Abstract
We used functional magnetic resonance imaging (fMRI) to determine whether neural activity can differentiate between true memory, false memory, and deception. Subjects heard a series of semantically related words and were later asked to make a recognition judgment of old words, semantically related nonstudied words (lures for false recognition), and unrelated new words. They were also asked to make a deceptive response to half of the old and unrelated new words. There were 3 main findings. First, consistent with the notion that executive function supports deception, 2 types of deception (pretending to know and pretending not to know) recruited prefrontal activity. Second, consistent with the sensory reactivation hypothesis, the difference between true recognition and false recognition was found in the left temporoparietal regions probably engaged in the encoding of auditorily presented words. Third, the left prefrontal cortex was activated during pretending to know relative to correct rejection and false recognition, whereas the right anterior hippocampus was activated during false recognition relative to correct rejection and pretending to know. These findings indicate that fMRI can detect the difference in brain activity between deception and false memory despite the fact that subjects respond with “I know” to novel events in both processes.
Keywords: false recognition, fMRI, lying, medial temporal lobe, prefrontal cortex
Introduction
The development of neuroimaging techniques has enabled us directly to measure brain activity associated with various cognitive functions. In recent years, much attention has been paid not only to clarifying the neural correlates of cognitive processes but also to ascertaining what someone is currently thinking by analyzing patterns of neural activity (Haynes and Rees 2006). In the context of this “brain reading,” discrimination between honest and deceptive responses is one of the most interesting topics in cognitive neuroscience.
The neural correlates of deception have gradually been delineated in studies using positron emission tomography (Abe et al. 2006, 2007), functional magnetic resonance imaging (fMRI; Spence et al. 2001; Langleben et al. 2002; Lee et al. 2002, 2005; Ganis et al. 2003; Kozel, Padgett, et al. 2004; Kozel, Revell, et al. 2004; Nunez et al. 2005; Phan et al. 2005; Mohamed et al. 2006; Gamer et al. 2007), event-related potential (Johnson et al. 2003, 2004, 2005, 2008), and, more recently, transcranial direct current stimulation (Priori et al. 2008). In addition, researchers have extended their findings to the application of fMRI or magnetoencephalography as a lie detector on the level of individual subjects (Davatzikos et al. 2005; Kozel et al. 2005; Langleben et al. 2005; Seth et al. 2006). However, it should be kept in mind that even if we can judge whether or not people are telling a lie, their “honest” responses do not always reflect “truthful” facts because of occasional memory errors.
It is known that human memory is prone to various kinds of distortions and illusions (Roediger 1996; Schacter 1999; Loftus 2003). Among these memory errors, many researchers have focused on false recognition, whereby people incorrectly claim that they have recently seen or heard a stimulus they have not encountered (Underwood 1965). In contrast to deception, false recognition is not accompanied by a subjective feeling that people are responding untruthfully, and therefore researchers need to be able to detect a difference between true and false recognition that is not apparent to the conscious mind. Despite this challenge, many neuroimaging studies have shown a difference in brain activities related to true and false recognition (Schacter et al. 1996, 1997; Heun et al. 2000, 2004; Cabeza et al. 2001; von Zerssen et al. 2001; Okado and Stark 2003; Kahn et al. 2004; Slotnick and Schacter 2004; Umeda et al. 2005; Garoff-Eaton et al. 2006, 2007; Kim and Cabeza 2007).
The main purpose of this study was directly to compare brain activity related to deception and memory distortion, both of which conceal the truth. Specifically, we focused on the processes of pretending to know (a type of deception) and false recognition, in both of which people respond “I know” to novel events. When distinguishing between these processes, it is not possible to rely on the difference between subjects' responses or the nature (“novel” in this situation) of the stimuli. The ability to judge objectively the truthfulness of someone's word based solely on their brain activations would help us to improve existing lie-detection systems on both theoretical and practical bases. In addition, imaging data may provide useful evidence for those involved in criminal investigation and prosecution in terms of the credibility of eyewitness testimony (Wells and Olson 2003). Despite this substantial implication, there has been no neuroimaging study directly comparing the patterns of brain activities associated with deception and false memory.
We performed an event-related fMRI study with a modified version of the set of word lists developed by Deese (1959) and Roediger and McDermott (1995) to produce false recognition with high probability. Before fMRI scanning, participants heard a number of lists consisting of semantic associates (e.g., moon, light, shine, bright, hot, gleam, etc). During a subsequent test phase with fMRI scanning, subjects were visually presented with previously studied “True targets” (e.g., hot), nonstudied “False targets” (e.g., sun) that were semantically related to the studied items (i.e., lures for false recognition), and unrelated “New targets” (e.g., building). The subjects' task was to make an old/new judgment in response to these stimuli. In addition to this procedure, the experimental condition of “Lie” was designed in which participants had to make deceptive responses to “True targets” and “New targets” (i.e., “do not know” responses for “True targets” and “know” responses for “New targets”).
Before starting this experiment, we presumed the following hypotheses related to brain activations associated with true memory, false memory, and deceptive responses. First, we expected that deception would be associated with increased brain activity in the lateral prefrontal cortex, which probably reflects executive function such as cognitive control (Spence et al. 2004; Hughes et al. 2005). Second, we expected that the regions responsible for auditory processing would be preferentially active during true versus false recognition due to sensory reactivation (Schacter and Slotnick 2004), because true memories engage perceptual encoding processes that are presumably not involved in the creation of false memories. In fact, recent neuroimaging studies have consistently shown that the regions engaged in encoding were reactivated during memory retrieval (e.g., Johnson and Rugg 2007; Ueno et al. 2007). The present experimental paradigm is suitable for this investigation because the participants were asked to make a recognition judgment to visually presented words that had been studied auditorily. Third, we expected that, compared with false recognition and correct rejection (baseline condition presenting novel stimuli to participants), the process of pretending to know would be associated with greater activity in the lateral prefrontal cortex due to the increased demand of executive function. Finally, we expected that, compared with pretending to know and correct rejection, false recognition would be associated with greater activity in the medial temporal lobe structures responsible for subjective familiarity and with no reactivation of regions involved in the sensory processing of auditory materials.
Materials and Methods
Participants
Twenty-eight volunteers were recruited to take part in this study. The criteria of recruitment for this study were 1) native Japanese speakers, 2) no history of neurological or psychiatric diseases, and 3) right-handedness on the Edinburgh Handedness Inventory (Oldfield 1971). All participants gave their written informed consent in accordance with the Declaration of Helsinki and the guidelines approved by the Ethical Committee of Tamagawa University.
Stimuli
For false recognition (Deese 1959; Roediger and McDermott 1995), we collated 81 word lists, each consisting of semantically related words, from the Japanese literature (Hamajima 2000; Takahashi 2001; Hoshino 2002; Miyaji and Yama 2002). By removing overlapping lists or words and including some lists with 1 or 2 words from the Japanese word association norms (Umemoto 1969), we eventually prepared 60 lists of semantic associates. Of these, 40 lists consisted of 1 theme word (e.g., sun) and 15 semantically related words (e.g., moon, light, shine, bright, hot, and so forth), and the remaining 20 lists consisted of 1 theme word and 10 semantically related words.
During 2 study phases, a total of 600 words (40 lists of 15 words each) prerecorded by a male speaker were presented to the participants through a personal computer.
During 2 test phases, a total of 200 words (80 “True targets,” 40 “False targets,” and 80 “New targets”) were presented. The 1st and 2nd strongest associates were used as “True targets” and the theme words as “False targets” in the 40 lists of 15 words each. In addition, the theme word and the 1st, 2nd, and 3rd strongest associates were used as “New targets” in the 20 lists of 10 words each. These stimuli were visually presented to participants in the MRI scanner. Each test word was presented in the center of a screen, and whichever finger of the right hand was to be used for old/new button pressing was presented on both sides of the word. All the “False targets” and half of the “True targets” and “New targets” were assigned to “Truth” blocks, in which subjects were asked to respond honestly to each stimulus. The remaining halves of the “True targets” and “New targets” were assigned to “Lie” blocks, in which subjects were asked to make a deceptive response to each stimulus. Due to the limited number of stimuli, “False targets” were not presented in the “Lie” blocks.
Tasks
The experiment consisted of 2 sessions, each of which included 1 study phase and 1 test phase (i.e., the 1st study phase without fMRI, the 1st test phase with fMRI, the 2nd study phase without fMRI, and the 2nd test phase with fMRI). Figure 1 illustrates 1 of the 2 task sessions.
Figure 1.
Depiction of 1 of the 2 task sessions (see Materials and Methods for details). The study-test phase was conducted twice with different stimulus sets. After participants heard the word lists of semantic associates in the study phase, they were asked to perform the recognition memory task (test phase) consisting of 4 “Truth” and 4 “Lie” blocks with fMRI scanning. In the “Truth” blocks, they were asked to respond honestly to “True targets” (old words from the study phase), “False targets” (nonstudied words that were semantically related to old words), and “New targets” (new words that were not semantically related to old words). In the “Lie” blocks, they were asked to dishonestly respond to “True targets” and “New targets.” In the “Truth blocks,” true recognition (TR) was defined as an “old” response to a “True target,” false recognition (FR) as an “old” response to a “False target,” and correct rejection (CR) as a “new” response to a “New target.” In the “Lie” blocks, lying to “True targets” (LT; i.e., pretending not to know) and lying to “New targets” (LN; i.e., pretending to know) were defined as deceptive responses to each target.
Before the experiment, the participants were given a thorough explanation of the task procedure, and familiarized with the task by completing a short practice session. During each study phase, subjects listened to a total of 300 words (20 lists of 15 words each) at a rate of 2 s per word. The theme words from the 20 lists were not presented during the study phase and were used as “False targets” for producing false recognition in the subsequent test phase. Words were presented in order of decreasing strength of association with the theme word, except for 2 words to be used as “True targets,” which were shifted to positions other than 1, 2, 14, and 15 to prevent primacy and recency effects. Subjects were instructed to remember the presented words for the later recognition memory test. Presentation of each list was separated by a 15-s interval during which subjects performed simple arithmetic (e.g., “5 plus 2”). The presentation order of the lists was randomized across subjects.
Approximately 10 min after the completion of the study phase, the test phase was initiated. During the test phase with fMRI scanning, subjects performed the recognition test, consisting of 4 “Truth” and 4 “Lie” blocks. The cues indicating the subsequent “Truth” or “Lie” block were of 15 s duration and were followed by the target words. In each “Truth” block, 5 “True targets,” 5 “False targets,” and 5 “New targets” were presented in randomized order, and subjects were asked to make old/new decisions as quickly as possible by pressing keys on the response box. In each “Lie” block, 5 “True targets” and 5 “New targets” were presented in randomized order, and subjects were asked to make deceptive responses (i.e., “new” responses for “True targets” and “old” responses for “New targets”). Subjects were asked to respond using the index or middle finger of their right hand. The assignment of these fingers for each old/new decision was counterbalanced across blocks. Each word was presented for 2 s, and the intervals between the words, during which cross-fixation was constantly presented, ranged between 2.5 s and 13.5 s to maximize the efficiency of the event-related design (Dale 1999).
In 1 test phase with fMRI scanning, participants responded to 20 “True,” 20 “False,” and 20 “New” target words in “Truth” blocks, and 20 “True” and 20 “New” trials in “Lie” blocks. Thus, across the 2 test phases, participants responded to 40 “True,” 40 “False,” and 40 “New” target words in “Truth” blocks, and 40 “True” and 40 “New” trials in “Lie” blocks.
Image Acquisition and Data Analysis
Whole-brain imaging was performed with a 1.5-Tesla MRI scanner (Magnetom Sonata; Siemens, Erlangen, Germany). A T2*-weighted echo planar imaging (EPI) sequence was used for functional imaging with the following parameters: time repetition = 2200 ms, time echo = 45 ms, flip angle = 90°, 64 × 64 acquisition matrix, field of view = 192 mm, 26 axial slices with 4 mm slice thickness and 1 mm interslice gap, 400 volume acquisitions per run. Head motion was restricted using firm padding that surrounded the head. The cognitive tasks during fMRI scanning were controlled using Cogent 2000 software (Wellcome Department of Imaging Neuroscience, London, UK). Visual stimuli were projected onto a screen and viewed through a mirror attached to a standard head coil. The subjects' responses were collected using a magnet-compatible response box.
Data preprocessing and statistical analyses were performed using Statistical Parametric Mapping 2 (Wellcome Department of Imaging Neuroscience). Preprocessing of the image volumes included realignment of head motions, slice-time correction with reference to the middle slice acquired in time, normalization to the EPI-template based on the Montreal Neurological Institute (MNI) reference brain (resampled voxel size 3 × 3 × 3 mm3), and spatial smoothing with a Gaussian kernel (8 mm at full-width half-maximum).
The fMRI data were analyzed using an event-related model. For each subject, activity associated with each experimental condition of interest (i.e., TR, true recognition to “True targets”; CR, correct rejection to “New targets”; FR, false recognition to “False targets”; LT, lying to “True targets”; LN, lying to “New targets”) was modeled using a canonical hemodynamic response function. Targets that were incorrectly classified (i.e., error responses) or for which a response was omitted were modeled as events of no interest, as were instructions presented during the onset of “Truth” and “Lie” blocks. A high-pass filter of 1/128 Hz was used to remove low-frequency noise, and an AR (1) model corrected for temporal autocorrelation. The resulting parameter estimates for each regressor at each voxel were then entered into a 2nd-level analysis where each participant served as a random effect in a repeated measures analysis of variance (ANOVA). Appropriate corrections were made for nonsphericity and correlated repeated measures (Friston, Glaser, et al. 2002; Friston, Penny, et al. 2002). The comparisons between experimental conditions were then performed by appropriately weighted linear contrasts and determined on a voxel-by-voxel basis. In addition to simple subtraction analyses, the above procedure allowed us to perform conjunction analyses at the 2nd level, and we identified activated regions with a conjunction using the minimum statistic (Friston et al. 2005), as suggested by Nichols et al. (2005). This procedure revealed areas in which all the contrasts entered into conjunction analysis were individually significant. For all the whole-brain subtraction analyses, the threshold of significance was set at P < 0.001 (uncorrected for multiple comparisons) with an extent threshold of 10 contiguous voxels. In the conjunction analysis, the same threshold was used (P < 0.001), but no extent threshold was applied. To extract the percent signal change of activated regions during each task, we also used MarsBaR software (Brett et al. 2002).
Results
Participants
Before the analysis of imaging data, 2 participants were excluded from the analysis due to excessive head motion during fMRI scanning (approximately 4 mm). An additional 6 participants were excluded due to poor task performance (i.e., less than 60% accuracy in “Truth” blocks, which was close to chance level) or an insufficient number of events in at least 1 of the conditions used in the imaging contrasts (i.e., fewer than 15), or both. Thus the results of the present study are based on the data from the remaining 20 subjects (11 males and 9 females, age range 19–28 years, mean age 21.9 years). These participants did not report any difficulty understanding the task procedure and performing each of the Truth and Lie tasks as instructed. There were no pathological findings on MRI of any of the subjects' brains.
Comparison between Truthful and Deceptive Responses
Behavioral Data
All the behavioral data are shown in Table 1. In this analysis, we used 2-way repeated measures ANOVA to analyze the behavioral data of 2 “Truth” and 2 “Lie” tasks except for the condition of FR. A main effect of stimulus type (“True targets” or “New targets”) was found in the accuracy of the response, as the average accuracy was significantly higher for “New targets” than for “True targets” (F1, 19 = 6.462, P < 0.05). A noticeable trend of response type (“Truth” or “Lie” tasks) qualified by a relatively higher accuracy in “Truth” conditions was also found (F1, 19 = 3.662, P = 0.071). For reaction times, a main effect of stimulus type was evident, as the average reaction time was significantly longer for “New targets” than for “True targets” (F1, 19 = 24.034, P < 0.0001). There was also a significant main effect of response type, characterized by a longer reaction time in “Lie” tasks (F1, 19 = 15.136, P < 0.005). There was no interaction between the 2 factors in terms of both accuracy and reaction time. These results indicate that the cognitive demand in “Lie” tasks was higher than that in “Truth” tasks.
Table 1.
Percent correct and reaction time for all conditions
| Percent correct |
Reaction time |
|||
| Mean | SD | Mean | SD | |
| Truth | ||||
| True targets | 71.8 | ±8.7 | 1652 | ±351 |
| New targets | 81.1 | ±9.4 | 1892 | ±417 |
| False targets | 28.9 | ±12.2 | 1647 | ±370 |
| Lie | ||||
| True targets | 70.6 | ±11.4 | 1959 | ±415 |
| New targets | 74.8 | ±14.3 | 2168 | ±533 |
Note: The accuracy of subjects' responses to “False targets” was assessed by the rate of correct rejection (“new” responses), but the reaction time was based on the trials of false recognition (“old” responses).
Brain Activation
To examine whether deception was associated with increased brain activity in the prefrontal cortex, we compared LT and LN with TR and CR (i.e., main effect of deception). As predicted, increased prefrontal activations were observed in this contrast. Table 2 summarizes these data for anatomical structures and Brodmann's area (BA), MNI coordinates, Z-values and cluster size of peak activations.
Table 2.
Brain regions showing main effect of making a deceptive responses {(LT − TR) + (LN − CR)}
| Region (BA) | MNI coordinates |
Z value | Cluster size | ||
| x | y | z | |||
| Rt medial prefrontal cortex (10) | 12 | 57 | −6 | 3.86 | 16 |
| Rt insula | 33 | 21 | 15 | 4.13 | 14 |
| Rt superior frontal gyrus (8) | 24 | 15 | 60 | 3.90 | 11 |
| Rt middle frontal gyrus (6) | 42 | 6 | 51 | 3.91 | 24 |
| Rt superior frontal gyrus (6) | 24 | −3 | 57 | 3.81 | 38 |
| Rt thalamus | 15 | −27 | 0 | 4.22 | 34 |
| Rt fusiform gyrus (19) | 24 | −69 | −12 | 3.80 | 16 |
| Rt cuneus (19) | 18 | −75 | 39 | 3.68 | 44 |
| Lt inferior frontal gyrus (47) | −45 | 48 | −15 | 3.58 | 14 |
| Lt supplementary motor area (6) | −12 | 21 | 57 | 4.07 | 73 |
| Lt insula | −30 | 21 | 9 | 3.52 | 17 |
| Lt insula | −48 | 9 | 6 | 3.67 | 16 |
| Lt middle frontal gyrus (6/8/9) | −27 | 3 | 60 | 4.01 | 140 |
| Lt postcentral gyrus (3) | −54 | −6 | 39 | 4.16 | 22 |
| Lt superior temporal sulcus/superior temporal gyrus (22) | −42 | −27 | 3 | 3.77 | 12 |
| Lt supramarginal gyrus (40) | −30 | −42 | 45 | 4.00 | 20 |
| Lt superior parietal lobule (7) | −27 | −57 | 54 | 4.12 | 35 |
| Lt angular gyrus (39) | −51 | −60 | 36 | 4.00 | 38 |
| Lt middle occipital gyrus (19) | −30 | −69 | 30 | 3.96 | 59 |
| Lt precuneus (7) | −9 | −78 | 45 | 3.86 | 49 |
Note: Only the most significant peaks within each area of activation are reported in this table.
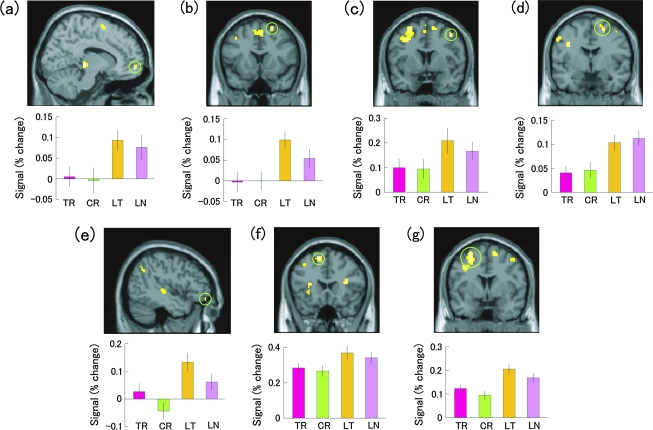
Second, to examine the influence of the familiarity of stimuli on fMRI signals in each activated frontal region and whether or not an interaction occurred, we performed the region of interest (ROI) analysis. The signal changes in each cluster were extracted and analyzed using 2-way ANOVA with the response to stimuli (“Truth,” “Lie”) and the familiarity of stimuli (“True targets,” “New targets”) as factors. Overall, there was no significant interaction between the 2 factors in the entire activated regions, indicating that these regions are commonly associated with the 2 types of deception. Results of the ANOVA for the right medial prefrontal cortex [12, 57, −6] showed a significant main effect of “Lie” (F1,19 = 18.281, P < 0.0005), but showed neither a main effect of familiarity of stimuli (F1,19 = 0.629, P = 0.438) nor an interaction between the 2 factors (F1,19 = 0.075, P = 0.787). ANOVA for the right superior frontal gyrus [24, 15, 60] yielded similar results: a significant main effect of “Lie” (F1,19 = 24.107, P < 0.0001), without a main effect of familiarity of stimuli (F1,19 = 1.943, P = 0.179) or an interaction (F1,19 = 1.805, P = 0.195). Results for the right middle frontal gyrus [42, 6, 51] showed a significant main effect of “Lie” (F1,19 = 13.112, P < 0.005) and a marginal main effect of familiarity of stimuli (“True targets” > “New targets”) (F1,19 = 3.425, P = 0.080), without an interaction (F1,19 = 0.746, P = 0.399). Results for the right superior frontal gyrus [24, −3, 57] showed a significant main effect of “Lie” (F1,19 = 23.866, P < 0.0005), but showed neither a main effect of familiarity of stimuli (F1,19 = 0.694, P = 0.415) nor an interaction (F1,19 = 0.021, P = 0.886). Results for the left inferior frontal gyrus [−45, 48, −15] showed a significant main effect of “Lie” (F1,19 = 20.538, P < 0.0005), and a main effect of familiarity of stimuli (“True targets” > “New targets”) (F1,19 = 10.911, P < 0.005) without an interaction (F1,19 = 0.002, P = 0.968). Results for the left supplementary motor area [−12, 21, 57] showed a significant main effect of the “Lie” (F1,19 = 21.678, P < 0.0005) and a marginal main effect of familiarity of stimuli (“True targets” > “New targets”) (F1,19 = 4.015, P = 0.060), without an interaction (F1,19 = 0.179, P = 0.677). Results for the left middle frontal gyrus [−27, 3, 60] showed a significant main effect of “Lie” (F1,19 = 24.276, P < 0.0001), and a main effect of familiarity of stimuli (“True targets” > “New targets”) (F1,19 = 12.683, P < 0.005), without an interaction (F1,19 = 0.139, P = 0.713). These results are illustrated in Figure 2.
Figure 2.
Regions showing greater activation during lying (LT and LN) relative to truth telling (TR and CR). The signal changes of the following 7 activated regions in the frontal lobe are depicted (error bars represent SEM). (a) Right medial prefrontal cortex [12, 57, −6], (b) right superior frontal gyrus [24, 15, 60], (c) right middle frontal gyrus [42, 6, 51], (d) right superior frontal gyrus [24, −3, 57], (e) left inferior frontal gyrus [−45, 48, −15], (f) left supplementary motor area [−12, 21, 57], (g) left middle frontal gyrus [−27, 3, 60]. TR, true recognition; CR, correct rejection; LT, lying to “True targets” (pretending not to know); LN, lying to “New targets” (pretending to know).
Comparison between True and False Recognition
Behavioral Data
In this analysis, we used 1-way repeated measures ANOVA to examine the behavioral data of TR, FR, and CR. Note that the accuracy of the task in which participants responded to nonstudied “False targets” was assessed by the rate of correct rejection in response to these stimuli. An ANOVA yielded a significant effect of target type in accuracy (F2, 38 = 140.698, P < 0.0001). A post hoc test (Scheffe) revealed significant differences between true recognition of “True targets” and correct rejection of “New targets” (P < 0.05), between correct rejection of “False targets” and correct rejection of “New targets” (P < 0.0001), and between true recognition of “True targets” and correct rejection of “False targets” (P < 0.0001). An ANOVA for reaction time also showed a significant effect of target type (F2, 38 = 13.731, P < 0.0001). A post hoc test (Scheffe) revealed significant differences between TR and CR (P < 0.0005) and between CR and FR (P < 0.0005). No difference was observed between TR and FR (P = 0.996). These results show a high rate of false recognition of nonpresented “False targets” (approximately 70%). The fact that there was no difference in reaction time between TR and FR indicates that the difference in brain activity associated with these processes cannot be ascribed to retrieval effort.
Brain Activation
Brain activity during TR was compared with that during FR. The results are shown in Table 3. Consistent with our hypothesis, activations in the temporal and parietal lobes were found in the bilateral hemisphere. To obtain more reliable evidence for the interpretation of sensory reactivation during TR, we performed conjunction analysis of TR versus FR and TR versus CR. If the difference between TR and FR were a true reflection of the reactivation of sensory information acquired during the encoding phase, the observed activations would also be detected in the contrast of TR versus CR. This conjunction analysis revealed that the activations in the left temporoparietal regions during TR relative to FR overlapped with the activations during TR relative to CR.
Table 3.
Brain regions showing greater responses during TR compared with FR
| Region (BA) | MNI coordinates |
Z value | Cluster size | ||
| x | y | z | |||
| Rt middle temporal gyrus (21/22) | 66 | −21 | −3 | 3.88 | 24 |
| *Rt/Lt medial superior frontal gyrus (10) | 0 | 60 | 24 | 3.53 | 18 |
| *Rt/Lt cerebellum | 0 | −45 | −42 | 3.97 | 17 |
| *Lt superior temporal sulcus/middle temporal gyrus (21) | −48 | −9 | −15 | 3.65 | 11 |
| *Lt middle temporal gyrus (21) | −60 | −18 | −12 | 3.88 | 12 |
| *Lt supramarginal gyrus (40) | −51 | −54 | 36 | 3.63 | 13 |
Note: Only the most significant peaks within each area of activation are reported in this table.
Indicates the region that includes the active voxels detected in the conjunction analysis of TR versus FR and TR versus CR.
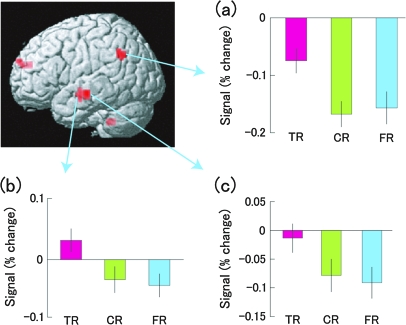
To confirm further this activation overlap, we extracted the signal change in each cluster of 3 left temporoparietal regions during each condition and analyzed it using 1-way repeated measures ANOVA. First, results of the ANOVA for the left superior temporal sulcus/middle temporal gyrus [−48, −9, −15] showed a significant difference (F2, 38 = 9.502, P < 0.001). A post hoc test (Scheffe) revealed that the activity of TR was higher than that of CR (P < 0.01) and FR (P < 0.005), whereas the difference between CR and FR was not significant (P = 0.873). Second, results of the ANOVA for the left middle temporal gyrus [−60, −18, −12] showed a significant difference (F2, 38 = 11.293, P < 0.0005). A post hoc test (Scheffe) revealed that the activity of TR was higher than that of CR (P < 0.005) and FR (P< 0.0005), whereas the difference between CR and FR was not significant (P = 0.770). Third, results of the ANOVA for the left supramarginal gyrus [−51, −54, 36] showed a significant difference (F2, 38 = 13.688, P < 0.0001). A post hoc test (Scheffe) revealed that the activity of TR was higher than that of CR (P < 0.0005) and FR (P < 0.001), whereas the difference between CR and FR was not significant (P = 0.853). These results are illustrated in Figure 3.
Figure 3.
Statistical parametric map of regions showing greater activation during TR than during CR and FR, displayed on a surface-rendered standard brain. The signal changes of the following 3 activated regions are depicted (error bars represent SEM). (a) Left supramarginal gyrus [−51, −54, 36], (b) left superior temporal sulcus/middle temporal gyrus [−48, −9, −15], (c) left middle temporal gyrus [−60, −18, −12]. TR, true recognition; CR, correct rejection; FR, false recognition.
Comparison between Deception and False Memory
Behavioral Data
In the analysis of the difference between deception and false memory, we used 1-way repeated measures ANOVA to compare the behavioral data of LN, FR, and CR. As mentioned above, the accuracy of the condition in which participants responded to nonstudied “False targets” was assessed by the rate of correct rejection of these stimuli. An ANOVA yielded a significant effect of accuracy (F2, 38 = 158.569, P < 0.0001). A post hoc test (Scheffe) revealed significant differences between correct rejection of “False targets” and correct rejection of “New targets” (P < 0.0001), and between lying in response to “New targets” and correct rejection of “False targets” (P < 0.0001). No difference was observed between correct rejection of “New targets” and lying in response to “New targets” (P = 0.152). An ANOVA for reaction time also showed a significant effect (F2, 38 = 33.483, P < 0.0001). A post hoc test (Scheffe) revealed that the reaction time for CR was longer than that for FR (P < 0.005), and that the reaction time for LN was longer than that for CR (P < 0.001) and that for FR (P < 0.0001). These data for reaction time indicate that although all the stimuli in these 3 conditions were novel to the participants, the process of classifying the stimuli as “New” (i.e., CR) was more complicated than that of classifying the stimuli as “Old” (i.e., FR). Furthermore, the longer reaction time for LN relative to that for CR suggests that the additional process of lying further enhanced cognitive demand during the recognition memory task.
Brain Activation
Comparison between LN and FR was the main purpose of this study. First, we compared the neural activities during LN with those during CR, and found significant activations in the prefrontal cortex. The results are summarized in Table 4. If the prefrontal activities found in LN versus CR were also detected in LN versus FR, those regions would be a reliable indicator of the process of intentional response manipulation characterizing deception. Conjunction analysis of these 2 contrasts revealed that activation in the left middle frontal gyrus detected in LN versus CR overlapped with the activation detected in LN versus FR.
Table 4.
Brain regions showing greater responses during LN compared with CR
| Region (BA) | MNI coordinates |
Z value | Cluster size | ||
| x | y | z | |||
| Rt superior frontal sulcus (8) | 24 | 15 | 39 | 4.37 | 14 |
| Rt thalamus | 18 | −27 | 3 | 3.49 | 12 |
| Lt supplementary motor area (6) | −12 | 21 | 54 | 3.32 | 11 |
| Lt insula | −27 | 21 | 12 | 3.75 | 16 |
| *Lt middle frontal gyrus (9) | −42 | 18 | 39 | 4.25 | 48 |
| Lt superior frontal sulcus (6) | −27 | 9 | 42 | 3.62 | 11 |
| *Lt supramarginal gyrus (40) | −51 | −57 | 36 | 3.68 | 22 |
| Lt middle occipital gyrus (19) | −33 | −69 | 33 | 3.84 | 12 |
Note: Only the most significant peaks within each area of activation are reported in this table.
Indicates the region that includes the active voxels detected in the conjunction analysis of LN versus CR and LN versus FR.
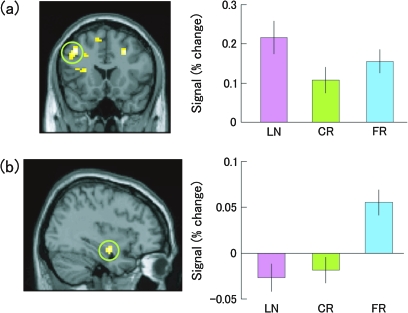
To further confirm this activation overlap, we extracted the signal change in the cluster of the left middle frontal gyrus [−42, 18, 39] during each condition and analyzed it using 1-way repeated measures ANOVA. Results of the ANOVA showed significant difference of signal change in this region (F2, 38 = 13.209, P < 0.0001). A post hoc test (Scheffe) revealed that the activity of LN was higher than that of CR (P < 0.0001) and FR (P < 0.05), and a trend was found between CR and FR (P = 0.092).
As for FR, we compared the neural activities during FR with those during CR, and found significant activation in the right anterior hippocampus. The results are summarized in Table 5. If the activations of the medial temporal lobe found in FR versus CR were also detected in FR versus LN, the region would be a reliable indicator of illusory familiarity characterizing false memory. Conjunction analysis of these 2 contrasts revealed that the activation in the right anterior hippocampus detected in FR versus CR overlapped with the activation detected in FR versus LN.
Table 5.
Brain regions showing greater responses during FR compared with CR
| Region (BA) | MNI coordinates |
Z value | Cluster size | ||
| x | y | z | |||
| *Rt basal forebrain/orbitofrontal cortex (12/25) | 9 | 6 | −9 | 4.23 | 35 |
| *Rt hippocampus | 36 | −9 | −15 | 3.98 | 12 |
| *Lt orbitofrontal cortex (11/12) | −9 | 33 | −18 | 3.61 | 25 |
| Lt orbitofrontal cortex (11) | −21 | 27 | −24 | 4.28 | 23 |
| Lt inferior frontal gyrus (45/47) | −42 | 27 | 0 | 3.59 | 15 |
| Lt superior frontal sulcus (8) | −24 | 18 | 39 | 3.83 | 25 |
| Lt insula | −27 | 18 | 12 | 4.16 | 18 |
| Lt caudate nucleus | −12 | 3 | 9 | 3.49 | 15 |
| Lt superior parietal lobule (7) | −27 | −63 | 54 | 4.05 | 43 |
| *Lt cuneus (18) | −18 | −63 | 21 | 4.04 | 127 |
Note: Only the most significant peaks within each area of activation are reported in this table.
Indicates the region that includes the active voxels detected in the conjunction analysis of FR versus CR and FR versus LN.
To confirm further this activation overlap, we extracted the signal change in the cluster of the right hippocampus [36, −9, −15] during each condition and analyzed it using 1-way repeated measures ANOVA. Results of the ANOVA showed significant difference of signal change in this region (F2, 38 = 10.826, P < 0.0005). A post hoc test (Scheffe) revealed that the activity of FR was higher than that of CR (P < 0.005) and LN (P < 0.001), whereas the difference between CR and LN was not significant (P = 0.907). In addition, as we expected, the comparison between FR versus CR revealed no reactivation of the regions responsible for processing the sensory information of auditorily presented word lists, such as language-processing areas in the left hemisphere. The signal change of the activation in the left middle frontal gyrus and the right hippocampus during each task is illustrated in Figure 4.
Figure 4.
The signal change of increased brain activity in (a) the left middle frontal gyrus [−42, 18, 39] during LN in comparison to during CR and FR, and (b) the right hippocampus [36, −9, −15] during FR in comparison to during CR and LN. Error bars represent standard error. The activation is superimposed onto MRIs of MNI templates. CR, correct rejection; FR, false recognition; LN, lying to “New targets” (pretending to know).
Discussion
In the present study, we aimed to clarify the neural correlates of true memory, false memory, and deception. As for the cerebral mechanisms underlying deception, the present data show that the process of intentional response manipulation in deception was characterized by prefrontal activity. These findings are highly consistent with those of previous neuroimaging studies, indicating a robust contribution of executive function to deception (Spence et al. 2004; Hughes et al. 2005). Significant activations were also detected in the supplementary motor area, which plays a role in higher motor control (Tanji 1994). This activity may reflect motor regulation during button pressing when making deceptive responses.
In relation to the difference in neural activity between veridical and illusory memories, the comparison of true recognition with false recognition revealed activations of the lateral temporal and parietal cortices. The conjunction analysis of TR versus FR and TR versus CR, which was performed to obtain more reliable evidence related to sensory reactivation of the regions engaged in encoding of the word lists, further showed left-lateralized activations of the supramarginal gyrus and the middle temporal gyrus. Previous neuroimaging studies employing similar word lists of semantic associates for producing false recognition have also reported comparable findings. For example, Schacter et al. (1996) reported increased blood flow in the left temporoparietal cortex (BA 42/22/40) during true recognition relative to false recognition, and Cabeza et al. (2001) reported a difference in activity in the left parietal cortex (BA 40/39) between true and false recognition. Considering the role of the left temporoparietal areas in the language processing of words presented auditorily, the left temporoparietal activity can be regarded as a reliable neural signature of true recognition.
The main purpose of this study was to compare the brain activity related to the process of pretending to know and false recognition in order to identify a reliable indicator of the brain activations associated with each process. We expected that the activations of the prefrontal cortex detected in the analysis of LN versus CR would also be detected in the analysis of LN versus FR, and that the activations of medial temporal lobe responsible for mnemonic processing detected in the analysis of FR versus CR would also be detected in the analysis of FR versus LN. To confirm these predictions, we performed 2 conjunction analyses (i.e., LN vs. CR conjunct with LN vs. FR, and FR vs. CR conjunct with FR vs. LN) and ROI analyses for each activation.
As we expected, activation of the left middle frontal gyrus, possibly reflecting the subjective, intentional cognitive process of response manipulation, was found in the conjunction analysis of LN versus CR and LN versus FR. The middle frontal gyrus, often referred to as the dorsolateral prefrontal cortex, can be regarded as a reliable indicator of pretending to know. Expanding on the previous neuroimaging studies on deception, our data suggest that prefrontal activity reflects not only the difference between deception and truth telling, but also the difference between deception and false memory.
Consistent with our hypothesis, conjunction analysis of FR versus CR and FR versus LN revealed activations of the right anterior hippocampus without reactivation of the regions responsible for language processing detected as true recognition-specific activity. This activation pattern indicates that the right anterior hippocampus is associated with “illusory” familiarity to novel stimuli. The results also appear to be consistent with the recent fMRI finding that medial temporal lobe activity is modulated not only by objective memory function but also by the subjective confidence level of recognition memory (Chua et al. 2006). One surprising result was that hippocampal activity was found not in the left but in the right hemisphere, despite the fact that verbal materials have to be retrieved. Although it is difficult to explain this finding from the available data, there is a possibility that the right anterior hippocampus plays a role in relatively rough judgment of episodic familiarity without access to memory fragments of perceptual traces stored in other cortical areas.
In conclusion, the present fMRI study provided evidence of neural activities differentiating between true memory, false memory, and deception. The most important finding is that the left prefrontal cortex was activated during pretending to know relative to both correct rejection and false recognition, whereas the right hippocampus was activated during false recognition relative to both correct rejection and pretending to know. Our approach to the comparison between deception and false memory demonstrated that what someone is currently thinking can only be judged on the basis of brain activity, rather than being able to rely on the subject's responses (“know” in the present study) or the nature of the stimuli (“novel” in the present study).
The limitations of the present study need to be mentioned. First, the difference between the neural activities associated with pretending not to know and forgetting (old-miss) could not be analyzed due to the insufficient number of old-miss trials. Because these processes, in both of which people respond “I don't know” to experienced events, often occur in various situations such as criminal investigations, it would be both intriguing and of practical value to clarify the difference between the neural activities associated with them. Second, probably because of the challenge inherent in our task, the subjects' performance level was relatively low and therefore the effect of guessing might have influenced the results. Methods such as the Remember/Know technique (Tulving 1985), source memory paradigm (Johnson et al. 1993), and recording the subjects' confidence level would be beneficial (e.g., Kim and Cabeza 2007). Third, simulated deception in laboratory experiments cannot be viewed as being the same as deception in real life. Replication of the current results in a more natural situation is warranted. Finally, the results in the present study cannot reveal whether the subjects tell a lie and whether the subjects retrieve veridical memory in the level of individual subject. Further study is needed to decode the brain activities related to these cognitive processes on a single-subject, trial-by-trial basis.
Funding
Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists to N.A. (05J04930); and 21st Century Center of Excellence (COE) Program by the Japan Society for the Promotion of Science to Tamagawa University.
Acknowledgments
We are grateful to Osamu Iizuka, Toshiyuki Ishioka, and Manami Yamamoto for their assistance and helpful comments. The experiment in this research was realized using Cogent 2000 developed by the Cogent 2000 team at the FIL and the ICN and Cogent Graphics developed by John Romaya at the LON, University College London. Conflict of Interest: None declared.
References
- Abe N, Suzuki M, Mori E, Itoh M, Fujii T. Deceiving others: distinct neural responses of the prefrontal cortex and amygdala in simple fabrication and deception with social interactions. J Cogn Neurosci. 2007;19:287–295. doi: 10.1162/jocn.2007.19.2.287. [DOI] [PubMed] [Google Scholar]
- Abe N, Suzuki M, Tsukiura T, Mori E, Yamaguchi K, Itoh M, Fujii T. Dissociable roles of prefrontal and anterior cingulate cortices in deception. Cereb Cortex. 2006;16:192–199. doi: 10.1093/cercor/bhi097. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. 2002 Neuroimage. 16 (Suppl. 1). (Abstract 497) [Google Scholar]
- Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proc Natl Acad Sci USA. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage. 2006;29:1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos C, Ruparel K, Fan Y, Shen DG, Acharyya M, Loughead JW, Gur RC, Langleben DD. Classifying spatial patterns of brain activity with machine learning methods: application to lie detection. Neuroimage. 2005;28:663–668. doi: 10.1016/j.neuroimage.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Deese J. On the prediction of occurrence of particular verbal intrusions in immediate recall. J Exp Psychol. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: applications. Neuroimage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J. Classical and Bayesian inference in neuroimaging: theory. Neuroimage. 2002;16:465–483. doi: 10.1006/nimg.2002.1090. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Gamer M, Bauermann T, Stoeter P, Vossel G. Covariations among fMRI, skin conductance, and behavioral data during processing of concealed information. Hum Brain Mapp. 2007;28:1287–1301. doi: 10.1002/hbm.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganis G, Kosslyn SM, Stose S, Thompson WL, Yurgelun-Todd DA. Neural correlates of different types of deception: an fMRI investigation. Cereb Cortex. 2003;13:830–836. doi: 10.1093/cercor/13.8.830. [DOI] [PubMed] [Google Scholar]
- Garoff-Eaton RJ, Kensinger EA, Schacter DL. The neural correlates of conceptual and perceptual false recognition. Learn Mem. 2007;14:684–692. doi: 10.1101/lm.695707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff-Eaton RJ, Slotnick SD, Schacter DL. Not all false memories are created equal: the neural basis of false recognition. Cereb Cortex. 2006;16:1645–1652. doi: 10.1093/cercor/bhj101. [DOI] [PubMed] [Google Scholar]
- Hamajima H. False memories created in laboratory experiments: the word lists in Japanese. [in Japanese]. Stud Inform Sci. 2000;11:175–193. [Google Scholar]
- Haynes JD, Rees G. Decoding mental states from brain activity in humans. Nat Rev Neurosci. 2006;7:523–534. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- Heun R, Jessen F, Klose U, Erb M, Granath DO, Grodd W. Response-related fMRI analysis during encoding and retrieval revealed differences in cerebral activation by retrieval success. Psychiatry Res. 2000;99:137–150. doi: 10.1016/s0925-4927(00)00060-3. [DOI] [PubMed] [Google Scholar]
- Heun R, Jessen F, Klose U, Erb M, Granath DO, Grodd W. Response-related fMRI of veridical and false recognition of words. Eur Psychiatry. 2004;19:42–52. doi: 10.1016/j.eurpsy.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hoshino Y. False recall created by learning related words: a comparison between blocked presentation and random presentation of related words. [in Japanese]. Jpn J Psychon Sci. 2002;20:105–114. [Google Scholar]
- Hughes CJ, Farrow TF, Hopwood MC, Pratt A, Hunter MD, Spence SA. Recent developments in deception research. Curr Psychiatry Rev. 2005;1:273–279. [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the reinstatement of encoding-related cortical activity. Cereb Cortex. 2007;17:2507–2515. doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Johnson M, Hashtroudi S, Lindsay D. Source monitoring. Psychol Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Johnson R, Jr, Barnhardt J, Zhu J. The deceptive response: effects of response conflict and strategic monitoring on the late positive component and episodic memory-related brain activity. Biol Psychol. 2003;64:217–253. doi: 10.1016/j.biopsycho.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Johnson R, Jr, Barnhardt J, Zhu J. The contribution of executive processes to deceptive responding. Neuropsychologia. 2004;42:878–901. doi: 10.1016/j.neuropsychologia.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Johnson R, Jr, Barnhardt J, Zhu J. Differential effects of practice on the executive processes used for truthful and deceptive responses: an event-related brain potential study. Brain Res Cogn Brain Res. 2005;24:386–404. doi: 10.1016/j.cogbrainres.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Johnson R, Jr, Henkell H, Simon E, Zhu J. The self in conflict: the role of executive processes during truthful and deceptive responses about attitudes. Neuroimage. 2008;39:469–482. doi: 10.1016/j.neuroimage.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Trusting our memories: dissociating the neural correlates of confidence in veridical vs. illusory memories. J Neurosci. 2007;27:12190–12197. doi: 10.1523/JNEUROSCI.3408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel FA, Johnson KA, Mu Q, Grenesko EL, Laken SJ, George MS. Detecting deception using functional magnetic resonance imaging. Biol Psychiatry. 2005;58:605–613. doi: 10.1016/j.biopsych.2005.07.040. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Padgett TM, George MS. A replication study of the neural correlates of deception. Behav Neurosci. 2004;118:852–856. doi: 10.1037/0735-7044.118.4.852. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Revell LJ, Lorberbaum JP, Shastri A, Elhai JD, Horner MD, Smith A, Nahas Z, Bohning DE, George MS. A pilot study of functional magnetic resonance imaging brain correlates of deception in healthy young men. J Neuropsychiatry Clin Neurosci. 2004;16:295–305. doi: 10.1176/jnp.16.3.295. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Loughead JW, Bilker WB, Ruparel K, Childress AR, Busch SI, Gur RC. Telling truth from lie in individual subjects with fast event-related fMRI. Hum Brain Mapp. 2005;26:262–272. doi: 10.1002/hbm.20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langleben DD, Schroeder L, Maldjian JA, Gur RC, McDonald S, Ragland JD, O'Brien CP, Childress AR. Brain activity during simulated deception: an event-related functional magnetic resonance study. Neuroimage. 2002;15:727–732. doi: 10.1006/nimg.2001.1003. [DOI] [PubMed] [Google Scholar]
- Lee TM, Liu HL, Chan CC, Ng YB, Fox PT, Gao JH. Neural correlates of feigned memory impairment. Neuroimage. 2005;28:305–313. doi: 10.1016/j.neuroimage.2005.06.051. [DOI] [PubMed] [Google Scholar]
- Lee TM, Liu HL, Tan LH, Chan CC, Mahankali S, Feng CM, Hou J, Fox PT, Gao JH. Lie detection by functional magnetic resonance imaging. Hum Brain Mapp. 2002;15:157–164. doi: 10.1002/hbm.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus EF. Make-believe memories. Am Psychol. 2003;58:518–537. doi: 10.1037/0003-066X.58.11.867. [DOI] [PubMed] [Google Scholar]
- Miyaji Y, Yama H. Making Japanese lists which induce false memory at high probability for the DRM paradigm [in Japanese] Jpn J Psychon Sci. 2002;21:21–26. [Google Scholar]
- Mohamed FB, Faro SH, Gordon NJ, Platek SM, Ahmad H, Williams JM. Brain mapping of deception and truth telling about an ecologically valid situation: functional MR imaging and polygraph investigation—initial experience. Radiology. 2006;238:679–688. doi: 10.1148/radiol.2382050237. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nunez JM, Casey BJ, Egner T, Hare T, Hirsch J. Intentional false responding shares neural substrates with response conflict and cognitive control. Neuroimage. 2005;25:267–277. doi: 10.1016/j.neuroimage.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Okado Y, Stark C. Neural processing associated with true and false memory retrieval. Cogn Affect Behav Neurosci. 2003;3:323–334. doi: 10.3758/cabn.3.4.323. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Phan KL, Magalhaes A, Ziemlewicz TJ, Fitzgerald DA, Green C, Smith W. Neural correlates of telling lies: a functional magnetic resonance imaging study at 4 Tesla. Acad Radiol. 2005;12:164–172. doi: 10.1016/j.acra.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Priori A, Mameli F, Cogiamanian F, Marceglia S, Tiriticco M, Mrakic-Sposta S, Ferrucci R, Zago S, Polezzi D, Sartori G. Lie-specific involvement of dorsolateral prefrontal cortex in deception. Cereb Cortex. 2008;18:451–455. doi: 10.1093/cercor/bhm088. [DOI] [PubMed] [Google Scholar]
- Roediger HL. Memory illusions. J Mem Lang. 1996;35:76–100. [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: remembering words not presented in lists. J Exp Psychol Learn Mem Cogn. 1995;21:803–814. [Google Scholar]
- Schacter DL. The seven sins of memory. Insights from psychology and cognitive neuroscience. Am Psychol. 1999;54:182–203. doi: 10.1037//0003-066x.54.3.182. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL, Koutstaal W, Dale AM, Rosen BR. Late onset of anterior prefrontal activity during true and false recognition: an event-related fMRI study. Neuroimage. 1997;6:259–269. doi: 10.1006/nimg.1997.0305. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Reiman E, Curran T, Yun LS, Bandy D, McDermott KB, Roediger HL., 3rd Neuroanatomical correlates of veridical and illusory recognition memory: evidence from positron emission tomography. Neuron. 1996;17:267–274. doi: 10.1016/s0896-6273(00)80158-0. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Slotnick SD. The cognitive neuroscience of memory distortion. Neuron. 2004;44:149–160. doi: 10.1016/j.neuron.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Seth AK, Iversen JR, Edelman GM. Single-trial discrimination of truthful from deceptive responses during a game of financial risk using alpha-band MEG signals. Neuroimage. 2006;32:465–476. doi: 10.1016/j.neuroimage.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. A sensory signature that distinguishes true from false memories. Nat Neurosci. 2004;7:664–672. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- Spence SA, Farrow TF, Herford AE, Wilkinson ID, Zheng Y, Woodruff PW. Behavioural and functional anatomical correlates of deception in humans. Neuroreport. 2001;12:2849–2853. doi: 10.1097/00001756-200109170-00019. [DOI] [PubMed] [Google Scholar]
- Spence SA, Hunter MD, Farrow TF, Green RD, Leung DH, Hughes CJ, Ganesan V. A cognitive neurobiological account of deception: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2004;359:1755–1762. doi: 10.1098/rstb.2004.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M. Experimental tests with the Japanese emotional word lists that create false memories. [in Japanese]. Seishin Stud. 2001;96:133–156. [Google Scholar]
- Tanji J. The supplementary motor area in the cerebral cortex. Neurosci Res. 1994;19:251–268. doi: 10.1016/0168-0102(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–12. [Google Scholar]
- Ueno A, Abe N, Suzuki M, Hirayama K, Mori E, Tashiro M, Itoh M, Fujii T. Reactivation of medial temporal lobe and occipital lobe during the retrieval of color information: a positron emission tomography study. Neuroimage. 2007;34:1292–1298. doi: 10.1016/j.neuroimage.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Umeda S, Akine Y, Kato M, Muramatsu T, Mimura M, Kandatsu S, Tanada S, Obata T, Ikehira H, Suhara T. Functional network in the prefrontal cortex during episodic memory retrieval. Neuroimage. 2005;26:932–940. doi: 10.1016/j.neuroimage.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Umemoto T. Renso Kijunhyo (free association norms) [in Japanese]. Tokyo: University of Tokyo Press; 1969. [Google Scholar]
- Underwood BJ. False recognition produced by implicit verbal responses. J Exp Psychol. 1965;70:122–129. doi: 10.1037/h0022014. [DOI] [PubMed] [Google Scholar]
- von Zerssen GC, Mecklinger A, Opitz B, von Cramon DY. Conscious recollection and illusory recognition: an event-related fMRI study. Eur J Neurosci. 2001;13:2148–2156. doi: 10.1046/j.0953-816x.2001.01589.x. [DOI] [PubMed] [Google Scholar]
- Wells GL, Olson EA. Eyewitness testimony. Annu Rev Psychol. 2003;54:277–295. doi: 10.1146/annurev.psych.54.101601.145028. [DOI] [PubMed] [Google Scholar]