Abstract
We have investigated the possibility of using element-tagged antibodies for protein detection and quantification in microplate format using Inductively Coupled Plasma Mass Spectrometry (ICP-MS), and compared the results to conventional immunoassays, such as Enzyme-Linked Immunosorbent Assay (ELISA) and Western blotting. The technique was further employed to detect low levels and measure DNA-binding activity of transcription factor p53 in leukemia cell lysates through its interaction with immobilized oligonucleotides and recognition by element-tagged antibodies. The advantages of ICP-MS detection for routine performance of immunoassays include increased sensitivity, wide dynamic range, minimal interference from complex matrices, and high throughput. Our approach advances the ICP-MS technology and demonstrates its applicability to proteomic studies through the use of antibodies directly labeled with polymer tags bearing multiple atoms of lanthanides. Development of this novel methodology will enable fast and quantitative identification of multiple analytes in a single well.
Keywords: ELISA, ICP-MS, element-tagged antibody, lanthanides, growth factors, transcription factor p53
1. Introduction
There is growing interest in measuring low levels of biological molecules in a complex matrix using affinity products, such as antibodies. A sensitive and dynamic method to quantify antigens is through inductively coupled plasma mass spectrometry (ICP-MS) using element-tagged antibodies. ICP-MS is extensively used as an analytical chemistry tool as well as to study natural and induced metal incorporation into bacteria (Li et al., 2005;Zhang et al., 2003a; Binet et al., 2003;Zhang et al., 2003b), plants (Sharma et al., 2005) and in metalloproteomics research (Prange and Profrock, 2005;Szpunar, 2005). In all of these cases, the bio-molecules being analyzed are already associated with a metal component. In contrast, the ICP-MS-linked immunoassays provide a means to determine the concentrations of proteins that do not necessarily contain a metal. To achieve this, ICP-MS immunoassays rely on the use of affinity products that include a metal containing tag (Careri et al., 2007;Zhang et al., 2001;Zhang et al., 2002). The most straightforward approach utilizes antibodies that have been raised specifically against the antigens (e.g. proteins) of interest and tagged with metal.
In this investigation, we used antibodies conjugated to a polymer tag containing multiple metal chelates loaded with lanthanide ions. Interchain disulfide bonds in the antibody were partially reduced, and the polymer was attached through its terminal maleimide group to sulfhydryl residues on the Fc portion of the antibody (Lou et al., 2007). Thus, the antigen recognition sites retained their specificity, while inclusion of multiple lanthanide atoms on the polymer increased instrument detection sensitivity. Multiplex analysis for a variety of surface biomarkers have been performed on cell samples using commercial and in-house prepared element-tagged antibodies (Ornatsky et al., 2006;Tanner et al., 2007;Lou et al., 2007). The present investigation focused on the evaluation and application of ICP-MS techniques utilizing element-tagged antibodies for the quantification of growth and transcription factors in cell lysates. The method is compared to the enzyme-linked immunosorbent assay (ELISA) and Western blotting. ELISA is considered the gold standard for measuring levels of a single analyte in a sample (Crowther J.R., 2001). We modified both direct and sandwich immunoassays to include lanthanide labeled antibodies rather than enzyme antibody conjugates.
The present study evaluated several model systems for the ICP-MS immunoassays: purified growth factor assay, p53 protein level analysis, and measurement of transcription factor p53 DNA-binding activity in human leukemia cell lysates. The first is the human platelet-derived growth factor (hPDGF), a major mitogenic factor found in serum with a role in connective tissue homeostasis (Claesson-Welsh and Heldin, 1989;Soma et al., 1992). hPDGF exists as a dimer, and has three distinct isoforms: hPDGF-AA, AB or BB. We used the human recombinant PDGF-AA isoform as the target growth factor in our assays. p53 was chosen primarily due to its presence in many cell lines, availability of well characterized antibodies that recognize wild-type and mutant protein, and reliable short hairpin RNA (shRNA) for human p53 silencing (Brummelkamp et al., 2002). p53 protein is a transcription factor involved in the regulation of cell growth, DNA repair, and apoptosis in response to environmental stress. Activating signals, such as ionizing radiation, cause the p53 protein to undergo a number of posttranslational modifications (e.g. phosphorylation on key Ser and Thr residues) which correlate with p53 protein accumulation, translocation into the nucleus and increased association with the consensus sequences of p53 responsive genes (Vogelstein et al., 2000).
ICP-MS offers the potential for multivariate biological analysis based on metal-tagged affinity assays. Significant advantages of the approach include universality of tagging antibodies; a wide variety of elemental tags from which to choose (up to 100 stable metal isotopes), and lack of sensitivity to light which allows for laboratory labelling under convenient ambient conditions. These antibody labeling features, combined with the analytical characteristics of ICP-MS instruments, including analyte quantification with high precision; low detection limits; large dynamic range, both for each antigen and between antigens; low matrix effects from other components of the biological sample (i.e. contaminating proteins in the sample have no effect on elemental analysis); low background from plastic plates (i.e., plastic containers do not cause interference on elemental detection as it can with fluorescence), and superior spectral resolution (abundance sensitivity) call for further development and application of this novel methodology in immunological studies.
Here we demonstrated the use of element-tagged ICP-MS immunoassays for protein quantification in microplate format and its equivalence to conventional immunoassays, such as ELISA and Western blotting. The technique is further employed to determine levels of activated transcription factor p53 through the measurement of its binding to p53 consensus oligonucleotides and recognition of the complex by element-tagged antibodies in nuclear lysates of leukemia cell.
2. Materials and Methods
2.1. Antibodies and Antibody Labeling
Monoclonal antibodies against human p53 (DO-1 and Pab1801; Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-human PDGF-AA, anti-GAPDH antibodies (GeneTex Inc., San Antonio, TX)) and mouse IgG1 (R&D Systems Inc., Minneapolis, MN) were obtained from commercial sources. Lanthanide tagging was performed according to published protocol (Lou X. et al, 2007). In brief, antibodies were partially reduced using TCEP, tris(2-carboxyethyl)phosphine (Pierce Biotechnology, Inc., Rockford IL) in 30K Nanosep centrifugal filters (Pall Canada Limited, Mississauga, ON), and incubated for 1 hour at 37°C with a 30-fold molar excess of ligand-bearing polymer, known as MAXPAR™ (DVS Sciences Inc., Richmond Hill, ON). Excess polymer was washed off in the same filters and a 100-fold molar excess of a lanthanide chloride solution was added. After 45 minute incubation at 37°C, excess metal was washed off and the antibodies were stored in TBS (Tris buffered saline) at a concentration of approximately 0.8 mg/mL and used in assays at dilutions 1:200 and 1:400.
2.2. Cell Lines
A431, 293T, KG1a and K562 human cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured according to manufacturer’s specifications at 37°C, 5% CO2 and 100% humidity. 293T cells are adenovirus-transformed human kidney cells that express the SV40 large T antigen. The Tex cell line is a human immortalized myeloid cell line derived from cord blood cells transduced with TLS-ERG oncogene. Tex cells were cultured in IMDM supplemented with 15% FCS, 20 ng/ml SCF and 2 ng/ml IL-3 (Amgen Inc., Thousand Oaks, CA) (Warner et. al, 2005). Tex cells were obtained from the laboratory of J.E.Dick (University Health Network, Toronto, Canada). Tex-ShRFP (control shRNA against Red Fluorescent Protein), Tex-Shp53, 293T-ShRFP and 293T-Shp53 cell lines were obtained by transduction of the parental cell lines with lentiviral vectors expressing shRNA targeting RFP or p53.
2.3. hPDGF-AA Immunoassays
2.3.1. Standard ELISA
Recombinant human PDGF-AA (R&D Systems Inc., Minneapolis, MN) was reconstituted in 4 mM HCl with 0.1% BSA and diluted with carbonate buffer pH 9.0 to concentrations from 0 – 5 µg/mL. 96-well BeactiBind maleic anhydride microplates (Pierce Biotechnology, Inc., Rockford, IL) were incubated with 50 µL protein solutions overnight at 4°C and blocked with 1% BSA/PBS with 20 µg/mL mouse IgG (Biomeda Co., Burlingame, CA). Plates were washed 5 times with 0.1% Tween-20/PBS. Unlabeled mouse anti-human PDGF-AA antibodies (R&D Systems Inc., Minneapolis, MN) (at 1:200 dilution of 0.5 mg/ml stock) were added to plates, and after 90 minutes of incubation at room temperature plates were washed several times. Horseradish peroxidase conjugated anti-mouse antibody (Cell Signaling Technology, Inc., Danvers, MA) was added to the plates and incubated for 30 minutes at 37°C. Excess antibody was washed away, and the TMB, 3,3’,5,5’-tetramethylbenzidine, (Sigma Chemical Co., St.Louis, MO) substrate with hydrogen peroxide were added to develop color. The reaction was allowed to proceed for 30 minutes at room temperature, and stopped with sulfuric acid. The optical density was determined in a microplate reader (Molecular Devices Co., Union City, CA) at 450 nm.
2.3.2. ICP-MS Detection
Microplates with recombinant human PDGF-AA were prepared similarly to the method described above for conventional ELISA. Terbium (Tb) labeled antibodies were added to wells and incubated for 2 hours at room temperature. Plates were washed, and the contents of each well were digested with 80 µl Ultrapure 37% HCl (SealStar Inc.) for 30 minutes at room temperature. An equal volume of 1 ppb Ir/10% HCl internal standard was added, and samples were analyzed on the ELAN DRCPlus™ ICP-MS instrument (PerkinElmer-SCIEX, Waltham, MA), according to published operating procedures (Baranov et al., 2002;Tanner et al., 2007;Ornatsky et al., 2006;Tanner et al., 2007) and are summarized in Table I. The sample uptake rate was adjusted depending on the particular experiment and sample size, typically 100 µl/min. A MicroFlow PFA-ST concentric nebulizer (Elemental Scientific, Inc) was used in all instances. Experiments were performed using an autosampler (Perkin Elmer AS 91) modified for operation with Eppendorf 1.5 ml tubes. Sample size varied from 150 to 300 µl. Standards were prepared from 1000 µg/mL PE Pure single-element standard solutions (PerkinElmer, Shelton, CT) by sequential dilution with high-purity deionized water (DIW) produced using a Elix/Gradient (Millipore, Bedford, MA) water purification system.
Table I.
Typical operating conditions of ICP-MS instrument ELAN DRCPlus™ (Perkin Elmer SCIEX). The instrument was optimized to provide standard operating conditions.
| Parameter | Value |
|---|---|
| Plasma power | 1400W |
| MicroFlow PFA-ST concentric nebulizer gas, Ar flow | 0.95 L/min |
| Plasma gas, Ar flow | 17 L/min |
| Auxiliary gas, Ar flow | 1.2 L/min |
| CeO+/Ce+ ratio in 10%HCl | <3% |
| Sensitivity 1ppb Ir in 10%HCl, typical 1ppb In in 10%HCl, typical |
104 cps 4×104 cps |
2.4. Preparation of Extracts
2.4.1. Cell Lysates
Cell lysates were prepared by incubation of cell pellets with lysis buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin, 1mM PMSF) on ice, followed by sonication. Lysates were cleared by centrifugation at 10000 × g for 10 minutes and the supernatant was stored at −80°C.
In order to measure the change in p53 protein expression, Tex-ShRFP and Tex-Shp53 cells were exposed to 3 Gy of ionizing radiation using the 137Cs irradiator (MDS Nordion, Ottawa, ON) at 1 Gy/min for 3 min, and were collected 5h and 24h post-irradiation. Non-irradiated cells were collected at the same time points. Cell suspensions were centrifuged and protein lysates were prepared as described above.
2.4.2. Nuclear Lysates
Nuclear lysates were prepared using a nuclear extract kit (Active Motif Inc., Carlsbad, CA) according to the manufacturer’s protocol. Cells were washed and collected in cold PBS with phosphatase inhibitors (5 mM NaF, 10 mM beta-glycerophosphate, 10 mM para-nitophenyl phosphate, 1 mM NaVO3), and incubated in hypotonic buffer (20 mM Hepes, pH 7.5, 5 mM NaF, 10 µM Na2MoO4, 0.1 mM EDTA) on ice for 15 minutes. Nonidet P-40 was added to a final concentration of 0.5%, and the suspension was centrifuged for 30 seconds at 14 000 × g, 4°C. The supernatant was removed and the nuclear pellet resuspended in the lysis buffer (with added DTT and protease inhibitor cocktail). The suspension was incubated for 30 minutes on ice on a rocking platform, and then centrifuged for 10 minutes at 14 000 × g, 4°C. The supernatant was collected in aliquots and stored at −80°C. Total protein concentration of all lysates was measured using a Bradford protein assay.
2.5. p53 ICP-MS Immunoassay
Polystyrene microplates pre-coated with anti-p53 antibody were purchased as part of a commercial p53 ELISA kit (Cell Signaling Technology, Inc., Danvers, MA). Cell lysates were diluted to a final protein concentration of 0.25 mg/mL in sample diluent provided in the kit, and 100 µL was added to duplicate wells. After overnight incubation at 4°C plates were washed 5 times with wash buffer. Element-tagged antibodies (p53(DO1)Tb and IgG1-Tb or p53(1801)Tm) were diluted 1:100 with 1% BSA/PBS and added and to wells. Following incubation for 2 hours at room temperature, plates were washed, and contents of each well were digested with concentrated 37% HCl for 30 minutes at room temperature. An equal volume of 1 ppb Ir in 10% HCl (Ir internal standard) was added to each sample as an internal standard before analysis with ICP-MS.
2.6. Immunoblotting
Cells were harvested from tissue culture flasks, washed twice in ice-cold PBS and lysed in RIPA buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 20mM Na2HPO4, 100mM NaCl, 20mM NaF, 0.2mM PMSF, 1mM DTT). The lysates were boiled in SDS sample buffer (62.5mM Tris, pH 6.8, 5% β–mercaptoethanol, 10% glycerol, 2% SDS, 0.001% bromophenol blue) and the cell extracts (15–30 µg total protein per lane) were fractionated by 10% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) using the Mini-Protean™ apparatus (Bio-Rad, Hercules, CA). The separated proteins were electrotransferred to PVDF membrane (Bio-Rad, Hercules, CA). The PVDF membrane was then soaked in a blocking solution (5% non-fat milk in TBST buffer (20mM Tris-HCl, pH 7.5, 0.15 M NaCl, 0.1% Tween-20) for 1 h at room temperature. The blocked PVDF membranes were incubated with primary monoclonal anti-p53 (DO-1) antibodies or thulium (Tm)-tagged antibodies (p53(DO1)Tm) and anti-GAPDH (glyceraldehyde 3-phosphate dehydrogenase) overnight at 4°C. Membranes were washed with TBST buffer three times for 15 min each and incubated at room temperature for 1 h in horseradish peroxidase-conjugated goat anti-mouse antibodies (HRP-conjugated secondary antibodies; Amersham Biosciences, Pittsburgh, PA). The membrane was washed with TBST buffer three times for 15 min each. Immunoblots were visualized by enhanced chemiluminescence (ECL Plus Kit, Amersham Biosciences, Pittsburgh, PA) and membranes were exposed to Kodak X-ray film (Eastman Kodak Co., Rochester, NY, USA) for 2–3 seconds.
2.7. p53 activation immunoassay
TransAM ™ p53 transcription factor activation kit (Active Motif Inc., Carlsbad, CA) was used according to the manufacturer’s protocol. Nuclear lysates were diluted with lysis buffer and 10 µg of total protein per well were added to plates coated with immobilized dsDNA containing the p53 consensus binding site (5’-GGACATGCCCGGGCATGTCC-3’). For competition experiments, 20 pmol of free oligonuleotide (either consensus (con-oligo) or scrambled (scr-oligo), was added to each well. After 1h incubation at room temperature, plates were washed and further incubated with rabbit anti-p53 antibody (1:1000) for another hour. In the case of colorimetric ELISA, diluted anti-rabbit horseradish peroxidase-conjugated antibody was then added to washed plates, followed by 4–5 min incubation with substrate solution. The reaction was stopped and absorbance read at 450 nm. For ICP-MS immunoassay, diluted anti-rabbit-Tm secondary antibodies (1:800) were applied to wells. Finally, the contents of washed wells were digested with concentrated HCl for 30 minutes and solution analysis for 169Tm was performed on a conventional ICP-MS instrument (ELAN DRCPlus™ (PerkinElmer-SCIEX, Waltham, MA). Assay performance was verified using positive control extracts from activated MCF-7 cells (5 µg per well). All reactions were performed in duplicate.
3. Results
3.1. Detection of growth factor PDGF-AA
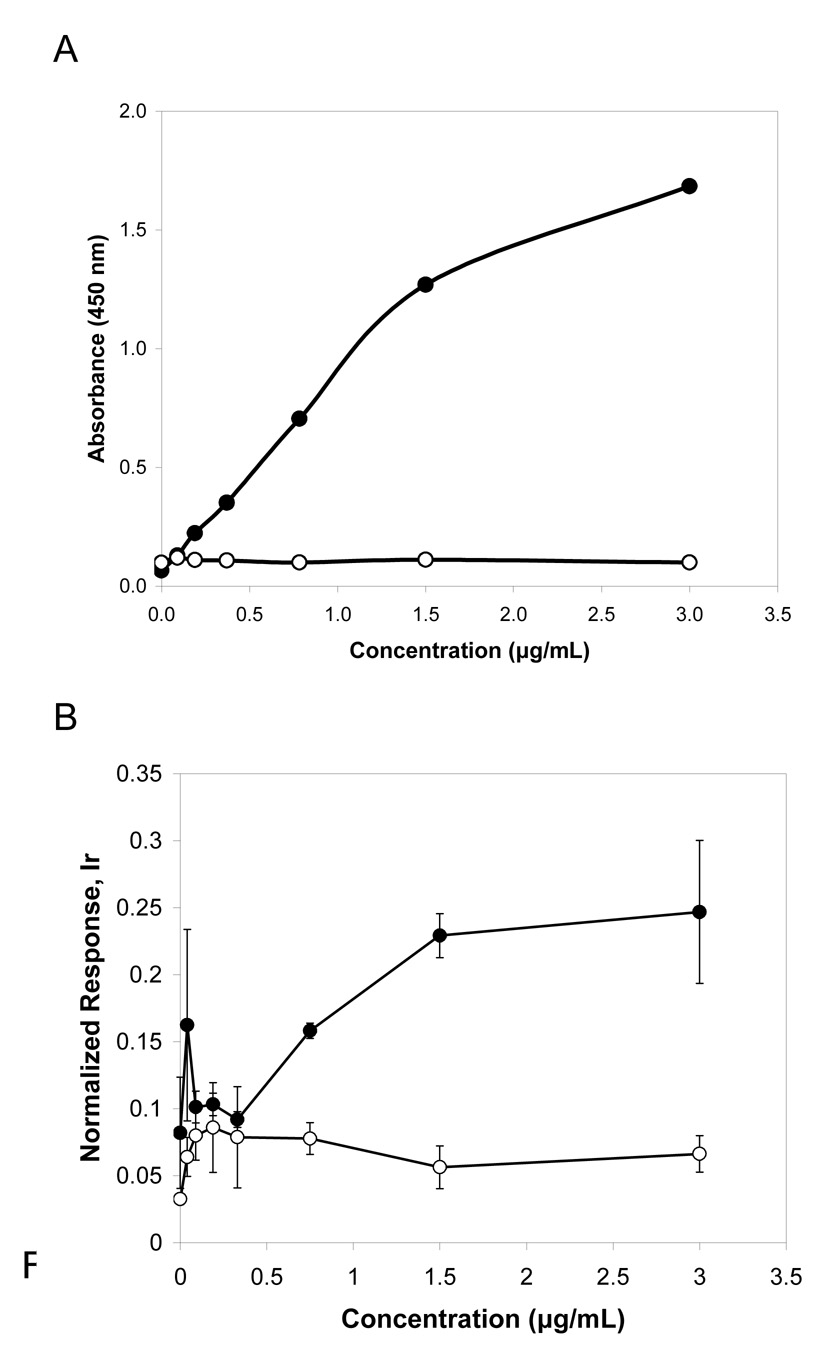
Immunoassays were performed using rhPDGF-AA directly attached to the bottom of maleic anhydrate microplates at 0 – 5 µg/ml. A primary monoclonal antibody against hPDGF-AA was used to measure specific biding, and a mouse IgG1 isotype was included as a control for non-specific binding. Detection was set up to measure color change after addition of HRP-labeled secondary antibody and substrate, and ICP-MS quantification of terbium (Tb) through the analysis of Tb-labeled primary antibody binding to rhPDGF-AA. The two methods showed comparable readouts (Figure 1) of specific binding of the anti-hPDGF-AA antibody increasing proportionally to growth factor concentration. The mouse IgG1 gave a low background signal in both methods. The difference in sensitivity may be explained by the fact that for the colorimetric detection we employed indirect ELISA with signal amplification through enzyme-linked secondary antibodies, whereas ICP-MS immunoassay used primary element-tagged antibodies (direct assay). To improve sensitivity of the ICP-MS assay in the lower concentration range, microtiter plates with rhPDGF-AA were additionally blocked with 5% BSA and 20 µg/mL mouse IgG in PBS, and the Tb-tagged primary antibody concentration was decreased four-fold. As a result, the assay sensitivity reached 0.5 µg/mL and signal variability and level of non-specific binding (Tb-tagged IgG1) were reduced (Figure 2A). The experiment shows that lanthanide labeled antibodies retain their specificity and behave similarly to non-tagged primary antibodies, thus, verifying the equivalence of the two detection methods. Measured responses for the rhPDGF-AA assay in both colorimetric and ICP-MS formats were studied using Pearson’s correlation coefficient. Results showed (Figure 2B) a strong linear relationship between the two detection methods, with correlation coefficient r2 = 0.9629.
Figure 1.
Comparison of colorimetric and ICP-MS immunoassays for growth factor hPDGF-AA. ReactiBind microplates were coated with recombinant hPDGF-AA at different concentrations. A) Indirect ELISA colorimetric method using anti-PDGF antibody, and mouse IgG for negative control (mIgG). Single measurements. B) ICP-MS using primary terbium (Tb) labeled antibodies against hPDGF-AA (anti-hPDGF-Tb), and mouse IgG tagged with Tb for negative control (mIgG-Tb). Response was normalized to 1 ppb Ir/10%HCl standard. Assay was performed in triplicate
Figure 2.
A) ICP-MS measurement of low amounts of hPDGF-AA. Plates were blocked with 5% BSA and 20 µg/mL IgG in PBS, then treated with terbium-tagged anti-PDGF-AA antibodies or terbium-tagged IgG1 immunoglobulins diluted 1:800. Response was normalized to 1 ppb Ir/10%HCl internal standard. Assay was performed in triplicate. B) Correlation analysis for conventional ELISA and ICP-MS measurements of rhPDGF-AA concentrations. Responses for the colorimetric and ICP-MS assay formats were compared at the same concentrations of growth factor.
3.2. p53 protein expression in cell lines
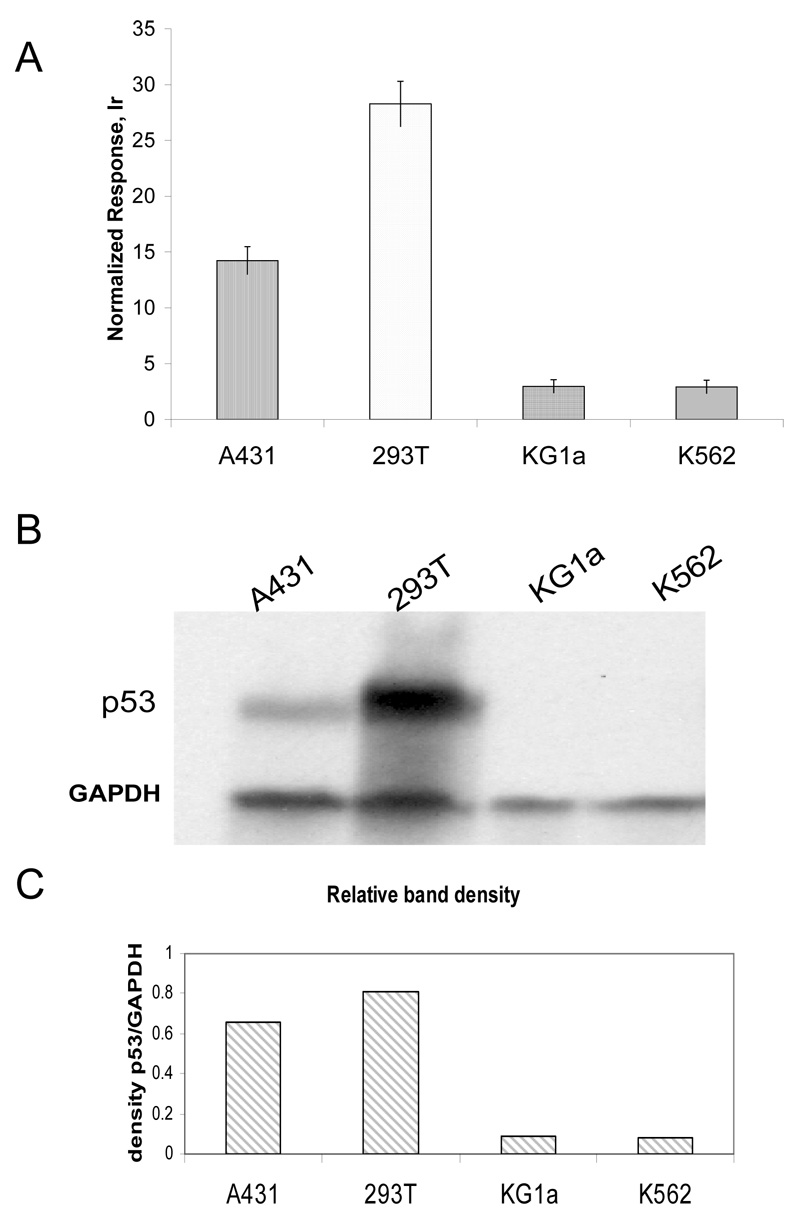
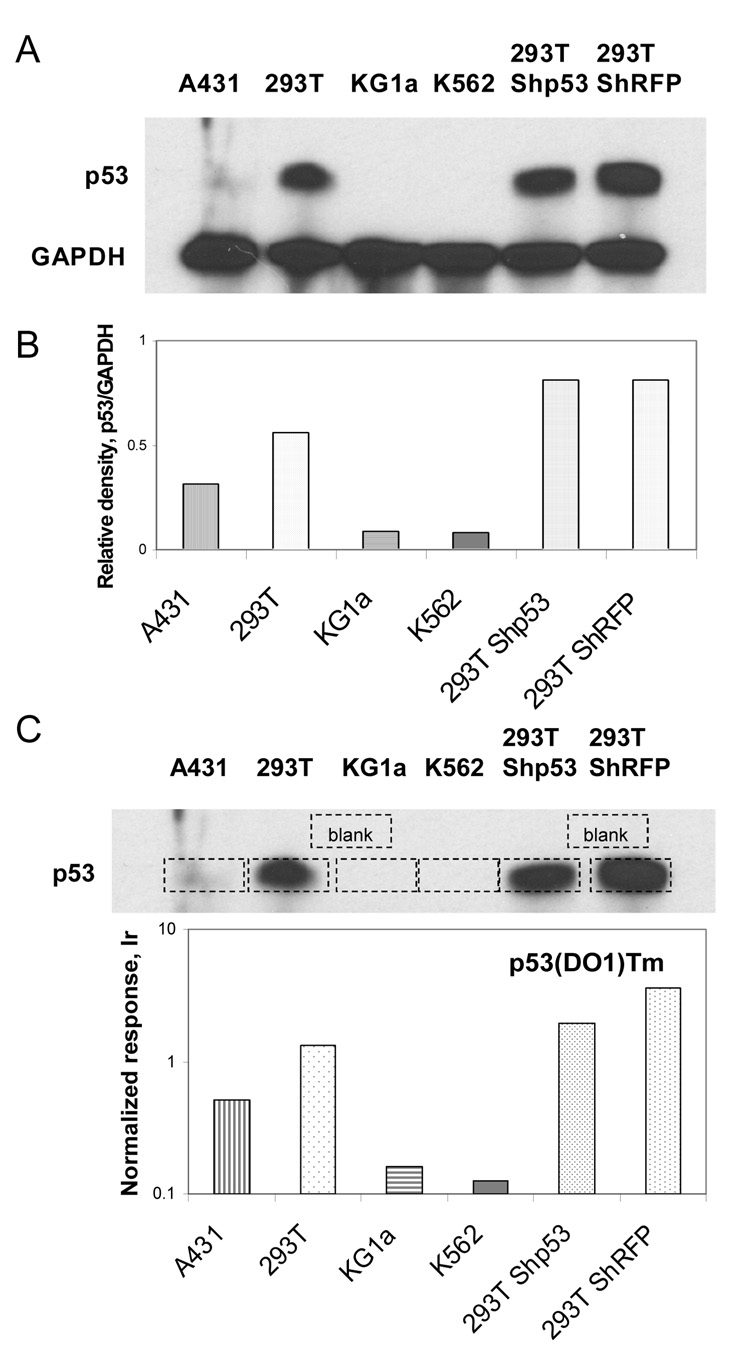
ICP-MS immunoassay was further tested using cell extracts for the possibility of detecting a specific protein in a complex matrix. Cell lysates from A431, 293T, KG1a, and K562 cells with varying p53 protein levels were distributed into microtiter plates coated with a rabbit anti-p53 antibody, and then probed with a monoclonal anti-p53 (DO-1) antibody labeled with Tb (sandwich ICP-MS immunoassay, Figure 3A). The expression of p53 in these cell lines differed significantly and the results of element-tagged assays demonstrated good correlation with analysis of the same cell lines by Western blotting (Figure 3B,C). GAPDH (glyceraldehyde 3-phosphate dehydrogenase), with a molecular weight at approximately 37 kDa, served as a control for equal total protein loading in SDS-PAGE. Due to the high levels of p53 in 293T cells, and absence of expression in K562 and KG1a cells, these lines were chosen as positive and negative controls, respectively. To prove that antibodies retain their specificity and affinity after element-tagging, PVDF membrane blots with cell extracts (A431, 293T, KG1a, K562, 293T-ShRFP (control) and 293T-Shp53 (shRNA targeting p53 results in a decreased levels of p53 protein expression) were probed with element-tagged primary anti-p53(DO1)Tm antibodies followed by secondary HRP-antibody. The chemiluminescent substrate was used to visualize bands on the X-ray film (Figure 4A). The film and PVDF membrane were superimposed (Figure 4C), and membrane pieces under the p53 bands and several blank areas were cut out with a sharp scalpel. Each membrane piece was placed into individual microfuge tubes with 80 µl HCl and soaked overnight to collect thulium dissociated from p53(DO1)Tm antibodies. The samples were then combined with an equal volume of Ir internal standard and analyzed by solution ICP-MS (Figure 4C). The experiment revealed that the element-tagged p53(DO1)Tm antibodies specifically bound to p53 electroblotted onto the membrane, and the element tag did not interfere with secondary HRP-labeled antibody binding. Moreover, the ICP-MS analysis indicates a small degree of silencing of p53 in the 293T-Shp53 cells compared to the control 293T-ShRFP cells, whereas densitometry of the Western blot (Figure 4B) failed to do so, presumably due to the very high amount of p53 protein in 293T cells resulting in the saturation of the film during the 2 second exposure.
Figure 3.
p53 protein detection in cell lines. A) ICP-MS immunoassay. Cell extracts were analyzed using Tb-labeled antibodies against p53. Response was normalized to 1 ppb Ir/10%HCl standard. B) Western blotting with anti-p53 and anti-GAPDH antibodies; C) Relative density of bands from exposed X-ray film.
Figure 4.
Demonstration of element-tagged p53(DO1)Tm antibody binding to p53 protein in different cell lysates electroblotted onto PVDF membrane. A) Western blotting and B) Relative density of bands from exposed X-ray film; C) Juxtaposition of X-ray film and membrane for delineation of immunoreactive bands (dashed line rectangles), which were cut out and soaked in concentrated HCl used ICP-MS analysis of Tm levels.
3.3. p53 DNA-binding immunoassay
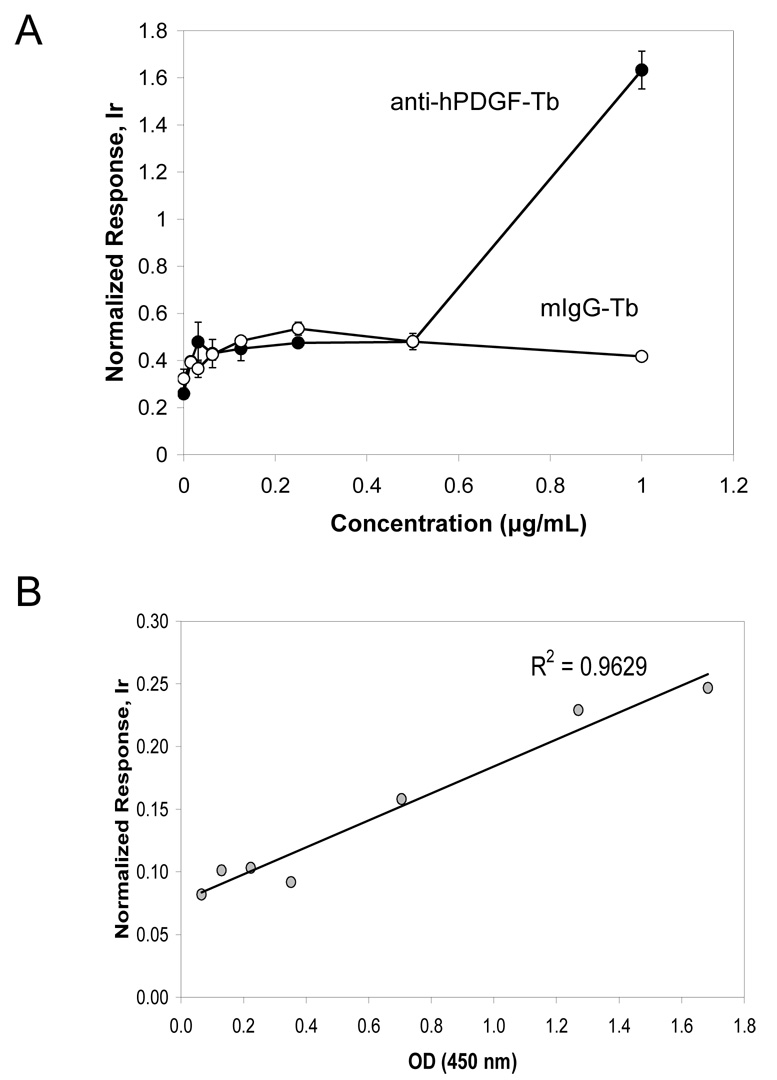
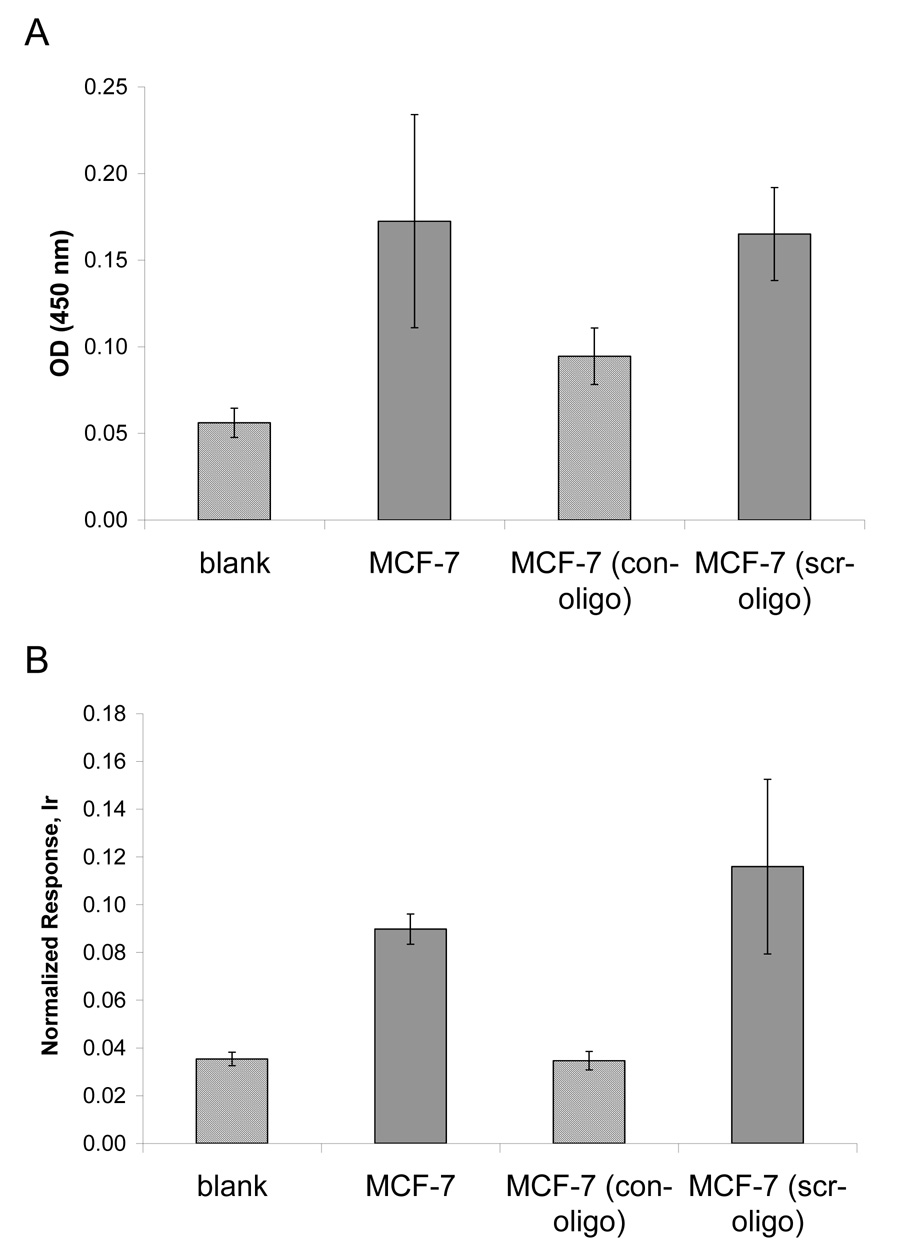
Element-tagged immunoassay for activated p53 was set up similarly to the ELISA p53 DNA-binding system suggested by Jagelska et al. (Jagelska et al., 2002). In this assay, the microplate wells were coated with a double strand DNA consensus binding sequence for activated p53, so that activated p53, arranged as a tetramer, could bind. The TransAM ™ p53 transcription factor activation kit (Active Motif Inc.) was used for the reactions. Levels of bound p53 were then measured by indirect ELISA (figure5A), or ICP-MS immunoassay through labeled secondary antibodies (Figure 5B). The assay functionality was tested using the nuclear lysate of MCF-7 cells provided by the manufacturer. When a free consensus oligonucleotide (con-oligo) was added together with the nuclear extract, low binding was seen due to competition for activated p53. p53 DNA binding activity was unchanged with the addition of scrambled oligonucleotide (scr-oligo). These experiments demonstrate the specificity of the assay for activated p53 for both the colorimetric and ICP-MS methods and concordance in measurements.
Figure 5.
Competition assays for the detection of activated p53 in MCF-7 cells. Nuclear extracts were treated with consensus (con-oligo) or scrambled (scr-oligo) oligonucleotides. A) Colorimetric ELISA. B) ICP-MS immunoassay; response was normalized to 1 ppb Ir/10%HCl internal standard. Assays were performed in duplicate.
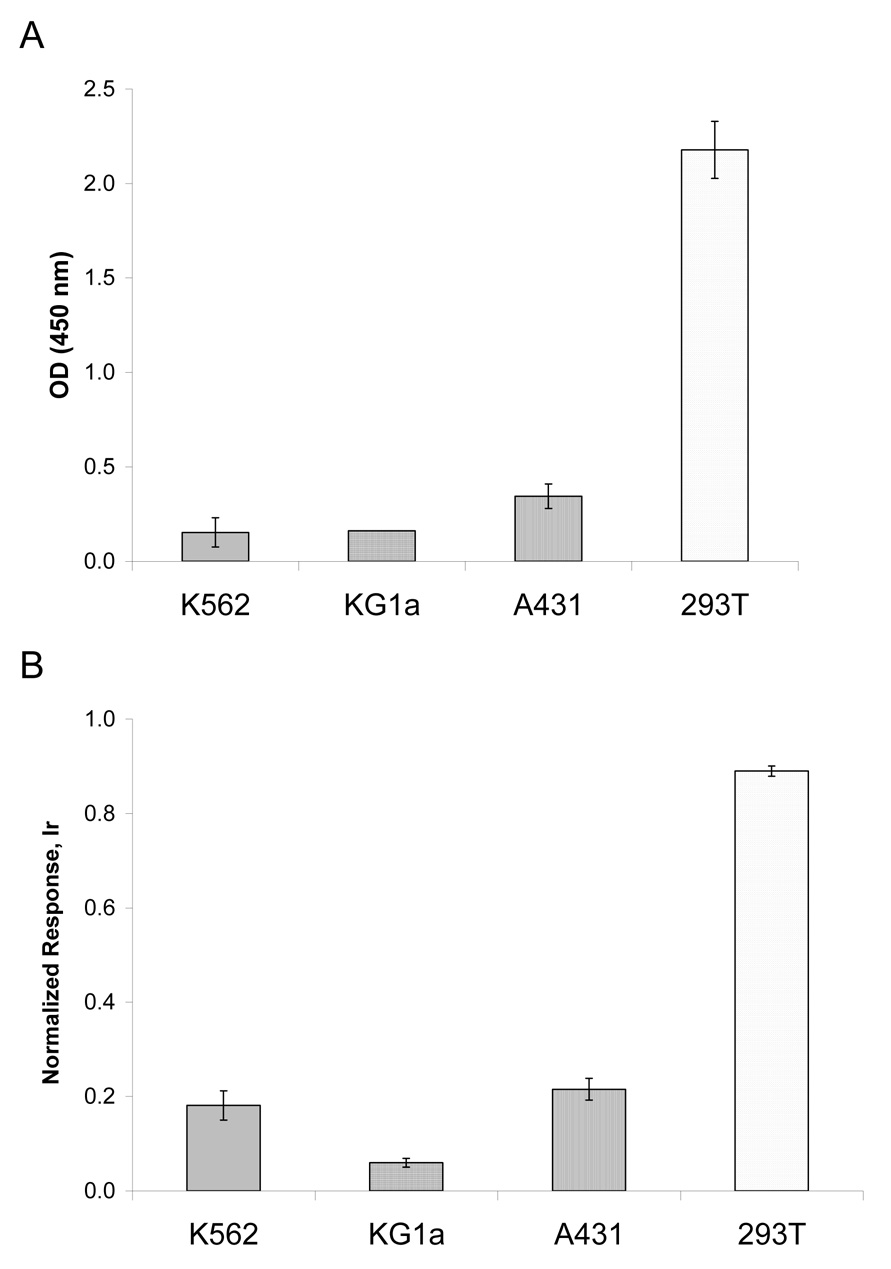
3.3.1 p53 DNA-binding activity in cell lines
Cell lines used previously (K562, KG1a, A431 and 293T) for measurement of total p53 by Western blotting and ICP-MS were analyzed for DNA-binding activity of p53 (Figure 6). A low response was seen in negative control cell lines K562 and KG1a, both of which do not carry the p53 gene. In 293T cells, the large T antigen has been shown to bind to and stabilize p53, thus causing SV40-transformed cells to accumulate large amounts of functionally inactive p53 (Ahuja et al., 2005;Oren et al., 1981). A431 cells are known to synthesize p53 with a point mutation in the DNA-binding domain (mutation at codon 273 results in an arginine to histidine substitution (Kwok et al., 1994)) which precludes robust interaction of p53 with the consensus oligo probes in the DNA-binding assay. This may explain the lower response in the activation assay compared to total p53 protein content measured in A431 cell extracts. The 293T cells displayed very high levels of p53 protein expression (Figure 3 and Figure 4) and at the same time demonstrated high DNA-binding ability in both colorimetric and ICP-MS methods (Figure 6). We interpret these results as being associated with the large amounts p53 protein in 293T, leading to non-specific binding with oligo probes under the conditions used. Addition of scr-oligo or con-oligo to 293T nuclear extracts decreased the measured response 3-fold in both cases (data not shown).
Figure 6.
DNA-binding activity of p53 in cell lines. A) Indirect ELISA; B) ICP-MS assay using secondary Tm-tagged antibodies. Response was normalized to 1 ppb Ir/10%HCl standard. Assay was performed in duplicate.
3.3.2 p53 DNA-binding activity in Tex cells
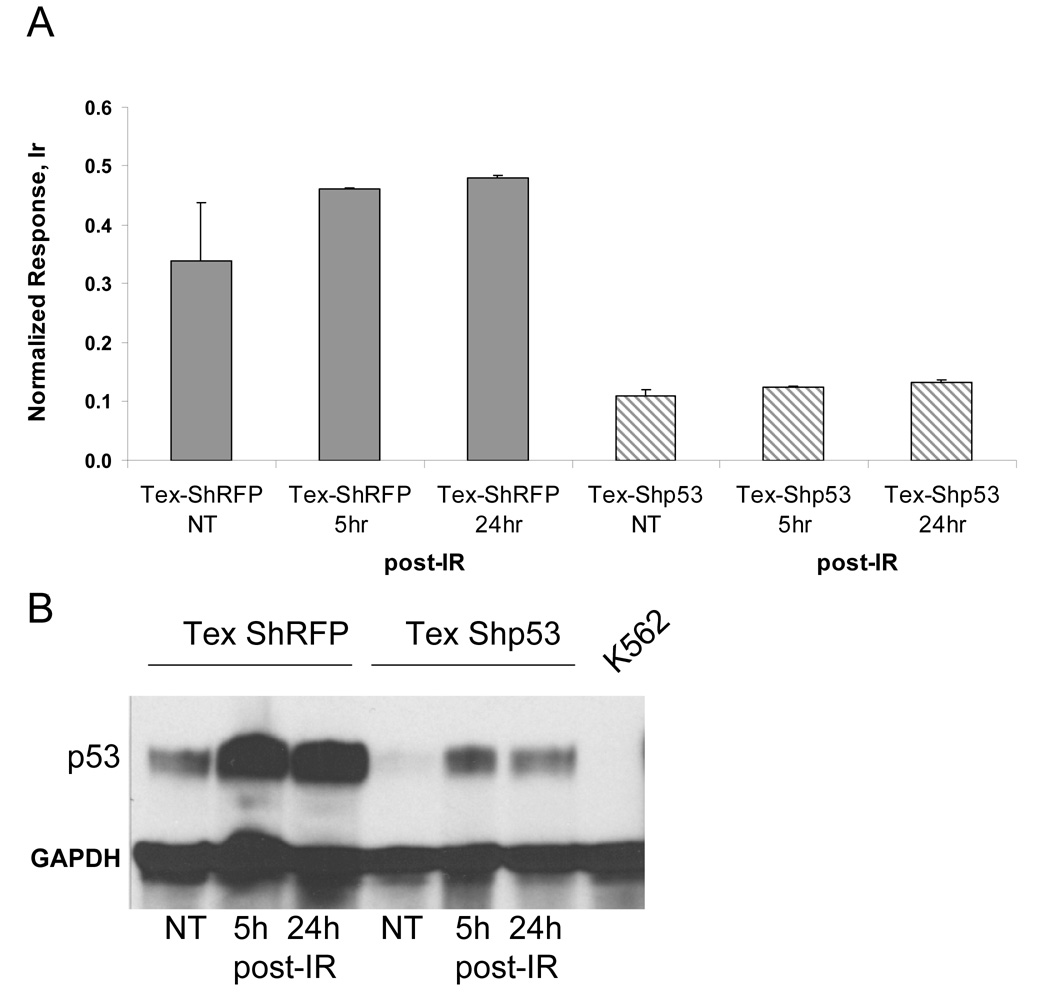
To confirm correlation between radiation and p53 activation as well as to analyze the consequences of p53 silencing on p53 activity, we designed an ICP-MS immunoassay to probe the activation state of p53 through its binding to the consensus sequence of target genes. Nuclear extracts from the Tex cells treated with ionizing radiation were used to probe for activated p53 by ICP-MS (Figure 7A). The TransAM p53 assay plate coated with consensus binding oligonucleotides was used. Total protein (10 µg/well) was applied to duplicate wells and incubated for 1 h with gentle shaking. Following blocking and several washing steps, primary anti-p53 antibodies were added. Finally, washed wells were probed with diluted (1:800) secondary Tm-tagged antibodies, and acidified contents of each well were subjected to ICP-MS analysis. As expected, a higher response to irradiation was seen in the control Tex-ShRFP NT cells, as opposed to those in which p53 was silenced (TexShp53). Thus, after irradiation there was an increase in activated p53 in ShRFP-transduced cells, whereas a non-significant change in p53 activity was noted in cells transduced with Shp53. Comparing these results to Western blots (Figure 8A), which show an increase of p53 protein levels after 5- and 24h post irradiation in control and silenced Tex cells, we come to the conclusion that activating stress led to the accumulation of small amounts of functionally inactive p53 in Tex-Shp53 cells.
Figure 7.
DNA-binding activity of p53 in Tex cells. A) Detection of activated p53 in Tex cells by ICP-MS using secondary Tm-tagged antibodies. Response was normalized to a 1 ppb Ir/10%HCL internal standard. Assay was performed in duplicate B) Western blot analysis of p53 protein expression in Tex cells. Control cells (Tex-ShRFP) and those blocked for p53 gene expression (Tex-Shp53) were irradiated and harvested 5 h and 24 h post-irradiation (post-IR) along with non-treated (NT) cells. K562 cell extract was included as negative control‥
4. Discussion
The present work provides evidence of the equivalency of ICP-MS immunoassay with traditional methods such as ELISA and Western immunoblotting. This novel technique was able to identify the presence and quantify low amounts of a target protein in a complex matrix. Element tagging and ICP-MS detection include advantages for routine performance of immunoassays such as (i) increased sensitivity, (ii) wide dynamic range, shown to be up to four orders of magnitude, and (iii) minimal interference from complex matrices. In addition, ICP-MS assays use antibodies directly labeled with element tags, thus increasing accuracy and throughput. Most importantly, ICP-MS detection also provides the opportunity for multiplex assays as was shown previously using secondary antibodies conjugated to europium-bearing chelates and gold nanoparticles (Quinn et al., 2002;Careri et al., 2007;Hu et al., 2007;Hutchinson et al., 2004). This is unattainable with colorimetric assays designed to measure one analyte in a single sample. Moreover, since there is no overlap between signals generated from element tags sophisticated compensation strategies, common in conventional multiparameter flow cytometry, are avoided and absolute quantification is achieved. Currently, our group is working on the demonstration of multiplex ICP-MS solid-phase immunoassays for the detection of ten analytes in a single well. ICP-MS element-tagged antibody technology is posed to dramatically improve the bio-analytical tool set for research, drug discovery/validation, and consequently to benefit health care.
Acknowledgments
This work was funded by Genome Canada through the Ontario Genomics Institute, the Ontario Cancer Research Network, NIH Grant (#5R01GM076127-02) and NSERC award to E.R. We thank Dr. Olga I. Gan for valuable suggestions and editing.
Abbreviations
- ICP-MS
Inductively Coupled Plasma Mass Spectrometry
- ELISA
Enzyme-Linked Immunosorbent Assay
- Tb
Terbium
- Tm
Thulium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 2.Baranov VI, Quinn ZA, Bandura DR, Tanner SD. The potential for elemental analysis in biotechnology. Journal of Analytical Atomic Spectrometry. 2002;17:1148. [Google Scholar]
- 3.Binet MRB, Ma RL, Mcleod CW, Poole RK. Detection and characterization of zinc- and cadmium-binding proteins in Escherichia coli by gel electrophoresis and laser ablation-inductively coupled plasma-mass spectrometry. Analytical Biochemistry. 2003;318:30. doi: 10.1016/s0003-2697(03)00190-8. [DOI] [PubMed] [Google Scholar]
- 4.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 5.Careri M, Elviri L, Mangia A, Mucchino C. ICP-MS as a novel detection system for quantitative element-tagged immunoassay of hidden peanut allergens in foods. Analytical and Bioanalytical Chemistry. 2007;387:1851. doi: 10.1007/s00216-006-1091-0. [DOI] [PubMed] [Google Scholar]
- 6.Claesson-Welsh L, Heldin CH. Platelet-derived growth factor. Three isoforms that bind to two distinct cell surface receptors. Acta Oncol. 1989;28:331. doi: 10.3109/02841868909111202. [DOI] [PubMed] [Google Scholar]
- 7.Crowther JR. The ELISA guidebook. Humana Press; 2001. [Google Scholar]
- 8.Hu SH, Zhang SC, Hu ZC, Xing Z, Zhang XR. Detection of multiple proteins on one spot by laser ablation inductively coupled plasma mass spectrometry and application to immuno-microarray with element-tagged antibodies. Analytical Chemistry. 2007;79:923. doi: 10.1021/ac061269p. [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson RW, Ma RL, Mcleod CW, Milford-Ward A, Lee D. Immunoassay with FI-ICP-MS detection - Measurement of free and total prostate specific antigen in human serum. Canadian Journal of Analytical Sciences and Spectroscopy. 2004;49:429. [Google Scholar]
- 10.Jagelska E, Brazda V, Pospisilova S, Vojtesek B, Palecek E. New ELISA technique for analysis of p53 protein/DNA binding properties. Journal of Immunological Methods. 2002;267:227. doi: 10.1016/s0022-1759(02)00182-5. [DOI] [PubMed] [Google Scholar]
- 11.Kwok TT, Mok CH, Menton-Brennan L. Up-regulation of a mutant form of p53 by doxorubicin in human squamous carcinoma cells. Cancer Res. 1994;54:2834. [PubMed] [Google Scholar]
- 12.Li FM, Armstrong DW, Houk RS. Behavior of bacteria in the inductively coupled plasma: Atomization and production of atomic ions for mass spectrometry. Analytical Chemistry. 2005;77:1407. doi: 10.1021/ac049188l. [DOI] [PubMed] [Google Scholar]
- 13.Lou X, Zhang G, Herrera I, Kinach R, Ornatsky O, Baranov V, Nitz M, Winnik MA. Polymer-Based Elemental Tags for Sensitive Bioassays. Angew. Chem. Int. Ed Engl. 2007;46:6111. doi: 10.1002/anie.200700796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oren M, Maltzman W, Levine AJ. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol. Cell Biol. 1981;1:101. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ornatsky O, Baranov V, Bandura DR, Tanner SD, Dick J. Multiple cellular antigen detection by ICP-MS. Journal of Immunological Methods. 2006;308:68. doi: 10.1016/j.jim.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Prange A, Profrock D. Application of CE-ICP-MS and CE-ESI-MS in metalloproteomics: challenges, developments, and limitations. Analytical and Bioanalytical Chemistry. 2005;383:372. doi: 10.1007/s00216-005-3420-0. [DOI] [PubMed] [Google Scholar]
- 17.Quinn ZA, Baranov VI, Tanner SD, Wrana JL. Simultaneous determination of proteins using an element-tagged immunoassay coupled with ICP-MS detection. Journal of Analytical Atomic Spectrometry. 2002;17:892. [Google Scholar]
- 18.Sharma NC, Sahi SV, Jain JC. Sesbania drummondii cell cultures: ICP-MS determination of the accumulation of Pb and Cu. Microchemical Journal. 2005;81:163. [Google Scholar]
- 19.Soma Y, Dvonch V, Grotendorst GR. Platelet-derived growth factor AA homodimer is the predominant isoform in human platelets and acute human wound fluid. FASEB J. 1992;6:2996. doi: 10.1096/fasebj.6.11.1644262. [DOI] [PubMed] [Google Scholar]
- 20.Szpunar J. Advances in analytical methodology for bioinorganic speciation analysis: metallomics, metalloproteomics and heteroatom-tagged proteomics and metabolomics. Analyst. 2005;130:442. doi: 10.1039/b418265k. [DOI] [PubMed] [Google Scholar]
- 21.Tanner SD, Ornatsky O, Bandura DR, Baranov VI. Multiplex bio-assay with inductively coupled plasma mass spectrometry: Towards a massively multivariate single-cell technology. Spectrochimica Acta Part B-Atomic Spectroscopy. 2007;62:188. [Google Scholar]
- 22.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Li FM, Houk RS, Armstrong DW. Pore exclusion chromatography-inductively coupled plasma-mass spectrometry for monitoring elements in bacteria: A study on microbial removal of uranium from aqueous solution. Analytical Chemistry. 2003a;75:6901. doi: 10.1021/ac0348017. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Wu FB, Zhang YY, Wang X, Zhang XR. A novel combination of immunoreaction and ICP-MS as a hyphenated technique for the determination of thyroid-stimulating hormone (TSH) in human serum. Journal of Analytical Atomic Spectrometry. 2001;16:1393. [Google Scholar]
- 25.Zhang C, Zhang ZY, Yu BB, Shi JJ, Zhang XR. Application of the biological conjugate between antibody and colloid Au nanoparticles as analyte to inductively coupled plasma mass spectrometry. Analytical Chemistry. 2002;74:96. doi: 10.1021/ac0103468. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YS, Zhang ZY, Maekawa T. Uptake and mass balance of trace metals for methane producing bacteria. Biomass & Bioenergy. 2003b;25:427. [Google Scholar]