Abstract
The supplanting of radical mastectomy by simple mastectomy and then by lumpectomy plus radiation, the use of adjuvant therapy to alter the natural course of breast and colorectal cancer, the use of tamoxifen for the prevention of breast cancer, and the dramatic improvement in survival demonstrated with the use of the monoclonal antibody trastuzumab in women with HER2-positive breast cancer are all the direct results of research that has been carried out over the past 50 years by the National Surgical Adjuvant Breast and Bowel Project. This National Cancer Institute-supported clinical cooperative trials group based in Pittsburgh, PA, currently has 200 member institutions and 700 satellite centers located throughout the United States, Canada, Puerto Rico, and Ireland. The NSABP’s mandate is to conduct large randomized phase III trials to evaluate therapies designed to improve the treatment and prevention of breast and colorectal cancer. Over the past half century, the NSABP has entered more than 150,000 patients and participants into clinical studies that have changed the treatment of colorectal cancer and have revolutionized the treatment and prevention of breast cancer.
Background
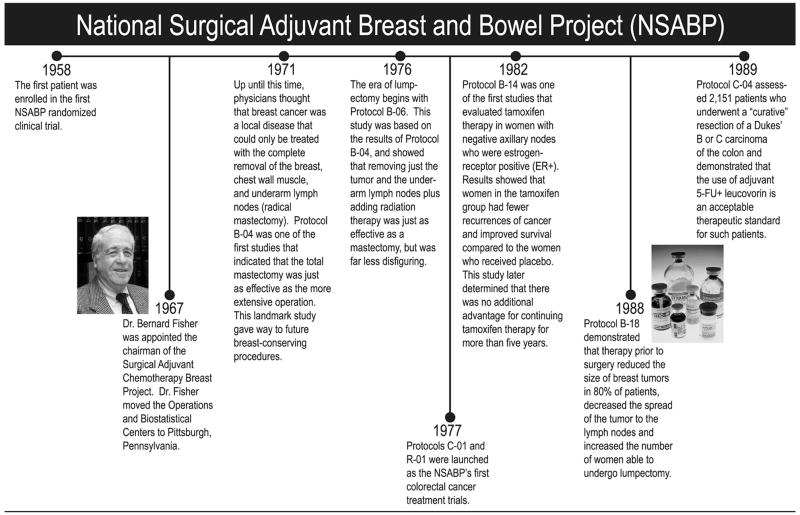
On April 4, 1958, the first patient was enrolled in the first randomized clinical trial conducted by an organization that was to become the National Surgical Adjuvant Breast and Bowel Project (NSABP). Now, more than 50 years later, the treatment of breast and bowel cancer has changed dramatically, thanks in large part to the trials conducted by the NSABP, which have had a profound impact on our understanding and treatment of these diseases (Fig. 1).
Figure 1.
NSABP timetable.
In the spring of 1957, I.S. Ravdin, MD, Chairman of the Department of Surgery at the University of Pennsylvania, invited 23 surgeons to attend a meeting to discuss the creation of the Surgical Adjuvant Chemotherapy Breast Project, the forerunner of the NSABP. Today there are 200 member institutions with 700 satellite centers located throughout the United States, and in Canada, Puerto Rico, and Ireland. The NSABP was among the first research groups to recognize the importance of including community-based investigators among its members. Today the majority of participating sites are located at non-university centers, allowing patients the opportunity to enter NSABP studies without the burden or cost of travel to academic centers. More than 5,000 surgeons, medical oncologists, radiation oncologists, pathologists, nurses, clinical research associates (CRAs), and other medical professionals participate in NSABP trials.
The initial studies of the NSABP focused on the effectiveness of Thiotepa, 5-FU (fluorouracil), radiation therapy, or oophorectomy in the adjuvant treatment of breast cancer patients following radical mastectomy.1 The group’s studies today continue to focus on the locoregional and adjuvant therapy of both breast and colorectal cancer, with the goal of improving survival and the quality of life of people with these diseases.
In 1967, Bernard Fisher, MD, Professor of Surgery at the University of Pittsburgh, was named the chairman of the NSABP, succeeding Rudolph Noer, MD, University of Louisville, and the group’s headquarters were moved to the University of Pittsburgh in 1970, beginning the “modern” era of the NSABP. Over the next 25 years, Dr. Fisher’s leadership catapulted the NSABP to the forefront of clinical cancer research. The current NSABP chairman is Norman Wolmark, MD, Professor of Human Oncology, Drexel University College of Medicine, and chairman of the Department of Human Oncology at Allegheny General Hospital in Pittsburgh. Joseph Costantino, DrPH, is the director of the NSABP’s Biostatistical Center, located at the University of Pittsburgh. The NSABP’s Operations Center is located in Pittsburgh and is affiliated with Allegheny General Hospital.
Breast Cancer Trials
Locoregional Treatment Studies
Among the best known NSABP studies are those that have evaluated locoregional therapies for invasive and non-invasive breast cancer. Results from these trials have been instrumental in changing the surgical management of both of these types of breast cancer, which previously was based on Halstedian principles of tumor growth and dissemination.
From radical mastectomy to simple mastectomy to lumpectomy
In 1971, NSABP protocol B-04 examined the question of whether local or regional treatments other than radical mastectomy in patients with operable breast cancer would result in outcomes similar to what was achieved with the radical procedure. This study of 1,765 women, the initial results of which were published in 1977, demonstrated no significant difference in treatment failure or survival among the various treatment groups.2 After 25 years of follow-up, the results continue to demonstrate no significant differences in clinical outcome between the clinically node-negative patients who underwent radical mastectomy and those who underwent total mastectomy with or without irradiation or between the clinically node-positive patients who underwent radical mastectomy and those who underwent total mastectomy with irradiation.3
The results of the B-04 trial had a profound impact on the surgical management of breast cancer. Following the publication of the B-04 results and the 1979 NIH Congress Conference on breast cancer, which announced that total mastectomy and axillary dissection should be recognized as the treatment standard, the use of radical mastectomy in the United States rapidly declined.4 The B-04 results also paved the way for the conduct of NSABP protocol B-06, which randomized patients with invasive breast cancers ≤4cm to receive a modified radical mastectomy, lumpectomy, or lumpectomy plus breast irradiation. Now after 20 years of follow-up, there continue to be no significant differences in overall survival, disease-free survival (DFS), or distant disease-free survival (DDFS) between any of the groups of patients.5,6
Breast conservation in DCIS
In the early 1980’s, the increasing use of screening mammography dramatically increased the diagnosis of small, localized, non-palpable ductal carcinoma in situ (DCIS). Despite the increasing use of lumpectomy for the treatment of invasive disease, DCIS was still routinely treated with mastectomy. The NSABP was the first group to test the value of breast conservation in patients with DCIS. Protocol B-17 compared lumpectomy alone to lumpectomy plus breast irradiation in patients with localized DCIS and demonstrated after 12 years of follow-up that radiotherapy significantly reduced the rate of both invasive and non-invasive ipsilateral breast tumor recurrence.7
Studies current and in follow-up in locoregional management
The NSABP continues to explore new therapies designed to improve the locoregional management of breast cancer. Several of our ongoing studies and those that have completed accrual and are in follow-up include:
B-32 - A Randomized, Phase III Clinical Trial to Compare Sentinel Node Resection to Conventional Axillary Dissection in Clinically Node-Negative Breast Cancer Patients
B-37 - A Randomized Clinical Trial of Adjuvant Chemotherapy for Radically Resected Locoregional Relapse of Breast Cancer (in collaboration with the International Breast Cancer Study Group)
B-39 - A Randomized Phase III Study of Conventional Whole Breast Irradiation (WBI) Versus Partial Breast Irradiation (PBI) for Women with Stage 0, I, or II Breast Cancer (in collaboration with the Radiation Therapy Oncology Group)
Adjuvant Therapy Trials for Breast Cancer
In parallel with the locoregional therapy studies, the NSABP has conducted an extensive series of adjuvant therapy trials that have evaluated systemic treatments in patients with node-negative or node-positive disease, as well as studies of neoadjuvant and adjuvant therapy in patients with DCIS.
Effect of adjuvant chemotherapy
Beginning in September 1972, 380 women with node-positive breast cancer were randomly assigned to receive either L-phenylalanine mustard (L-PAM) or placebo in the NSABP’s B-05 trial. Results documented that postoperative adjuvant therapy could impact the natural history of breast cancer and reduce the risk of recurrence. Subsequent trials 8 have studied combination chemotherapy, incorporating anthracyclines (AC) and sequential therapy (AC→T).
Effect of adjuvant chemotherapy plus endocrine therapy
Protocol B-09, which began in 1977, compared combination chemotherapy with and without tamoxifen and also included a quality assurance program for estrogen receptor analysis.9 The results of this study demonstrated that the addition of tamoxifen to chemotherapy improved outcome in node-positive, receptor-positive patients.
For postmenopausal women with hormone-receptor positive breast cancer, tamoxifen is rapidly being replaced by the use of selective aromatase inhibitors (AIs). Among the unanswered questions concerning the use of AIs is the optimum duration of therapy. NSABP Protocol B-42, A Clinical Trial to Determine the Efficacy of Five Years of Letrozole Compared to Placebo in Patients Completing Five Years of Hormonal Therapy Consisting of an Aromatase Inhibitor (AI) or Tamoxifen Followed by an AI in Prolonging Disease-Free Survival in Postmenopausal Women with Hormone-Receptor-Positive Breast Cancer is currently accruing patients and will address the AI duration issue.
Effect of adjuvant chemotherapy plus monoclonal antibody
Recently in Protocol B-31 the NSBAP evaluated the use of trastuzumab (Herceptin®) plus adjuvant chemotherapy in node-positive patients with HER2 positive breast cancer. The results of that study were combined with those from NCCTG trial 9831 and demonstrated a 12% absolute improvement in DFS and a 33% reduction in the risk of death with the use of trastuzumab.10
Findings from other NSABP trials
NSABP node-negative trials began in 1981–82 with the start of B-13, in which we evaluated chemotherapy in node-negative, ER-negative patients and B-14, in which we evaluated tamoxifen alone in node-negative, ER-positive patients. Both studies demonstrated improvement in DFS and survival in favor of the active treatment.11,12 B-14 also evaluated the optimum duration of tamoxifen administration, showing that continuing treatment beyond 5 years provided no additional advantage.13 Subsequent NSABP trials in node-negative patients have evaluated other combinations of therapy, including the combination of chemotherapy and tamoxifen.
Effect of neoadjuvant therapy
The first NSABP neoadjuvant trial (B-18) began in 1998 and tested 4 cycles of Adriamycin and cyclophosphamide delivered either postoperatively or preoperatively. While the preoperative group showed no overall improvement in outcome, they underwent significantly more lumpectomies (than mastectomies), and those in the preoperative group who had a complete pathologic response did have an improved outcome.14 Current neoadjuvant trials (B-40 and B-41) are evaluating the use of the targeted therapies bevacizumab or Lapatinib in patients with operable breast cancer.
Effect of endocrine therapy in DCIS
NSABP protocol B-24, begun in 1991, tested the use of tamoxifen in patients with DCIS treated by lumpectomy and breast irradiation. Tamoxifen significantly reduced the risk of invasive breast cancer events, although the benefit was largely restricted to patients with ER-positive DCIS included in the trial.15 Protocol B-35 is now being conducted in a similar group of 3,000 DCIS patients randomized to receive either tamoxifen or anastrozole. This study has completed the accrual phase and follow-up continues, but no results are yet available. Concepts for additional DCIS trials are in development.
Chemoprevention Trials
In 1992 the NSABP expanded its research agenda to include the concept of chemoprevention. Since that time, the group has completed two large breast cancer chemoprevention studies, the Breast Cancer Prevention Trial (P-1/BCPT), and the Study of Tamoxifen and Raloxifene (STAR). These studies screened more than ¼ million and randomized more than 33,000 healthy women at increased risk for the future development of breast cancer. Both studies focused on the use of selective estrogen receptor modulators (SERMs) as a method to reduce the development of primary invasive breast cancer. In the BCPT, between 1992 and 1997, more than 13,000 women were randomized to receive either tamoxifen 20 mg or placebo daily for 5 years. The results demonstrated a highly statistically significant 49% reduction in invasive breast cancer.16 However, tamoxifen also increased the risk of uterine malignancy, thromboembolic events, and cataracts.
The STAR trial began in 1999 and enrolled more than 19,000 women. This study randomly assigned women to take either tamoxifen or raloxifene, a SERM approved in the United States for the treatment and prevention of osteoporosis. Women taking raloxifene for fracture prevention had been noted to have a decrease in receptor-positive breast cancers with no excess in endometrial cancers.
The results of the STAR trial, published in 2006, documented that tamoxifen and raloxifene were equally effective in reducing the risk of invasive breast cancer.17 Although raloxifene was not as effective as tamoxifen in preventing non-invasive breast cancer, its use resulted in fewer endometrial cancers, fewer venous thromboembolic events, and no excess of cataracts, making it an attractive option for the chemoprevention of breast cancer in postmenopausal women at increased risk.
The NSABP is currently developing concepts for additional chemoprevention studies of both breast and colorectal cancers.
NSABP Colorectal Cancer Studies
Since initiating its adjuvant therapy program for colorectal cancer in 1977, the NSABP has randomized approximately 14,500 patients into phase III colon and rectal cancer clinical trials. The data from these trials have had a significant influence on the treatment of stage II and III colorectal cancer. Most notably, Protocol C-03, which was initiated in 1987, established 5-FU + leucovorin as a new standard treatment for patients with carcinoma of the colon.18 With 10 years of follow-up,19 this study demonstrated an absolute advantage of 10% in both disease-free and overall survival for 5-FU + leucovorin compared to a contemporary control arm of methyl-CCNU, vincristine, and 5-FU (MOF).
The value of 5-FU + leucovorin was further supported by the results of Protocol C-04, which compared this regimen to 5-FU + levamisole or 5-FU + leucovorin to which levamisole was added.20 The study was initiated in 1989, and the results at 12 years of follow-up showed a trend toward an advantage in disease-free and overall survival in favor of 5-FU + leucovorin over the 5-FU + levamisole combination. The addition of levamisole to 5-FU + leucovorin provided no incremental advantage, and levamisole was therefore dropped from subsequent studies of colon cancer adjuvant therapy.
Initiated in 2000, Protocol C-07 addressed the value of adding oxaliplatin to the standard 5-FU + leucovorin regimen used in previous protocols. The results of this study demonstrated a significant improvement in DFS for the oxaliplatin-containing regimen.21 Protocol C-07 also allowed a thorough characterization of patient-reported oxaliplatin neurotoxicity.22 In Protocol C-08, which completed patient accrual in 2006, we are evaluating the addition of targeted anti-angiogenesis therapy with bevacizumab to an infusional regimen of 5-FU combined with leucovorin and oxaliplatin.
Since 1990 the standard of care for adjuvant therapy of patients with stage II and III colon cancer changed from surgery alone to 5-FU + levamisole to 5-FU + leucovorin to 5-FU + leucovorin + oxaliplatin with a commensurate improvement in patient outcome. The NSABP’s studies, as noted from the above, contributed significantly to these advances.
A series of parallel studies has been carried out for carcinoma of the rectum. These trials have contributed to a better understanding of the role of radiotherapy in the adjuvant setting. Protocol R-01, which compared postoperative radiotherapy and chemotherapy with MOF to a no-treatment control, indicated that radiotherapy could reduce the incidence of locoregional recurrence but had no effect on DFS or overall survival.23 The chemotherapy group, when compared with the group treated by surgery alone, demonstrated an overall improvement in DFS (P=.006) and in survival (P=.05).
Protocol R-02 was significant in that it assessed the value of postoperative radiotherapy in a setting in which all patients received adjuvant chemotherapy as well. This study once again demonstrated that radiotherapy could reduce the incidence of locoregional recurrence, but there was no prolongation in DFS or overall survival.24
Protocol R-03 compared preoperative chemoradiotherapy with leucovorin-modulated 5-FU to the same regimen given in the traditional postoperative setting. Although only 267 patients were randomized into the R-03 trial, the data provide an intriguing glimpse of the efficacy of preoperative therapy.25 Sixty-nine percent of the preoperative therapy group demonstrated objective tumor response; 26% demonstrated a complete response. A pathologic complete response in which there was no histologic evidence of residual tumor was present in 17%. There was concomitant downstaging in the proportion of patients with positive nodes (45% in the postoperative cohort vs 32% in those receiving preoperative therapy) and an increase in the proportion of patients who had sphincter-saving procedures (42% in the postoperatively treated group vs 52% in those who received preoperative therapy). The results of this study were instrumental in the design of Protocol R-04, which was initiated in 2004 and in collaboration with several other cooperative groups is evaluating oral capecitabine versus infusional 5-FU, with or without oxaliplatin, given preoperatively. NSABP’s rectal cancer studies are contributing to the current multidisciplinary strategy of optimizing preoperative systemic therapy in conjunction with surgery and pelvic radiation therapy.
NSABP Behavioral and Health Outcomes (BAHO)/Quality of Life (QOL) Activities
The measurement of health-related quality of life (HRQOL) in cancer clinical trials accelerated in the late 1980s and early 1990s. The NSABP’s first active trial with HRQL endpoints was the BCPT, the protocol for which was developed in 1991. The trial began in 1992. A QOL committee was appointed to develop this component of the protocol, and thus began our first effort at measuring patient-reported outcomes (PROs). The decision to include HRQL in the BCPT was critical; in contrast to women receiving adjuvant treatment for breast cancer, any adverse side effects or changes in HRQL in these high-risk women could affect adherence to the study medication and to subsequent success in the dissemination of tamoxifen for breast cancer prevention. Results from this placebo-controlled HRQOL study26–29 have been important for healthy women considering tamoxifen for chemoprevention as well as for women receiving this treatment in the adjuvant therapy of breast cancer. Many symptoms that were thought to be related to tamoxifen (e.g., depression, weight gain, nausea) were not increased in the women who took the active drug compared with placebo. Subsequently, the strategies used to measure HRQL in the BCPT were successfully employed in the STAR trial.30 In the late 1990s, the NSABP established a treatment-focused QOL committee and began to include HRQL endpoints in several treatment trials.31,32 By 2001, we had decided to revamp the committee to further develop efforts related to PROs and HRQL, in particular to facilitate the integration of additional important outcomes into our treatment and prevention trials. The Behavioral and Health Outcomes (BAHO) committee was launched that year and was established as a standing committee at the level of the Breast and Colorectal Cancer Committees. BAHO members represent a broad array of scientific disciplines including medical oncology, psychology, internal medicine, surgery, nursing, and biostatistics. A formal process was established to have all new protocols, from early concept inception through final development, reviewed by members of BAHO to determine whether the protocol has a substantial question that merits additional data collection (often directly from the study participant) or other biomedical measurements that will enhance interpretation of the study questions.
Specific BAHO studies that have been completed or are still ongoing include the measurement of neurotoxicity in adjuvant therapy with oxaliplatin in colon cancer (NSABP C-07)22,33 the assessment of arm function after sentinel node biopsy (NSABP B-32); the assessment of amenorrhea in association with adjuvant chemotherapy in breast cancer (NSABP B-30 and B-36); and the assessment of HRQL in a comparison of different adjuvant treatment regimens (NSABP B-33, B-35 and B-36). We have also launched our first long-term survivor study (LTS-01, supported by an extramural grant from the American Cancer Society), which is examining late effects of treatments used in our R-02, R-03, C-05, C-06 and C-07 trials in colorectal cancer patients beyond 5 years after diagnosis.
BAHO endpoints have been very successfully integrated into NSABP trials, and through careful coordination by staff of the NSABP Biostatistical Center and the commitment of NSABP members, these outcomes now are regularly measured and augment and enhance the science of the treatment protocols.
Tissue Bank and Correlative Science Efforts of the NSABP
NSABP Tissue Bank
The NSABP Tissue Bank arose out of the group’s quality assurance program for diagnostic pathology. While the intended requirement was to collect H&E stained slides for central confirmation of cancer diagnosis, many sites decided to send tumor tissue blocks (or sometimes blocks of the entire case) as an alternative. This resulted in the unplanned procurement of blocks from 30–40% of the NSABP trial cohort before 1996. There was no government funding available for tissue banking effort at that time.
In 1996, a new initiative under the current leadership of the NSABP expanded the tissue bank and formally launched correlative science within the group. Currently, our tissue bank houses tumor tissue blocks from more than 70,000 cases of breast and colorectal cancer from patients who participated in NSABP trials. Microarrays of tissue from all of the key trials we have conducted are now available in this tissue bank.
Open Access System
The NSABP Tissue Bank operates under the principle of completely open access to investigators. Any investigator with scientific ideas can send in a letter of intent to conduct research using the resources of the bank. After the concept described in the letter is reviewed by an external scientific review panel and approved, the NSABP works jointly with the investigator to develop a protocol.
Correlative Science Efforts
While over the past five decades great achievements have been made in the treatment of breast and colorectal cancer through clinical trials, the future holds the promise of moving beyond strictly empirical approaches to the idea of trials designed to address the treatment and prevention of cancer based on biomarkers and genetic makeup.
We believe that the current trial mechanism of including patients from different risk categories in a single trial should be examined to achieve a better selection process for patients participating in clinical trials. For example, we can employ banked materials from completed trials to develop context-specific prognostic and predictive markers that not only predict response to therapy but that also provide an assessment of baseline risk of recurrence.
Along these lines, we have developed a context-specific marker for ER-positive, node-negative tamoxifen-treated patients to determine baseline risk that can be used in the decision of whether a patient should be offered chemotherapy. The OncotypeDX (Genomic Health, Inc., Redwood City, Calif.) ™ test, based on measurement of the mRNA expression levels in 21 genes34 marks two significant paradigm shifts for correlative science studies: the test is a continuous predictor for individual risk of recurrence, and there is now recognition of the significance of the linearity of the assay. The OncotypeDX assay has set a benchmark for our current correlative science efforts. We have also developed a robust method for microarray gene expression profiling of formalin-fixed, paraffin-embedded tumor tissue blocks. This has virtually eliminated barriers to the interrogation of gene expression levels in archived materials in our tissue bank.
The NSABP is now engaged in developing context-specific markers that may help in the management of each stage and subtype of breast and colorectal cancer using gene expression profiling.
Conclusion
Over the past 50 years, randomized clinical trials conducted by the NSABP have resulted in dramatic improvements in the treatment and prevention of both breast and colorectal cancers. These advances in our understanding of the biology of these diseases and in patient treatment and care would not have been possible without the willingness of the women and men who participate in these studies. We are grateful to the more than 150,000 individuals who have entered NSABP trials over the past 50 years and to those participating in our current studies.
Acknowledgments
Supported in part by: NCI Grants U10-CA37377, U10-CA69974, U10-CA69651, U10-CA12027, and U24-CA114732.
The authors thank Barbara C. Good, PhD, for editorial assistance and Holly A. McCalmon for preparation of the timetable.
Footnotes
From the National Surgical Adjuvant Breast and Bowel Project; East Commons Professional Building; Four Allegheny Center - 5th Floor, Pittsburgh, PA 15212; Phone: 412-330-4600 - Fax: 412-330-4661.
Financial disclosure obligations: D. Lawrence Wickerham, MD, Eli Lilly and Company (consulting) and AstraZeneca
Pharmaceuticals (honorarium); Walter M. Cronin, MPH, AstraZeneca Pharmaceuticals (compliance advisory board); Soonmyung Paik, MD, Glaxo Smith Kline and Genentech (honorarium); Eleftherios P. Mamounas, MD, MPH, Aventis (consulting, honorarium), Genentech (consulting, honorarium), Genomic Health (honorarium), Roche (consulting), Glaxo Smith Kline (consulting), Eli Lilly (consulting).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
D. Lawrence Wickerham, Associate Chairman, NSABP, Pittsburgh, PA.
Michael J. O’Connell, Associate Chairman, NSABP, Pittsburgh, PA.
Joseph P. Costantino, Director, NSABP Biostatistical Center, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA.
Walter M. Cronin, Associate Director, NSABP Biostatistical Center; Soonmyung Paik, MD, Director, Division of Pathology, NSABP, Pittsburgh, PA.
Charles E. Geyer, Jr., Director, NSABP Medical Affairs, Pittsburgh, PA.
Patricia A. Ganz, Professor, UCLA School of Medicine and Public Health, Jonsson Comprehensive Cancer Center, Los Angeles, CA.
Nicholas Petrelli, Medical Director, Helen F. Graham Cancer Center, Newark, DE.
Eleftherios P. Mamounas, Medical Director, Aultman Hospital Cancer Center, Canton, OH.
Thomas B. Julian, Associate Director, NSABP Medical Affairs, Pittsburgh, PA.
Norman Wolmark, Chairman, NSABP, and Chairman, Department of Human Oncology, Allegheny General Hospital, Pittsburgh, PA.
References
- 1.Fisher B. Status of adjuvant therapy: Results of the National Surgical Adjuvant Breast Project studies on oophorectomy, postoperative radiation therapy, and chemotherapy. Other comments concerning clinical trials. Cancer. 1971;28:1654–8. doi: 10.1002/1097-0142(197112)28:6<1654::aid-cncr2820280648>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Montague E, Redmond C, et al. Comparison of radical mastectomy with alternative treatments for primary breast cancer. A first report of results from a prospective randomized clinical trial. Cancer. 1977;39:2827–39. doi: 10.1002/1097-0142(197706)39:6<2827::aid-cncr2820390671>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Jeong J, Anderson S, et al. Twenty-five year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–75. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 4.Moxley J, 3rd, Allegra JC, Durant JR, et al. The treatment of primary breast cancer: Management of local disease. [Accessed 2007, July, 26];National Institutes of Health Consensus Development Conference Statement Online June 5. 1979 :29–30. http://consensus.nih.gov/1979/1979PrimaryBreastCancer015html.htm. [PubMed]
- 5.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–73. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Land S, Mamounas E, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ: An update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400–18. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Carbone P, Economou SG, et al. 1-Phenylalanine mustard (L-PAM) in the management of primary breast cancer. A report of early findings. N Engl J Med. 1975;292:117–22. doi: 10.1056/NEJM197501162920301. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Redmond C, Brown A, et al. Treatment of primary breast cancer with chemotherapy and tamoxifen. N Engl J Med. 1981;305:1–6. doi: 10.1056/NEJM198107023050101. [DOI] [PubMed] [Google Scholar]
- 10.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–84. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Redmond C, Wickerham DL, et al. Systemic therapy in patients with node-negative breast cancer: A commentary based on two National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trials. Ann Intern Med. 1989;111:703–12. doi: 10.7326/0003-4819-111-9-703. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–42. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 14.Mamounas EP. Overview of National Surgical Adjuvant Breast Project neoadjuvant chemotherapy studies. Semin Oncol. 1998;25(2 suppl 3):31–5. [PubMed] [Google Scholar]
- 15.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomized controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 17.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 18.Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: Results from National Surgical Adjuvant Breast and Bowel Project Protocol C-03. J Clin Oncol. 1993;11:1879–87. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell MJ, Wolmark N, Yothers G, et al. Durable improvement in disease-free survival (DFS) and overall survival (OS) for stage II or III colon cancer treated with leucovorin-modulated fluorouracil (FL): 10-year follow-up of National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol C-03. Proc Am Soc Clin Oncol. 2005;23:3511. abstr. [Google Scholar]
- 20.Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17:3353–59. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 21.Keubler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol. 2007;25:2198–204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 22.Land SR, Kopec JA, Cecchini RS, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol. 2007;25:2205–11. doi: 10.1200/JCO.2006.08.6652. [DOI] [PubMed] [Google Scholar]
- 23.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: Results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–9. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 24.Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388–96. doi: 10.1093/jnci/92.5.388. [DOI] [PubMed] [Google Scholar]
- 25.Roh MS, Colangelo L, Wieand S, et al. Response to preoperative multimodality therapy predicts survival in patients with carcinoma of the rectum. Proc Am Soc Clin Oncol. 2004;22:3505. doi: 10.1200/JCO.2009.22.0467. abstr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganz PA, Day R, Ware JE, et al. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995;87:1372–82. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 27.Day R, Ganz PA, Costantino JP, et al. Health-related quality of life and tamoxifen in breast cancer prevention: A report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–69. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 28.Ganz PA, Day R, Costantino J. Compliance with quality of life data collection in the National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial. Stat Med. 1998;17:613–22. doi: 10.1002/(sici)1097-0258(19980315/15)17:5/7<613::aid-sim808>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Day R, Ganz PA, Costantino JP. Tamoxifen and depression: More evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93:1615–23. doi: 10.1093/jnci/93.21.1615. [DOI] [PubMed] [Google Scholar]
- 30.Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–51. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 31.Land SR, Kopec JA, Yothers G, et al. Health related quality of life in axillary node-negative, estrogen receptor-negative breast cancer patients undergoing AC versus CMF chemotherapy: Findings from the National Surgical Adjuvant Breast and Bowel Project B-23. Breast Cancer Res Treat. 2004;86:153–64. doi: 10.1023/B:BREA.0000032983.87966.4e. [DOI] [PubMed] [Google Scholar]
- 32.Kopec JA, Yothers G, Ganz PA, et al. Quality of life in operable colon cancer patients receiving oral compared with intravenous chemotherapy: Results from National Surgical Adjuvant Breast and Bowel Trial C-06. J Clin Oncol. 2007;25:424–30. doi: 10.1200/JCO.2005.05.2597. [DOI] [PubMed] [Google Scholar]
- 33.Kopec JA, Land SR, Cecchini RS, et al. Validation of a self-reported neurotoxicity in patients with operable colon cancer receiving oxaliplatin. J Support Oncol. 2006;4:W1–W8. [Google Scholar]
- 34.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. New Engl J Med. 2004;30:2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]