Abstract
Extending transplant criteria to include livers obtained from donor after cardiac death (DCD) could increase the liver donor pool, but conventional simple cold storage of these ischemic organs can lead to poor graft function after transplantation. Experimental normothermic machine perfusion has previously proven to be useful for the recovery and preservation of DCD livers, but it is more complicated than conventional cold storage, and is therefore perhaps not practical during the entire preservation period. In clinical situations, the combined use of simple cold storage and normothermic perfusion preservation of DCD livers might be more realistic, but even a brief period of cold storage prior to normothermic preservation has been suggested to have a negative impact on graft viability. In this study we show that rat livers subjected to 45 minutes of ex-vivo warm ischemia followed by 2 hours of simple cold storage can be reclaimed by 4 hours of normothermic machine perfusion. These livers could be orthotopically transplanted into syngeneic recipients with 100% survival after 4 weeks (N=10), similar to the survival of animals that received fresh livers that were stored on ice in University of Wisconsin (UW) solution for 6 hours (N=6). On the other hand, rats that received ischemic livers preserved on ice in UW solution for 6 hours (N = 6) all died within 12 hours after transplantation. These results suggest that normothermic perfusion can be used to reclaim DCD livers subjected to an additional period of cold ischemia during hypothermic storage.
The current shortage of suitable donor livers could be significantly alleviated by extending liver graft criteria to include livers obtained from donors after cardiac death (DCD). However, conventional with simple cold storage (SCS) of these DCD livers is associated with a higher risk of primary non-function and delayed graft failure (1–3). Normothermic Extracorporeal Liver Perfusion (NELP) has been suggested as an alternative (1–3). However, if machine perfusion of donor organs is to be realized clinically, a brief period of static cold storage between donor organ recovery and normothermic preservation is desirable. This period would enable the recovery of the organ as usual, after which it could be transported to a specialized transplantation center, where subsequent recovery of the organ with NELP can occur. The addition of an initial period of SCS prior to NELP, however, has been suggested to negatively impact the viability of these DCD grafts due to their increased sensitivity to cold ischemia (4,5). To assess the feasibility of this hybrid approach, we have evaluated post-operative graft function of orthotopically transplanted ischemic rat livers that were preserved with a combination of cold storage in University of Wisconsin (UW) solution and normothermic machine perfusion. Recipients of these livers all survived beyond 4 weeks. By comparison, animals that received warm ischemic livers preserved with simple cold storage alone all died within 24 hours after transplantation.
EXPERIMENTAL PROCEDURES
Normothermic Extracorporeal Perfusion
Perfusion took place in a circuit that consisted of a perfusion chamber, a peristaltic pump, a membrane oxygenator, a heat exchanger/bubble trap and a hollow fiber dialyzer. Continuous dialysis of the perfusate took place throughout the duration of the perfusion. Temperature within the system was maintained at 37.5°C. The total perfusate volume used for one liver perfusion was 55–60ml. Details of the perfusion system and technique can be found elsewhere (6).
Perfusion medium consisted of a combination of Williams Medium E cell culture medium (Sigma Chemical) autologous erythrocytes (16–18% hct) and plasma (25 % v/v). To this were added: insulin (2 u/l, Humulin, Eli Lily), penicillin (40,000 u/l) /streptomycin (40,000 µg/l) (Gibco, Invitrogen), l-glutamine (0.292 g/l, Gibco), hydrocortisone (10mg/l, solu cortef, Pharmacia &Upjohn) and heparin (1000 u/l APP). Dialysate was similar to the perfusate but without erythrocytes or plasma.
Surgical Procedures
Experiments were performed using male Lewis rats weighing 250–300 g (Charles River Labs). The animals were maintained in accordance with National Research Council guidelines and the experimental protocols were approved by the Subcommittee on Research Animal Care, Committee on Research, Massachusetts General Hospital. All surgical procedures were performed under aseptic conditions using isoflurane anesthesia. The liver was harvested and transplanted as previously described (6).
DCD model
The liver was placed in a chamber filled with saline and maintained at 34.0 (±0.1) °C for 45 minutes, after which it was flushed with 10 ml of ice cold UW solution and placed on melting ice in a bowl containing UW solution 2 hrs, or for the entire 6 hr preservation period.
Analysis
Perfusate samples were collected every hour and analyzed using a Piccolo blood chemistry analyzer (Abaxis) to determine ALT and AST. Bile was collected and weighed every hour. For analysis of the hepatic oxygen uptake rate (OUR) samples were taken from the in-and outflow (PV and IVC) of the liver and analyzed immediately using a blood gas analyzer (Rapidlab). The total concentration of oxygen in the samples and OUR were determined as described previously (6). For post-operative levels of ALT, AST urea and total bilirubin, 200 µl of blood was collected on post-operative days 1, 3, 5 and 7 from tail vein puncture under isoflurane anesthesia. The samples were immediately analyzed using a Piccolo blood chemistry analyzer.
RESULTS
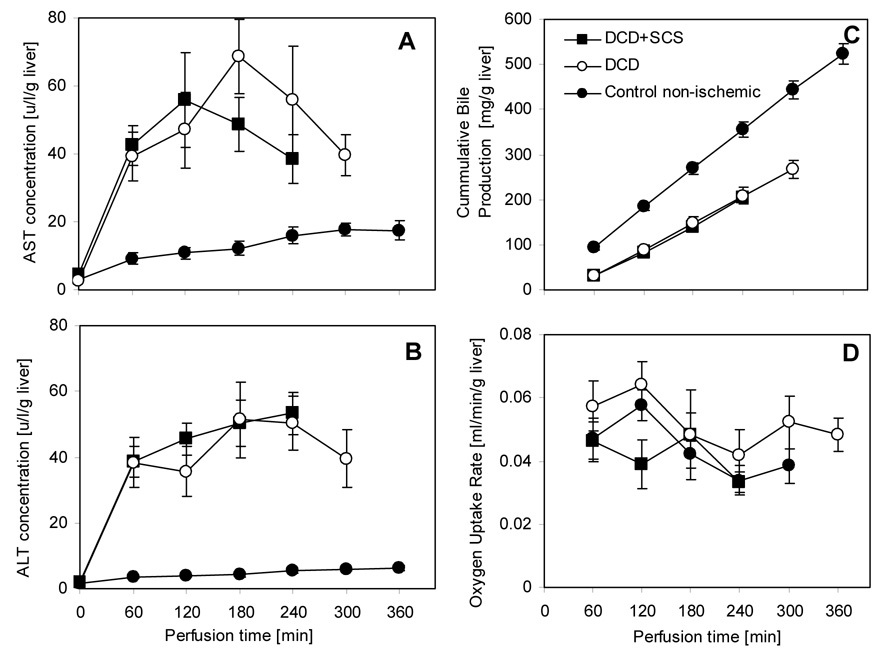
ALT and AST levels were 3–4 fold higher during perfusion (p< 0.01) for the experiment groups, as compared to perfused non-DCD livers (Figure 1). The values were very similar to the values obtained in perfusion of 1-hr warm ischemic livers without any intermittent cold storage (Tolboom et al, manuscript submitted). ALT levels reached a plateau after the first hour in all cases, and AST levels started to decline after the third hour. ALT or AST were not detected in the dialysate. Bile production rate was constant through perfusion, indicating consistent metabolic activity, but was lower than that of non-DCD livers (p<0.01). The oxygen uptake rate of the livers that were subjected to warm ischemia remained roughly constant during perfusion at an average value of 0.04 ml/min g liver, though a slight decrease was observed. The oxygen uptake rate of the fresh livers followed the same pattern, was slightly but not significantly higher.
Figure 1.
Integrity and metabolic function of warm ischemic livers during NELP. The DCD group was subjected to 1 hr warm ischemia. The DCD+SCS group was subjected to 45 min of warm ischemia and 2 hrs of SCS. The control group consisted of freshly isolated livers with no warm ischemia. Data from the DCD and control groups are from Tolboom et al. (submitted), and ref. (8), respectively. (A) AST and (B) ALT levels in perfusate samples collected hourly from the primary circuit. (C) Total bile secreted. (D) Oxygen uptake rate. All values are normalized to the wet weight of the liver. Data shown are averages of 6 warm ischemic livers ± SEM. ALT & AST values for the warm ischemic livers are significantly higher than the controls (p <0.01), but no difference was observed between DCD and DCD+SCS groups. OURs were not statistically different among the groups (p >0.1). Bile production was significantly reduced (p <0.01) in both groups of warm ischemic livers compared to control freshly isolated livers.
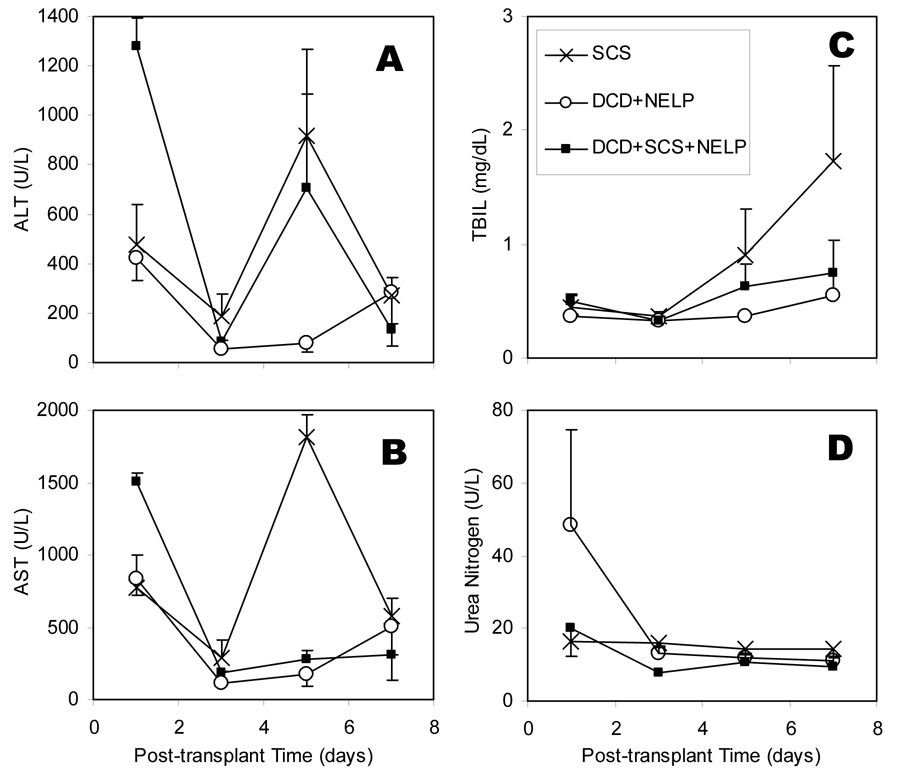
Transplant surgery was without complications in all cases. The animals that received perfused ischemic livers (N=10) and the control group receiving fresh cold stored livers (N=6), survived beyond one month (100% survival in both groups). Post-operative enzyme levels of combined DCD+SCS+NELP liver recipients were elevated on day 1 but remained below SCS liver recipients beyond that point (Figure 2). Bilirubin and urea levels were normal and similar across all cases. The animals receiving cold stored ischemic livers all died within 12 hours postoperatively (N=6; 0% survival). Upon examination of the abdomen the liver appeared patchy and serous fluid was present in the abdomen.
Figure 2.
Values of ALT (A), AST (B), Bilirubin (C), and Blood Urea Nitrogen (D) measured on days 1, 3, 5 and 7 after transplantation of perfused warm ischemic livers compared to healthy perfused livers (controls) and healthy cold-stored livers. Data shown are the averages of 3 recipients per group ± SEM. Overall values for recipients of DCD livers treated with NELP post cold storage were statistically indifferent from other DCD livers. However, on day 1 both ALT and AST levels are significantly higher in DCD+SCS group. BUN was lower when compared to recipients of NELP treated normal livers.
CONCLUSIONS
We have shown that livers subjected to 45 minutes of ex vivo warm ischemia followed by two hours of cold storage in UW solution can be resuscitated and preserved with normothermic extracorporeal perfusion. These livers showed functional recovery during normothermic perfusion, although overall hepatic metabolism was lower than that of fresh perfused livers. Survival after transplantation of perfused livers was far superior to that of animals receiving ischemically damaged livers preserved with SCS for even a brief period of time, and was similar to that of animals receiving fresh livers. The results of this study indicate that ischemic rat livers can be recovered and preserved with NELP even after a brief period of cold storage.
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health (R01 DK43371, R01 DK59766, K99 DK080942), the Shriners Hospitals for Children, and the Harvard University William F. Milton Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Reddy S, Zilvetti M, Brockmann J, McLaren A, Friend P. Liver transplantation from non-heart-beating donors: current status and future prospects. Liver Transpl. 2004;10(10):1223. doi: 10.1002/lt.20268. [DOI] [PubMed] [Google Scholar]
- 2.Maathuis J, Leuvenink H, Ploeg R. Perspectives in Organ Preservation. Transplantation. 2007;83(10):1289. doi: 10.1097/01.tp.0000265586.66475.cc. [DOI] [PubMed] [Google Scholar]
- 3.Schon MR, Kollmar O, Wolf S, et al. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surgery. 2001;233:114. doi: 10.1097/00000658-200101000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy SP, Bhattacharjya S, Maniakin N, et al. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion. Transplantation. 2004;77(9):1328. doi: 10.1097/01.tp.0000119206.63326.56. [DOI] [PubMed] [Google Scholar]
- 5.Reddy S, Greenwood J, Maniakin N, et al. Non-Heart-Beating Donor Porcine Livers: The Averse Effect of Cooling. Liver Transpl. 2005;11(1):355. doi: 10.1002/lt.20287. [DOI] [PubMed] [Google Scholar]
- 6.Tolboom H, Pouw R, Uygun K, et al. A Model for Normothermic Preservation of the Rat Liver. Tissue Eng. 2007;13(8):2143. doi: 10.1089/ten.2007.0101. [DOI] [PubMed] [Google Scholar]