Abstract
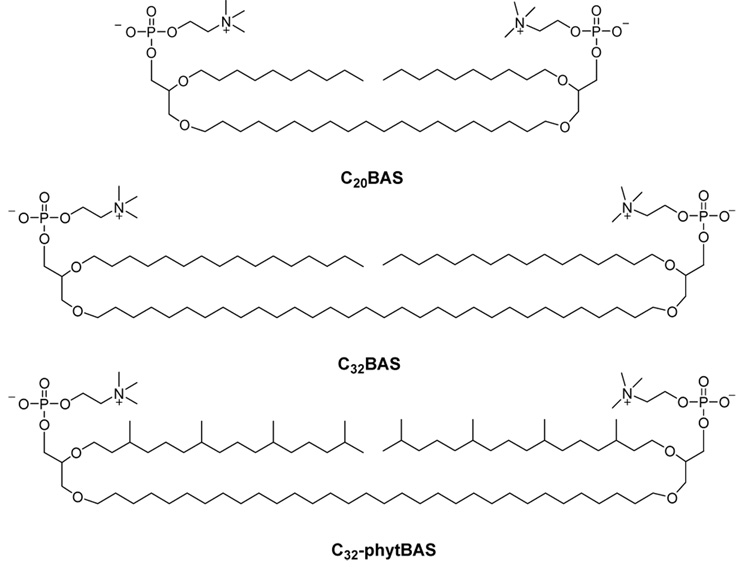
Three bipolar archaeal-type diglycerophosphocholine tetraether lipids (a.k.a., bolalipids) have been prepared to determine 1) the influence of molecular structure on the physical properties of bolalipid membranes and 2) their impact on the functional reconstitution of Ste14p, a membrane-associated isoprenylcysteine carboxyl methyltransferase from Saccharomyces cerevisiae. The three bolalipids synthesized were: C20BAS, C32BAS, and C32phytBAS. These bolalipid structures differ in that the C20BAS derivative has a short sn-1 glyceryl diether C20H40 transmembrane alkyl chain and two ether-linked sn-2 n-decyl chains, whereas the C32BAS and C32phytBAS derivatives have a longer sn-1 diether C32H64 membrane-spanning chain and two ether-linked sn-2 n-hexadecyl or phytanyl chains, respectively. Differential scanning calorimetry and temperature-dependent 31P NMR was used to determine the gel-to-liquid crystalline phase transition temperatures of the bolalipids (C32BAS Tm > 85 °C; C32phytBAS Tm = 14 °C; C20BAS Tm = 17°C). The bolalipid lateral diffusion coefficients, determined by fluorescence recovery after photobleaching at 20 °C, were 1.5 × 10−8 and 1.8 × 10−9 cm2/s for C20BAS and C32phytBAS, respectively. The mobility of C32BAS could not be measured at this temperature. Ste14p activity was monitored by an in vitro methyltransferase assay in reconstituted vesicle dispersions composed of DMPC, C20BAS:E. coli polar lipid, C20BAS:POPC, C32phytBAS:E. coli polar lipid, and C32phytBAS:POPC. Ste14p activity was lost in vesicles composed of 75–100 mol% C20BAS and 0–100 mol% C32BAS, but retained in vesicles with 0–50 mol% C20BAS and 0–100 mol% C32phytBAS. Confocal immunofluorescence microscopy confirmed the presence of Ste14p in 100 mol% C20BAS and 100 mol% C32phytBAS vesicle dispersions, even though the lamellar liquid crystalline phase thickness of C20BAS is only 32 Å. Since Ste14p activity was not affected by either the gel to liquid-crystal phase transition temperature of the lipid or the temperature of the assay, the low activity observed in 75–100 mol% C20BAS membranes can be attributed to hydrophobic mismatch between this bolalipid and the hydrophobic surface of Ste14p.
Bolalipids, also called bolaamphiphiles, are a class of bipolar lipids found in the cell membranes of some Archaea. In many cases, these organisms can survive in extreme environments due to the presence of isoprenoid-based tetraether bolalipids in their membranes (1–5). The membrane-spanning bipolar structural motif confers increased stability to these membranes by effectively cross-linking the apposed leaflets of a bilayer membrane, thereby producing a monolayer membrane that functionally mimics a conventional membrane bilayer. This membrane stabilization strategy blocks membrane failure via delamination in the physically and chemically challenging environments that are often preferred by this class of organism. Archaeal bolalipid structures are characterized by ether linkages to each glycerol backbone, nonitol or phosphate polar headgroups, and transmembrane chains that contain combinations of methyl branching and cyclopentane rings to help maintain membrane fluidity across a wide range of environmental temperatures (6–7). Studies of biogenic and synthetic variants of these unusual membrane materials demonstrate that bolalipid membranes are less permeable and more durable than membranes composed of monopolar lipids (8–13). This unique combination of properties has ignited interest in their use as non-polymeric membrane stabilizing agents in applications such as drug delivery and membrane protein-based biosensors (5,12,14,15).
Biosensor and drug delivery applications of bolalipids will require large quantities of material. Unfortunately, bolalipids derived from Archaea are difficult to obtain in pure form on even the 1 mg scale. We report three synthetic archaeal bolalipid mimics that retain key structural motifs of naturally occurring bolalipids, yet are easier to produce in >100 mg quantities: 2,2’-di-O-decyl-3,3’-di-O-(1”,20”-eicosanyl)-bis-(rac-glycero)-1,1’-diphosphocholine (C20BAS), 2,2’-di-O-hexadecyl-3,3’-di-O-(1”,32”-dotriacontanyl)-bis-(rac-glycero)-1,1’-diphosphocholine (C32BAS) and 2,2’-di-O-(3,7,11,15-tetramethylhexadecyl)-3,3’-di-O-(1”,32”-dotriacontanyl)-bis-(rac-glycero)-1,1’-diphosphocholine (C32phytBAS) (Figure 1). Each of these bolalipid structures is acyclic, symmetric, contains glycerophosphocholine headgroups, and possesses either 20 or 32 carbon transmembrane chains to stabilize the hydrophobic domains of integral membrane proteins. The hydrophobic chains at each sn-2 position of the bolalipid glycerol backbones are both n-alkyl or phytanyl, with lengths that are half that of the transmembrane chain. Methyl branching was designed into the C32phytBAS structure, since is was expected that the phytanyl chains would lower the bolalipid gel to liquid-crystalline phase transition temperature relative to C32BAS by disrupting the alkyl chain packing within the membrane.
Figure 1.
Structure of the synthetic bolalipids used in this study: C20BAS, C32BAS and C32phytBAS.
In this report, the first successful reconstitution of an integral membrane protein in synthetic bolalipid vesicles and the correlation of its activity with the physical properties of the membrane is demonstrated. The only prior reports of integral membrane protein enzymes in bolalipid vesicles described the use of the polar lipid extract from the thermophile S. acidocaldarius to functionally reconstitute beef heart cytochrome c-oxidase (16) and the leucine transporter of Lactococcus lactis (17). These studies, however, did not provide insight into the role of bolalipid structure and physical properties on the function of these membrane proteins.
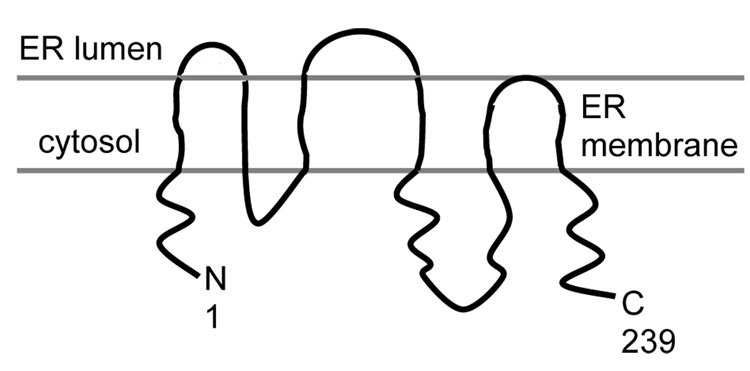
The isoprenylcysteine carboxyl methyltransferase (Icmt) from Saccharomyces cerevisiae, Ste14p, was chosen for functional reconstitution into bolalipid membranes. Ste14p is a 26 kDa integral membrane protein with six transmembrane domains and a predicted topology as shown in Scheme 1 (18). It is a polytopic endoplasmic reticulum membrane-localized Icmt enzyme that is responsible for carboxyl methylation of C-terminal CaaX sequences (C is cysteine, “a” is generally an aliphatic residue, X can be one of many amino acids). Carboxyl methylation of CaaX sequences is essential for the proper localization and transformation ability of oncogenic Ras proteins, thus making it an important target for chemotherapeutic agent discovery. We used the 37 kD His10myc3-tagged variant of Ste14p as a model enzyme for human Icmt in this study because 1) they are functional homologs (19) and 2) it has previously been successfully purified and functionally reconstituted in conventional lipids (20). A supported membrane sensor constructed from human Icmt-containing bolalipids may be useful for screening Icmt inhibitor candidates, therefore, reconstitution of functional Ste14p in bolalipid vesicles is an essential first step toward the development of a sensor based on this enzyme. Unfortunately, to date, attempts at purifying and functionally reconstituting human and other mammalian Icmts have met with limited to no success (21–24).
Scheme 1.
Topology model of Ste14p, adapted from (18).
This contribution describes the characterization of C20BAS, C32BAS and C32phytBAS by differential scanning calorimetry (DSC), temperature-dependent 31P NMR, and fluorescence recovery after photobleaching (FRAP). The impact of bolalipid structure and membrane composition on Ste14p activity, monitored by an in vitro vapor diffusion methyltransferase assay (20), was then determined using vesicles of differing lipid composition, gel to liquid crystalline phase transition temperatures, and lipid mobility. Confocal immunofluorescence microscopy and protein assays were used to determine the extent and distribution of Ste14p in reconstituted vesicle membranes. Our results show that Ste14p activity is sensitive to hydrophobic mismatch between the bolalipid membrane and the hydrophobic surface of Ste14p, however, it is not sensitive to the phase state of the host membrane. These findings suggest that C32phytBAS is an excellent candidate for a rugged host membrane material for Ste14p sensor production.
MATERIALS AND METHODS
Materials
The synthesis of C20BAS has been previously described (25,26). C32phytBAS and C32BAS were synthesized as described below. POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine), DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine), cholesterol (Chol), E. coli polar lipid extract and 1-oleoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-sn-glycero-3-phosphocholine (NBD-OPPC) were purchased from Avanti Polar Lipids (Alabaster, AL). Fluorescein-labeled anti-myc-FITC mouse antibodies were obtained from Invitrogen (Carlsbad, CA). N-Acetyl-S-farnesyl-L-cysteine (AFC) was provided by Dr. Richard Gibbs (Purdue University). S-Adenosyl-L-[14C-methyl] methionine (14C-SAM) was purchased from Amersham Biosciences (Uppsala, Sweden). 1-Dodecyl-β-D-maltopyranoside (DDM) was obtained from Anatrace, Inc. (Maumee, OH). Triton® X was obtained from Sigma (St. Louis, MO). HPLC grade chloroform (CHCl3), diethyl ether (Et2O), and methanol (MeOH) were supplied by Mallinckrodt-Baker (Paris, KY). All chemicals for the synthesis of C32phytBAS and C32BAS were purchased from Aldrich except for 2-chloro-2-oxo-1,3,2-dioxaphospholane (Fluka, Milwaukee, WI). Reaction solvents were reagent grade and were dried by distillation from an appropriate dessicant under N2 before use: tetrahydrofuran (THF) from sodium benzophenone ketyl; dichloromethane (CH2Cl2), toluene (PhCH3), acetonitrile (CH3CN), and triethylamine (Et3N) from CaH2. All reactions were performed under dry Ar or N2 gas. NMR spectra were recorded on a Varian 300 MHz spectrometer using 1H and 13C solvent peaks as the internal reference. Column chromatography was performed with 230–400 mesh silica gel using HPLC grade eluents. Electrospray mass spectrometry (ESI MS) was performed using a Waters ZQ spectrometer. Thin layer chromatography was performed using Baker-flex IB-F plates (J.T. Baker) and visualized using UV, I2 adsorption, KMnO4/heat, and/or H2SO4/heat.
Synthesis of C32phytBAS
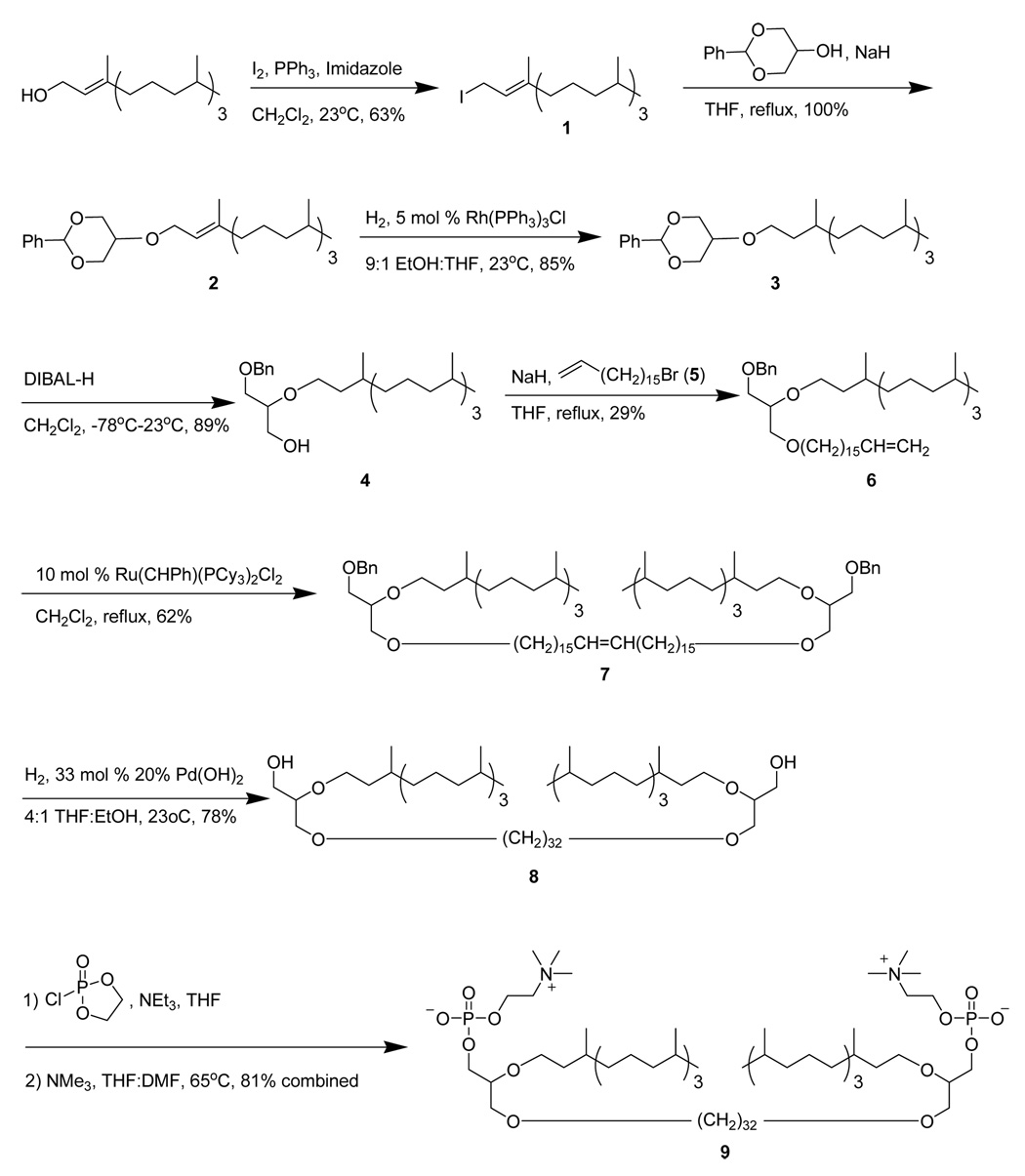
The synthesis route for C32phytBAS (Scheme 2) was adapted from a method described for the preparation of C20BAS (25,26).
Scheme 2.
Synthesis pathway for C32phytBAS.
(E)-1-Iodo-3,7,11,15-tetramethylhexadec-2-ene (1)
A flame dried flask was charged with PPh3 (1.0557 g, 4.04 mmol) followed by 11 mL of CH2Cl2 and imidazole (0.2776 g, 4.04 mmol). The solution was cooled to 0 °C, I2 crystals (1.0273 g, 4.04 mmol) added slowly, and the mixture stirred at 25 °C for 15 min under Ar. Phytol (1.0 g, 3.37 mmol) in 3 mL CH2Cl2 was then added and the mixture stirred at 25 °C under Ar for an additional 2 h. Petroleum ether (200 mL) was then added and the mixture filtered twice through a 1” pad of silica gel. After solvent removal, 0.874 g of 1 (63% yield) was obtained as a light yellow oil. 1H NMR (300 MHz, CDCl3): 0.88 (t, 12 H, CH3); 0.99–1.41 (m, 19 H, CH+CH2 aliphatic); 1.63 (s, 3 H, CH3 vinylic); 1.98 (t, 2 H, CH2 allylic); 3.94 (d, 2H, CH2I); 5.532 (t, 1 H, CH vinylic). 13C NMR (75 MHz, CDCl3): 4.10, 15.71, 19.71, 19.77, 22.63, 22.73, 24.47, 24.80, 24.91, 27.98, 32.66, 32.80, 36.43, 37.29, 37.36, 37.44, 39.38, 39.88, 121.67, 142.88. ESI+ MS: m/z (M+H) 461.
(E)-2-Phenyl-5-O-(3,7,11,15-tetramethylhexadec-2-enyl)-1,3-dioxane (2)
A flame-dried flask was charged with 2-phenyl-5-hydroxy-1,3-dioxane (0.322 g, 1.79 mmol) followed by 4 mL THF before cooling to 0 °C. NaH dispersion (80% in oil, 0.1485 g, 4.95 mmol) was slowly added and the mixture stirred at 25 °C for 1 h. Compound 1 (0.874 g, 2.15 mmol) was dissolved in 3 mL THF and slowly added to the alkoxide solution at 0 °C prior to heating at reflux overnight under Ar. The unreacted NaH was quenched at 0 °C with deionized H2O. The aqueous phase was extracted with Et2O (3 × 25 mL), the organic phases combined, dried over MgSO4, filtered, and the solvent removed by rotary evaporation. The crude mixture was separated via column chromatography using gradient elution: 100% hexane to 1:1 hexane:CH2Cl2. Fractions containing the desired product were pooled, the solvent removed by rotary evaporation, and the residue dried under vacuum to give 0.879 g (100% yield) of 2 as a clear oil. 1H NMR (300 MHz, CDCl3): 0.86 (t, 12 H, CH3); 1.05–1.57 (m, 19 H, CH+CH2 aliphatic); 1.65 (s, 3 H, CH3 vinylic); 1.99 (m, 2 H, CH2 allylic), 3.29 (s, 1 H, CH-O); 4.01 (dd, 2 H, OCH2); 4.15 (dd, 2 H, OCH2 allylic), 4.31 (dd, 2 H, OCH2), 5.39 (t, 1 H, CH vinylic), 5.53 (s, 1 H, OCHO acetal), 7.32 (dd, 3 H, phenyl), 7.51 (dd, 2 H, phenyl). 13C NMR (75 MHz, CDCl3): 16.33, 19.62, 22.53, 22.62, 24.35, 24.69, 24.97, 27.85, 32.57, 32.67, 36.58, 37.18, 37.26, 37.32, 39.26, 39.80, 64.92, 68.97, 69.16, 101.14, 120.66, 126.10, 127.97, 128.64, 138.04, 140.29. ESI+ MS: m/z (M+) 458.
2-Phenyl-5-O-(3,7,11,15-tetramethylhexadecyloxy)-1,3-dioxane (3)
A flask was charged with 2 (0.12 g, 0.262 mmol) followed by 27 mL of a 9:1 EtOH:THF solution and Wilkinson’s catalyst (Rh(PPh3)3Cl, 10 mg, 0.01 mmol). The mixture was stirred under 1 atm of H2 at 25 °C overnight and filtered through a 0.5” pad of alumina, followed by a ~100 mL Et2O rinse. The solvent was removed on a rotary evaporator and the crude product purified via column chromatography using hexane:CH2Cl2 as eluent. Fractions containing the product were pooled and the solvent removed on a rotary evaporator to give 0.103 g of 3 (85% yield) as a clear oil. 1H NMR (300 MHz, CDCl3): 0.854 (q, 15 H, CH3); 1.04–1.59 (m, 22 H, CH+CH2 aliphatic); 1.68–1.72 (m, 2 H, CH2 β to O); 3.25 (s, 1 H, CH-O); 3.58 (m, 2H, OCH2); 4.03 + 4.33 (dd, 4 H, OCH2); 5.54 (s, 1 H, CH acetal); 7.34 + 7.51 (dd, 5 H, phenyl). 13C NMR (75 MHz, CDCl3): 19.73, 22.60, 22.70, 24.33, 24.46, 24.77, 27.94, 29.89, 32.77, 36.70, 36.78, 37.26, 37.29, 37.42, 37.47, 39.33, 67.23, 68.97, 69.05, 70.57, 101.29, 126.16, 128.11, 128.78, 138.14. ESI+ MS: m/z (M+H) 461.
1-O-Benzyl-2-O-(3,7,11,15-tetramethylhexadecyl)-rac-glycerol (4)
A flame-dried flask was charged with 3 (2.98 g, 6.47 mmol) followed by 14 mL CH2Cl2, and then cooled to −78 °C. Diisobutylaluminum hydride (DIBAL-H, 7.12 mL of a 1 M solution in CH2Cl2, 7.12 mmol) was added dropwise via syringe and the reaction stirred at −78 °C for 1 h under Ar before slowly warming to 25 °C and stirring overnight. The reaction was then cooled to 0 °C, quenched with 600 µL of MeOH, and NaOH solution added to disperse the gel (10 mL of a 5 N NaOH solution). The aqueous phase was extracted with Et2O (3 × 50 mL), the organic phases combined, dried over MgSO4, filtered, evaporated, and the residue dried under vacuum to give 2.676 g of 4 (89% yield) as a colorless oil. 1H NMR (300 MHz, CDCl3): 0.86 (t, 15 H, CH3); 1.23 (m, 22 H, CH+CH2 aliphatic); 1.56 (m, 2 H, CH2 β to O); 2.34 (t, 1 H, OH), 3.63 (m, 7 H, CH-O + OCH2), 4.53 (s, 2 H, OCH2Ph), 7.29 (bs, 5H, phenyl). 13C NMR (75 MHz, CDCl3): 19.53, 19.62, 19.68, 22.55, 22.64, 24.27, 24.40, 24.71, 27.87, 29.74, 32.70, 37.00, 37.08, 37.21, 37.24, 37.27, 37.31, 37.37, 37.42, 39.29, 62.67, 68.58, 69.92, 73.40, 78.54, 127.51, 127.56, 128.28, 137.96. ESI+ MS: m/z (M+H) 463.
17-Bromo-1-heptadecene (5)
This precursor was prepared as described by Peanasky et al. (27). 1H NMR (300 MHz, CDCl3): 1.25 (s, 24 H, -CH2), 1.85 (q, 2 H, -CH2), 2.25 (q, 2 H, allylic –CH2), 3.40 (t, 2 H, -CH2Br), 4.95 (dd, 2 H, vinyl CH2), 5.8 (m, 1 H, vinyl CH). 13C NMR (75MHz, CDCl3): 28.4, 29.0, 29.2, 29.4, 29.7, 29.7, 29.8, 29.8, 29.9, 29.9, 33.1, 34.0, 34.2, 76.8, 77.2, 77.7, 114.3, 139.5. ESI+ MS: m/z (M+H) 317/319.
1-Benzyl-2-O-(3,7,11,15-tetramethylhexadecyl)-3-O-(16’-heptadecenyl)-rac-glycerol (6)
A flame-dried flask was charged with 4 (2.676 g, 5.78 mmol) in 10 mL THF and cooled to 0 °C. NaH (0.1457 g, 6.07 mmol) was added slowly and stirred at 25 °C for 1 h under Ar. A solution of 5 (5.68 g, 0.0179 mol) in 10 mL THF was slowly added at 0 °C and stirred at 25 °C for 2 d under Ar. Water (25 mL) was added and the aqueous phase extracted with Et2O (3 × 50 mL). The organic phase was separated, dried over MgSO4, filtered and evaporated on a rotary evaporator. The crude product was purified by column chromatography using gradient elution: 1:1 hexane:CH2Cl2 to 100% CH2Cl2. The fractions containing the desired product were pooled, evaporated and the residue dried under vacuum to give 1.187 g of 6 (29% yield). 1H NMR (300 MHz, CDCl3): 0.86 (t, 15 H, CH3); 1.21 (m, 46 H, CH+CH2 aliphatic); 1.57 (m, 4 H, CH2 β to O); 2.03 (q, 2 H, CH2 allylic), 3.54 (m, 9 H, CH-O + OCH2), 4.55 (s, 2 H, OCH2Ph), 4.95 (dd, 2 H, CH2 vinyl), 5.81 (m, 1 H, CH vinyl), 7.29 (bs, 5 H, phenyl). 13C NMR (75 MHz, CDCl3): 19.74, 22.61, 22.71, 24.35, 24.47, 24.79, 26.12, 27.95, 28.94, 29.15, 29.50, 29.68, 29.78, 32.78, 33.81, 37.38, 37.45, 39.35, 68.85, 70.26, 70.72, 71.64, 73.33, 77.92, 114.06, 126.04, 127.47, 127.56, 127.67, 128.28, 139.20. ESI+ MS: m/z (M + H) 699.
1,1’-Di-O-benzyl-2,2’-di-O-(3,7,11,15-tetramethylhexadecyl)-3,3’-di-O-(1”,32”-dotriacont-16”-enyl)-bis-(rac-glycerol) (7)
A flame dried flask was charged with 6 (1.927 g, 2.76 mmol) and 6 mL CH2Cl2. Grubbs catalyst (Ru(CHPh)(PCy3)3Cl2, 0.2268 g, 0.276 mmol) in 5 mL CH2Cl2 was slowly added before heating the mixture at reflux overnight. After cooling the reaction mixture, the catalyst was quenched with 10 mL H2O and the aqueous phase extracted with CH2Cl2 (3 × 10 mL). The organic phase was then dried over MgSO4, filtered and the solvent evaporated on a rotary evaporator. The crude product was purified via column chromatography using gradient elution: 9:1 to 6:4 hexane:Et2O. Fractions containing the desired product were pooled, evaporated and the residue dried under vacuum to give 1.177 g of 7 (62% yield) as a light brown oil. 1H NMR (300 MHz, CDCl3): 0.86 (t, 30 H, CH3); 1.25 (m, 92 H, CH+CH2 aliphatic); 1.57 (m, 8 H, CH2 β to O); 1.96 (c, 4 H, CH2 allylic), 3.52 (m, 18 H, CH-O + OCH2), 4.55 (s, 4 H, OCH2Ph), 5.37 (m, 2 H, CH2 vinyl), 7.29 (bs, 10 H, phenyl). 13C NMR (75 MHz, CDCl3): 19.83, 19.91, 19.96, 22.84, 22.93, 24.57, 24.70, 25.01, 26.35, 28.17, 29.40, 29.72, 29.76, 29.89, 29.92, 30.00, 32.82, 33.00, 33.31, 37.39, 37.50, 37.62, 37.67, 37.72, 39.58, 69.05, 70.52, 70.97, 71.85, 73.55, 78.17, 127.66, 127.75, 128.47, 130.06, 130.53, 138.64. ESI+ MS: m/z (M + Na+) 1393.
2,2’-Di-O-(3,7,11,15-tetramethylhexadecyl)-3,3’-di-O-(1”,32”-dotriacontanyl)-bis-(rac-glycerol) (8)
A flask was charged with 7 (1.094 g, 0.798 mmol) followed by 25 mL of a 4:1 THF:EtOH solvent mixture, 20% Pd(OH)2 on carbon catalyst (0.168 g, 0.234 mmol) and the mixture stirred under 1 atm H2 at 25 °C overnight. The catalyst was removed by filtration through a 0.5” pad of Celite, followed by a ~100 mL CHCl3 rinse. The solvent was removed by rotary evaporation and the crude product purified by column chromatography using 3:1 hexane:Et2O as eluent. Fractions containing the desired product were pooled, evaporated, and the residue dried under vacuum to give 0.738 g (78% yield) of 8 as an ivory wax. 1H NMR (300 MHz, CDCl3): 0.87 (t, 30 H, CH3); 1.25 (m, 100 H, CH+CH2 aliphatic); 1.57 (m, 8 H, CH2 β to O); 2.22 (t, 2 H, OH), 3.57 (m, 18 H, CH-O + OCH2). 13C NMR (75 MHz, CDCl3): 19.97, 22.85, 22.95, 24.58, 24.71, 25.03, 26.33, 28.20, 29.72, 29.86, 29.94, 33.03, 37.37, 37.52, 37.69, 39.60, 63.32, 68.86, 71.15, 72.08, 78.51. ESI+ MS: m/z (M + H) 1192.
2,2’-Di-O-(3,7,11,15-tetramethylhexadecyl)-3,3’-di-O-(1”,32”-dotriacontanyl)-bis-(rac-glycero)-1,1’-diphosphocholine (C32phytBAS, 9)
A flame dried flask was charged with 8 (0.150 g, 0.125 mmol), followed by the addition of 0.25 mL THF, 2-oxo-2-chloro-1,3,2-dioxaphospholane (0.15 mL, 1.13 mmol), and Et3N (0.12 mL, 0.565 mmol). The turbid mixture was diluted with 2 mL THF and 1 mL DMF. The reaction mixture was then transferred to a flame-dried pressure tube and 1 mL Me3N condensed into the tube. The tube was then sealed and heated to 70 °C with stirring for 2 d. The reaction vessel was then slowly cooled to 0 °C, the pressure carefully released, and the product mixture concentrated on a rotary evaporator. The product was isolated by silica gel column chromatography using step gradient elution: 80:20:0, 0:100:0, 65:35:5, 60:40:10 CHCl3:MeOH:H2O. Suspended silica particles were removed from the fractions using a 0.22 µm PTFE filter, the solvent evaporated, and the residue dried under vacuum to give 0.1903 g of 9 (81% yield) as a wax. 1H NMR (300 MHz, CDCl3:CD3OD): 0.86 (t, 30 H, CH3); 1.19 (m, 100 H, CH+CH2 aliphatic); 1.53 (m, 8 H, CH2 β to O); 3.23 (s, 18 H, NMe3), 3.49 (m, 18 H, CH-O + OCH2), 3.90 (t, 4 H, CH2N), 4.25 (bs, 4 H, CH2OP). 13C NMR (75 MHz, CDCl3:CD3OD): 21.16, 21.24, 24.12, 24.20, 26.04, 26.37, 27.67, 29.52, 31.28, 34.37, 38.84, 39.01, 40.94, 55.76, 60.52, 66.69, 68.08, 70.43, 70.62, 72.17, 73.33. 31P NMR (124 MHz, CDCl3:CD3OD): single peak (unreferenced) confirms the presence of a single phosphorus bonding motif. ESI+ MS: m/z (M + H) 1522.
Synthesis of C32BAS
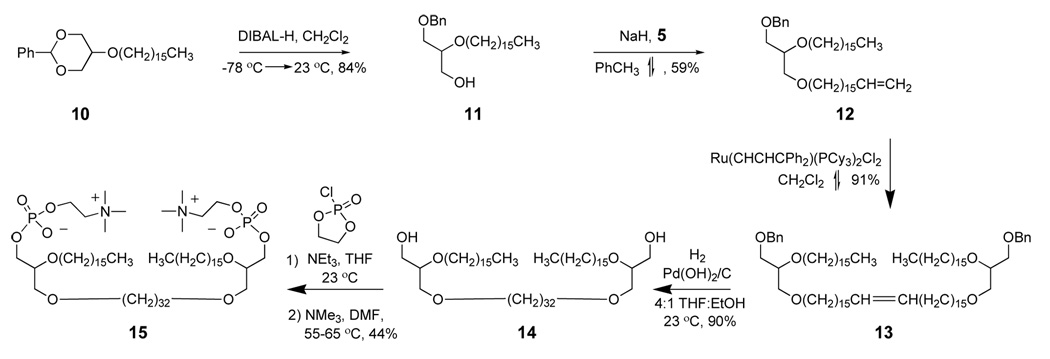
The synthesis route for C32BAS is shown in Scheme 3.
Scheme 3.
Synthesis pathway for C32BAS.
1-O-Benzyl-2-hexadecyloxy-3-O-(16’-heptadecenoxy)-rac-glycerol (12)
A flame dried flask was charged with 95% NaH dispersion in oil (0.159 g, 6.6 mmol) followed by 30 mL toluene and cooled to 0 °C. A solution of 11 (1.51 g, 3.7 mmol) (25,26) in toluene (30 mL) was added via syringe and the mixture stirred for 1 h. A solution of 5 (2.1 g, 6.6 mmol) dissolved in 20 mL toluene was added at 25 °C via syringe and the mixture heated at reflux overnight. Water (50 mL) was added and the aqueous phase extracted with diethyl ether (3 × 50 mL), dried over MgSO4, filtered, and evaporated on a rotary evaporator. The crude product was purified by column chromatography using 10:1 hexane:Et2O as eluent. The fractions collected at Rf = 0.2 were pooled, evaporated, and the residue dried under vacuum to give 2.5 g of 12 (59% yield) as a clear oil. 1H NMR (300 MHz, CDCl3): 0.89 (t, 3 H, -CH3), 1.26 (s, 50 H, -CH2), 1.57 (m, 4 H, -CH2 β to O), 2.03 (c, 2 H, vinyl CH2), 3.45 (m, 9 H, -OCH2), 4.56 (s, 2 H, -OCH2-Ph), 4.95 (dd, 2 H, vinyl CH2), 7.33 (m, 5 H, -C6H5). 13C NMR (75 MHz, CDCl3): 14.3, 22.9, 22.9, 26.3, 26.4, 26.4, 29.2, 29.4, 29.6, 29.7, 29.9, 30.3, 31.8, 32.2, 34.1, 70.5, 70.8, 71.0, 71.2, 71.9, 73.6, 78.2, 114.3, 127.7, 127.8, 128.5, 138.7, 139.5. Mass (M + H) = 643 by EI/CI.
1,1’-Di-O-benzyl-2,2’-di-O-hexadecyl-3,3’-di-O-(16”-dotriacontenyl)-bis-(racglycerol) (13)
This compound was prepared as described for 7 from 12. The crude product was purified via column chromatography using 7:2 hexane:diethyl ether. Fractions at Rf = 0.3 were combined, evaporated, and the residue dried under vacuum to give 0.4007 g of 13 (91% yield). 1H NMR (300 MHz, CDCl3): 0.89 (t, 6 H, -CH3), 1.26 (s, 25 H, -CH2), 1.57 (m, 8 H, -CH2 β to O), 1.97 (m, 4 H, vinyl CH2), 3.43 (m, 18 H, -OCH2), 4.56 (s, 4 H, -OCH2-Ph), 5.39 (m, 2 H, vinyl CH2), 7.33 (m, 10 H, -C6H5). 13C NMR (75 MHz, CDCl3): 14.1, 22.7, 23.6, 26.1, 29.2, 29.4, 29.5, 29.7, 30.1, 31.9, 32.6, 70.3, 70.6, 70.7, 71.7, 73.3, 77.9, 127.5, 127.6, 128.3, 130.3. Mass (M + Na)= 1279 by MALDI.
2,2’-Di-O-hexadecyl-3,3’-di-O-(dotriacontyl)-bis-(rac-glycerol) (14)
This compound was prepared as described for 8 starting from 13. The crude product was purified via column chromatography eluting with a gradient solvent system from 4:1 to 0:100 hexane:Et2O, followed by a column flush with 5:1 CHCl3:MeOH to give 14 as a white solid (0.557 g, 90% yield). 1H NMR (300 MHz, CDCl3): 0.89 (t, 6 H, -CH3), 1.25 (s, 108 H, -CH2), 1.56 (m, 8 H, -CH2 β to O), 1.89 (bs, 2 H, -OH), 3.37–3.74 (m, 18 H, -OCH2, -OCH). 13C NMR (75 MHz, CDCl3): 14.1, 22.7, 26.1, 26.2, 29.2, 29.3, 29.5, 29.6, 29.7, 30.1, 31.9, 63.0, 70.4, 70.9, 71.8, 78.3. Mass (M + Na)= 1102 by MALDI.
2,2’-Di-O-hexadecyl-3,3’-di-O-(dotriacontyl)-bis-(rac-glycerol)-1,1’-diphosphocholine (C32BAS, 15)
This compound was prepared as described for 9 using 14 as the precursor. The crude yellow solid obtained was purified via silica gel column chromatography using a step gradient elution with 80:20:0, 50:50:0, 0:100:0, 65:35:5, and 60:40:10 CHCl3:MeOH:H2O. The fractions containing product were combined and extracted using the Bligh-Dyer method. The organic phase was dried with MgSO4, concentrated and further dried under vacuum to give 0.0581 g of 15 (44% yield); the remaining material was the starting diol, 14. 1H NMR (300 MHz, CDCl3:CD3OD): 0.89 (t, 6 H, -CH3), 1.28 (s, 108 H, -CH2), 1.57 (t, 8 H, -CH2 β to O), 3.23 (s, 18 H, N(CH3)3), 3.45–3.52 (m, 6 H, -OCH2, -OCH), 3.58–3.64 (m, 12 H, -OCH2, -OCH), 3.91 (t, 4 H, -CH2-NMe3), 4.29 (bs, 4 H, -CH2-OP). 13C NMR (75 MHz, CDCl3:CD3OD): 24.4, 27.8, 31.1, 31.3, 31.4, 31.8, 33.6, 55.9, 72.4. 31P NMR (124 MHz, CDCl3:CD3OD): one unreferenced peak. Mass (M + H) = 1411 by MALDI.
DSC
Calorimetric experiments were performed on a TA DSC-Q10 instrument using 1 mg lipid/4 mg H2O in stainless steel pans. All thermograms were run using a reference pan with H2O. Indium was used as a calibration standard. Thermograms were collected in the heating mode using a scan rate of 1 °C/min. The temperatures scanned ranged from −20 to 85 °C. Three thermograms were collected and the average values for the transition temperature (determined at the transition peak) reported.
31 P NMR chemical shift anisotropy
Proton-decoupled 31P NMR spectra were acquired at temperatures from −5 to 30°C to determine the change in chemical shift anisotropy of the 31P powder pattern lineshape for C32phytBAS. The sample was prepared by hydrating a dry film of C32phytBAS at a concentration of 20 mg/mL in D2O. Spectra were obtained using a Bruker DRX 500 spectrometer equipped with a 5 mm probe. A period of 15 min was allowed for temperature equilibration before spectral acquisition at each temperature studied. All spectra were acquired using the following parameters: 1600 scans, relaxation delay of 1 sec, 90° pulse duration of 6 µsec, acquisition time of 0.2 sec, and 200 Hz line broadening. The peak width at half the peak height was measured for each spectrum and the results plotted in ppm vs. temperature. The intersection of the two linear regions in this plot was taken as the Tm.
FRAP
Standard coverslips were cleaned with warm 7:1 Triton® X100:H2O for 10 min, rinsed extensively with deionized H2O, dried under a stream of N2 and baked at 400 °C for 5 h. Vesicles of POPC, POPC:C20BAS (100 nm nominal diameter), C20BAS, and C32phytBAS (200 nm nominal diameter) were prepared by extrusion as described below, except that 18 MΩ H2O was used instead of buffer and the extrusions were done at 65 °C. Total lipid concentrations were 5 µmol/mL at lipid ratios of 99.5% lipid + 0.5% NBD-OPPC. The vesicle solutions were mixed with 300 mM NaCl (1:1 vol/vol) and transferred to a perfusion chamber mounted on the coverslips. After approximately 1 min, the coverslips were dipped into a 18 MΩ H2O bath and a cover glass sandwich prepared. The sandwich was quickly placed on a microscope holder with H2O pools on both sides. A detailed description of this method for preparing supported lipid bilayers from vesicles has been reported elsewhere (28,29).
A Nikon TE200U fluorescence microscope coupled to a silicon avalanche photodiode and a continuous wave argon ion laser (488 nm emission, 25 mW) was used to both bleach and excite the NBD-OPPC probe (λex = 460 nm, λem = 534 nm). A neutral density filter was placed in the beam path to reduce the laser intensity to 250 nW to avoid constant photobleaching of the probe. A shutter in the beam path was pulsed to enable detection of the initial fluorescence counts for ~40 s. Upon removal of the filter from the beam path, the shutter sends a single photobleaching laser pulse for ≤1 s (bleach radius = 13 µm). The recovery of fluorescence intensity in this photobleached area is then monitored as a function of time. Diffusion coefficients of NBD-OPPC in various membrane compositions were determined by measuring the half-time to recovery using a modified Bessel function as described by Soumpasis (30). All of the data were fit as a single exponential and compared to POPC as standard. Diffusion coefficients determined in this manner are accurate to ±15%. All FRAP measurements were conducted at 25°C.
Vesicle Preparation
Dry lipid (10 mg) was dissolved in 1:1 CHCl3:MeOH in a cryovial and a thin film produced from it by evaporating the solvent under a gentle stream of Ar. The solvent was further removed under a 100 µm vacuum overnight. After addition of 1 mL 100 mM TRIS HCl pH 7.5, the solution was subjected to five cycles of freeze (liquid N2 bath), thaw (water bath at 35 °C for 5 min), and vortex (30 s) to give a polydisperse MLV suspension as described by DiMeglio et al. (31). This dispersion was extruded twenty times at 25 °C through two stacked 100 nm pore track-etch filters to produce an almost transparent solution. All samples were prepared similarly and were analyzed immediately after preparation.
Purification of Ste14p from Membranes
Ste14p with ten histidine residues and three repeats of the myc epitope at the N-terminus was expressed and purified as previously described (20). The histidines were used to facilitate Ste14p purification and the myc epitopes were used for immunodetection. Briefly, His10myc3-tagged Ste14p was expressed in S. cerevisiae, extracted from crude membranes using 1% (w:v) 1-β-D-dodecylmaltopyranoside (DDM), and purified using Talon™ metal affinity chromatography. The activity of the pure enzyme was measured using AFC as the model substrate in an in vitro vapor diffusion methyltransferase assay (see below). Protein concentrations were determined by an amido black protein assay (32).
Reconstitution of Ste14p in Mixed Lipid Vesicles
Purified Ste14p was reconstituted by rapid dilution in the presence of lipid dispersions as previously described (20). Briefly, ~1 µg of protein was added to 10 µL of a 10 mg/mL vesicle solution and a subsequent 20-fold rapid dilution was performed by addition of 100 mM Tris-HCl pH 7.5 buffer at 5 °C.
In Vitro Vapor Diffusion Methyltransferase Assay
The assay was performed as previously described by Anderson et al. (20) with the exception that the reactions contained less than 0.9 µg of purified protein. The buffer, vesicle solution, protein and assay reagents were temperature-equilibrated on ice prior to the addition of 1 µg Ste14p to 10 µL of vesicle solution followed by addition of 200 µM AFC. The samples were incubated on ice for 5 minutes followed by rapid dilution with buffer and the addition of 20 µM [14C]-SAM to a final volume of 60 µL. The reaction solution was incubated in a water bath at 30°C and the reaction allowed to progress for 30 min. The methyl transfer reaction was quenched by the addition of 50 µL of 1 M NaOH/1% SDS. The mixture (100 µL) was then spotted on filter paper and placed in the neck of a scintillation vial that contained 10 mL BioSafe II scintillation cocktail. After 2.5 h, the filters were removed and the NaOH-labile counts determined by liquid scintillation. The results are reported in pmol of methyl groups transferred/mg protein/min of reaction.
Temperature-Dependent Methyltransferase Assays
In vitro vapor diffusion methyltransferase assays were performed at different temperatures to test the effect of the lipid phase transition temperature on protein activity. Ste14p was reconstituted at 32°C into E. coli polar lipid, DMPC and C20BAS liposomes. In vitro vapor diffusion methyltransferase experiments were done as previously described with the exception that the incubation temperature was changed to 10°C, 16°C, 32°C, or 44°C as needed to probe Ste14p activity above and below the phase transition temperature of the host membrane.
Confocal Immunofluorescence Microscopy
Ste14p (10 µL of a 0.012 µg/µL in 500 mM imidazole elution buffer) was diluted to a final volume of 200 µL in 100 mM Tris-HCl pH 7.5 buffer containing unextruded vesicles (1 µL of a 10 µg/µL). The protein was stained by the addition of an anti-myc monoclonal mouse antibody followed by the addition of a fluorescein isothiocyanate-conjugated goat-anti mouse secondary antibody (0.1 µL of 1 µg/µL). Nile Blue was then added to stain the membrane and the samples were imaged at 60× magnification.
RESULTS
Synthesis
C32phytBAS was prepared as shown in Scheme 2 based on the previously described syntheses of C20BAS and C32BAS (24–26). Phytanyl iodide, prepared by iodination of phytol in the presence of triphenylphosphine and imidazole, was coupled with 2-phenyl-5-hydroxy-1,3-dioxane to produce ether 2 in quantitative yield. The phytanyl olefin was hydrogenated in the presence of Wilkinson’s catalyst in 85% yield. Reductive ring opening of the dioxane ring with DIBAL-H (33) gave the 2-phytanyl-1-benzyl-rac-glycerol diether 4 in 89% yield. Alkylation of the primary alcohol in 4 with 5 (27) gave the corresponding benzyl-protected glyceryl triether 6 in 29% yield. Dimerization of 6 via olefin metathesis using the first-generation Grubbs catalyst (34) gave 7 in 62% yield. Simultaneous olefin reduction and deprotection of 7 was achieved using Pd(OH)2-catalyzed hydrogenolysis. The phosphocholine headgroup was installed in the final step using a 2-chloro-2-oxo-1,3,2-dioxaphospholane esterification/trimethylamine ring opening sequence (35,36), to give the methyl substituted bolalipid, C32-phytBAS (9) in 81% yield (2% overall yield).
The synthesis route for C32BAS (Scheme 3) followed a similar path. 2-Phenyl-5-hexadecyloxy-1,3-dioxane (10), prepared as described by Patwardhan and Thompson (25,26) in 79% yield, was subjected to reductive ring opening with DIBAL-H (33) to give 11 in 84% yield. 17-Bromo-1-heptadecene (5) was used to alkylate 11 in 59% yield. Intermediate 13 was obtained in 91% yield via dimerization of 12 using Grubbs catalyst (34). Simultaneous deprotection and reduction of this precursor gave the tetraether bisglycerol intermediate 14 in 90% yield. Installation of the phosphocholine headgroups as before produced C32BAS (15) in 44% yield (14% overall yield).
Phase transition behavior of C32phytBAS and C32BAS dispersions
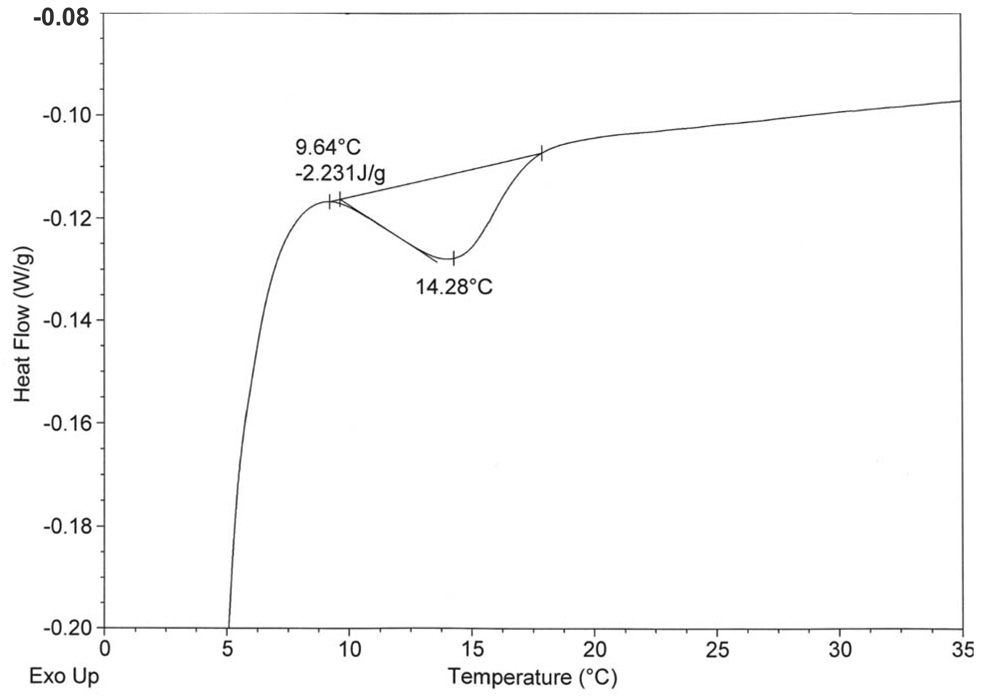
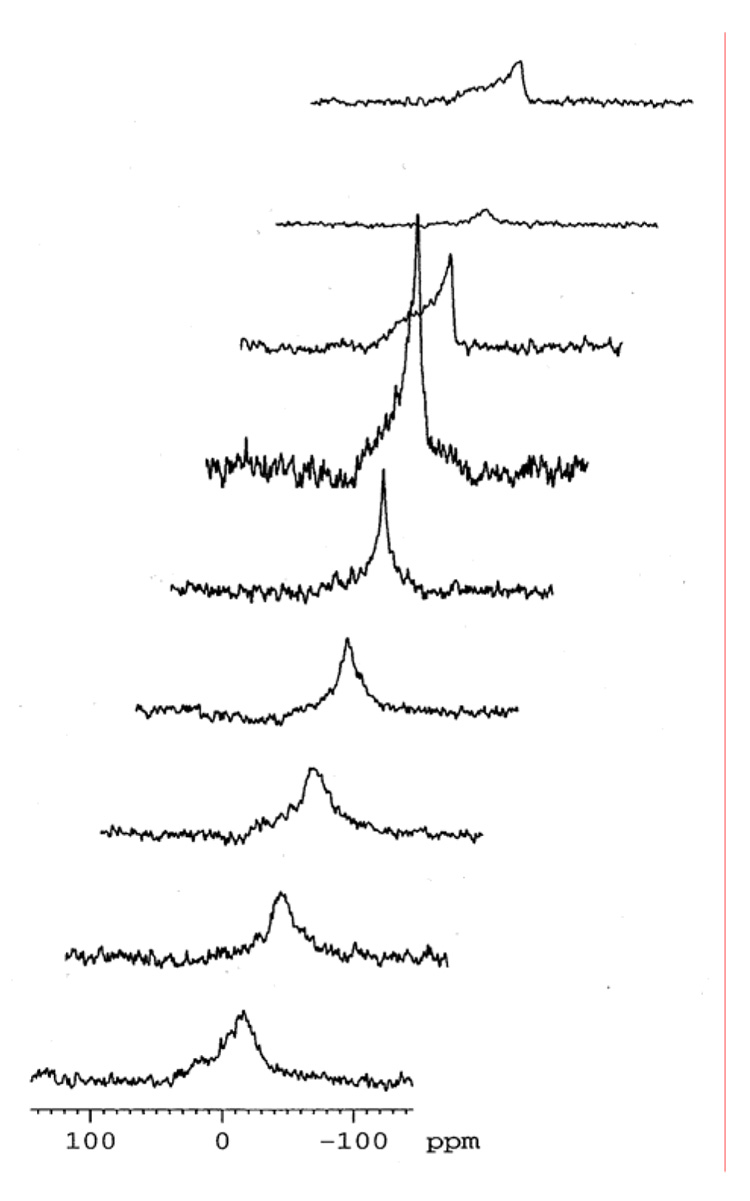
The DSC data obtained for hydrated C32phytBAS, C20BAS, and C32BAS in sealed pans is summarized in Table 1. The C20BAS was previously reported to have a Tm = 17 °C as determined by DSC and temperature-dependent Raman spectroscopy (31). DSC experiments revealed that the gel-to-liquid crystalline phase transition of C32phytBAS was 14 °C (Figure 2A) and was undetectable up to 85 °C for C32BAS (data not shown). Changes in the chemical shift anisotropy of C32phytBAS, determined by temperature-dependent 31P NMR (Figure 2B), also indicated that the Tm for this bolalipid was 14 °C.
Table 1.
Gel-to-liquid crystalline phase transition temperatures for hydrated bolalipids determined by differential scanning calorimetry.
| Bolalipid | Tm (°C) |
|---|---|
| C32phytBAS | 14 ± 0.1 |
| C20BAS | 17 ± 0.3 |
| C32BAS | > 85 |
Figure 2.
Phase transition behavior of C32phytBAS.
A: DSC of C32phytBAS dispersion (1 mg lipid/4 µL H2O) in sealed stainless steel pans, heating rate of 1°C/min. One out of three trials is shown. The phase transition temperature (Tm = 14.3 °C) and transition enthalpy (−2.23 J/g) are displayed.
B: Temperature-dependent proton-decoupled 31P NMR spectra of C32phytBAS in D2O. Top to bottom: 30, 25, 20, 15, 12, 10, 5, 0, −5 °C. The lipid was hydrated (20 mg lipid/1 mL D2O) and extruded through 200 nm track-etch membranes. Typically, 1600 scans were acquired with a relaxation delay of 1 s, 90° pulse of 6 µsec, acquisition time of 0.2 s. The spectra were processed with a line broadening of 200 Hz.
Lateral diffusion rates of C32phytBAS dispersions
Work by Thompson and coworkers (37) has shown that the lateral diffusion coefficients for POPC, C20BAS:POPC, C20BAS, and 7:3 C20BAS:Chol on detergent cleaned and baked cover glass are 8 × 10−8 cm2/s, 4 × 10−8 cm2/s, 1.5 × 10−8 cm2/s, and 1.2 × 10−9 cm2/s, respectively. Our FRAP data for glass-supported bolalipid membranes showed that the lateral diffusion rate of pure C32phytBAS (1.8 × 10−9 cm2/s, Table 2) was an order of magnitude slower than that observed for the unbranched shorter chain variant, C20BAS (1.5 × 10−8 cm2/s, Table 2) and similar to that observed for 7:3 C20BAS:Chol (1.2 × 10−9 cm2/s, Table 2).
Table 2.
Lateral diffusion coefficients of NBD-OPPC in lipid membranes on glass determined by fluorescence recovery after photobleaching.
| Lipid | D (× 108 cm2/s) |
|---|---|
| POPC | 8.0 ± 2.2 |
| C20BAS:POPC | 3.6 ± 0.3 |
| C20BAS | 1.5 ± 0.1 |
| 7:3 C20BAS:chol | 0.12 ± 0.03 |
| C32phytBAS | 0.18 ± 0.03 |
In vitro vapor diffusion methyltransferase assays
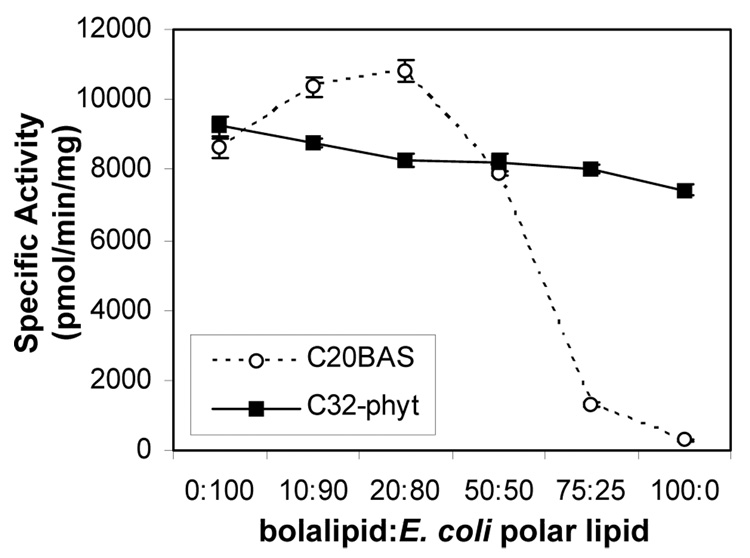
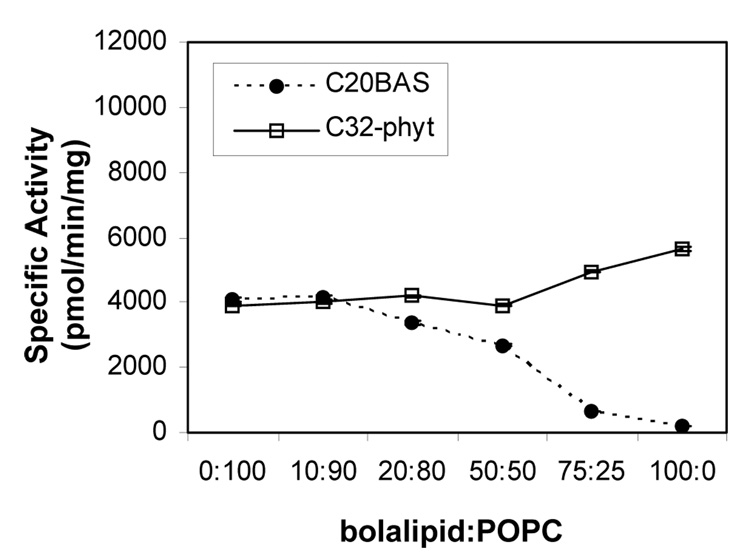
In vitro vapor diffusion methyltransferase assays (Scheme 4) demonstrated that when the molar ratio of C20BAS was increased in E. coli polar lipid membranes, Ste14p activity was retained until the C20BAS content in the membrane exceeded 50 mol% (Figure 3A). At 3:1 C20BAS:E. coli polar lipid, only 15% methyltransferase activity remained, whereas only 3% activity remained in pure C20BAS. A similar variation of membrane composition using C32phytBAS showed that Ste14p activity was retained in all molar ratios tested, such that pure C32phytBAS vesicles possessed 80% of the Ste14p methyltransferase activity found in Ste14p vesicles reconstituted with pure E. coli polar lipid extract.
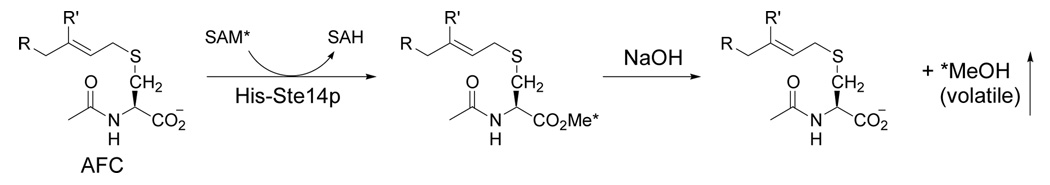
Scheme 4.
Vapor Diffusion Assay Reaction.
Figure 3.
Ste14p methyltransferase activity versus bolalipid:E. coli polar lipid composition (A), bolalipid:POPC composition (B). Ste14p (~1 µg) was added to 10 µL of vesicle dispersion followed by 200 µM AFC and rapid dilution by the addition of 20 µM [14C]-SAM. The samples were then incubated for 30 min at 30°C before quenching with 50 µL of 1 M NaOH/1% SDS. The mixture was spotted on a filter paper and analyzed by the vapor diffusion assay for 2.5 h as described in the Materials and Methods section. Specific activities were plotted as pmol of methyl groups transferred/mg protein/min.
When POPC was used as the host membrane lipid instead of E. coli polar lipid, the Ste14p activity dropped to 47% of its level in 100% E. coli polar lipid (Figure 3B). Ste14p activity gradually dropped as the C20BAS composition in POPC increased to 1:1 C20BAS:POPC. Further increases in the C20BAS molar ratio, however, caused a drop in the methyltransferase activity to 16% and 4% of the pure POPC level for 3:1 and 100:0 C20BAS:POPC compositions, respectively. When the same bolalipid composition variation experiment was performed with C32phytBAS in POPC, Ste14p activity was retained at levels similar or greater than that of pure POPC dispersions regardless of C32phytBAS content in the membrane. It should be noted that the slight difference in Ste14p specific activity observed in both 100% C32phytBAS dispersions (Figures 3A & B) is due to the different Ste14p preparations used in these two series of experiments. Nonetheless, all bolalipid:E. coli polar lipid data were collected with a single Ste14p preparation, whereas all bolalipid:POPC data were collected with another Ste14 preparation to eliminate this difference as a variable within a set of lipid mixtures.
To further probe the influences of lipid physical properties on Ste14p function, we monitored its methyltransferase activity in lipids above and below their gel to liquid crystalline phase transition after reconstitution at a single temperature (32°C) (Table 3). For E. coli polar lipid (control), Ste14p activity remained essentially constant when the assay temperature was maintained at 10, 16, 32, or 44 °C. The methyltransferase activity in DMPC was unchanged below (16 °C) and above (32 °C) its phase transition temperature (Tm = 23 °C), yielding activity levels that were the same as the E. coli polar lipid values within experimental error. Dramatically different behavior was found for C20BAS, however. In this case, the methyltransferase activity was only 5% of that observed for E. coli polar lipid above (32 °C) and below (10 °C) the phase transition temperature of C20BAS (Tm =17 °C).
Table 3.
Specific activity of Ste14p (pmol/min/mg) as a function of lipid type and their gel-to-liquid crystalline phase transition temperatures. Ste14p reconstitutions in all lipid types were all conducted at 32 °C; the methyltransferase reactions were subsequently incubated at the temperatures indicated in parentheses for each lipid measured. The precision of the specific activity measurements are ± 3%.
| Lipid | Tm (°C) | Specific Activity, Below Tm (Assay Temp, °C) |
Specific Activity, Above Tm (Assay Temp, °C) |
|---|---|---|---|
| E. coli polar lipid | 6–24† | 6240 (10) | -- |
| “ | “ | -- | 6820 (30) |
| “ | “ | -- | 7140 (32) |
| “ | “ | -- | 6560 (44) |
| C20BAS | 17 | 290 (10) | 310 (32) |
| DMPC | 23 | 6450 (16) | 6630 (32) |
M. Esfahani, et al., Proc. Natl. Acad. Sci. USA (1971) 68, 3180.
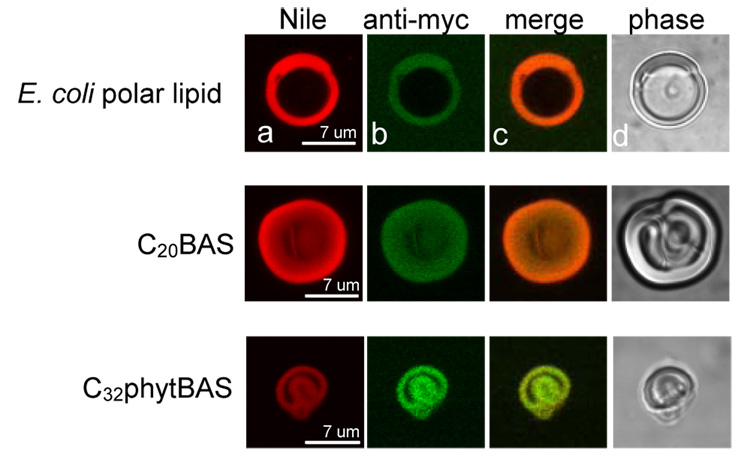
Confocal immunofluorescence assays were performed to determine whether Ste14p was actually incorporated within the membrane dispersions where low methyltransferase activity was observed (Figure 4). Fluorescence and phase contrast images were collected on reconstituted vesicle samples prepared as described above, except that mouse anti-myc primary antibodies and FITC-labeled goat anti-mouse secondary antibodies were added to fluorescently-label Ste14p and Nile Blue was added as a membrane-localizing fluorophore. As the data in Figure 4 demonstrate, Ste14p was present in all reconstituted vesicle compositions, including those showing low methyltransferase activity (i.e., C20BAS).
Figure 4.
Confocal immunofluorescence microscopy of giant vesicles of E. coli polar lipid (top), C20BAS (middle row), and C32phytBAS (bottom row). Nile Blue was added to stain the membranes (column A) and Ste14p was stained with mouse anti-myc/goat FITC-anti-mouse antibodies (column B). The images from A and B were merged (column C); the phase contrast images appear in column D. Ste14p was reconstituted into unextruded vesicles for these experiments and imaged at 60 × magnification.
DISCUSSION
The goal of this study was to determine the optimal host membrane material in which to functionally reconstitute the Icmt enzyme, Ste14p, for use in a stable supported membrane sensor. The design rules for producing stable integral membrane protein-containing membranes are not yet clear. For example, some integral membrane protein enzymes, such as Ca2+-ATPase, do not retain their functional conformation in gel phase lipid bilayers (38). However, not all membrane proteins display higher activities in the liquid-crystalline phase membranes, particularly when the liquid crystalline membrane phase is too thin to accommodate the entire hydrophobic surface of the protein. This is the case with diacylglycerol kinase and Na+, K+-ATPase (39,40), both of which undergo activity enhancements when the host membrane thickens upon transition from the liquid crystalline to gel phase. In some other cases, the proteins themselves affect the transition temperature of the surrounding lipids to relieve hydrophobic mismatch between the membrane and the integral membrane protein (41). The function of bacteriorhodopsin (bR)-containing membranes, however, is highly dependent on membrane fluidity. bR in polymerized synthetic bilayer membranes is inactive (42,43), while bR in bilayers composed of naturally-occurring lipids shows a strong dependence on the site and degree of acyl chain unsaturation (44). Taken together, these studies show that integral membrane protein activity can be strongly influenced in unpredictable ways by the phase behavior, physical dimensions and fluidity of the host membrane.
An existing bolalipid, C20BAS, and two newly synthesized bolalipids, C32BAS and C32phytBAS (prepared in 14% and 2% overall yield, respectively), were used to probe the structure-performance relationship between bolalipid structure and Ste14p activity. DSC and 31P NMR data show that C32phytBAS and C20BAS form liquid crystalline membranes at 25 °C, however, C32BAS is in the gel phase at this temperature and did not have an observable melting transition over the temperature range studied (15–85 °C). Methyl branching of the sn-2 chains in C32phytBAS disrupts the alkyl chain packing of this bolalipid, thereby increasing membrane fluidity and lowering the gel to liquid crystalline phase transition temperature and the transition enthalpy relative to C32BAS. The observed phase transition temperature of C32phytBAS, Tm = 14 °C, is in reasonable agreement with the value of 8 °C reported by Kinoshita and co-workers, who also prepared this bolalipid (45,46). Differences in sample preparation methods, the presence of contaminants, or different thermal history of the samples may account for the lower temperature reported in the previous work. The Tm of C20BAS has been previously reported to be 17 °C (31,37). The absence of methyl branching in C20BAS and the shorter membrane-spanning alkyl chain length (C20 vs. C32) are factors that compensate each other to produce a bolalipid with a modest phase transition temperature in this case. The Tm values measured for this bolalipid series are summarized in Table 1.
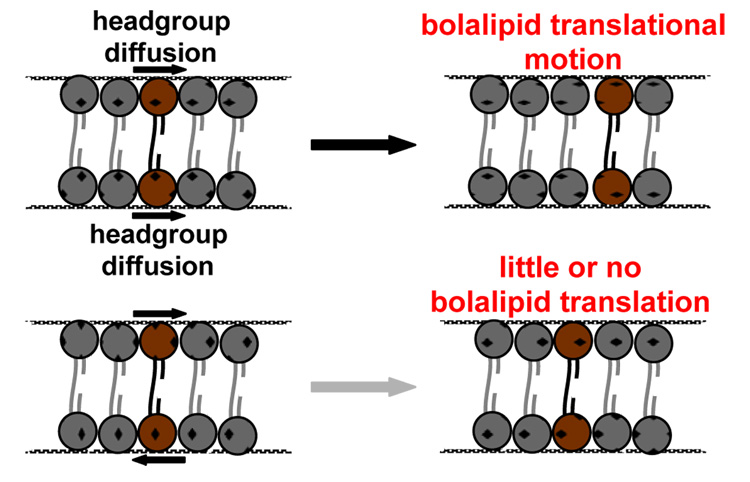
Lateral diffusion rates of the liquid-crystalline bolalipids, determined using the FRAP technique for supported membrane samples, are summarized in Table 2 along with the control lipid, POPC. The diffusion rate obtained for C20BAS was slower by a factor of five relative to POPC under the same conditions. When 30 mol% Chol was incorporated in the C20BAS membrane, the observed lateral diffusion rate decreased by an order of magnitude, giving a value similar to that obtained for the longer chain C32phytBAS derivative. FRAP measurements were not possible with C32BAS. This bolalipid produced glass-supported membranes where only punctate fluorescence was observed, indicative of fluorphore-rich domains that were phase separated from the gel phase bolalipid. We infer from these results that the reduced rates of diffusion in bolalipid membranes, relative to monopolar lipids that form bilayer membranes, is caused by coupled diffusion of headgroups at opposing lipid-water interfaces in the monolayer membranes formed by bolalipids (Figure 5). An alternative explanation is based on the consideration of lipid size. Since methyl branching increases the lipid molecular area at the lipid-water interface relative to its unbranched counterpart (45), the diffusion path length of these larger diameter molecules is effectively increased, thereby decreasing the net diffusion rate. Our observed diffusion rate of 1.8 × 10−9 cm2/s for C32phytBAS is in reasonable agreement with the reported value of 7 × 10−9 cm2/s for a biogenic bolalipid extract (9:1 bolalipid:monopolar lipid), determined at 30 °C by 2D exchange 31P NMR (47). A key finding from these experiments is that the slower bolalipid lateral diffusion rates did not significantly impact methyltransferase activity in the functional reconstitution of Ste14p within bolalipid vesicles. While there is no literature on this subject as far as we know, we anticipated that a change in lipid lateral diffusion rate would lead to changes in membrane microviscosity that would ultimately impact Ste14p activity. Our data suggest that Ste14p may not be sensitive to these types of membrane perturbations.
Figure 6.
Conceptual diagram of headgroup-coupled diffusion of bolalipids indicating the different outcomes of cooperative and uncooperative headgroup motions at the opposing membrane-water interfaces.
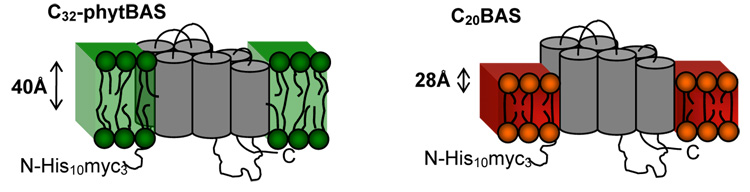
Confocal immunofluorescence microscopy further shows that the properties of the lipid do not alter the capacity for Ste14p incorporation within the host membrane (i.e., Ste14p is incorporated within both gel and liquid crystalline phase membranes as well as hydrophobically-mismatched membranes). Consistent with this finding, protein assay results confirmed the presence of Ste14p in the membrane, regardless of lipid type. Since the hydrated membrane thicknesses for C20BAS, C32BAS, and C32phytBAS are 32 Å (31), 56 Å and 50 Å, respectively, we conclude that the lower levels of methytransferase activity observed in C20BAS vesicles is caused by mismatch of the hydrophobic domain of Ste14p (anticipated to be on the order of 40 Å thick) and the ~28 Å thick hydrophobic region of the C20BAS membrane (Figure 6). This hydrophobic mismatch may lead to misfolded and/or denatured Ste14p segments in the thinner C20BAS membranes, thereby accounting for the lower methyltransferase activity observed.
Figure 7.
Illustration of the hydrophobic mismatch and match between C20BAS and C32phytBAS bolalipids and Ste14p, respectively.
CONCLUSIONS
Hrycyna and co-workers (20) have previously shown that Ste14p can be reconstituted in vesicles with a variety of different monopolar lipid types while retaining greater than 60% of the activity present in E. coli polar lipid extract vesicles. We now report that a large amount of Ste14p activity was retained (65% or higher) in membranes composed of ≤50 mol% C20BAS, however, methyltransferase activity decreases rapidly at higher molar ratios of C20BAS. Ste14p activity levels were maintained across the full 0–100 mol% range of C32phytBAS:E. coli polar lipid extract compositions. Surprisingly, gel phase lipids do not appear to negatively affect methyltransferase activity, based on our findings that gel phase lipids (DMPC vesicles at 16 °C) and liquid crystalline phase lipids (C32phytBAS and DMPC vesicles at 25 °C and 32 °C, respectively) yield similar activities at membrane compositions of ≤50 mol% bolalipid. The thickness of the hydrophobic region of the membrane, however, does appear to have a significant impact on Ste14p activity, with the thinner C20BAS membrane producing a less active reconstituted Ste14p vesicle dispersion. Additional experiments to probe this question and extension of these studies to other classes of integral membrane proteins are in progress to determine the generality of these findings.
Supplementary Material
The 1H, 13C, and 31P NMR spectra of the compounds produced during the C32phytBAS synthesis are available, free of charge, via the internet at http://pubs.acs.org.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the assistance of Dr. Jong-Mok Kim (bolalipid preparation), Kalani Seu and Prof. Jennifer Hovis (FRAP measurements), Prof. Richard Gibbs (AFC substrate), and Jennifer Sturgis (confocal microscopy).
ABBREVIATIONS
- AFC
N-acetyl-S-farnesyl-L-cysteine
- 14C-SAM
S-adenosyl-L-[14C-methyl] methionine
- C20BAS
2,2’-di-O-decyl-3,3’-di-O-(1”,20”-eicosanyl)-bis-(rac-glycero)-1,1’-diphosphocholine
- C32BAS
2,2’-di-O-hexadecyl-3,3’-di-O-(1”,32”-dotriacontanyl)-bis-(rac-glycero)-1,1’-diphosphocholine
- C32phytBAS
2,2’-di-O-(3,7,11,15-tetramethylhexadecyl)-3,3’-di-O-(1”,32”-dotriacontanyl)-bis-(rac-glycero)-1,1’-diphosphocholine
- Chol
cholesterol
- DDM
1-dodecyl-β-D-maltopyranoside
- DMF
N,N-dimethylformamide
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DSC
differential scanning calorimetry
- E. coli polar lipid
commercial polar lipid extract from E. coli
- FRAP
fluorescence recovery after photobleaching
- Ste14p
His10myc3-tagged Ste14p Icmt isolated from S. cerevisiae
- Icmt
isoprenylcysteine carboxyl methyltransferase
- NBD-OPPC
1-oleoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-sn-glycero-3-phosphocholine
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- SDS
sodium dodecylsulfate
- THF
tetrahydrofuran
- Tris
tris(hydroxymethyl)aminomethane
Footnotes
This work was supported by NIH CA112427, the NIH “Diversity in Biomedical Science Program” at Purdue University, and the Indiana 21st Century Fund.
REFERENCES
- 1.Langworthy TA. In: The Bacteria Vol. VIII. Woese CR, Wolfe RS, editors. New York: Academic Press; 1985. pp. 459–497. [Google Scholar]
- 2.Damsté JSS, Schouten S, Hopmans EC, van Duin ACT, Geenevasen JAJ. Crenarchaeol: the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeota. J. Lipid Res. 2002;43:1641–1651. doi: 10.1194/jlr.m200148-jlr200. [DOI] [PubMed] [Google Scholar]
- 3.De Rosa M, Gambacorta A. The lipids of archaebacteria. Prog. Lipid Res. 1988;27:153–175. doi: 10.1016/0163-7827(88)90011-2. [DOI] [PubMed] [Google Scholar]
- 4.Kates M. Adventures with membrane lipids. Biochem. Soc. Trans. 1995;23:697–709. doi: 10.1042/bst0230697. [DOI] [PubMed] [Google Scholar]
- 5.Patel GB, Sprott GD. Archaeobacterial ether lipid liposomes (archaeosomes) as novel vaccine and drug delivery systems. Crit. Rev. Biotech. 1999;19:317–357. doi: 10.1080/0738-859991229170. [DOI] [PubMed] [Google Scholar]
- 6.Gräther O, Arigoni D. Detection of regioisomeric macrocyclic tetraethers in the lipids of methanobacterium-thermoautotrophicum and other archaeal organisms. Chem. Comm. 1995;4:405–406. [Google Scholar]
- 7.Hopmans EC, Schouten S, Pancost RD, van der Meer MTJ, Damsté JSS. Analysis of intact tetraether lipids in archaeal cell material and sediments by high performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Comm. Mass Spec. 2000;14:585–589. doi: 10.1002/(SICI)1097-0231(20000415)14:7<585::AID-RCM913>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Thompson DH, Wong K, Humphry-Baker R, Wheeler J, Kim J-M, Rananavare SB. Tetrather bolaform amphiphiles as models of archaebacterial membrane-lipids -Raman-spectroscopy, P-31 NMR, X-Ray scattering, and electron-microscopy. J. Am. Chem. Soc. 1992;114:9035–9042. [Google Scholar]
- 9.Elferink MGL, de Wit JG, Driessen AJM, Konings WN. Stability and proton-permeability of liposomes composed of archaeal tetraether lipids. Biochim. Biophys. Acta. 1994;1193:247–254. doi: 10.1016/0005-2736(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 10.Arakawa K, Kano H, Eguchi T, Nishiyama Y, Kakinuma K. Significance of the 72-membered macrocyclic structure found in archaeal membrane lipids: Model studies of the macrocyclic tetraether diphospholipids by calorimetric, P-31 NMR, and electron microscopic analyses. Bull. Chem. Soc. Jpn. 1999;72:1575–1581. [Google Scholar]
- 11.Arakawa K, Eguchi T, Kakinuma K. Highly thermostable liposome from 72-membered macrocyclic tetraether lipid: Importance of 72-membered lipid for archaea to thrive under hyperthermal environments. Chem. Lett. 2001;5:440–441. [Google Scholar]
- 12.Kim J-M, Patwardhan A, Bott A, Thompson DH. Preparation and electrochemical behavior of gramicidin-bipolar lipid monolayer membranes supported on gold electrodes. Biochim. Biophys. Acta. 2003;1617:10–21. doi: 10.1016/j.bbamem.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Thompson DH, Patwardhan AP, Di Meglio C, Kim J-M, Haynes R, Burden DL, Rananavare SB. unpublished results. [Google Scholar]
- 14.Cornell BA, Braach-Maksvytis VLB, King LG, Osman PDJ, Raguse B, Wieczorek L, Pace RJ. A biosensor that uses ion-channel switches. Nature. 1997;387:580–583. doi: 10.1038/42432. [DOI] [PubMed] [Google Scholar]
- 15.Wiess-Wichert C, Smetazko M, Valina-Saba M, Schalkhammer T. A new analytical device based on gated ion channels: A peptide-channel biosensor. J. Biomol. Screening. 1997;2:11–18. [Google Scholar]
- 16.Elferink MGL, de Wit JG, Demel R, Driessen AJM, Konings WN. Functional reconstitution of membrane-proteins in monolayer liposomes from bipolar lipids of Sulfolobus acidocaldarius. J. Biol. Chem. 1992;267:1375–1381. [PubMed] [Google Scholar]
- 17.In’t Veld G, Elferink MGL, Driessen AJM, Konings WN. Reconstitution of the leucine transport-system of Lactococcus lactis into liposomes composed of membrane-spanning lipids from Sulfolobus acidocaldarius. Biochemistry. 1992;31:12493–12499. doi: 10.1021/bi00164a028. [DOI] [PubMed] [Google Scholar]
- 18.Romano JD, Michaelis S. Topological and mutational analysis of Saccharomyces cerevisiae Ste14p, founding member of the isoprenylcysteine carboxyl methyltransferase family. Mol. Biol. Cell. 2001;12:1957–1971. doi: 10.1091/mbc.12.7.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai Q, Choy E, Chiu V, Romano J, Slivka SR, Steitz SA, Michaelis S, Philips MR. Mammalian prenylcysteine carboxyl methyltransferase is in the endoplasmic reticulum. J. Biol. Chem. 1998;273:15030–15034. doi: 10.1074/jbc.273.24.15030. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JL, Frase H, Michaelis S, Hrycyna CA. Purification, functional reconstitution, and characterization of the Saccharomyces cerevisiae isoprenylcysteine carboxylmethyltransferase Ste14p. J. Biol. Chem. 2005;280:7336–7345. doi: 10.1074/jbc.M410292200. [DOI] [PubMed] [Google Scholar]
- 21.Pillinger MH, Volker C, Stock JB, Weissmann G, Philips MR. Characterization of a plasma membrane-associated prenylcysteine-directed alpha carboxyl methyltransferase in human neutrophils. J Biol Chem. 1994;269:1486–1492. [PubMed] [Google Scholar]
- 22.Stephenson RC, Clarke S. Characterization of a rat liver protein carboxyl methyltransferase involved in the maturation of proteins with the -CXXX C-terminal sequence motif. J. Biol. Chem. 1992;267:13314–13319. [PubMed] [Google Scholar]
- 23.Boivin D, Gingras D, Beliveau R. Purification and characterization of a membrane-bound protein carboxyl methyltransferase from rat kidney cortex. J Biol Chem. 1993;268:2610–2615. [PubMed] [Google Scholar]
- 24.Yoo BC, Kang MS, Kim S, Lee YS, Choi SY, Ryu CK, Park GH, Han JS. Partial purification of protein farnesyl cysteine carboxyl methyltransferase from bovine brain. Exp Mol Med. 1998;30:227–234. doi: 10.1038/emm.1998.33. [DOI] [PubMed] [Google Scholar]
- 25.Patwardhan AP, Thompson DH. Efficient synthesis of 40-and 48-membered tetraether macrocyclic bisphosphocholines. Org. Lett. 1999;1:241–244. doi: 10.1021/ol990567o. [DOI] [PubMed] [Google Scholar]
- 26.Patwardhan AP, Thompson DH. Novel flexible and rigid tetraether acyclic and macrocyclic bisphosphocholines: synthesis and monolayer properties. Langmuir. 2000;16:10340–10350. [Google Scholar]
- 27.Peanasky J, Schneider HM, Granick S, Kessel CR. Self-assembled monolayers on mica for experiments utilizing the surface forces apparatus. Langmuir. 1995;11:953–962. [Google Scholar]
- 28.Groves JT, Boxer SG. Electric field-induced concentration gradients in planar supported bilayers. Biophys. J. 1995;69:1972–1975. doi: 10.1016/S0006-3495(95)80067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cremer PS, Boxer SG. Formation and spreading of lipid bilayers on planar glass supports. J. Phys. Chem. B. 1999;103:2554–2559. [Google Scholar]
- 30.Soumpasis DM. Theoretical-analysis of fluorescence photobleaching recovery experiments. Biophys. J. 1983;41:95–97. doi: 10.1016/S0006-3495(83)84410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Meglio C, Rananavare S, Svenson S, Thompson DH. Bolaamphiphilic phosphocholines: Structure and phase behavior in aqueous media. Langmuir. 2000;16:128–133. [Google Scholar]
- 32.Schaffner W, Weissmann C. Rapid, sensitive, and specific method for determination of protein in dilute-solution. Anal. Biochem. 1973;56:502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber SL, Wang Z, Schulte G. Group selective reduction of acetals related to the ansa chain of the streptovaricins –conformational and stereochemical analysis. Tet. Lett. 1988;29:4085–4088. [Google Scholar]
- 34.Schwab P, France MB, Ziller JW, Grubbs RH. A series of well-defined metathesis catalysts –synthesis of [RuCl2(=CHR’)(PR3)2] and its reactions. Angew. Chem. Int. Ed. Engl. 1995;34:2039–2041. [Google Scholar]
- 35.Thompson DH, Svendsen CB, Di Meglio C, Anderson BC. Synthesis of chiral diether and tetraether phospholipids –regiospecific ring-opening of epoxy alcohol intermediates derived from asymmetric epoxidation. J. Org. Chem. 1994;59:2945–2955. [Google Scholar]
- 36.Svenson S, Thompson DH. Facile and efficient synthesis of bolaamphiphilic tetraether phosphocholines. J. Org. Chem. 1998;63:7180–7182. doi: 10.1021/jo9803176. [DOI] [PubMed] [Google Scholar]
- 37.Febo-Ayala W, Holland DP, Bradley SA, Thompson DH. doi: 10.1021/la063720d. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee AG. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta. 2003;1612:1–40. doi: 10.1016/s0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 39.Pilot JD, East JM, Lee AG. Effects of phospholipid headgroup and phase on the activity of diacylglycerol kinase of Escherichia coli. Biochemistry. 2001;40:14891–14897. doi: 10.1021/bi011333r. [DOI] [PubMed] [Google Scholar]
- 40.Cornelius F. Modulation of Na,K-ATPase and Na-ATPase activity by phospholipids and cholesterol. I. Steady-state kinetics. Biochemistry. 2001;40:8842–8851. doi: 10.1021/bi010541g. [DOI] [PubMed] [Google Scholar]
- 41.Killian JA. Hydrophobic mismatch between proteins and lipids in membranes. Biochim. Biophys. Acta. 1998;1376:401–416. doi: 10.1016/s0304-4157(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 42.Tyminski PN, Latimer LH, O’Brien DF. Rhodopsin in polymerized bilayer-membranes. J. Amer. Chem. Soc. 1985;107:7769–7760. [Google Scholar]
- 43.Tyminski PN, Latimer LH, O’Brien DF. Reconstitution of rhodopsin and the cGMP cascade in polymerized bilayer-membranes. Biochemistry. 1988;27:2696–2705. doi: 10.1021/bi00408a009. [DOI] [PubMed] [Google Scholar]
- 44.Gibson NJ, Brown MF. Lipid headgroup and acyl chain composition modulate the MI-MII equilibrium of rhodopsin in recombinant membranes. Biochemistry. 1993;32:2438–2454. doi: 10.1021/bi00060a040. [DOI] [PubMed] [Google Scholar]
- 45.Yamauchi K, Yamada K, Kinoshita M, Kamikawa T. Archaebacterial lipids –surface pressure-surface area isotherms of 1,1’-(1,32-dotriacontanediyl)bis[2-[3RS, 7R, 11R)-3,7,11,15-Tetramethyl-hexadecyl]sn-glycero-3-phosphocholine. Bull. Chem. Soc. Jpn. 1991;64:2088–2090. [Google Scholar]
- 46.Yamauchi K, Sakamoto Y, Moriya A, Yamada K, Hosokawa T, Higuchi T, Kinoshita M. Archaebacterial lipid models –highly thermostable membranes from 1,1’-(1,32-dotriacontamethylene)bis(2-phytanyl-sn-glycero-3-phosphocholine) J. Am. Chem. Soc. 1990;112:3188–3191. [Google Scholar]
- 47.Jarrell HC, Zukotynski DA, Sprott GD. Lateral diffusion of the total polar lipids from Thermoplasma acidophilum in multilamellar liposomes. Biochim. Biophys. Acta. 1998;1369:259–266. doi: 10.1016/s0005-2736(97)00228-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The 1H, 13C, and 31P NMR spectra of the compounds produced during the C32phytBAS synthesis are available, free of charge, via the internet at http://pubs.acs.org.