Abstract
Anemia is a significant co-morbidity in patients with heart failure (HF) irrespective of EF (EF) and is routinely quantified by hemoglobin concentration. Hemodilution as a cause of anemia has been described in systolic HF. This study aims to further investigate the effects of plasma volume in HF patients by 1) assessing prevalence of dilutional anemia in patients with anemia and a preserved EF and 2) exploring the relation between hemoglobin and red cell volume in these patients. Forty-six anemic patients (as determined by standard hemoglobin measurement): 22 with HF and low EFs (HFLEF) and 24 with HF and a preserved EFs (HFPEF) all underwent plasma volume measurement with I-131 labeled albumin. Hemoglobin values did not differ between subjects with HFLEF and HFPEF (10.8±1.0 vs. 11.0±1.0 gm/dl, p=0.55) but a red cell deficit was found in 88% of patients with HFPEF as compared with 59% of HFLEF (p=0.04). This was the result of a higher prevalence of an expansion of plasma volume in HFLEF patients (100%) as compared with HFPEF patients (71%). Among all patients, no correlation was found between hemoglobin and red cell volume (r = 0.09, p=0.54), but a correlation did exist among patients with normal blood volumes (r=0.55, p = 0.02). In conclusion, dilutional anemia resulting from an expansion in plasma volume without a red cell deficit occurs more commonly in HFLEF than HFPEF patients and hemoglobin does not correlated with red cell volume in anemic HF patients.
Keywords: Anemia, Heart Failure, Plasma Volume
Introduction
The etiology of anemia in subjects with heart failure (HF) is multi-factorial1–3 and several therapies have been employed with varying success.2 While hemoglobin values are typically employed to diagnose anemia, low hemoglobin, on a physiologic basis, can be the result of either: (1) a true red cell deficit, or (2) hemodilution with plasma volume expansion.4 While hemoglobin or hematocrit provides a good estimate of red cell volume in healthy patients,5 discordance between hemoglobin and red cell volume has been described in patients with polycythemia vera6 , liver disease,7, 8 and in the presence of splenomegaly,9 due to the confounding effects of alterations in plasma volume.5, 9–11 Among patients with advanced HF and low ejection fraction (EF), almost half were reported to have anemia on the basis of hemodilution with a normal red cell volume.4 Whether a similar percentage of HF and a preserved EF (HFPEF) patients have such dilutional anemia is unclear. Given the mixed success in treatment of anemia,2, 12 issues surrounding measurement of anemia deserve clarification. Accordingly, the purpose of this study was 2-fold: (1) to explore the correlation between hemoglobin values and red cell volume in anemic HF patients and (2) to determine whether anemic patients with HFPEF have a similar prevalence of dilutional anemia as patients with HF with a low EF (HFLEF).
Methods
Subjects were outpatients referred for evaluation and treatment to the Columbia University Medical Center Heart Failure Center. Subjects greater than 21 years of age with HF for at least 3 months’ duration with stable symptoms were enrolled. All subjects were anemic (World Health Organization criteria: hemoglobin <13.0 g/dL in men and <12.0 g/dL in women).2 Criteria for exclusion were acute decompensated HF, severe renal dysfunction (serum creatinine >3.5 mg/dl or history of nephrotic syndrome), severe hepatic dysfunction (serum liver enzymes >3 times the upper limits of normal or history of cirrhosis). Cardiac medications included diuretics, digoxin, renin-angiotensin system inhibitors, and/or β-adrenergic receptor antagonists that were stable prior to measurement of blood volume. Forty-six non-edematous ambulatory patients with HF were studied; 24 patients with HFPEF and 22 patients with HFLEF. Subjects with HF were dichotomized into those with HFLEF and HFPEF by an EF of < or ≥ 45%, thus patients with HFLEF had systolic heart failure. Blood volume measurements4, 13, 14 and the degree of hemodilution in subjects with HFLEF have been reported previously;4 however, none of the previous analyses included a population of HFPEF and none focused on the relation between blood volume components and hemoglobin as will be presented herein. The Institutional Review Board at Columbia University Medical Center approved the protocol. All subjects gave written informed consent before participation.
Plasma volume was determined after intravenous administration of iodine-131–labeled albumin, as has been described previously.4, 13, 15 Blood volume and red blood cell volumes were calculated from the plasma volume measurement, the measured hematocrit corrected for trapped plasma, and mean body hematocrit, and then compared with normal values for age, sex, height, and weight based on the ideal weight system.16 In addition to reporting absolute values, we report percent deviation from expected based on the ideal weight system. Normovolemia was prospectively defined as a measured blood volume within 8% of the predicted normal value; while hypervolemia and hypovolemia were defined as blood volume greater than 8% and less than 8% of predicted respectively.4 True anemia was defined as having <95% predicted red blood cell volume based on the individual study participant’s sex and body size.4 Hemoglobin was measured as part of a routine complete blood count from the hospital core lab (Sysmex XE 2100).
Data are expressed as mean ± standard deviation, unless otherwise noted. Dichotomous variables were compared by chi-square analysis with fisher’s exact test where appropriate while continuous variables were compared by unpaired 2-tailed T tests. Simple linear regression analysis was used to determine correlation between hemoglobin and red blood cell volumes. A p value of 0.05 was considered significant. SAS 9.1 (Cary, NC, USA) was used for all analyses.
Results
The demographic characteristics of the patient populations are shown in Table 1. Subjects with HFLEF were predominately male, while HFPEF subjects were older and predominately female. The groups did not differ with regards to body size, functional state (New York Heart Association class), renal function and hemoglobin, but the HFLEF patients were taking a higher daily dose of loop diuretics.
Table 1.
Demographic and clinical characteristics in anemic patients with HF and low EF (HFLEF) and HF with a preserved EF (HFPEF).
| Variable | HFLEF (n=22) | HFPEF (n=24) | P values |
|---|---|---|---|
| Age (years) | 63±11* | 73±14 | 0.009 |
| Women | 5(23%)* | 18(75%) | <0.001 |
| Ejection Fraction (%) | 26±10* | 60±7 | <0.001 |
| Weight (kg) | 83±23 | 85±21 | 0.744 |
| Body Surface Area (m2) | 1.9±0.2 | 1.9±0.2 | 0.591 |
| Creatinine (mg/dl) | 1.5±0.6 | 1.6±0.8 | 0.758 |
| Gender Glomerular Filtration Rate (mL/min) | 67±41 | 55±29 | 0.340 |
| Blood Urea Nitrogen (mg/dl) | 36±22 | 35±21 | 0.839 |
| Hemoglobin (g/dl) | 10.8±1.0 | 11.0±1.0 | 0.55 |
| Hematocrit (%) | 33±3 | 33±3 | 0.576 |
| Iron (ug/dL) | 67±55 | 53±20 | 0.274 |
| Ferritin (ng/mL) | 195±200 | 124±174 | 0.279 |
| New York Heart Association Class | 2.7±0.7 | 2.5±0.5 | 0.475 |
| Diuretic Dose (mg of furosemide or equivalent) | 177±127* | 68±91 | 0.022 |
p<0.05. Values expressed as ± one standard deviation.
Despite similar mean hemoglobin values, blood volume measurements differed between HFLEF patients and those with HFPEF (Table 2). With respect to blood volume, of the HFPEF patient, 38% were hypovolemic, 42% were normovolemic 20% were hypervolemic. In the HFLEF patients, none were hypovolemic, 36% were normovolemic, and 64% were hypervolemic.
Table 2.
Hematologic Characteristics in anemic patients with systolic heart failure (HFLEF) and heart failure with a preserved EF (HFPEF).
| Variable | HFLEF | Reference Values | HFPEF | Reference Values |
|---|---|---|---|---|
| Blood Volume (mL) | 5809±925*┼ | 4940±568 | 4487±1170 | 4620±791 |
| Blood Volume Volume/Kg (mL) | 73.9±17 | - | 54.1±15 | - |
| Blood Volume Deviation (mL) | 865±824* | - | −132±1091 | - |
| Blood Volume Deviation (%) | 18.0%* | - | −2.2% | - |
| Red Cell Volume (mL) | 1760±338*┼ | 1958±266 | 1317±340┼ | 1712±326 |
| Red Cell Volume/Kg (mL) | 22.1±5.0* | - | 15.9±4.4 | - |
| Red Cell Volume Deviation (mL) | −199±335* | - | −395±323 | - |
| Red Cell Volume Deviation (%) | −9.3%* | - | −22.4% | - |
| Plasma Volume (mL) | 4049±650*┼ | 2986±327 | 3170±867 | 2908±487 |
| Plasma Volume/Kg (mL) | 51.8±13* | - | 38.2±11 | - |
| Plasma Volume Deviation (mL) | 1063±599* | - | 263±842 | - |
| Plasma Volume Deviation (%) | 36.2%* | - | 10.1% | - |
p<0.05 HFLEF versus HFPEF.
< 0.05 for HFLEF or HFPEF versus reference values.
Deviation (%) refers to percent difference from reference values based on sex and body size. Values expressed as ± one standard deviation.
Plasma volume excess was found more frequently in the HFLEF group (100%) then in the HFPEF group (71%) and the average plasma volume excess was significantly larger in the HFLEF group (p<0.001). Red blood cell deficit was more pronounced in HFPEF patients and plasma volume expansion was less pronounced in comparison to the HFLEF cohort. The incidence of dilutional anemia was 41% in the HFLEF group and 12% in the HFPEF group (p=0.04).
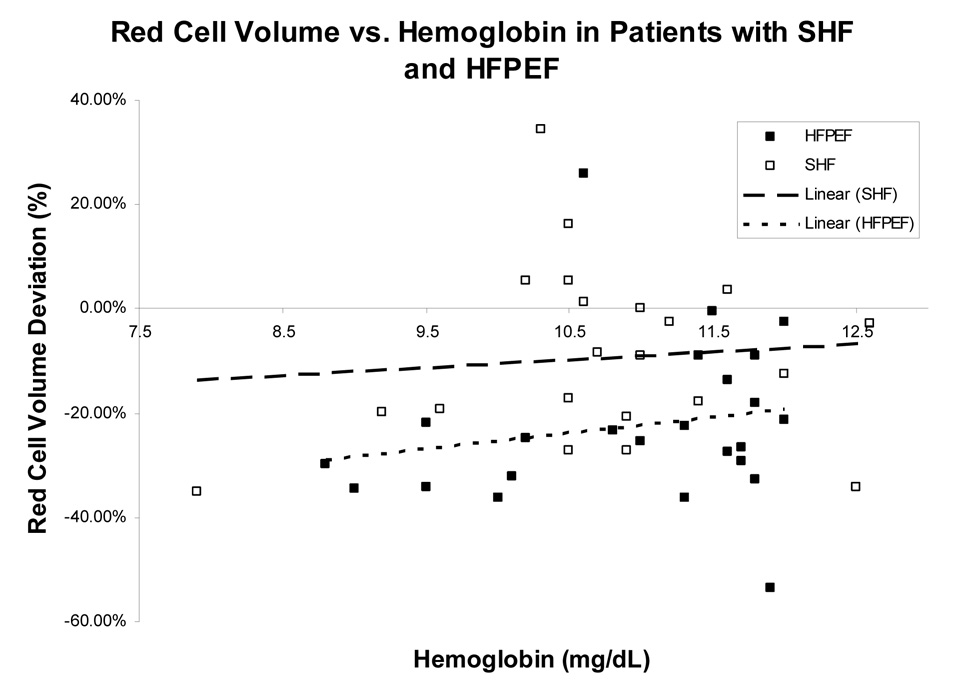
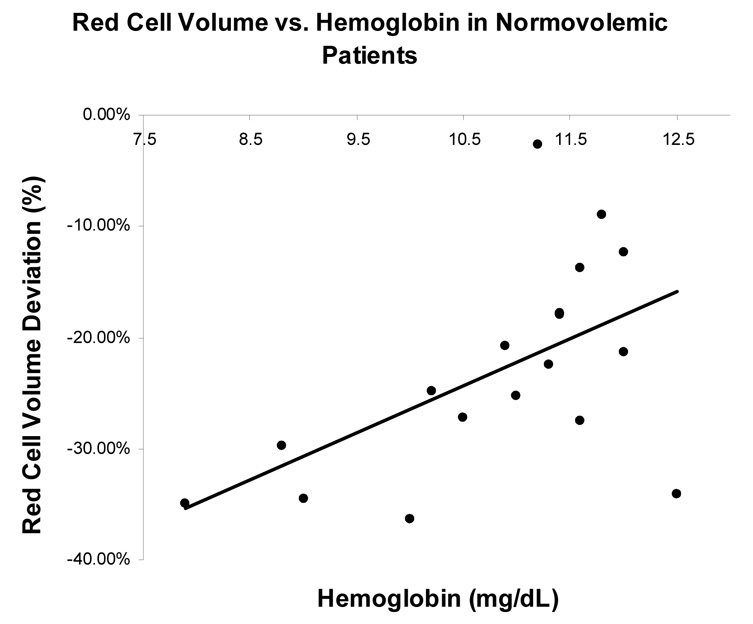
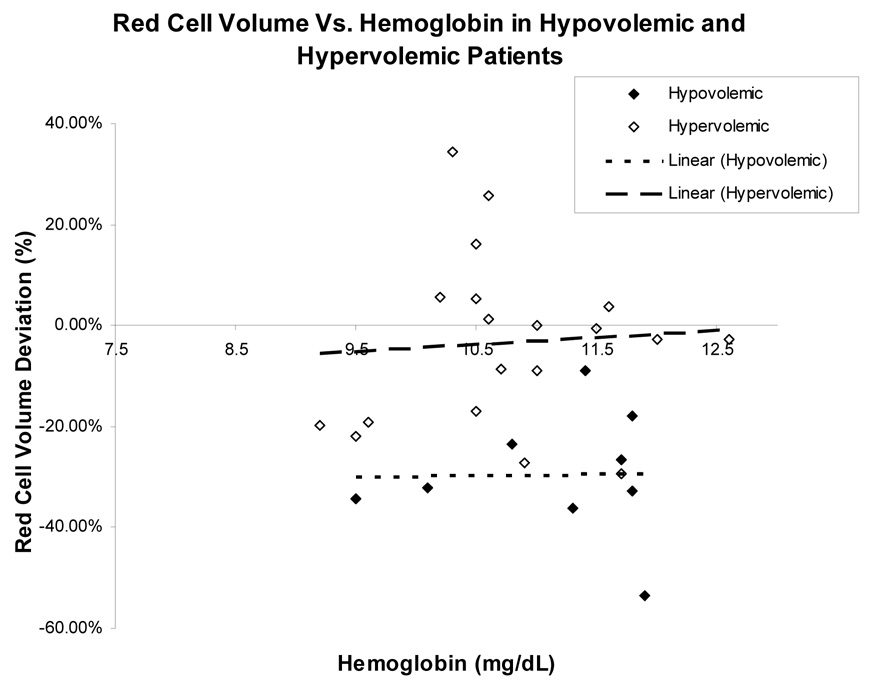
Among all HF patients studied, there was no significant association found between hemoglobin and percent red cell volume deviation from reference values (r = 0.09, p=0.54). This was found for both patients with HFLEF and HFPEF (r=0.09, p=0.69 and r = 0.19, p =0.38) (Figure 1, panel A). However, when subjects were classified by volume status, normovolemic patients had a stronger association between hemoglobin and red cell volume (r=0.55, p = 0.02) than either hypovolemic or hypervolemic patients, who had a non-significant association (r = 0.016, p =0.97 and r=0.067, p=0.79 respectively), (Figure 1, Panel B and C).
Figure 1.
Figure 1(A): The relationship between red blood cell volume deviation and hemoglobin in patients with HF and a low EF (HFLEF) and HF with preserved EF (HFPEF).
Figure 1 (B) and 1 (C): The relationship between red blood cell volume deviation and hemoglobin graphed by volume status. Normovolemia is blood volume within 8% of predicted. Hypervolemia is blood volume greater than 8% of predicted. Hypovolemia is blood volume less than 8% of predicted. Blood volume deviation refers to percent difference between predicted and determined by blood volume analysis.
Discussion
The principal findings of this study are 2-fold. First, among anemic HF subjects, the prevalence of a red cell deficit differs among patients with a normal as compared to a reduced EF. Specifically, most of subjects with HFPEF (88% in this series) had a red cell deficit while slightly more than half of subjects with HFLEF had a red cell deficit. Second, the correlation of hemoglobin, the standard measure by which clinicians diagnose and manage anemia in patients with HF, with red cell volume is poor irrespective of the EF. This is because of the confounding effects of alterations in plasma volume, which are common in patients with HF, resulting from either the underlying disease or the concomitant use of diuretic therapy. Blood volumes in HF patients have been evaluated in multiple studies in patient with HFLEF, with alterations of both blood volume contraction and expansion described.13, 14 Blood volumes have not been described in patients with HFPEF, and our findings suggest that compensated HFPEF patients can likewise be hypo-, normo-, or hypervolemic.
The discordance between hemoglobin and red cell volume found in this study is in accordance with literature from other patient populations where the poor association has been attributed to alterations in plasma volume.5, 9–11 Much of the previous literature in this area has been focused on polycythemia vera, where plasma volume derangements and splenomegaly confound the utility of employing hemoglobin (or hematocrit) as a surrogate for red cell volume.9 Both splenomagely17, 18 and plasma volume expansion13 have been described in HF patients. Given the relevance of plasma volume to the hemoglobin-red cell volume relationship, some have noted that hematocrit (or hemoglobin) is no more a marker of red cell volume than serum sodium is of total body sodium.9 Our data support this explanation; the association between hemoglobin and red cell volume was stronger in normovolemic patients than in those with hypovolemia or hypervolemia. Plasma volume alterations, whether leading to hemodilution or hemoconcentration therefore appear to influence hemoglobin determination.
These observations may have implications for treatment of anemia in HF. Two main therapies have been employed to treat anemia in HF, intravenous iron19, 20 and erythropoetic stimulators21 either alone or in combination,22, 23 and data on treatment has been mixed.2, 12 Both therapies have been shown in small single center studies to increase hemoglobin, quality of life, and exercise tolerance in anemic HF patients with systolic dysfunction.21, 23–26 However, a larger multi-center study, STAMINA-HeFT trial did not meet its primary end-point of exercise time and quality of life changes.27
Potential explanations for the discrepant results include that treatment for anemia in these trials may have been applied to subjects with a dilutional anemia as well as to those with a red cell deficit. In the only study in which blood volume was measured during erythropoietin treatment,21 the limited data suggest that that erythropoietin may have direct effects on both components of total blood volume (red cell volume and plasma volume). Erythropoietin increased hemoglobin in heart failure patients with both true and dilutional anemia through different mechanisms; while both groups had an increase in red cell volume, the patients with dilutional anemia also experienced a reduction in plasma volume.21 Although the effects on plasma volume are poorly understood, they have been observed in dialysis patients as well.28 While erythropoietin may therefore benefit HF patients both with a normal and reduced red cell volume, perhaps diuresis may be the safer and cheaper approach to dilutional anemia patients without predisposing patients to the side effects attributed to erythropoietin.
There are several limitations to our study. Our study population consisted of patients with advanced HF receiving care at a tertiary center, which may not represent the general HF population. All patients were stable outpatient on high dose chronic diuretic therapy; hence the alterations of plasma volume observed need to be interpreted in this context and the relevance of these measures to the pathophysiology of the acutely decompensated state is limited. Splenomegaly was not assessed in our patients. The data measurements, including hemoglobin and blood volume measures, were taken at a single point in time. Blood volume measurements were done with I-131-labeled albumin and we did not directly measure red cell volume. However, the validity of our approach for measuring red cell volume indirectly versus the chromium labeling technique has been demonstrated, with only small differences found between chromium labeling and the albumin dilution technique.29
Acknowledgements
Dr. Maurer is supported by a grant from the NIH/NIA (R01AG027518-01A1)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Iversen PO, Woldbaek PR, Tonnessen T, Christensen G. Decreased hematopoiesis in bone marrow of mice with congestive heart failure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R166–R172. doi: 10.1152/ajpregu.2002.282.1.R166. [DOI] [PubMed] [Google Scholar]
- 2.Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. 2006;113:2454–2461. doi: 10.1161/CIRCULATIONAHA.105.583666. [DOI] [PubMed] [Google Scholar]
- 3.Felker GM, Adams KF, Jr, Gattis WA, O'Connor CM. Anemia as a risk factor and therapeutic target in heart failure. J Am Coll Cardiol. 2004;44:959–966. doi: 10.1016/j.jacc.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 4.Androne AS, Katz SD, Lund L, Lamanca J, Hudaihed A, Hryniewicz K, Mancini DM. Hemodilution is common in patients with advanced heart failure. Circulation. 2003;107:226–229. doi: 10.1161/01.cir.0000052623.16194.80. [DOI] [PubMed] [Google Scholar]
- 5.Erslev AJ. Clinical erythrokinetics: a critical review. Blood Rev. 1997;11:160–167. doi: 10.1016/s0268-960x(97)90011-4. [DOI] [PubMed] [Google Scholar]
- 6.Johansson PL, Safai-Kutti S, Kutti J. An elevated venous haemoglobin concentration cannot be used as a surrogate marker for absolute erythrocytosis: a study of patients with polycythaemia vera and apparent polycythaemia. Br J Haematol. 2005;129:701–705. doi: 10.1111/j.1365-2141.2005.05517.x. [DOI] [PubMed] [Google Scholar]
- 7.Pati HP, Dayal S, Kashyap R, Padhy AK. Masked polycythaemia vera in a patient with extrahepatic portal venous obstruction. Eur J Gastroenterol Hepatol. 1998;10:883–885. doi: 10.1097/00042737-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Cirera I, Elizalde JI, Pique JM, Feu F, Casadevall M, Goldin E, Teres J, Bosch J, Rodes J. Anemia worsens hyperdynamic circulation of patients with cirrhosis and portal hypertension. Dig Dis Sci. 1997;42:1697–1702. doi: 10.1023/a:1018861415259. [DOI] [PubMed] [Google Scholar]
- 9.Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100:4272–4290. doi: 10.1182/blood-2001-12-0349. [DOI] [PubMed] [Google Scholar]
- 10.Lorberboym M, Rahimi-Levene N, Lipszyc H, Kim CK. Analysis of Red Cell Mass and Plasma Volume in Patients With Polycythemia. Archives of Pathology & Laboratory Medicine. 2005;129:89–91. doi: 10.5858/2005-129-89-AORCMA. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A. Diagnosing polycythemia vera: a paradigm shift. Mayo Clin Proc. 1999;74:159–162. doi: 10.4065/74.2.159. [DOI] [PubMed] [Google Scholar]
- 12.Murphy NF, McDonald K. Treatment of anaemia in chronic heart failure optimal approach still unclear. Eur Heart J. 2007;28:2185–2187. doi: 10.1093/eurheartj/ehm331. [DOI] [PubMed] [Google Scholar]
- 13.Androne AS, Hryniewicz K, Hudaihed A, Mancini D, Lamanca J, Katz SD. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. Am J Cardiol. 2004;93:1254–1259. doi: 10.1016/j.amjcard.2004.01.070. [DOI] [PubMed] [Google Scholar]
- 14.Feigenbaum MS, Welsch MA, Mitchell M, Vincent K, Braith RW, Pepine CJ. Contracted plasma and blood volume in chronic heart failure. J Am Coll Cardiol. 2000;35:51–55. doi: 10.1016/s0735-1097(99)00530-6. [DOI] [PubMed] [Google Scholar]
- 15.Manzone TA, Dam HQ, Soltis D, Sagar VV. Blood volume analysis: a new technique and new clinical interest reinvigorate a classic study. J Nucl Med Technol. 2007;35:55–63. doi: 10.2967/jnmt.106.035972. [DOI] [PubMed] [Google Scholar]
- 16.Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation. 1977;56:605–612. doi: 10.1161/01.cir.56.4.605. [DOI] [PubMed] [Google Scholar]
- 17.O'Reilly RA. Splenomegaly in 2,505 patients in a large university medical center from 1913 to 1995. 1913 to 1962: 2,056 patients. West J Med. 1998;169:78–87. [PMC free article] [PubMed] [Google Scholar]
- 18.O'Reilly RA. Splenomegaly in 2,505 patients at a large university medical center from 1913 to 1995. 1963 to 1995: 449 patients. West J Med. 1998;169:88–97. [PMC free article] [PubMed] [Google Scholar]
- 19.Toblli JE, Lombrana A, Duarte P, Di GF. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50:1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Beck-da-Silva L, Rohde LE, Pereira-Barretto AC, de AD, Bocchi E, Vilas-Boas F, Moura LZ, Montera MW, Rassi S, Clausell N. Rationale and design of the IRON-HF study: a randomized trial to assess the effects of iron supplementation in heart failure patients with anemia. J Card Fail. 2007;13:14–17. doi: 10.1016/j.cardfail.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Mancini DM, Katz SD, Lang CC, Lamanca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- 22.Palazzuoli A, Silverberg DS, Iovine F, Calabro A, Campagna MS, Gallotta M, Nuti R. Effects of beta-erythropoietin treatment on left ventricular remodeling, systolic function, and B-type natriuretic peptide levels in patients with the cardiorenal anemia syndrome. Am Heart J. 2007;154:645–715. doi: 10.1016/j.ahj.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, Brosh D, Laniado S, Schwartz D, Yachnin T, Shapira I, Gavish D, Baruch R, Koifman B, Kaplan C, Steinbruch S, Iaina A. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 24.Francis GS, Kanderian A. Anemia and heart failure a new pathway? J Am Coll Cardiol. 2007;50:1666–1667. doi: 10.1016/j.jacc.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg DS, Wexler D, Sheps D, Blum M, Keren G, Baruch R, Schwartz D, Yachnin T, Steinbruch S, Shapira I, Laniado S, Iaina A. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001;37:1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- 26.Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369:381–388. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 27.Ghali JK, Anand IS, Abraham WT, Fonarow GC, Greenberg B, Krum H, Massie BM, Wasserman SM, Trotman ML, Sun Y, Knusel B, Armstrong P. Randomized Double-Blind Trial of Darbepoetin Alfa in Patients With Symptomatic Heart Failure and Anemia. Circulation. 2008;117:526–535. doi: 10.1161/CIRCULATIONAHA.107.698514. [DOI] [PubMed] [Google Scholar]
- 28.Macdougall IC, Davies ME, Hutton RD, Cavill I, Lewis NP, Coles GA, Williams JD. The treatment of renal anaemia in CAPD patients with recombinant human erythropoietin. Nephrol Dial Transplant. 1990;5:950–955. doi: 10.1093/ndt/5.11.950. [DOI] [PubMed] [Google Scholar]
- 29.Dworkin HJM, Premo MBCA, Dees SC. Comparison of Red Cell and Whole Blood Volume as Performed Using Both Chromium-51-Tagged Red Cells and Iodine-125-Tagged Albumin and Using I-131-Tagged Albumin and Extrapolated Red Cell Volume. [Report] American Journal of the Medical Sciences. 2007;334:37–40. doi: 10.1097/MAJ.0b013e3180986276. [DOI] [PubMed] [Google Scholar]