Abstract
Electrophysiological studies have suggested that the activity of the primary motor cortex (M1) during ipsilateral hand movement reflects both the ipsilateral innervation and the transcallosal inhibitory control from its counterpart in the opposite hemisphere, and that their asymmetry might cause hand dominancy. To examine the asymmetry of the involvement of the ipsilateral motor cortex during a unimanual motor task under frequency stress, we conducted block-design functional magnetic resonance imaging with 22 normal right-handed subjects. The task involved visually cued unimanual opponent finger movement at various rates. The contralateral M1 showed symmetric frequency-dependent activation. The ipsilateral M1 showed task-related deactivation at low frequencies without laterality. As the frequency of the left-hand movement increased, the left M1 showed a gradual decrease in the deactivation. This data suggests a frequency-dependent increased involvement of the left M1 in ipsilateral hand control. By contrast, the right M1 showed more prominent deactivation as the frequency of the right-hand movement increased. This suggests that there is an increased transcallosal inhibition from the left M1 to the right M1, which overwhelms the right M1 activation during ipsilateral hand movement. These results demonstrate the dominance of the left M1 in both ipsilateral innervation and transcallosal inhibition in right-handed individuals.
Keywords: fMRI, inhibition, ipsilateral, motor control, motor cortex, negative BOLD
Introduction
The involvement of the primary motor cortex (M1) in ipsilateral hand movement is complex. Under transcranial magnetic stimulation (TMS), the recruitment of M1 activity during ipsilateral movement is observed more often in the M1 in the left hemisphere (the left M1) than in the M1 in the right hemisphere (the right M1) in right-handed subjects (Muellbacher et al. 2000; Ziemann and Hallett 2001; Ghacibeh et al. 2006). This finding indicates the asymmetric recruitment of neural activity at the cortical level through the uncrossed corticospinal tract. The ipsilateral motor cortex receives an inhibitory signal from the other side of the motor cortex (Allison et al. 2000). This transcallosal inhibition seems to play a crucial role in suppressing mirror activation of the ipsilateral motor cortex during unilateral hand motor tasks (Nass 1985). This inhibitory effect is asymmetric, such that the left motor cortex has a relatively greater effect on the right motor cortex (Netz et al. 1995). Based on these findings, Ziemann and Hallett (2001) concluded that the asymmetric ipsilateral innervation and transcallosal inhibitory control of the M1 closely interact to produce left hemisphere dominancy over hand control.
Functional magnetic resonance imaging (fMRI) studies have revealed asymmetry of the positive (Kim et al. 1993; Rao et al. 1993) and negative (Nirkko et al. 2001; Newton et al. 2005) blood oxygen level–dependent (BOLD) response in the motor cortex during ipsilateral unimanual motor control. Although the relationship between the negative BOLD response and the electrophysiological inhibitory effect is still controversial, the fMRI results are generally concordant with the electrophysiological findings in terms of asymmetry. Thus, the asymmetric response of the motor cortex during ipsilateral hand movement probably represents the asymmetry of the summation of the ipsilateral innervation and transcallosal inhibitory control (Spraker et al. 2007).
The asymmetry of ipsilateral innervation and transcallosal inhibitory control of the M1 can be observed in bimanual coordination. Repetitive bimanual asymmetric finger-tapping movements tend to spontaneously shift their phase to more stable, symmetrical patterns under frequency stress (Kelso 1984). The nondominant hand is more prone to this phase shift than the dominant hand (Semjen et al. 1995; Kennerley et al. 2002; Aramaki et al. 2006a).
The phenomenon of spontaneous phase transition has been successfully described by the neural cross-talk model (Cattaert et al. 1999). The concept of intermanual cross-talk maintains that 2 independent motor plans exist (Marteniuk and MacKenzie 1980). The lowest level of cross-talk supposedly occurs downstream from the specification of movement parameters, possibly through the ipsilateral corticospinal tract (Cattaert et al. 1999). Some of the signals sent to the contralateral hand also descend ipsilaterally in a mirror image. The ipsilaterally mediated signal activates homologous muscles, in conflict with the primary signal for that hand, which originates contralaterally during asymmetric coordination (Kagerer et al. 2003). As the frequency of movement increases, the conflict appears to become larger. Using TMS, Kagerer et al. (2003) showed that participants with stronger ipsilateral innervation demonstrated a greater instability of asymmetric bimanual movement than those with weaker innervation. Thus, asymmetric ipsilateral innervation of the hand affects bimanual coupling and the phase transition.
During bimanual coordination, there appears to be interhemispheric cross-talk. Using fMRI, Aramaki et al. (2006b) found that the right M1 showed less prominent activation during mirror symmetric hand movement than during unimanual left hand movement at the same frequency (3 Hz), which was close to the phase-transition frequency (around 4 Hz). The left M1 did not show such a change. Aramaki and colleagues suggested that the reduced right M1 activity during the mirror movements might have been caused by increased transcallosal inhibition from the left M1 over the right M1.
These previous studies led us to hypothesize that asymmetric ipsilateral innervation and transcallosal control are frequency dependent; thus, as the sum of these activities, the BOLD responses in M1 during ipsilateral unimanual hand movement should also be frequency dependent. A movement rate-dependent increase of the BOLD signal in the contralateral M1 during finger movement has been reported in several previous studies (Rao et al. 1996; Schlaug et al. 1996; Sadato et al. 1997; Jancke et al. 1998a, 1998b; Khushu et al. 2001; Agnew et al. 2004). However, the rate dependency of ipsilateral motor activity during finger movement has not been investigated comprehensively. The rate effect represents the increased processing demand on M1 (Lutz et al. 2005)—that is, executing rapid movements of the contralateral hand. Similarly, the rate effect in the ipsilateral M1, if there is any, should represent the summation of the effects of ipsilateral innervation and transcallosal inhibition. In the present study, we examined the extent to which the rate effect of the ipsilateral motor cortex during unimanual motor tasks showed hemispheric asymmetry.
Materials and Methods
Subjects
In total, 22 healthy volunteers (13 male, 9 female) aged 21–31 years participated in the fMRI study. All of the subjects were right handed according to the Edinburgh handedness inventory (Oldfield 1971). None of the subjects had a history of neurological or psychiatric illness. The protocol was approved by the Ethical Committee of the National Institute for Physiological Sciences, and all of the subjects gave their written informed consent for participation in the study.
Magnetic Resonance Imaging
A time-course series of 54 volumes was acquired using T2*-weighted gradient-echo echo-planar imaging sequences using a 3-Tesla MR imager (Allegra, Siemens, Erlangen, Germany). Every volume consisted of 34 oblique slices, each 4.0 mm in thickness, with no interslice gap, in order to cover the entire cerebral and cerebellar cortex. The time interval between 2 successive acquisitions of the same slice was 2000 ms with a flip angle of 80° and a 30 ms echo time. The field of view was 192 × 192 mm. The digital in-plane resolution was 64 × 64 pixels with a pixel dimension of 3.0 × 3.0 mm. The head motion was minimized by placing comfortable, but tight-fitting, foam padding around each subject's head. The subjects rested their wrists and arms comfortably on towels. High-resolution whole-brain MR images were also obtained using a T1-weighted 3-dimensional (3D) magnetization-prepared rapid-acquisition gradient-echo sequence (voxel size = 0.9 × 0.9 × 1.0 mm).
Task
Unimanual opponent movements of the right and left index fingers were performed with visual pacing at frequencies of 0.25, 0.5, 1, 1.5, 2, and 4 Hz (Sadato et al. 1997). The orders of the frequency conditions and the hand performance were counterbalanced among the subjects. The participants were taught how to perform a brisk and precise touch of the index finger to the tip of the thumb in response to each stimulus, which was immediately followed by a return to the resting position. Before scanning, all of the subjects practiced this movement, and we confirmed that they could execute the task correctly. All of the sessions consisted of 5 epochs, each 20 s in duration, which comprised 2 alternating epochs for right and left hand performance and 3 rest epochs. During each data acquisition series, the frequency of the movement was kept constant. Each subject performed each frequency condition twice: 1 session was performed starting with the right hand, and the other session was performed starting with the left hand.
The pace-making cue was projected by a liquid crystal display projector (DLA-M2000L, Victor, Yokohama, Japan) onto a half-transparent screen. The screen was viewed by the subjects through a mirror. We confirmed that all of the subjects were able to see the screen at the center of their view. The visual cue was a small circle that blinked on and off at the center of the screen. The visual angle of the cue was about 1 degree. The subjects were required to fixate on the cue circle throughout the session. During the rest period, a white closed circle was presented. During the task period, a white circle filled with red was presented. Presentation software (Neurobehavioral System, Albany, CA) was used to provide the pace-making cue. Throughout the sessions, hand movement was monitored on-line through a color television camera (WV-GP110, Panasonic, Osaka, Japan) placed in the MRI scanner room, and was recorded by a digital video cassette recorder (GV-D1000, Sony, Tokyo, Japan).
We did not record electromyograms mainly for technical and instrumental reasons. As negative M1 responses were found during ipsilateral hand movements, covert muscular contraction was unlikely.
Data Analysis
The 1st 4 volumes of each fMRI session were discarded because of unsteady magnetization, and the remaining 50 volumes per session (a total of 600 volumes per subject) were used for the analysis. The data were analyzed using statistical parametric mapping (SPM5; Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (Mathworks, Sherborn, MA; Friston et al. 1995a, 1995b). Following realignment, all of the images were coregistered to the high-resolution 3D T1-weighted MRI images. The parameters for affine and nonlinear transformation into a template of T1-weighted images that was already fitted to a standard stereotaxic space (Montreal Neurological Institute [MNI] template) were estimated based on the high-resolution 3D T1-weighted MR images using the least-square means (Friston et al. 1995b). The parameters were applied to the coregistered fMRI data. The anatomically normalized fMRI data were filtered using a Gaussian kernel of 8 mm full width at half maximum in the x, y, and z axes.
Individual Analysis
The signal time-course of each subject was modeled with a boxcar function convolved with a hemodynamic-response function, high-pass filtering (128 s) for detrending purpose, and session effects. The 2 regressors were set at the onsets of the right and left hand task periods independently. The individual task-related activity was evaluated using a general linear model (Friston et al. 1995b). The resulting set of voxel values for each comparison constituted a SPM of the t statistic [SPM{t}]. Global mean scaling was not applied, so as not to induce type II errors in the assessment of negative BOLD responses (Aguirre et al. 1998).
To plot the amplitude of the brain activity in M1, we calculated the beta value for each frequency, and the hand conditions in the right M1 and the left M1, where the contralateral local maximum was defined by the main effect contrast for each hand. The beta value is a regression coefficient in the general linear model. In the entire data analysis, we used the same amplitude and form for the regressors. Therefore, the beta value could be used as a measure of the change in the brain activity from the baseline condition (Aramaki et al. 2006b). The right and left M1 were defined in each individual by the local maximum of the SPM{t}, which was highlighted by the main effect contrast for each hand movement across all frequencies. The statistical threshold was set at a family-wise error (FWE) corrected P < 0.05 value (Friston et al. 1996). Each local maximum was confirmed to be located on either the anterior or the posterior bank of the central sulcus (CS) around the motor-hand knob (Yousry et al. 1997) by referring to the individual's high-resolution anatomical MRI data. Three-way repeated measures analysis of variance (ANOVA) was conducted to evaluate the main effects of hemisphere, hand, and movement frequency, and their interaction. The statistical threshold was set at P < 0.05 with a Greenhouse–Geisser correction.
Group Analysis with the Random Effect Model
The summary data for each individual were incorporated into the 2nd-level analysis using a random effect model, in order to make inferences at a population level. The weighted sum of the parameter estimates in the individual analysis constituted “contrast” images, which were used for the group analysis (Friston et al. 1999). The contrast images obtained via the individual analysis represented the task-related increment of the MR signal of each subject. For the contrasts of all frequency and hand performances, a repeated measures 2-way ANOVA was performed for every voxel within the brain, in order to obtain population inferences. The resulting set of voxel values for each contrast constituted the SPM{t}. The threshold was set at FWE-corrected P < 0.05 at the voxel level (Friston et al. 1996). Clusters larger than 40 voxels were reported. The results of the group analysis are shown in Supplementary Figure 1.
Results
Task Performance
All of the subjects performed the finger movements correctly in accordance with the visual cues, as confirmed by on-line observation and retrospective inspection of the video-recorded hand movements.
fMRI Results
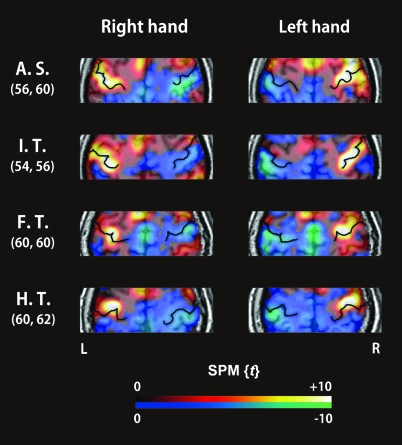
The main effect for each hand showed significant activation in the contralateral M1 (FWE-corrected P < 0.05) in all of the subjects. The coordinates defined as the local maximum of the M1 were in almost symmetrical locations in the right and left hemispheres: the MNI coordinates (average ± standard deviation) of the right and left M1 were x = 38 ± 3.8, y = −22 ± 3.4, z = 58 ± 7.4, and x = −39 ± 5.1, y = −22 ± 6.0, z = 58 ± 6.0, respectively (Table 1). Figure 1 shows representative individual activation maps around the CS for the performance of each task with each hand.
Table 1.
Individual coordinates of the M1
| Subject | Right M1 |
Left M1 |
||||||
| x | y | z | Δy | x | y | z | Δy | |
| S1 | 34 | −18 | 60 | +4 | −42 | −20 | 56 | +4 |
| S2 | 30 | −20 | 60 | +3 | −34 | −22 | 60 | +1 |
| S3 | 40 | −22 | 60 | 0 | −44 | −20 | 64 | 0 |
| S4 | 34 | −22 | 48 | +2 | −34 | −18 | 52 | +4 |
| S5 | 38 | −20 | 62 | +1 | −34 | −20 | 60 | +3 |
| S6 | 34 | −24 | 56 | −1 | −38 | −30 | 54 | 0 |
| S7 | 42 | −26 | 62 | +1 | −40 | −28 | 60 | 0 |
| S8 | 46 | −22 | 54 | +1 | −42 | −24 | 50 | +2 |
| S9 | 38 | −18 | 66 | +1 | −38 | −24 | 60 | 0 |
| S10 | 38 | −22 | 70 | +4 | −38 | −28 | 68 | +5 |
| S11 | 38 | −24 | 50 | −1 | −36 | −30 | 50 | +1 |
| S12 | 40 | −20 | 58 | +4 | −42 | −26 | 54 | −1 |
| S13 | 40 | −28 | 68 | +2 | −46 | −20 | 64 | +2 |
| S14 | 44 | −26 | 58 | −1 | −36 | −30 | 52 | +2 |
| S15 | 42 | −24 | 68 | −3 | −34 | −22 | 70 | 0 |
| S16 | 42 | −14 | 50 | +6 | −42 | −2 | 58 | +6 |
| S17 | 36 | −24 | 48 | +1 | −34 | −24 | 50 | 0 |
| S18 | 42 | −20 | 68 | +2 | −54 | −20 | 56 | −3 |
| S19 | 36 | −24 | 54 | −2 | −40 | −18 | 66 | +1 |
| S20 | 38 | −18 | 52 | +2 | −38 | −20 | 52 | +5 |
| S21 | 38 | −24 | 46 | +1 | −34 | −24 | 56 | 0 |
| S22 | 36 | −26 | 50 | 0 | −32 | −22 | 54 | 0 |
| Mean ± SD | 38 ± 3.8 | −22 ± 3.4 | 58 ± 7.4 | +1 ± 2.2 | −39 ± 5.1 | −22 ± 6.0 | 58 ± 6.0 | +1 ± 2.3 |
Note: All of the coordinates are represented in standard stereotaxic space. Δy represents the distance from the CS (+ indicates anterior to the CS; − indicates posterior to the CS).
Figure 1.
Representative SPM{t} values from around the CS during finger movements (main effect of hand movement) for all frequencies superimposed on the individual's high-resolution MRI data. In the color scale, red to yellow represent positive t-values (an increase in the BOLD signal), whereas blue to green represent negative t-values (a decrease in the BOLD signal). The enhanced black lines on the activation maps represent the CS. The numbers on the left indicate the z coordinates of the 2 axial slices (mm), which represent the distance from the transaxial plane including the anterior commissure–posterior commissure (AC–PC) line.
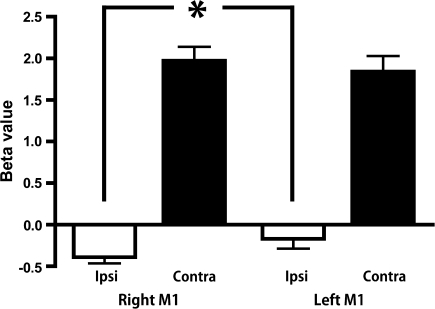
To evaluate the main effects of hemisphere (right/left) and hand (ipsilateral/contralateral), the task-related activity of M1 was collapsed across all of the frequencies. Two-way repeated measures ANOVA revealed significant effects of hand (F1, 21 = 161.866, P < 0.001), and the hemisphere × hand interaction (F1, 21 = 5.402, P = 0.030). The effect of hemisphere (F1, 21 = 0.287, P = 0.598) was not significant. The predefined contrast showed significant suppression of the right M1 compared with the left M1 during ipsilateral hand movement (F1, 21 = 5.693, P = 0.027), whereas the differences during contralateral hand movement were not significant (F1, 21 = 0.951, P = 0.341; Fig. 2).
Figure 2.
Task-related BOLD signal changes of the left and right M1 during ipsilateral and contralateral hand movements. *P = 0.027 (F1, 21 =5.693, 1-way repeated measures ANOVA with predefined contrast).
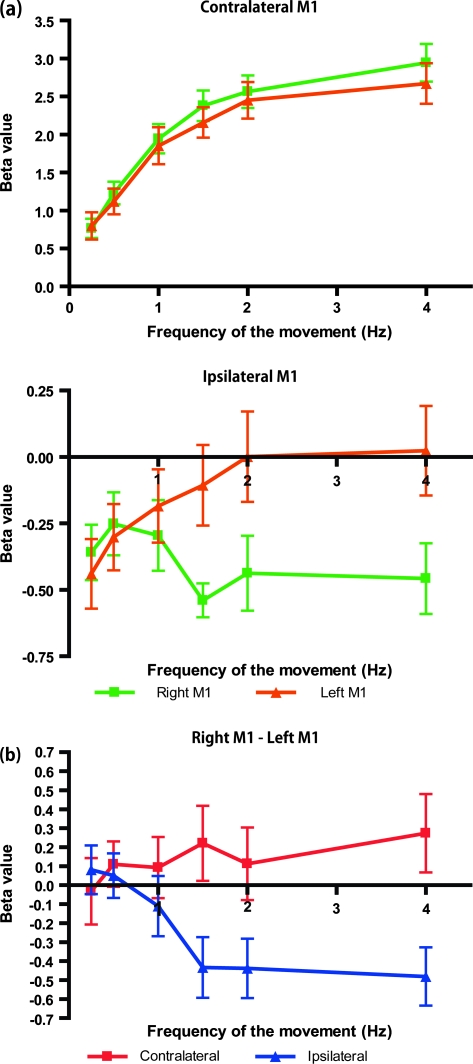
The averages of the individual beta values for each frequency condition are shown in Figure 3a. The contralateral M1 showed a frequency-dependent increase without a hemispheric effect (2-way repeated measures ANOVA; [right/left M1] × [frequency]; F5, 105 = 0.686, P = 0.635). By contrast, the ipsilateral M1 showed an asymmetric frequency-dependent change (2-way repeated measures ANOVA; (right/left M1) × (frequency); F5, 105 = 4.372, P = 0.001). The M1 ipsilateral to the performance hand showed task-related deactivation (a negative BOLD response) at 0.25 and 0.5 Hz without laterality. The left M1 showed a gradual decrease of deactivation as the frequency of the left hand increased. The right M1 showed more prominent deactivation as the frequency of the right hand increased. To demonstrate how these asymmetries of the beta value changed, the subtraction of the beta value (right M1 − left M1) is shown in Figure 3b. The asymmetry of the ipsilateral M1 activity was enhanced as the frequency of the movement increased; by contrast, the contralateral M1 did not show such a change. A 3-way repeated measures ANOVA (hand: contralateral and ipsilateral) × (hemisphere: right and left) × (frequency of movement: 0.25, 0.5, 1, 1.5, 2, and 4 Hz) showed significant main effects of hand (F1, 21 = 161.866, P < 0.001) and frequency (F3.214, 67.499 = 33.223, P < 0.001), and the interactions of (hand × hemisphere; F1, 21 = 5.402, P = 0.030), (hand × frequency; F2.378, 49.937 = 68.160, P < 0.001), and (hand × hemisphere × frequency; F5, 105 = 2.877, P = 0.018). The main effects of hemisphere and the interaction of (hemisphere × frequency) were not significant (hemisphere: F1, 21 = 0.287, P = 0.598; hemisphere × frequency: F2.710, 56.901 = 1.608, P = 0.201).
Figure 3.
Average beta values for each frequency condition. (a) The beta values measured at the right (green) and left (orange) M1 areas, contralateral (top) and ipsilateral (bottom) to the task hand. (b) Subtraction of the beta values (right M1 − left M1) for the contralateral and ipsilateral M1 areas shown in (a). The ipsilateral M1 showed an asymmetric activity pattern, whereas that of the contralateral M1 was symmetric. All data represent the mean ± SEM.
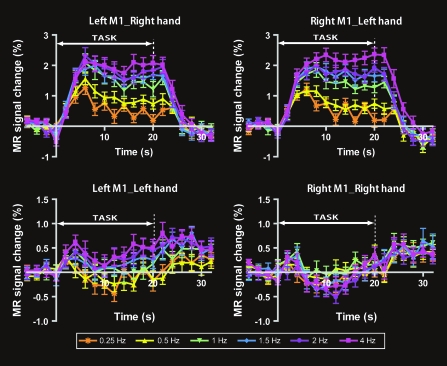
Figure 4 shows the averaged time-course of the M1 activity. The M1 contralateral to the task hand showed a positive hemodynamic change in all frequency conditions. By contrast, the ipsilateral M1 showed a negative or slightly positive MR signal change.
Figure 4.
Average time-courses across all subjects (N = 22) for each M1 area during task performance with the right and left hands. The M1 contralateral to the task hand showed a positive hemodynamic response. By contrast, the right M1 during right-hand movement showed a negative response. The percent MRI signal change compared with the average value of the 4 time points just before starting the task epoch were averaged across subjects for each time point. These data represent the mean ± SEM.
To depict the area where there was a frequency-dependent change of the BOLD signal, a whole-brain 2-way repeated measures ANOVA was conducted. The contrast of the main effect of frequency during right-hand movement highlighted the contralateral M1 and the ipsilateral cerebellum (Supplementary Fig. 1, top). By contrast, the left hand movement highlighted the thalamus and the secondary somatosensory cortex (S2), in addition to the contralateral M1 and the ipsilateral cerebellum (Supplementary Fig. 1, bottom). A plot of the beta values revealed a monotonic increase in the signal change (Supplementary Fig. 1, graphs).
Discussion
BOLD Response
The positive fMRI BOLD signal is coupled with the cerebral metabolic rate of oxygen (CMRO2; Smith et al. 2002; Stefanovic et al. 2004) and the neural activity measured by the local field potential (Logothetis 2002; Mukamel et al. 2005). The negative BOLD signal is also tightly coupled with cerebral blood flow (CBF) and CMRO2 in the human visual cortex (Shmuel et al. 2002) and M1 (Stefanovic et al. 2004), and with cerebral blood volume in the primary visual cortex (V1) of anesthetized cats (Harel et al. 2002). Recent simultaneous fMRI and electrophysiological measurements in the monkey V1 revealed that both the positive and negative BOLD responses were strongly correlated with neuronal activity (Shmuel et al. 2006). Hence, a negative BOLD response in the ipsilateral M1 represents a decrease in neural activity.
Region of Interest Analysis
We defined the M1 of each subject by the local maximal response to the contralateral hand movement across all of the frequencies, under anatomical constraints (Table 1). This was because the group analysis with spatial normalization and the voxel-by-voxel calculation of the task-related activation had lost the specific characteristics of the ipsilateral representation of M1 (Nirkko et al. 2001).
Negative Response in the Ipsilateral M1
We showed that the ipsilateral M1 was deactivated (Fig. 1), and that this phenomenon was more prominent during right-hand execution than left hand execution (Fig. 2). This was consistent with the results of Nirkko et al. (2001), who showed that the anterior and posterior wall of the CS was exclusively activated by contralateral hand movement, and deactivated by ipsilateral hand movement. The anterior part of the precentral gyrus, corresponding to the premotor cortex, was activated by contralateral and ipsilateral distal hand movements. By contrast, proximal shoulder movement activated the M1 bilaterally (Nirkko et al. 2001). Regarding the positive BOLD response of the ipsilateral M1 reported previously (Kim et al. 1993), Nirkko et al. (2001) suggested that poor spatial resolution had made it difficult to differentiate the deactivation of the M1 and the activation of the premotor cortex. They also argued that tonic proximal activity could be a source of contamination, because there was a tendency for subjects to lift their hands from the surface when finger tapping and to relax their hands during rest periods. Several recent studies have shown a negative BOLD response in the right M1 during ipsilateral hand movement (Allison et al. 2000; Hamzei et al. 2002; Stefanovic et al. 2004; Newton et al. 2005). These findings led us to conclude that the ipsilateral M1 was deactivated during distal hand movement.
Frequency-Dependent Response in M1
Contralateral M1
A monotonous increase of the BOLD signal change was observed in the contralateral M1 without laterality. Many previous reports described a similar relationship between the repetition rate of finger movement and the activity change in the contralateral motor cortex, as measured by positron-emission tomography (PET; VanMeter et al. 1995; Blinkenberg et al. 1996; Sadato et al. 1996; Sadato et al. 1997) and fMRI (Rao et al. 1996; Schlaug et al. 1996; Sadato et al. 1997; Jancke et al. 1998a, 1998b; Khushu et al. 2001; Agnew et al. 2004). This “rate effect” has been attributed to the increasing processing demands in M1 (Lutz et al. 2005). The symmetric frequency dependency is consistent with a previous study in which subjects executed thumb flexions at a variable rate with their right and left hands (Agnew et al. 2004).
Ipsilateral M1
During ipsilateral hand movement, across all of the frequencies examined, there was no significant positive response in M1 on either side. This was consistent with the notion that transcallosal inhibition is crucial in suppressing the mirror activation of the ipsilateral motor cortex during intended unilateral hand motor tasks (Nass 1985; Allison et al. 2000). In children up to 10 years old, mirror movement is common (Connolly and Stratton 1968), although this phenomenon gradually disappears thereafter (Nass 1985; Muller et al. 1997; Heinen et al. 1998; Mayston et al. 1999). This fact has been attributed to maturation of the transcallosal inhibitory system (Danek et al. 1992). The evidence suggests that interhemispheric inhibition suppresses the M1 coactivation, allowing independent movement and unimanual control.
At lower frequencies, M1 in both hemispheres showed symmetrical negative responses. As the frequency of the movement increased, the left M1 gradually increased up to a baseline, whereas the right M1 showed a gradual decrease in the task-related signal response. Hence, there was an asymmetric frequency dependency of the M1 response during ipsilateral hand movement.
The ipsilateral innervation of the left M1 was more prominent than that of the right M1. Previous repetitive TMS studies demonstrated that transient disturbance of the left M1 had a greater effect on ipsilateral motor control during the execution of complex finger movements (Chen et al. 1997). A lesion study revealed that left hemisphere stroke disrupted ipsilateral motor performance more severely than right hemisphere stroke (Haaland and Harrington 1994). This indicated that left motor cortex activation was required not only for contralateral motor control but also for ipsilateral motor control through the ipsilateral uncrossed corticospinal projection.
Another component that might affect ipsilateral M1 activity is interhemispheric interaction between the right and left motor areas, which could be mediated via the corpus callosum (Di Lazzaro et al. 1999). Netz et al. (1995) reported the asymmetry of the inhibitory effects by applying TMS conditioning stimuli contralateral to the task hand, followed by a test stimulus on the other side of the motor cortex. Netz and colleagues showed that the left hemisphere had a greater inhibitory effect. Liepert et al. (2001) showed that the interhemispheric interaction between the motor areas depends on the type of unilateral pinch grip performed. Tonic contractions enhanced the MEPs in the homologous muscles, particularly during higher force conditions, consistent with previous studies, including Stinear et al. (2001). On the other hand, low-force phasic pinch grips induced a decrease in the TMS-induced MEPs. Liepert et al. (2001) speculated that the decreased excitability during phasic pinch grip could improve the capacity to perform fine finger movements, which are usually carried out unilaterally. This finding indicates that the specific type of natural movement also induces interhemispheric inhibition. In imaging studies, these areas showed a reduction in the neural response to the low-force pinch-grip task (Hamzei et al. 2002; Stefanovic et al. 2004). Newton et al. (2005) conducted fMRI while subjects made a button press with their thumb. They found a decrease in BOLD signal in the ipsilateral M1. The generation of low-force hand grips has been shown to reduce the cortical excitability of M1 ipsilateral to the movement in response to TMS test pulses (Ferbert et al. 1992; Liepert et al. 2001) and also to reduce the BOLD signal measured from this area (Hamzei et al. 2002; Stefanovic et al. 2004). Considering the similarity of the tasks, Newton et al. (2005) speculated that “the observed negative BOLD responses may reflect a reduction in cortical excitability in M1 as a consequence of button pressing with the ipsilateral thumb”. Using TMS, Waldvogel et al. (2000) showed that the no-go condition of a go/no-go task inhibits the primary motor cortex. This inhibition evoked no measurable change in the BOLD signal in the motor cortex. The authors concluded that inhibition is less metabolically demanding, and thus the positive BOLD response results from excitation rather than inhibition. From these previous studies, the observed negative BOLD response likely reflects the reduction of cortical excitability.
This interpretation is consistent with the model proposed to explain the cortical activation ipsilateral to an active hand (Ghacibeh et al. 2006). The model assumes that distal hand movements are initially generated bilaterally, and only during the final preparation phase does the movement become unilateral due to transcallosal inhibition (Rossini et al. 1988; Britton et al. 1991). The dominant hemisphere exerts a more potent action on the nondominant hemisphere via asymmetric interhemispheric inhibition (Ziemann and Hallett 2001). This model is supported by electroencephalography studies of the Bereitshaftspotential, which initially develops bilaterally and only becomes lateralized a few hundred milliseconds prior to movement onset (Shibasaki and Nagae 1984; Kristeva et al. 1991). Because of the poor temporal resolution of fMRI compared with EEG, the activity of the ipsilateral M1 might represent the net effect of transcallosal inhibition and activation by the ipsilateral hand movement. At lower frequencies, both M1s were suppressed during ipsilateral movement. The left M1 showed a gradual increase of signal up to the baseline as the frequency of the left hand increased. This might indicate increased involvement of the left M1 in ipsilateral hand control in a frequency-dependent manner. The “rate” effect refers to the frequency-dependent recruitment of the left ipsilateral M1, from which the neural signal is sent to the ipsilateral hand through the ipsilateral corticospinal tract. By contrast, the right M1 showed a further decrease of signal as the frequency of the right hand increased. This suggests increased transcallosal inhibition to the right M1, which surpassed the involvement of the right M1 in ipsilateral hand control. Here we assume that the ipsilateral innervation is independent from the interhemispheric inhibition. This assumption is supported by an electrophysiological study (Lee et al. 2007), which suggests that the transcallosal fibers mediating interhemispheric inhibition and the corticospinal output system arise from different neuronal populations. These results demonstrate the dominance of the left M1 in both ipsilateral innervation and transcallosal inhibition under frequency-stress conditions (Fig. 5).
Figure 5.
Possible scheme for asymmetric ipsilateral motor systems originating from M1. For low-frequency movements (top row), the ipsilateral M1 is suppressed symmetrically by an inhibitory system, such as transcallosal inhibition from the contralateral M1 (horizontal line). For high-frequency movements of the right hand (bottom left), both the control of the contralateral hand and the transcallosal inhibition from the left M1 increase, resulting in a frequency-dependent increase of contralateral activation and ipsilateral deactivation. During left hand movement at high frequencies (bottom right), the ipsilateral activation of the left M1 increases, apparently canceling out the transcallosal inhibition from the right M1.
Cerebral Dominance
Ziemann and Hallett (2001) proposed 2 different, although not mutually exclusive, models to explain the functional differences of the human cerebral hemispheres. One model assumes that asymmetrical motor performance is a consequence of intrinsic hemispheric specialization. The other proposes that both motor cortices have identical motor capabilities in controlling the contralateral hands, but that hemispheric differences occur due to asymmetric inhibitory interactions between the 2 motor cortices. Our study revealed asymmetry of the rate-dependent change of the ipsilateral M1 response, such that the left M1 was dominant for both interhemispheric inhibition and ipsilateral innervation. These findings support the 2nd model, which proposes that the asymmetric interaction between the primary motor cortices contributes to cerebral dominance, at least in right-handed subjects.
Sensory Feedback
The task-related activity of M1 might be affected by tactile feedback associated with hand movement. Using functional MRI, Hlushchuk and Hari (2006) showed that the unilateral touching of the fingers is associated with deactivation of the ipsilateral primary somatosensory cortex and M1 in both hemispheres. However, the suppression did not show any frequency dependency or asymmetry (Hlushchuk and Hari 2006). Therefore, it is not likely that a suppressive sensory-evoked response induced by finger movement can explain the asymmetric frequency-dependent change in the M1 during finger movement in the present study.
Other components included in finger movements.
There are several parameters which were not measured in the present study, but still worth consideration, such as amplitude, force, and precision of the movement.
Amplitude and Force
The frequency of movement, force, and the amplitude may covary. In the current study, we defined “performance” as “the correct movement made in time with the frequency of the cues,” and manipulated movement pacing parametrically with visual pacing cues. We used videotape to confirm that the finger movements were well-synchronized with the visual pacing cues. The present study replicated the previous PET and fMRI studies investigating the frequency dependency of the response of M1 to contralateral finger tapping, and thus the experimental setup used was appropriate.
It is possible that a higher frequency of this movement might require more force than the same movement performed at a lower frequency. Dettmers et al. (1995) investigated the ipsilateral right M1 activity during various force levels for the right hand. They showed that lower finger pressure reduced the regional CBF, but increased the value compared with the baseline at a higher force level. This was associated with electromyographic evidence of contraction of the left shoulder muscles at the highest force levels in some of the participants. Dettmers and colleagues interpreted the negative response at the lower force level as transcallosal inhibition, which is essential for the execution of a unilateral task.
Recently, Spraker et al. (2007) conducted an fMRI study with a pinch grip task at forces which ranged from 5 to 80% of the maximum voluntary contraction. The region of interest analysis revealed that a portion of the M1/S1 was activated and the other portion was deactivated. The activated voxels in the ipsiltateral M1/S1 showed a significant positive force effect, the slope of which was smaller than that of the contralateral M1/S1. The deactivation in the ipsilateral M1/S1 was more prominent than that in the contralateral M1/S1, and did not show any force effect. Spraker et al. (2007) interpreted this finding as comprising 2 distinct mechanisms, that is, the ipsilateral corticospinal innervation and transcallosal inhibition. The activated portion of the ipsilateral M1/S1 is related to the regulation of the smaller motor units that control force through the ipsilateral innervation. The deactivated portion of the ipsilateral M1/S1 was interpreted as the asymmetric transcallosal inhibition. These results indicate that the ipsilateral S1/M1 deactivation is not sensitive to the force level, at least in the lower force ranges.
In the present study, we examined the full range of movement frequencies from 0.25 Hz up to 4 Hz (Sadato et al. 1996). Previously, Inui et al. (1998) recorded the force while subjects performed the tapping task with the index finger at various frequencies. The generated forces were 0.95 N at 1.2 Hz, 0.48 N at 2.6 Hz, and 0.48 N at 4.3 Hz. Kuhtz-Buschbeck et al. (2001) recorded the static grip forces while holding a small object with precision. Normal grip generated a force of 1.83 N, which was 2.8% of the maximum voluntary grip force. These previous data suggest that the force range during opponent finger tapping is likely to be relatively narrow at low levels. As the ipsilateral S1/M1 deactivation is not sensitive to force levels, at least in the low-force range, the correlational changes found in the present study likely reflect the effects of frequency rather than force. This speculation should be examined in future studies.
Precision of the Movement
In the present study, we did not measure precision which could be measured as the deviation from the ideal intertap interval (ITI, Aramaki et al. 2006b). There is an asymmetric capability of finger movement. The dominant hand showed shorter ITIs (range from 130 to 180 ms; corresponds to from 7.7 to 5.5 Hz) than the nondominant hand (range from 160 to 200 ms; corresponds to from 6.25 to 5 Hz) (Jancke et al. 2004; Lutz et al. 2005). In the present study, the highest ITI was 250 ms (4 Hz), indicating that both hands potentially have a capability to execute current task correctly.
To make these points clearer, it needs further studies with simultaneous measurement of force, amplitude, and timing of the movement.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Grant-in Aid for Scientific Research (S#17100003) to N.S. from the Japan Society for the Promotion of Science.
Supplementary Material
Acknowledgments
We would like to thank K. Izuma for assistance with fMRI data acquisition. Conflict of Interest: None declared.
References
- Agnew JA, Zeffiro TA, Eden GF. Left hemisphere specialization for the control of voluntary movement rate. Neuroimage. 2004;22:289–303. doi: 10.1016/j.neuroimage.2003.12.038. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D'Esposito M. The inferential impact of global signal covariates in functional neuroimaging analyses. Neuroimage. 1998;8:302–306. doi: 10.1006/nimg.1998.0367. [DOI] [PubMed] [Google Scholar]
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC. Functional MRI cerebral activation and deactivation during finger movement. Neurology. 2000;54:135–142. doi: 10.1212/wnl.54.1.135. [DOI] [PubMed] [Google Scholar]
- Aramaki Y, Honda M, Okada T, Sadato N. Neural correlates of the spontaneous phase transition during bimanual coordination. Cereb Cortex. 2006a;16:1338–1348. doi: 10.1093/cercor/bhj075. [DOI] [PubMed] [Google Scholar]
- Aramaki Y, Honda M, Sadato N. Suppression of the non-dominant motor cortex during bimanual symmetric finger movement: a functional magnetic resonance imaging study. Neuroscience. 2006b;141:2147–2153. doi: 10.1016/j.neuroscience.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Blinkenberg M, Bonde C, Holm S, Svarer C, Andersen J, Paulson OB, Law I. Rate dependence of regional cerebral activation during performance of a repetitive motor task: a PET study. J Cereb Blood Flow Metab. 1996;16:794–803. doi: 10.1097/00004647-199609000-00004. [DOI] [PubMed] [Google Scholar]
- Britton TC, Meyer BU, Benecke R. Central motor pathways in patients with mirror movements. J Neurol Neurosurg Psychiatr. 1991;54:505–510. doi: 10.1136/jnnp.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaert D, Semjen A, Summers JJ. Simulating a neural cross-talk model for between-hand interference during bimanual circle drawing. Biol Cybern. 1999;81:343–358. doi: 10.1007/s004220050567. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Hallett M, Cohen LG. Involvement of the ipsilateral motor cortex in finger movements of different complexities. Ann Neurol. 1997;41:247–254. doi: 10.1002/ana.410410216. [DOI] [PubMed] [Google Scholar]
- Connolly K, Stratton P. Developmental changes in associated movements. Dev Med Child Neurol. 1968;10:49–56. doi: 10.1111/j.1469-8749.1968.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Danek A, Heye B, Schroedter R. Cortically evoked motor responses in patients with Xp22.3-linked Kallmann's syndrome and in female gene carriers. Ann Neurol. 1992;31:299–304. doi: 10.1002/ana.410310312. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol. 1995;74:802–815. doi: 10.1152/jn.1995.74.2.802. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Ferrara L, Saturno E, Pilato F, Tonali P. The diagnostic value of motor evoked potentials. Clin Neurophysiol. 1999;110:1297–1307. doi: 10.1016/s1388-2457(99)00060-7. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Poline J, Frith C, Heather J, Frackowiak R. Spatial registration and normalization of images. Hum Brain Mapp. 1995a;2:165–189. [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995b;2:189–210. [Google Scholar]
- Ghacibeh GA, Mirpuri R, Drago V, Jeong Y, Heilman KM, Triggs WJ. Ipsilateral motor activation during unimanual and bimanual motor tasks. Clin Neurophysiol. 2006;7:7. doi: 10.1016/j.clinph.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Limb-sequencing deficits after left but not right hemisphere damage. Brain Cogn. 1994;24:104–122. doi: 10.1006/brcg.1994.1006. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Dettmers C, Rzanny R, Liepert J, Buchel C, Weiller C. Reduction of excitability (“inhibition”) in the ipsilateral primary motor cortex is mirrored by fMRI signal decreases. Neuroimage. 2002;17:490–496. doi: 10.1006/nimg.2002.1077. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Heinen F, Glocker FX, Fietzek U, Meyer BU, Lucking CH, Korinthenberg R. Absence of transcallosal inhibition following focal magnetic stimulation in preschool children. Ann Neurol. 1998;43:608–612. doi: 10.1002/ana.410430508. [DOI] [PubMed] [Google Scholar]
- Hlushchuk Y, Hari R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci. 2006;26:5819–5824. doi: 10.1523/JNEUROSCI.5536-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui N, Ichihara T, Minami T, Matsui A. Interactions: timing and force control of finger-tapping sequences. Percept Motor Skills. 1998;86:1395–1401. doi: 10.2466/pms.1998.86.3c.1395. [DOI] [PubMed] [Google Scholar]
- Jancke L, Peters M, Schlaug G, Posse S, Steinmetz H, Muller-Gartner H. Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Brain Res Cogn Brain Res. 1998a;6:279–284. doi: 10.1016/s0926-6410(98)00003-2. [DOI] [PubMed] [Google Scholar]
- Jancke L, Specht K, Mirzazade S, Loose R, Himmelbach M, Lutz K, Shah NJ. A parametric analysis of the ‘rate effect’ in the sensorimotor cortex: a functional magnetic resonance imaging analysis in human subjects. Neurosci Lett. 1998b;252:37–40. doi: 10.1016/s0304-3940(98)00540-0. [DOI] [PubMed] [Google Scholar]
- Jancke L, Steinmetz H, Benilow S, Ziemann U. Slowing fastest finger movements of the dominant hand with low-frequency rTMS of the hand area of the primary motor cortex. Exp Brain Res. 2004;155:196–203. doi: 10.1007/s00221-003-1719-7. [DOI] [PubMed] [Google Scholar]
- Kagerer FA, Summers JJ, Semjen A. Instabilities during antiphase bimanual movements: are ipsilateral pathways involved? Exp Brain Res. 2003;151:489–500. doi: 10.1007/s00221-003-1496-3. [DOI] [PubMed] [Google Scholar]
- Kelso JA. Phase transitions and critical behavior in human bimanual coordination. Am J Physiol. 1984;246:R1000–R1004. doi: 10.1152/ajpregu.1984.246.6.R1000. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Diedrichsen J, Hazeltine E, Semjen A, Ivry RB. Callosotomy patients exhibit temporal uncoupling during continuous bimanual movements. Nat Neurosci. 2002;5:376–381. doi: 10.1038/nn822. [DOI] [PubMed] [Google Scholar]
- Khushu S, Kumaran SS, Tripathi RP, Gupta A, Jain PC, Jain V. Functional magnetic resonance imaging of the primary motor cortex in humans: response to increased functional demands. J Biosci. 2001;26:205–215. doi: 10.1007/BF02703644. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993;261:615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Kristeva R, Cheyne D, Deecke L. Neuromagnetic fields accompanying unilateral and bilateral voluntary movements: topography and analysis of cortical sources. Electroencephalogr Clin Neurophysiol. 1991;81:284–298. doi: 10.1016/0168-5597(91)90015-p. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Ehrsson HH, Forssberg H. Human brain activity in the control of fine static precision grip forces: an fMRI study. Eur J Neurosci. 2001;14:382–390. doi: 10.1046/j.0953-816x.2001.01639.x. [DOI] [PubMed] [Google Scholar]
- Lee H, Gunraij C, Chen R. The effects of inhibitory and facilitatory intracortical circuits on interhemispheric inhibition in the human motor cortex. J Physiol. 2007;580:1021–1032. doi: 10.1113/jphysiol.2006.126011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Dettmers C, Terborg C, Weiller C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol. 2001;112:114–121. doi: 10.1016/s1388-2457(00)00503-4. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz K, Koeneke S, Wustenberg T, Jancke L. Asymmetry of cortical activation during maximum and convenient tapping speed. Neurosci Lett. 2005;373:61–66. doi: 10.1016/j.neulet.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Marteniuk RG, MacKenzie CL. Information processing in movement organization and execution. In: Nickerson R, editor. Attention and performance VIII. Hillsdale: Erlbaum; 1980. pp. 29–57. [Google Scholar]
- Mayston MJ, Harrison LM, Stephens JA. A neurophysiological study of mirror movements in adults and children. Ann Neurol. 1999;45:583–594. doi: 10.1002/1531-8249(199905)45:5<583::aid-ana6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol. 2000;111:344–349. doi: 10.1016/s1388-2457(99)00243-6. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Muller K, Kass-Iliyya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol. 1997;42:705–711. doi: 10.1002/ana.410420506. [DOI] [PubMed] [Google Scholar]
- Nass R. Mirror movement asymmetries in congenital hemiparesis: the inhibition hypothesis revisited. Neurology. 1985;35:1059–1062. doi: 10.1212/wnl.35.7.1059. [DOI] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Homberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res. 1995;104:527–533. doi: 10.1007/BF00231987. [DOI] [PubMed] [Google Scholar]
- Newton JM, Sunderland A, Gowland PA. fMRI signal decreases in ipsilateral primary motor cortex during unilateral hand movements are related to duration and side of movement. Neuroimage. 2005;24:1080–1087. doi: 10.1016/j.neuroimage.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Nirkko AC, Ozdoba C, Redmond SM, Burki M, Schroth G, Hess CW, Wiesendanger M. Different ipsilateral representations for distal and proximal movements in the sensorimotor cortex: activation and deactivation patterns. Neuroimage. 2001;13:825–835. doi: 10.1006/nimg.2000.0739. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rao SM, Bandettini PA, Binder JR, Bobholz JA, Hammeke TA, Stein EA, Hyde JS. Relationship between finger movement rate and functional magnetic resonance signal change in human primary motor cortex. J Cereb Blood Flow Metab. 1996;16:1250–1254. doi: 10.1097/00004647-199611000-00020. [DOI] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD, et al. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–2318. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Zarola F, Stalberg E, Caramia M. Pre-movement facilitation of motor-evoked potentials in man during transcranial stimulation of the central motor pathways. Brain Res. 1988;458:20–30. doi: 10.1016/0006-8993(88)90491-x. [DOI] [PubMed] [Google Scholar]
- Sadato N, Ibanez V, Campbell G, Deiber MP, Le Bihan D, Hallett M. Frequency-dependent changes of regional cerebral blood flow during finger movements: functional MRI compared to PET. J Cereb Blood Flow Metab. 1997;17:670–679. doi: 10.1097/00004647-199706000-00008. [DOI] [PubMed] [Google Scholar]
- Sadato N, Ibanez V, Deiber MP, Campbell G, Leonardo M, Hallett M. Frequency-dependent changes of regional cerebral blood flow during finger movements. J Cereb Blood Flow Metab. 1996;16:23–33. doi: 10.1097/00004647-199601000-00003. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Sanes JN, Thangaraj V, Darby DG, Jancke L, Edelman RR, Warach S. Cerebral activation covaries with movement rate. Neuroreport. 1996;7:879–883. doi: 10.1097/00001756-199603220-00009. [DOI] [PubMed] [Google Scholar]
- Semjen A, Summers J, Cattaert D. Hand coordination in bimanual circle drawing. J Exp Psychol Hum Percept Perform. 1995;21:1139–1157. [Google Scholar]
- Shibasaki H, Nagae K. Mirror movement: application of movement-related cortical potentials. Ann Neurol. 1984;15:299–302. doi: 10.1002/ana.410150317. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36:1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol. 2007;98:821–834. doi: 10.1152/jn.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Pike GB. Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage. 2004;22:771–778. doi: 10.1016/j.neuroimage.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Walker KS, Byblow WD. Symmetric facilitation between motor cortices during contraction of ipsilateral hand muscles. Exp Brain Res. 2001;139:101–105. doi: 10.1007/s002210100758. [DOI] [PubMed] [Google Scholar]
- VanMeter JW, Maisog JM, Zeffiro TA, Hallett M, Herscovitch P, Rapoport SI. Parametric analysis of functional neuroimages: application to a variable-rate motor task. Neuroimage. 1995;2:273–283. doi: 10.1006/nimg.1995.1035. [DOI] [PubMed] [Google Scholar]
- Waldvogel D, van Gelderen P, Muellbacher W, Ziemann U, Immisch I, Hallett M. The relative metabolic demand of inhibition and excitation. Nature. 2000;406:995–998. doi: 10.1038/35023171. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus: a new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M. Hemispheric asymmetry of ipsilateral motor cortex activation during unimanual motor tasks: further evidence for motor dominance. Clin Neurophysiol. 2001;112:107–113. doi: 10.1016/s1388-2457(00)00502-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.