Abstract
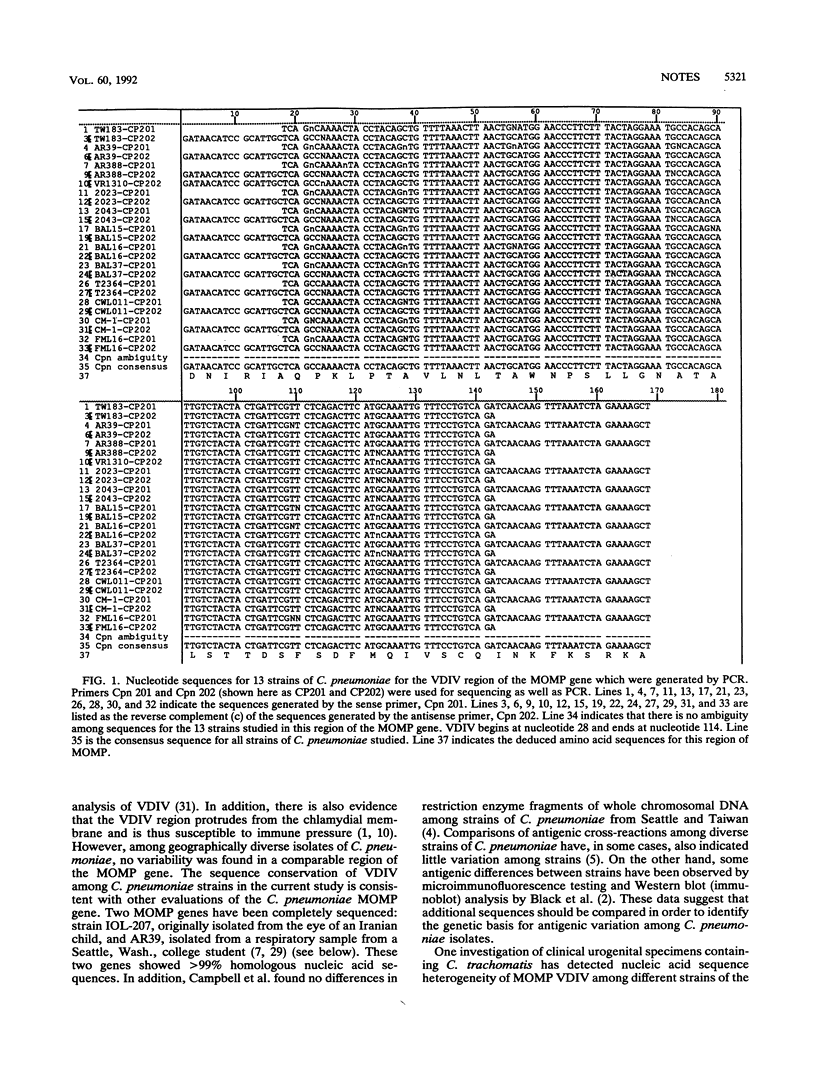
DNA was amplified by polymerase chain reaction from the gene encoding the major outer membrane protein (MOMP) of Chlamydia pneumoniae in order to examine the relatedness of strains isolated from diverse geographical regions. Primers for this reaction were chosen to span a 207-bp region comparable to that of the fourth variable segment of the MOMP gene of Chlamydia trachomatis. Among C. trachomatis, sequence heterogeneity is characteristic within variable sequence domain IV (VDIV) and correlates with serovar type. In contrast, sequence analysis of polymerase chain reaction products from 13 C. pneumoniae isolates indicated that all tested strains were identical in this segment of the MOMP gene. The predicted amino acid sequences from the C. pneumoniae VDIV gene products shared only 13.3 to 30% homology with published VDIV regions from serovars of C. trachomatis. Homology of these VDIV amino acid sequences with sequences from strains of C. psittaci ranged from 45.7 to 60%. The sequence conservation of the VDIV region of the MOMP gene indicates that C. pneumoniae strains may be more genetically homogeneous than C. trachomatis or Chlamydia psittaci strains. Future investigations of antigenic diversity among C. pneumoniae strains should be aimed at the evaluation of variation in other regions of the C. pneumoniae genome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. M., Johnson J. E., Farshy C. E., Brown T. M., Berdal B. P. Antigenic variation among strains of Chlamydia pneumoniae. J Clin Microbiol. 1991 Jul;29(7):1312–1316. doi: 10.1128/jcm.29.7.1312-1316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. F., Barnes R. C., Kozarsky P. E., Spika J. S. Culture-confirmed pneumonia due to Chlamydia pneumoniae. J Infect Dis. 1991 Aug;164(2):411–413. doi: 10.1093/infdis/164.2.411. [DOI] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Grayston J. T. Characterization of the new Chlamydia agent, TWAR, as a unique organism by restriction endonuclease analysis and DNA-DNA hybridization. J Clin Microbiol. 1987 Oct;25(10):1911–1916. doi: 10.1128/jcm.25.10.1911-1916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Perez Melgosa M., Hamilton D. J., Kuo C. C., Grayston J. T. Detection of Chlamydia pneumoniae by polymerase chain reaction. J Clin Microbiol. 1992 Feb;30(2):434–439. doi: 10.1128/jcm.30.2.434-439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. W., al-Mahdawi S. A., Giles I. G., Treharne J. D., Ward M. E., Clark I. N. Nucleotide sequence and taxonomic value of the major outer membrane protein gene of Chlamydia pneumoniae IOL-207. J Gen Microbiol. 1991 Mar;137(3):465–475. doi: 10.1099/00221287-137-3-465. [DOI] [PubMed] [Google Scholar]
- Chirgwin K., Roblin P. M., Gelling M., Hammerschlag M. R., Schachter J. Infection with Chlamydia pneumoniae in Brooklyn. J Infect Dis. 1991 Apr;163(4):757–761. doi: 10.1093/infdis/163.4.757. [DOI] [PubMed] [Google Scholar]
- Cles L. D., Stamm W. E. Use of HL cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1990 May;28(5):938–940. doi: 10.1128/jcm.28.5.938-940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D., Patton M., Stephens R. S. Direct sequence evaluation of the major outer membrane protein gene variant regions of Chlamydia trachomatis subtypes D', I', and L2'. Infect Immun. 1991 Apr;59(4):1579–1582. doi: 10.1128/iai.59.4.1579-1582.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G. D., Fine M., Orloff J., Arisumi D., Yu V. L., Kapoor W., Grayston J. T., Wang S. P., Kohler R., Muder R. R. New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine (Baltimore) 1990 Sep;69(5):307–316. doi: 10.1097/00005792-199009000-00004. [DOI] [PubMed] [Google Scholar]
- Frost E. H., Deslandes S., Veilleux S., Bourgaux-Ramoisy D. Typing Chlamydia trachomatis by detection of restriction fragment length polymorphism in the gene encoding the major outer membrane protein. J Infect Dis. 1991 May;163(5):1103–1107. doi: 10.1093/infdis/163.5.1103. [DOI] [PubMed] [Google Scholar]
- Gaydos C. A., Bobo L., Welsh L., Hook E. W., 3rd, Viscidi R., Quinn T. C. Gene typing of Chlamydia trachomatis by polymerase chain reaction and restriction endonuclease digestion. Sex Transm Dis. 1992 Nov-Dec;19(6):303–308. [PubMed] [Google Scholar]
- Gaydos C. A., Quinn T. C., Eiden J. J. Identification of Chlamydia pneumoniae by DNA amplification of the 16S rRNA gene. J Clin Microbiol. 1992 Apr;30(4):796–800. doi: 10.1128/jcm.30.4.796-800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T., Campbell L. A., Kuo C. C., Mordhorst C. H., Saikku P., Thom D. H., Wang S. P. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990 Apr;161(4):618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- Grayston J. T. Chlamydia pneumoniae, strain TWAR. Chest. 1989 Mar;95(3):664–669. doi: 10.1378/chest.95.3.664. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Mordhorst C., Bruu A. L., Vene S., Wang S. P. Countrywide epidemics of Chlamydia pneumoniae, strain TWAR, in Scandinavia, 1981-1983. J Infect Dis. 1989 Jun;159(6):1111–1114. doi: 10.1093/infdis/159.6.1111. [DOI] [PubMed] [Google Scholar]
- Hahn D. L., Dodge R. W., Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991 Jul 10;266(2):225–230. [PubMed] [Google Scholar]
- Haidl S., Ivarsson S., Bjerre I., Persson K. Guillain-Barré syndrome after Chlamydia pneumoniae infection. N Engl J Med. 1992 Feb 20;326(8):576–577. [PubMed] [Google Scholar]
- Hamilton P. T., Malinowski D. P. Nucleotide sequence of the major outer membrane protein gene from Chlamydia trachomatis serovar H. Nucleic Acids Res. 1989 Oct 25;17(20):8366–8366. doi: 10.1093/nar/17.20.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S. M., Gaydos C. A., Quinn T. C. Detection and differentiation of Chlamydia trachomatis, Chlamydia psittaci, and Chlamydia pneumoniae by DNA amplification. J Infect Dis. 1990 Oct;162(4):984–987. doi: 10.1093/infdis/162.4.984. [DOI] [PubMed] [Google Scholar]
- Kaltenboeck B., Kousoulas K. G., Storz J. Two-step polymerase chain reactions and restriction endonuclease analyses detect and differentiate ompA DNA of Chlamydia spp. J Clin Microbiol. 1992 May;30(5):1098–1104. doi: 10.1128/jcm.30.5.1098-1104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Chen H. H., Wang S. P., Grayston J. T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986 Dec;24(6):1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Grayston J. T. A sensitive cell line, HL cells, for isolation and propagation of Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990 Sep;162(3):755–758. doi: 10.1093/infdis/162.3.755. [DOI] [PubMed] [Google Scholar]
- Miller S. T., Hammerschlag M. R., Chirgwin K., Rao S. P., Roblin P., Gelling M., Stilerman T., Schachter J., Cassell G. Role of Chlamydia pneumoniae in acute chest syndrome of sickle cell disease. J Pediatr. 1991 Jan;118(1):30–33. doi: 10.1016/s0022-3476(05)81839-6. [DOI] [PubMed] [Google Scholar]
- Perez Melgosa M., Kuo C. C., Campbell L. A. Sequence analysis of the major outer membrane protein gene of Chlamydia pneumoniae. Infect Immun. 1991 Jun;59(6):2195–2199. doi: 10.1128/iai.59.6.2195-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Markoff B. A., de la Maza L. M. The major outer membrane protein nucleotide sequence of Chlamydia trachomatis, serovar E. Nucleic Acids Res. 1990 Jun 11;18(11):3414–3414. doi: 10.1093/nar/18.11.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole E., Lamont I. Chlamydia trachomatis serovar differentiation by direct sequence analysis of the variable segment 4 region of the major outer membrane protein gene. Infect Immun. 1992 Mar;60(3):1089–1094. doi: 10.1128/iai.60.3.1089-1094.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P., Vekris A., de Barbeyrac B., Dutilh B., Bonnet J., Bebear C. Typing of Chlamydia trachomatis by restriction endonuclease analysis of the amplified major outer membrane protein gene. J Clin Microbiol. 1991 Jun;29(6):1132–1136. doi: 10.1128/jcm.29.6.1132-1136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikku P., Leinonen M., Tenkanen L., Linnanmäki E., Ekman M. R., Manninen V., Mänttäri M., Frick M. H., Huttunen J. K. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study. Ann Intern Med. 1992 Feb 15;116(4):273–278. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Mullenbach G., Sanchez-Pescador R., Agabian N. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J Bacteriol. 1986 Dec;168(3):1277–1282. doi: 10.1128/jb.168.3.1277-1282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Thom D. H., Grayston J. T., Wang S. P., Kuo C. C., Altman J. Chlamydia pneumoniae strain TWAR, Mycoplasma pneumoniae, and viral infections in acute respiratory disease in a university student health clinic population. Am J Epidemiol. 1990 Aug;132(2):248–256. doi: 10.1093/oxfordjournals.aje.a115654. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Morrison S. G., Caldwell H. D., Baehr W. Cloning and sequence analysis of the major outer membrane protein genes of two Chlamydia psittaci strains. Infect Immun. 1989 May;57(5):1621–1625. doi: 10.1128/iai.57.5.1621-1625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


