pigment granule-bound myosin motors. To do this, we isolated pigment granules from melanophores and compared their movement along the actin filaments, fluorescently labeled with rhodamine-phalloidin, in the presence or absence of jasplakinolide in an in vitro motility assay. In both control and jasplakinolide-treated samples, pigment granules frequently attached to actin filaments and moved along them (Fig. 3A and Supplemental Videos 9 and 10). In both samples, these transport events occurred with similar frequencies and at similar velocities (3.47±0.17 and 3.93±0.12 μm/min, in the control and jasplakinolide-treated samples, respectively, Fig. 3 B). To rule out the possibility that in our motility assays actin filaments bound jasplakinolide and therefore removed it from solution, we also examined movement of pigment granules along the jasplakinolide-stabilized actin filaments assembled from rhodamine-actin. We found that in these experimental conditions pigment granules moved with velocity 3.69±0.17 μm/min similar to control samples. Therefore, suppression of pigment granule transport in jasplakinolide-treated cells cannot be explained by inhibition of myosin Va activity or its capacity to drive organelle transport along the actin filaments.
Figure 3. Jasplakinolide does not affect myosin-driven movement of isolated pigment granules along actin filaments examined in in vitro motility assay.
A, Successive images of fluorescently labeled actin filaments and pigment granules acquired in the absence (top panel) or in the presence (bottom panel) of jasplakinolide (1 μM); in both control and jasplakinolide-containing samples pigment granules often attached to actin filaments and moved along them, which indicates that jasplakinolide does not significantly inhibit myosin-based transport of pigment granules; numbers indicate time in s; bar, 0.5 μm. B, Frequency histograms of movement velocities of pigment granules along the actin filaments in vitro in the absence (left) or presence (right) of jasplakinolide; in both the presence or the absence of jasplakinolide the movement velocities peaked at about 3 μm/min, which indicates that the drug has no detectable effect on the activity of myosin Va. See also Supplemental Videos 9-10.
Thus, the experiments shown above demonstrate that inhibition of dynamics of actin filaments inhibits organelle transport along them without affecting the density or distribution of the actin filament tracks or the properties of the myosin motor. A possible explanation for this effect is that continuous growth and shortening of actin filaments creates temporal openings between the adjacent actin filaments and removes physical obstacles in the granule paths to prevent their trapping in the actin filament network. If this is the case, stabilization of actin filaments should affect the overall ability of granules to move through the cytoplasm, including their microtubule-based transport. While our initial experiments show that jasplakinolide treatment does not affect global pigment redistribution along microtubules during pigment aggregation (Fig. 1A) and therefore argue against such a possibility, it is still possible that local changes in microtubule-based transport are observed in jasplakinolide-treated cells. To address this possibility, we examined the effect of stabilization of actin filaments by jasplakinolide on the parameters of bidirectional movement of individual pigment granules along microtubules during pigment aggregation. Measurement and analysis of individual granule displacement showed that suppression of actin filament dynamics by jasplakinolide did not affect the rates or durations of uninterrupted runs of pigment granules along microtubules, or the duration of pauses (Supplemental Figure 1). Thus, stabilization of actin filaments during jasplakinolide treatment does not induce pigment granule trapping.
To further test the trapping ability of stabilized actin filaments, we tested whether jasplakinolide treatment affects the diffusion of soluble cytoplasmic particles that are not a cargo for myosin-driven movement, and quantified the movement of fluorescent latex beads microinjected into melanophores in the presence or absence of the drug (see Supplemental Videos 11 and 12). We found that diffusion coefficients of latex beads in the presence or absence of jasplakinolide were very similar (0.41 × 10−3, and 0.39 × 10−3 μm2/s, respectively). Therefore stabilization of actin filaments does not restrain the movement of cytoplasmic particles regardless of the basis of their motility. The results of these experiments suggest that actin filament dynamics facilitates myosin-based transport of pigment granules by a specific mechanism that affects the ability of the actin tracks themselves to support organelle movement.
To test whether this actin dynamics-dependent transport mechanism apples to the transport of organelles different from pigment granules, we tested the effect of stabilization of actin filaments on the transport of lysosomes labeled with fluorescent LysoTracker dye (see Supplemental Videos 13 and 14). Similar to pigment granules, lysosomes move along actin tracks by means of a myosin motor [16]. Decomposition of lysosome trajectories using the Multiscale Trend Analysis showed that linear actin runs were shorter and the time spent on actin was reduced in jasplakinolide-treated cells. Although the movement of lysosomes was affected to a lesser extent than the movement of pigment granules, presumably because of a smaller contribution of actin-based movement to the transport of lysosomes, inhibition of the lysosome motility was substantial (Table 1). Therefore, the requirement for actin dynamics is not a specific property of the pigment granule transport, but appears to be a universal property for the actin-based movement of membrane organelles.
Our results suggest that the actin-based component of the organelle transport involves a population of dynamic actin filaments that lose actin subunits off the minus (“pointed”) ends and add them to the plus (“barbed”) ends. Analysis of the FRAP data (see Supplemental Data) suggests that the average rate of growth of actin filaments in the cytoplasm is about 4.3 μm/min, which is higher than velocities of movement of pigment granules or lysosomes along the actin filaments (Table 1). Such faster filament growth compared to the velocity of the myosin-based movement suggests that myosin motors that move organelles to the growing “barbed” ends of actin filaments never reach the ends of transport tracks. Thus, growth of actin filaments should significantly increase the distances traveled by organelles by increasing the effective transport tracks length (Fig. 4). Inhibition of actin dynamics inhibits addition of subunits to the “barbed” ends actin filaments, blocking the elongation of the actin tracks ahead of the moving organelles. An organelle that reaches the end of such a non-dynamic track would be forced to stop or switch to an adjacent actin track. For this switching to occur, however, the density of actin filaments in the cytoplasm should be sufficient for each organelle to be in close proximity (touching distance) to multiple actin filaments at a time. Examination of the stereo EM images of melanophores (see Supplemental Figure 2) indicates, however, that despite an apparently high density (Fig. 2D) most of the actin filaments do not make close contact with pigment granule surface, suggesting that switching of organelles to adjacent actin tracks is far more infrequent than their continued transport along the same filament. Therefore, organelles arriving at the ends of stable actin tracks would be forced to come off into the cytoplasm and stop moving. On a mass scale, such inhibition is expected to slow down the entire actin-based movement in the cytoplasm, as seen in our experiments.
Figure 4. Role of actin dynamics in the myosin-based transport of pigment granules.
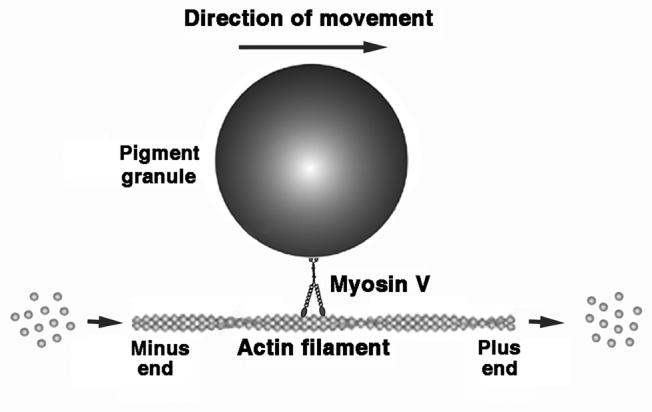
Myosin Va moves pigment granule towards the plus ends of actin filaments, which grow at the same time. Therefore growth of actin filaments should increase distance traveled by pigment granule along them.
Our hypothesis about the role of actin dynamics in intracellular transport sheds light onto the general mechanisms of transport of all types of membrane organelles that use myosin motors for the movement along the actin filaments. Actin filaments are highly dynamic in all types of eucaryotic cells examined so far. The published half-times for the turnover of actin filaments in mammalian cells vary from a few seconds to several minutes (see e.g. refs. [17-20]), and are therefore in about the same range as in melanophores (∼2 min; Fig. 2B). This suggests that membrane organelles, such as mitochondria [21], synaptic vesicles [22], and secretory granules [23], always move along the dynamic actin filaments. Most myosins transport cargoes to the “barbed” ends of actin filaments with the velocities comparable to the actin filament growth rates at physiological actin concentrations [2, 5], therefore the filament growth should be a significant factor that determines the distances traveled by membrane organelles in the cytoplasm. Analysis of the role of actin dynamics in the transport of specific types of membrane organelles is an exciting new direction for the future experiments.
It is widely accepted that actin-based movement of membrane organelles and cytoplasmic particles involves two independent mechanisms. While some organelles, such as pigment granules, are transported along the actin filaments by myosin motors [2], others are pushed through the cytoplasm by the actin filaments that assemble at the surface and often form the characteristic “comet tails” [24-28]. The actin filament assembly-dependent transport mechanism is responsible for the movement of intracellular pathogens [25, 27, 29], and organelles such as endosomes [28] or mitochondria [24]. In this mechanism, the assembly of actin filaments plays a central role because it provides the driving force for the transport. Our data show for the first time that dynamics of actin filaments is indispensable for the myosin-based transport as well. Therefore dynamic behavior is a fundamental property of actin filaments, which plays an essential role in all types of the actin-dependent intracellular transport mechanisms.
Supplementary Material
Acknowledgements
The authors would like to thank Oleg Nadiarnykh for critical reading of the manuscript, Dr. Vladimir Gelfand for a gift of dominant-negative myosin Va construct, and P.Sterling for permission to use his JEOL 1200EX electron microscope. This work was supported by NIH grant GM62290 to V.I.R.
References
- 1.Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell. 2001;12:780–794. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krendel M, Mooseker MS. Myosins: tails (and heads) of functional diversity. Physiology (Bethesda) 2005;20:239–251. doi: 10.1152/physiol.00014.2005. [DOI] [PubMed] [Google Scholar]
- 3.Mooseker MS, Cheney RE. Unconventional myosins. Annu Rev Cell Dev Biol. 1995;11:633–675. doi: 10.1146/annurev.cb.11.110195.003221. [DOI] [PubMed] [Google Scholar]
- 4.Tuxworth RI, Titus MA. Unconventional myosins: anchors in the membrane traffic relay. Traffic. 2000;1:11–18. doi: 10.1034/j.1600-0854.2000.010103.x. [DOI] [PubMed] [Google Scholar]
- 5.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 6.Nascimento AA, Roland JT, Gelfand VI. Pigment cells: a model for the study of organelle transport. Annu Rev Cell Dev Biol. 2003;19:469–491. doi: 10.1146/annurev.cellbio.19.111401.092937. [DOI] [PubMed] [Google Scholar]
- 7.Rodionov VI, Hope AJ, Svitkina TM, Borisy GG. Functional coordination of microtubule-based and actin-based motility in melanophores. Curr Biol. 1998;8:165–168. doi: 10.1016/s0960-9822(98)70064-8. [DOI] [PubMed] [Google Scholar]
- 8.Rogers SL, Gelfand VI. Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr Biol. 1998;8:161–164. doi: 10.1016/s0960-9822(98)70063-6. [DOI] [PubMed] [Google Scholar]
- 9.Rogers SL, Karcher RL, Roland JT, Minin AA, Steffen W, Gelfand VI. Regulation of melanosome movement in the cell cycle by reversible association with myosin V. J Cell Biol. 1999;146:1265–1276. doi: 10.1083/jcb.146.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- 11.Cramer LP. Role of actin-filament disassembly in lamellipodium protrusion in motile cells revealed using the drug jasplakinolide. Curr Biol. 1999;9:1095–1105. doi: 10.1016/s0960-9822(99)80478-3. [DOI] [PubMed] [Google Scholar]
- 12.Slepchenko BM, Semenova I, Zaliapin I, Rodionov V. Switching of membrane organelles between cytoskeletal transport systems is determined by regulation of the microtubule-based transport. J Cell Biol. 2007;179:635–641. doi: 10.1083/jcb.200705146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dancker P, Low I, Hasselbach W, Wieland T. Interaction of actin with phalloidin: polymerization and stabilization of F-actin. Biochim Biophys Acta. 1975;400:407–414. doi: 10.1016/0005-2795(75)90196-8. [DOI] [PubMed] [Google Scholar]
- 14.Gross SP, Tuma MC, Deacon SW, Serpinskaya AS, Reilein AR, Gelfand VI. Interactions and regulation of molecular motors in Xenopus melanophores. J Cell Biol. 2002;156:855–865. doi: 10.1083/jcb.200105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaliapin I, Semenova I, Kashina A, Rodionov V. Multiscale trend analysis of microtubule transport in melanophores. Biophys J. 2005;88:4008–4016. doi: 10.1529/biophysj.104.057083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordonnier MN, Dauzonne D, Louvard D, Coudrier E. Actin filaments and myosin I alpha cooperate with microtubules for the movement of lysosomes. Mol Biol Cell. 2001;12:4013–4029. doi: 10.1091/mbc.12.12.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theriot JA, Mitchison TJ. Actin microfilament dynamics in locomoting cells. Nature. 1991;352:126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- 18.Theriot JA, Mitchison TJ. Comparison of actin and cell surface dynamics in motile fibroblasts. J Cell Biol. 1992;119:367–377. doi: 10.1083/jcb.119.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGrath JL, Tardy Y, Dewey CF, Jr., Meister JJ, Hartwig JH. Simultaneous measurements of actin filament turnover, filament fraction, and monomer diffusion in endothelial cells. Biophys J. 1998;75:2070–2078. doi: 10.1016/S0006-3495(98)77649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy K, Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol. 2005;15:724–731. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 21.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bridgman PC. Myosin-dependent transport in neurons. J Neurobiol. 2004;58:164–174. doi: 10.1002/neu.10320. [DOI] [PubMed] [Google Scholar]
- 23.Eichler TW, Kogel T, Bukoreshtliev NV, Gerdes HH. The role of myosin Va in secretory granule trafficking and exocytosis. Biochem Soc Trans. 2006;34:671–674. doi: 10.1042/BST0340671. [DOI] [PubMed] [Google Scholar]
- 24.Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol. 2007;17:502–510. doi: 10.1016/j.tcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Frischknecht F, Way M. Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol. 2001;11:30–38. doi: 10.1016/s0962-8924(00)01871-7. [DOI] [PubMed] [Google Scholar]
- 26.Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol. 2006;4:423–434. doi: 10.1038/nrmicro1413. [DOI] [PubMed] [Google Scholar]
- 27.Cameron LA, Giardini PA, Soo FS, Theriot JA. Secrets of actin-based motility revealed by a bacterial pathogen. Nat Rev Mol Cell Biol. 2000;1:110–119. doi: 10.1038/35040061. [DOI] [PubMed] [Google Scholar]
- 28.Taunton J. Actin filament nucleation by endosomes, lysosomes and secretory vesicles. Curr Opin Cell Biol. 2001;13:85–91. doi: 10.1016/s0955-0674(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 29.Stevens JM, Galyov EE, Stevens MP. Actin-dependent movement of bacterial pathogens. Nat Rev Microbiol. 2006;4:91–101. doi: 10.1038/nrmicro1320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.