Abstract
Activation of motor-related areas has consistently been found during various motor imagery tasks and is regarded as the central mechanism generating motor imagery. However, the extent to which motor execution and imagery share neural substrates remains controversial. We examined brain activity during preparation for and execution of physical or mental finger tapping. During a functional magnetic resonance imaging at 3 T, 13 healthy volunteers performed an instructed delay finger-tapping task either in a physical mode or mental mode. Number stimuli instructed subjects about a finger-tapping sequence. After an instructed delay period, cue stimuli prompted them either to execute the tapping movement or to imagine it. Two types of planning/preparatory activity common for movement and imagery were found: instruction stimulus–related activity represented widely in multiple motor-related areas and delay period activity in the medial frontal areas. Although brain activity during movement execution and imagery was largely shared in the distributed motor network, imagery-related activity was in general more closely related to instruction-related activity than to the motor execution–related activity. Specifically, activity in the medial superior frontal gyrus, anterior cingulate cortex, precentral sulcus, supramarginal gyrus, fusiform gyrus, and posterolateral cerebellum likely reflects willed generation of virtual motor commands and analysis of virtual sensory signals.
Keywords: emulation, motor areas, motor planning, neuroimaging, stimulus response linkage
Introduction
Motor imagery has been defined as an ability to simulate or emulate bodily movement, without being overtly manifested as physical movement (Jeannerod 1994; Decety 1996; Crammond 1997; Grush 2004). This cognitive process is considered to underlie a variety of cognitive and motor behaviors including action observation or understanding (Grafton et al. 1996; Iacoboni et al. 1999), cognitive mental operations (Parsons et al. 1995; Frak et al. 2001; Hanakawa, Honda et al. 2003), and planning of overt movement (Stephan et al. 1995; Deiber et al. 1998).
Physiologically, motor imagery may correspond to activation of the neural correlates of motor representations (Rizzolatti et al. 2002), probably involving subthreshold activation of descending motor pathways. The neural substrates of motor imagery have been extensively studied with neuroimaging. Positron emission tomography and functional magnetic resonance imaging (fMRI) studies on motor imagery have consistently disclosed activity in cortical and subcortical motor areas, which substantially overlap the neural substrates of motor execution (Grezes and Decety 2001). However, the extent to which motor execution and imagery share neural substrates is not yet completely understood. Direct comparison of motor imagery with motor execution demonstrated partially segregated brain regions (Deiber et al. 1998; Gerardin et al. 2000; Hanakawa, Immisch, et al. 2003; Hanakawa et al. 2005). For example, the primary motor cortex (M1) and primary somatosensory cortex (S1) may show mild activity during kinesthetic-type motor imagery (Porro et al. 1996; Ehrsson et al. 2003). However, activity in these areas is typically much greater during motor execution than during motor imagery (Gerardin et al. 2000; Hanakawa, Immisch, et al. 2003; Dechent et al. 2004).
We previously compared neural correlates during a sequential movement and imagery (SMI) task, in which subjects kept track of a tapping sequence according to visually presented numbers in either a mental or movement mode (Hanakawa, Immisch, et al. 2003). In the SMI task, activity common to execution and imagery was revealed in the widely distributed frontoparietal network where Gerardin et al. (2000) found imagery-predominant activity. To obtain objective confirmation of task performance during motor imagery, however, a relatively high-level sensory–cognitive processing component was introduced to the SMI task. A possibility remained that activity required for sensory–cognitive processing was superimposed on “pure” activity for motor execution and imagery, and hence, the disparity between the 2 might have been blurred. To explore this issue, a delayed version of the SMI (dSMI) task was employed in the present study for dissociating sensory–cognitive processing from actual performance of movement or that of motor imagery. In practice, an instruction stimulus (IS) specifying a tapping pattern was followed by an instructed delay period (D1) during which subjects were fully aware of the tapping pattern but did not know which performance mode (movement or imagery) would be demanded. After a variable delay period, a cue stimulus prompted actual performance of the tapping in either an execution or imagery mode. This task period was then followed by another delay period (D2), during which subjects kept in mind a finger to determine the tapping pattern in combination with the IS of the following trial. Similarly to the previous task, subjects were required to keep track of a finger-tapping sequence continuously throughout an experimental run.
Here, the time course and statistical parametric maps of brain activity were investigated during the dSMI task, using a time-resolved fMRI. The aims were 3-fold: 1) to reevaluate movement- and imagery-related activities after separating sensory–cognitive processing from actual motor or imagery performance, 2) to compare movement- and imagery-related activities with sensory–cognitive activity for behavioral planning during IS presentation, and 3) to examine activity corresponding to a preparatory process applicable to both execution and imagery performance during the D1 delay period.
Materials and Methods
Subjects
Thirteen healthy volunteers (6 men and 7 women; mean age ± standard deviation [SD] = 30 ± 8 years; age range = 21–48 years) participated in the study, after giving written informed consent. All subjects were right handed as assessed by Edinburgh Handedness Inventory (Oldfield 1971), and eight of them had participated in the previous study with the SMI task (Hanakawa, Immisch, et al. 2003). None had previous history of any neuropsychiatric disorders. The study protocol was approved by the institutional review board.
SMI Task
Subjects 1st learned the basic principles of the SMI task (Hanakawa, Immisch, et al. 2003). In brief, the subjects 1st memorized a tapping sequence (thumb, index, middle, ring, little, ring, middle, index). Next, they practiced sequences of finger tapping where the number of finger taps was instructed by the visual presentation of an Arabic numeral of 1, 2, or 3. The numerals were presented visually 1 by 1 at a rate of 1.5 Hz for a total of 15 presentations. The stimulus presentation was controlled by SuperLab (Cedrus, Phoenix, AZ) on a Power Macintosh computer (Apple Computer Inc., Cupertino, CA). Subjects always started a full tapping sequence from the thumb. Each IS was selected pseudorandomly from the numerals 1, 2, or 3, which instructed the subjects to proceed with the sequence for that number of taps. For example, suppose that a number series of 3–1–2 is presented. One should tap the thumb, index finger, and middle finger in response to the number 3; next the ring finger in response to the number 1; and then the little and ring fingers in response to the number 2. In the original SMI task, subjects were required to keep track of the tapping sequence throughout a single fMRI run in either a movement or an imagery mode. As long as the subjects completely followed the instructions, they would know the specific finger to move next in the sequence after each performance. The subjects reported the “next finger” after each block, and the report served as a behavioral measure of task performance in both the movement and imagery runs.
dSMI Task
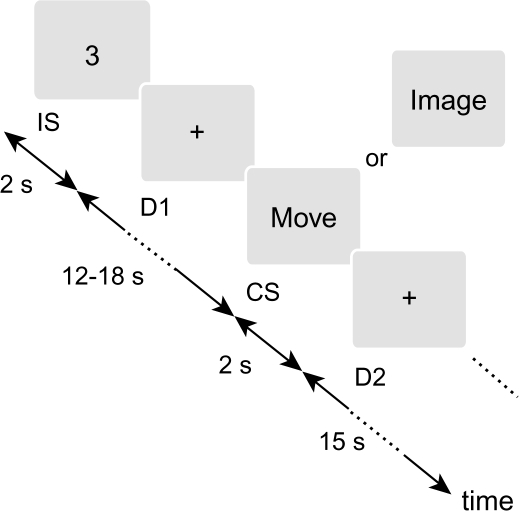
In the dSMI task employed in the present study, subjects were also required to keep track of the tapping sequence throughout an fMRI run. However, each run incorporated both performance modes (movement and imagery) in a pseudorandom manner. Two different classes of visual stimuli were alternately presented (Figure 1). An instruction stimulus (IS) was an Arabic numeral semirandomly selected among 1, 2, and 3 and visually presented for 2 s. Subjects were instructed to withhold any performance and to wait for the next stimulus. After a variable 1st delay period (D1) lasting 12, 15, or 18 s, a cue stimulus was presented for 2 s. The cue stimulus was a visual word stimulus, either MOVE or IMAGE, which told subjects to perform the instructed sequential tapping in either the movement or imagery mode, respectively. Specifically, subjects were required to press response buttons corresponding to the instructed sequence in response to the MOVE stimulus (MOVE trials). In response to the IMAGE stimulus, subjects were asked to imagine the corresponding finger movement as performed by them (1st-person perspective) without actually moving their fingers (IMAGE trials). The MOVE and IMAGE stimuli were pseudorandomly assigned to the cue stimuli, so that subjects were unable to predict the forthcoming performance mode. The cue stimulus was followed by the 2nd delay period (D2) lasting 15 s, during which subjects had to keep in mind the finger from which they would resume tapping. The delay periods were set long enough to allow a time-course analysis dissociating between the 2 classes of stimulus-related hemodynamic changes, and the D1 was set to variable length to reduce an anticipation effect.
Figure 1.
dSMI task. Subjects were asked to remember the instruction stimuli (IS), which were presented for 2 s and specified a segment of the learned finger-tapping sequence. During a variable delay period (D1) for 12–18 s, subjects waited for the presentation of a cue stimulus (CS), which were presented for 2 s and specified a mode of performance, actual movement, or motor imagery. When the word “MOVE” was presented, subjects pressed buttons corresponding to the instructed tapping sequence. When the word “IMAGE” was presented, subjects imagined the same movement as being performed by them. Subjects were required to keep track of the tapping sequence continuously throughout a single experimental run. During a 2nd delay period (D2) for 15 s, subjects therefore remembered the finger from which they would resume tapping for the next set of stimuli.
As in the original SMI task, subjects should be able to press the correct buttons during the MOVE trials, as long as they had accurately performed the preceding imagery trials. Hence, the button responses in response to each MOVE stimulus allowed us to confirm not only the performance of that MOVE trial but also that of the preceding IMAGE trial, whereby an objective measure of imagery performance was obtained in the present experiment.
Although subjects were instructed to withhold any performance during the presentation of IS, it was more likely that they immediately associated the stimuli with the requisite tapping sequence and completed planning of tapping. This process would be a semiautomatic process difficult to suppress. That is, the IS would instantaneously let subjects “know” the movement pattern to perform in response to the next cue stimulus, yet subjects did not know which performance mode to select. Therefore, we assumed that the IS should evoke activity associated primarily with retrieval of the starting finger information, integration of the starting finger and the IS information to establish an action plan, and encode it into a memory, in addition to recognition of visual number stimuli. This process includes several subprocesses of behavior, which may be collectively called “motor planning” (Hoshi and Tanji 2007), although the planning in the present study is atypical as it also has to be applicable to imagery. During D1, subjects held the instructed movement in memory as a generic “motor” memory in a form that allowed the subjects to prepare for both movement execution and imagery. In response to the cue stimuli, subjects would recognize the word stimulus, select the performance mode, retrieve the motor memory from the short-term buffer, and then execute the movement or imagine it. During D2, subjects would need to remember the next finger to resume tapping. This temporal memory during the D2 served as partial information of motor planning, which needed to be completed by the IS to fully constrain the tapping pattern within the possibilities.
The comparison between the 2 modes should more purely reflect the difference between motor execution and motor imagery than our previous SMI study (Hanakawa, Immisch, et al. 2003). Furthermore, D1 minus D2 contrast would demonstrate activity specific to short-term storage of the “generic behavioral plan” in flexible preparation for both physical execution and cognitive imagery because general short-term memory components were controlled across the delay periods.
Image Data Acquisition
Functional image data were obtained using a 3-T scanner with a standard head coil (GE Medical Systems, Milwaukee, WI). Subjects lay supine on the scanner bed and viewed visual stimuli back-projected onto a screen through a mirror built into the head coil. The subjects’ hands were invisible to them during the fMRI experiment. To reduce head motion during scanning, a bite bar made of a dental impression material was custom made for each subject and fixed to a cradle of the head coil. Blood oxygen level–dependent (BOLD) fMRI data were obtained with a T2*-sensitive, 3-dimensional (3D) transverse functional sequence based on principles of echo shifting with a train of observations (PRESTO) (van Gelderen et al. 1995). Acquisition parameters were as follows: effective repetition time of 1 s/volume, data matrix = 38 × 48 × 24, voxel size = 5 × 5 × 5 mm3. This fMRI sequence was chosen because of its fine temporal resolution and relative insensitivity to inflow effects, despite relatively poor in-plane resolution (Bushara et al. 2003; Hanakawa et al. 2006) to take a closer look at temporal dynamics of task-related activity in a set of brain regions based on the previous experiment (Hanakawa, Immisch, et al. 2003). Both high-resolution PRESTO images (1.67 × 1.67 × 1.67 mm3) and T1-weighted, 3D, fast spoiled gradient-recalled at steady-state images (data matrix = 256 × 256 × 124, voxel size = 0.94 × 0.94 × 1.5 mm3) were acquired for anatomical coregistration. Each experiment consisted of 12 scanning runs (156 runs in total for 13 subjects), and each run consisted of 260 functional images (scanning time = 4 min 20 s). Each run included 8 sets of the IS and cue stimuli; therefore, 96 events associated with the IS and 48 events associated with each performance mode were observed for each subject.
Behavioral Data Acquisition
An MRI-compatible response unit with 5 buttons (Psychology Software Tools Inc., Pittsburgh, PA), each corresponding to 1 finger of the right hand, was attached to the subjects. Responses to the “MOVE” stimuli were recorded using SuperLab, and the response time (reaction time plus partial movement time) and accuracy were assessed according to the 1st response in each trial. The behavioral criterion was to reject fMRI runs with 2 or more erroneous responses (i.e., the accuracy was more than or equal to 75% in each run). This criterion was based on our previous experiment with the SMI task, yielding accuracy of approximately 70% for imagery performance on average (Hanakawa, Immisch, et al. 2003).
Surface electromyograms (EMGs) were monitored during actual MRI scanning for 6 subjects primarily to confirm the absence of muscle activity during the imagery events. EMGs were obtained from the right hand and forearm muscles using fMRI-compatible equipment (Ives et al. 1993;Hanakawa, Immisch, et al. 2003). Pairs of Grass gold electrodes were placed on the right abductor pollicis brevis, flexor digiti minimi, extensor digitorum communis, and flexor digitorum superficialis muscles. Interelectrode distance was approximately 3 cm. EMGs were amplified, digitized (sampling rate, 250 Hz), filtered with a bandpass of 30 Hz to 70 Hz, and rectified for subsequent review. For the rest of the subjects (7 subjects) whose online surface EMGs were not available, EMGs were checked in a separate session using a similar setup.
Preprocessing of Image Data
From the data of all subjects, 13 runs out of 156 (8.3%) were excluded from the image analysis on the basis of the behavioral criterion. The maximum number of the runs excluded from a single subject was 3. The 1st 10 volumes from each run were discarded to allow for T1 equilibration effects. The image data were analyzed using SPM99 and, in part, by SPM2 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (MathWorks Inc., Natick, MA). Alignment for the difference in time for acquiring each MRI slice was not performed because of 3-D acquisition of the PRESTO images (i.e., sampling rate was 1 Hz). All volumes were realigned to the 1st volume. The realigned volumes were spatially normalized (Ashburner and Friston 1999) to fit to an in-house PRESTO template consistent with the Montreal Neurological Institute (MNI) reference brain provided by SPM. During the spatial normalization process, the volumes were resampled into 2 × 2 × 2 mm3 voxels in the x (left to right), y (posterior to anterior), and z (inferior to superior) directions, respectively, in reference to the anterior commissure–posterior commissure line (Talairach and Tournoux 1988). The normalized data were smoothed with a 10-mm full width at half maximum (FWHM) isotropic Gaussian kernel. Considering the data as time series, the smoothed volumes were high-pass filtered to 0.01 Hz. A minimum low-pass filter (FWHM = 2 s) was applied to compensate for autocorrelation of the data (Friston et al. 2000). Difference in global signal was removed by scaling the data to a grand mean of 100 over all voxels and scans within a run.
Statistical Parametric Mapping Analysis
Three event types were defined: 1) IS related, 2) imagery related, and 3) movement related. Statistical analysis was performed in a 2-stage, mixed-effect procedure (Holmes and Friston 1998). In the 1st-stage analysis, the BOLD response for each event type was modeled with the canonical hemodynamic response function and its temporal derivative. These functions were convolved with an event train of short boxcar functions starting at each stimulus onset and lasting for the duration of stimulus presentation (2 s) to create a covariate in a general linear model. Two types of design matrix were made for each subject. First, baselines were not explicitly specified to detect event-related activities (i.e., D1 and D2 delay periods together served as an implicit baseline). Second, the D1 and D2 delay periods were modeled separately as 2 different epochs, using boxcar functions for the duration of each delay period. In this design, D1 was used as a baseline to detect the 2 cue stimulus–related activities (movement or imagery event) and D2 was used to detect the IS-related activity. The effects of generic motor plan and preparation were tested using a subject-specific contrast representing D1 versus D2. A constant term for each run was also modeled. Parameter estimates for each covariate were calculated from the least mean square fit of the time series. Images of the parameter estimates for the covariates were created by subject-specific contrasts (collapsing across sessions within subjects). Because the results for the event-related activities were virtually the same irrespective of the selection of the baseline, only those from the split baseline design will be reported hereafter for simplicity.
These “summary” images from the parameter estimate images comprised the data for the 2nd-stage analysis, treating subjects as a random variable (SPM2). A pairwise contrast on the canonical parameter images allowed 1-sample t-tests on differences in the magnitude of each event- and epoch-related response (t values were subsequently converted into Z values). Three types of planned contrast were tested: 3 event-related activities, differential activity between the events (number vs. imagery and movement vs. imagery), and differential epoch-related activity between the D1 and D2. SPMs from the 2nd-stage analysis were 1st thresholded at the height threshold of uncorrected P < 0.001, and the extent threshold of P < 0.05 with correction for multiple comparisons was used as the final statistical criteria for significance. Activity with magnitude that exceeded the voxelwise uncorrected P < 0.001 but not the extent threshold was regarded as a trend. To explore areas commonly involved across the movement and imagery events or the IS and imagery events, a conjunction analysis based on the global null hypothesis was used as implemented in SPM99/2 (Price and Friston 1997; Friston et al. 2005). The locations of brain activity are reported as the MNI coordinates and are linked to the system of Brodmann (Brodmann 1909) only when the SPM Anatomy Toolbox (http://www.fz-juelich.de/inb/inb-3//spm_anatomy_toolbox) was available.
Time-Course Analysis
By capitalizing on fine temporal resolution of the imaging sequence, we designed the experiment to allow for assessment of temporal profile of brain activity. The primary motivation of this analysis was to clarify how each brain region represented task-relevant activity over different task periods (i.e., IS-D1-cue-D2). This approach gives different information than the mapping analysis that visualizes spatial distribution of activity at a given timing, which needs to be explicitly modeled in the design matrix. Time-course data were retrieved from volumes of interest (VOIs) on a subject-by-subject basis, which allowed us to discriminate neighboring activities that might be blurred in the group-level analysis. For this purpose, we only selected the frontoparietal and cerebellar areas known for their roles in motor control because motor imagery was hypothesized to be represented in the distributed motor network with a functional gradient.
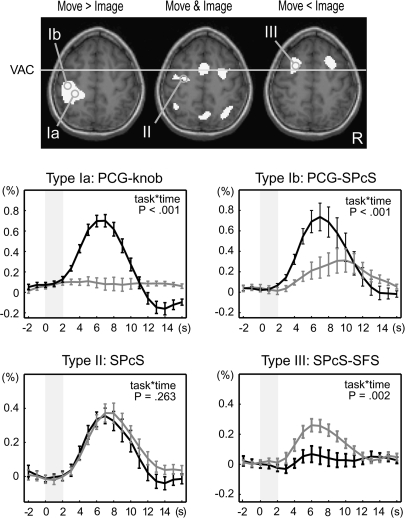
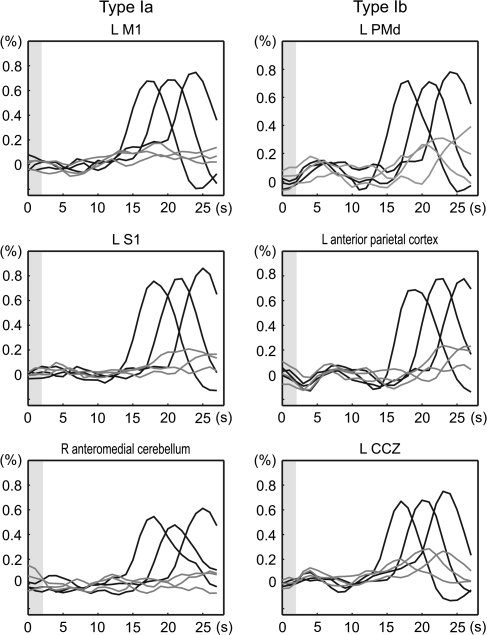
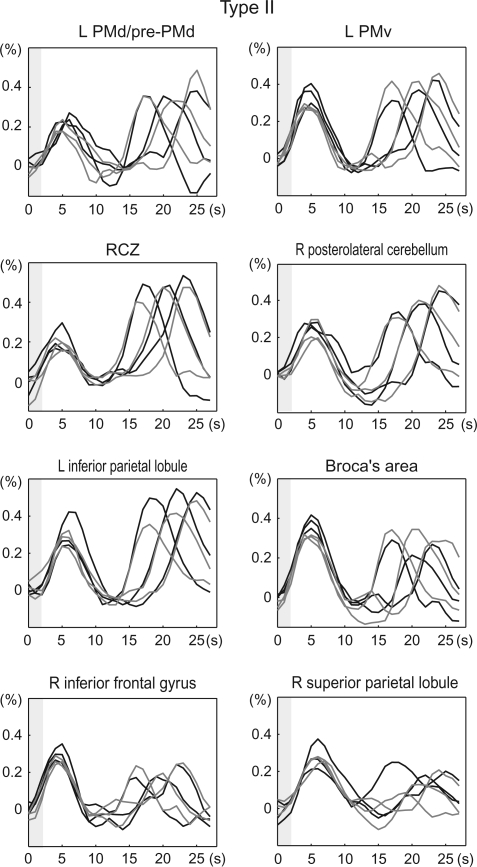
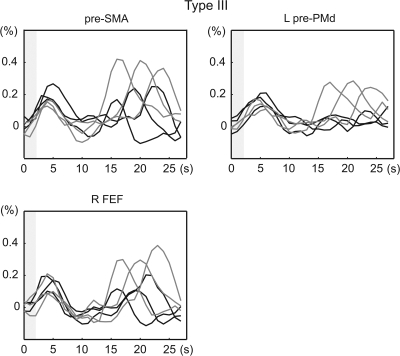
A set of brain areas showing cue stimulus-related activity was chosen on the basis of our previous study (Hanakawa, Immisch, et al. 2003). A spherical VOI (5-mm radius) was set up for each region for each subject in reference to the individual's anatomy and activation maps from the 1st-stage subject-specific contrast. The location of sampling was determined according to the contrast of the movement- and imagery-predominant activities as well as the conjunction of the 2 cue stimulus–related activities (Figure 2). The retrieved time-course data were averaged across voxels that survived uncorrected P < 0.001 in each contrast and were converted to percent signal changes, by treating 2 volumes scanned before each IS presentation as a baseline. These normalized time-course data were 1st aligned to the cue stimulus onset for the classification based on the cue stimulus–related activity. Activity was classified into 3 types: type I activity showing greater activity for the MOVE than the IMAGE events (movement predominant); type II activity showing comparable activity for the MOVE and IMAGE events (movement and imagery); and type III showing greater activity for the IMAGE than for the MOVE events (imagery predominant). A confirmatory repeated-measures analysis of variance (RM-ANOVA) with Greenhouse–Geisser correction for nonsphericity was performed to test the interaction of the time course with the task at the group level. According to the classification by the response to the cue stimuli, the time-course data were realigned to the IS onset. The temporally realigned data were averaged across trials by considering the 3 different durations of the D1 periods and were then averaged across subjects.
Figure 2.
Classification of cue stimulus–related activity for time-course analysis. Cue stimulus–related activity was classified into 3 types: type I showing movement-predominant activity, type II showing cue stimulus–related activity similarly for movement and imagery events, and type III showing imagery-predominant activity. Activation maps from a representative subject (P < 0.001, uncorrected) were overlain onto an axial slice (z = 56 mm) of the individual's own anatomical image. Gray circles are examples of the sampled VOIs including the “hand knob” of the precentral gyrus (PCG-knob, Ia), rostrolateral part of the PCG juxtaposed to the superior precentral sulcus (PCG-SPcS, Ib), SPcS (SPcS, II), and SPcS extending into a posterior part of the superior frontal sulcus (SPcS-SFS, III). Time-course data were sampled from predetermined sets of areas according to each individual's anatomy and activation maps as such. Signal changes from VOIs were aligned to the cue stimulus onset (time 0), converted into percent signal changes, and averaged across subjects. Type I activity was further divided into 2 subtypes: type Ia (e.g., PCG-knob) showing clear movement-related activity (black) with almost no imagery-related activity (gray) and type Ib (e.g., PCG-SPcS) showing salient movement-related activity with modest (ca. 0.3%) imagery-related activity. Activity in the SPcS (type II) and the SPcS-SFS (type III) is shown in the same format. P values indicate task-by-time interaction by repeated-measures ANOVA, and the error bars indicate standard errors of mean. The x-axis represents time in seconds after the cue stimulus onset, and the y-axis represent percent signal changes. Gray shades indicate the period of time during which the cue stimuli were presented.
In the time-course analysis, the individual's VOIs were localized as precisely as possible to the functional subdivisions of motor-related areas (see Figure 2). The hand M1 was defined as the precentral hand knob (Yousry et al. 1997). The superior precentral and superior frontal sulci were used to define the rostral and caudal dorsal premotor cortex (pre-PMd and PMd, respectively) and the frontal eye fields (FEFs) (Paus 1996; Desmurget et al. 2000; Hanakawa et al. 2002; Amiez et al. 2006). The rostral and caudal parts of the supplementary motor areas (pre-SMA and SMA, respectively) and the rostral and caudal cingulate zones (RCZ and CCZ, respectively) were defined in reference to the cingulate and paracingulate sulci (Paus et al. 1996; Picard and Strick 1996).
Results
Behavioral Data
The mean response time for the dSMI task was 1095.6 (SD = 198.3) ms, and the mean accuracy was 92.6% (SD = 5.2), which guaranteed that the subjects were mostly able to keep track of the sequence. This result also indicated, although indirectly, that the subjects successfully performed the IMAGE trials in between the MOVE (response) trials. The accuracy after exclusion of the unreliable runs was 96.1% (SD = 2.5), which endorsed high-quality imagery performance in the data used for the image analysis. There was no muscle activity during imagery performance detectable in 6 subjects with online EMG monitoring or 7 subjects with offline monitoring.
Statistical Parametric Mapping Analysis of Event-Related Activities
IS-Related Activity
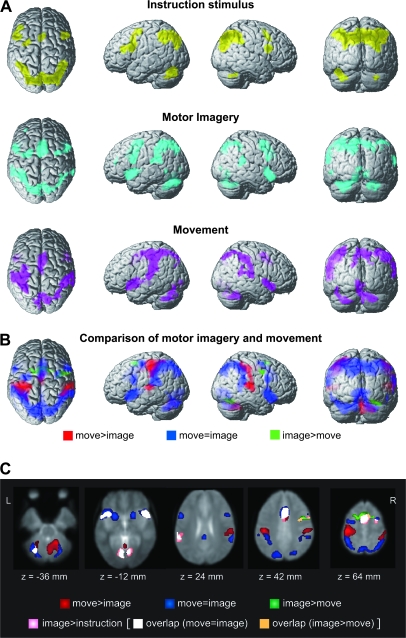
The activity related to the IS events was observed in the inferior frontal gyrus, middle frontal gyrus, superior precentral sulcus, medial aspects of the superior frontal gyrus, anterior cingulate cortex, inferior parietal lobule, superior occipital gyrus, and posterolateral cerebellum (Table 1A, Figure 3A). Activity was mostly bilaterally symmetric in the frontoparietal areas.
Table 1.
Event-related activity
| Regions (BA) | Cluster-level P corrected | Volume (mm3) | Coordinates |
Z value | |||
| x | y | z | |||||
| A. IS-related activity | |||||||
| 1 | L posterolateral cerebellum | 0.001 | 2872 | −40 | −72 | −28 | 4.97 |
| 2 | R inferior frontal gyrus (BA 44 at 50%) | 0.006 | 1992 | 52 | 14 | 26 | 4.93 |
| 3 | L superior precentral sulcus (BA 6 at 30%) | 0.05 | 1248 | −28 | 0 | 62 | 4.53 |
| 4 | L inferior parietal lobule | 0.000 | 20 248 | −32 | −56 | 44 | 4.22 |
| R inferior parietal lobule | 42 | −56 | 58 | 4.09 | |||
| R superior occipital gyrus | 28 | −76 | 44 | 4.06 | |||
| 5 | L inferior frontal gyrus (BA 44 at 40%) | 0.001 | 2688 | −54 | 10 | 30 | 3.93 |
| L middle frontal gyrus (BA 6/44 at 20/10%) | −50 | 6 | 42 | 3.44 | |||
| 6 | L anterior cingulate cortex | 0.015 | 1656 | −2 | 12 | 42 | 3.89 |
| L medial superior frontal gyrus (BA 6 at 70%) | −4 | 10 | 52 | 3.49 | |||
| B. Imagery-related activity | |||||||
| 1 | R supramarginal gyrus | 0.002 | 2480 | 58 | −44 | 34 | 5.55 |
| 2 | L inferior frontal gyrus (BA 44/6 at 50/10%) | 0.000 | 3280 | −56 | 10 | 26 | 5.48 |
| 3 | L posterolateral cerebellum | 0.000 | 8224 | −44 | −70 | −28 | 5.33 |
| 4 | R anterior cingulate cortex | 0.000 | 10 112 | 6 | 14 | 40 | 5.22 |
| L medial superior frontal gyrus (BA 6 at 80%) | −2 | 2 | 58 | 5.12 | |||
| 5 | L inferior parietal lobule | 0.000 | 6840 | −40 | −52 | 58 | 5.13 |
| 6 | L superior frontal gyrus (BA 6 at 30%) | 0.000 | 2656 | −20 | 4 | 60 | 5.11 |
| L precentral gyrus (BA 6 at 30%) | −38 | −4 | 58 | 4.36 | |||
| 7 | L frontal and temporal opercular areas | 0.000 | 8424 | −44 | 14 | −12 | 5.05 |
| 8 | R superior precentral sulcus (BA 6 at 20%) | 0.032 | 1320 | 34 | −2 | 56 | 4.60 |
| 9 | R frontal and temporal opercular areas | 0.000 | 6416 | 56 | 14 | −14 | 4.51 |
| 10 | R superior parietal lobule | 0.022 | 1448 | 12 | −74 | 50 | 4.03 |
| C. Movement-related activity | |||||||
| 1 | R anteromedial cerebellum/dentate nucleus | 0.000 | 10 480 | 18 | −52 | −32 | 5.56 |
| Cerebellar vermis | 6 | −64 | −32 | 3.61 | |||
| 2 | L postcentral gyrus (BA 2/1 at 50/10%) | 0.000 | 24 152 | −48 | −30 | 46 | 5.38 |
| L precentral gyrus (BA 4/3 at 70/30%) | −36 | −26 | 56 | 4.61 | |||
| L superior precentral sulcus (BA 6 at 50%) | −36 | −8 | 62 | 4.25 | |||
| 3 | L medial superior frontal gyrus (BA 6 at 80%) | 0.000 | 10 824 | −2 | 2 | 50 | 5.28 |
| 4 | L posterolateral cerebellum | 0.000 | 3560 | −28 | −62 | −42 | 4.83 |
| 5 | R superior temporal gyrus | 0.000 | 7960 | 66 | −22 | 12 | 4.75 |
| 6 | L globus pallidus | 0.000 | 13 848 | −26 | −10 | −2 | 4.58 |
| L putamen | −28 | 8 | −6 | 4.58 | |||
| 7 | R superior parietal lobule | 0.000 | 5640 | 32 | −68 | 56 | 4.54 |
| 8 | R frontal and parietal opercular areas | 0.000 | 4728 | 52 | 8 | −4 | 4.52 |
| 9 | L thalamus | 0.012 | 1880 | −10 | −24 | −6 | 4.26 |
Note: BA, Brodmann area (with probability determined by SPM Anatomy Toolbox when available); medial superior frontal gyrus, medial aspect of the superior frontal gyrus.
Figure 3.
Statistical parametric maps of event-related activity. (A) IS-related activity (yellow) was observed bilaterally in the frontoparietal areas and cerebellum. Motor imagery-related activity (cyan) was seen in the similar frontoparietal areas, with greater emphasis on the supplementary motor areas, ventral and dorsal premotor areas, frontal and temporal opercular areas, inferior parietal areas, and cerebellum. Movement-related activity (magenta) was marked in the left central areas in addition to the frontoparietal areas. For a display purpose, the activity was theresholded at P < 0.001 (uncorrected), and clusters with more than 50 voxles are surface rendered onto a standard brain. (B) The comparison of movement-related and imagery-related activities. Movement-predominant activity (red) was found in the left central area, bilateral parietotemporal junctions, and right anterior cerebellum. Imagery-predominant activity (green) was found in the left superior frontal sulcus, bilateral superior precentral sulcus, medial aspects of the superior frontal gyrus, and right occipital cortex. The common movement-and-imagery activity (blue) from a conjunction analysis was widely distributed in the frontoparietal areas, occipital cortex, and cerebellum. (C) Imagery-related activity greater than the IS-related activity (pink) was observed in the right posterolateral cerebellum (z = −36 mm), bilateral frontal and temporal opercular areas and fusiform gyri (z = −12 mm), left supramarginal gyrus (z = 24 mm), anterior cingulate cortex and right precentral sulcus (z = 42 mm), and medial superior frontal gyrus (z = 64 mm). This activity mostly overlapped with the common movement-and-imagery activity (overlap in white) and slightly with imagery-predominant activity in the right precentral sulcus (overlap in orange). The other part of the movement-and-imagery activity (blue), imagery-predominant (green), and movement-predominant (red) activities are shown for reference. Activity is overlain onto the PRESTO template image.
Cue Stimulus–Related Activity
Substantially overlapping brain areas were detected for the 2 cue stimulus–related activities (Table 1B and C, Figure 3A). Motor imagery–related activity was seen in the frontoparietal areas, with greater emphasis on the supplementary motor areas, dorsal and ventral premotor areas, frontal and temporal opercular areas, inferior parietal areas, and cerebellum. A nonsignificant trend was found in the left dorsolateral prefrontal cortex (x, y, z = −36, 44, 24; Z = 4.11). Movement-related activity was most marked in the left central areas in addition to the frontoparietal areas similar to the imagery-related activity.
The conjunction analysis of movement-related and imagery-related activities revealed widely distributed frontoparietal cortical areas, medial aspect of the superior frontal gyrus, anterior cingulate cortex, frontal and temporal opercular areas, occipital areas, and posterolateral cerebellum (Table 2, Figure 3B). This activity pattern was similar to the common movement-and-imagery activity in our previous experiment with the SMI task (Hanakawa, Immisch, et al. 2003).
Table 2.
Conjunction of the movement- and imagery-related activities
| Regions (BA) | Volume (mm3) | Coordinates |
Z value | |||
| x | y | z | ||||
| 1 | L posterolateral cerebellum | 4720 | −44 | −70 | −28 | Inf |
| 2 | R anterior cingulate cortex | 11 808 | 6 | 12 | 44 | Inf |
| L medial superior frontal gyrus (BA 6 at 70%) | −2 | −2 | 54 | Inf | ||
| 3 | R frontal and temporal opercular areas | 6512 | 56 | 16 | −14 | Inf |
| 4 | R posterolateral cerebellum | 2600 | 32 | −68 | −36 | Inf |
| 5 | L frontal and temporal opercular areas | 12 512 | −46 | 14 | −10 | Inf |
| L globus pallidus | −24 | −4 | −2 | 5.82 | ||
| 6 | L inferior frontal gyrus (BA 44 at 60%) | 1968 | −54 | 12 | 26 | Inf. |
| L inferior precentral gyrus (BA 6 at 50%) | −56 | 6 | 38 | 4.12 | ||
| 7 | L inferior parietal lobule | 7432 | −40 | −52 | 58 | 7.65 |
| L superior parietal lobule | −28 | −66 | 58 | 5.59 | ||
| L supramarginal gyrus | −62 | −38 | 22 | 5.34 | ||
| 8 | R globus pallidus | 2968 | 20 | 2 | 4 | 7.60 |
| 9 | R superior parietal lobule | 16 816 | 14 | −76 | 52 | 7.21 |
| R supramarginal gyrus | 64 | −42 | 28 | 6.64 | ||
| 10 | L superior frontal sulcus (BA 6 at 40%) | 1512 | −36 | −6 | 60 | 7.42 |
| 11 | L primary visual cortex (BA 17 at 90%) | 6432 | −4 | −88 | 2 | 6.37 |
| 12 | R superior frontal sulcus | 1200 | 34 | 0 | 60 | 6.25 |
Note: BA, Brodmann area (with probability determined by SPM Anatomy Toolbox when available); Inf, inferior; medial frontal gyrus, medial aspect of the superior frontal gyrus.
The direct comparison between the movement- and imagery-related activities highlighted a segregated aspect of the 2 (Table 3, Figure 3B), with a sharper contrast than that from the previous SMI experiment. Movement-predominant areas were composed of the precentral and postcentral gyri, anterior superior parietal cortex, middle cingulate cortex, temporoparietal junction, basal ganglia, thalamus, and also cerebellum. The precentral and postcentral gyral activities were contralateral to the movement side, whereas the cerebellar activity was predominantly ipsilateral to it. Imagery-predominant activity was observed in the medial superior frontal gyrus extending into the superior frontal sulci anterior to the vertical anterior commissure (VAC) line (Talairach and Tournoux 1988), right precentral sulcus at the level of the middle frontal gyrus, and right fusiform gyrus. The imagery-predominant superior frontal sulcus activity was located rostral and medial to the common movement-and-imagery activity at the similar z coordinate level (ca. 60 mm) (Figure 3B and C). The other imagery-predominant activity in the precentral gyrus/sulcus was located more ventrally (z coordinate = ca. 40 mm). A trend toward imagery predominance was found in the left precentral gyrus (x, y, z = −42, 2, 38; Z = 3.73) and left fusiform gyrus (x, y, z = –26, –74, –16; Z = 4.45).
Table 3.
Comparison between the movement- and imagery-related activities
| Regions (BA) | Cluster-level P corrected | Volume (mm3) | Coordinates |
Z value | |||
| x | y | z | |||||
| MOVEMENT > IMAGERY (movement-predominant) | |||||||
| 1 | L precentral gyrus (BA 4/1 at 70/30%) | 0.000 | 14 336 | −34 | −28 | 64 | 6.19 |
| L postcentral gyrus (BA 2 at 60%) | −48 | −28 | 46 | 6.08 | |||
| L precentral sulcus (BA 4/6 at 60/40%) | −38 | −18 | 60 | 4.47 | |||
| L anterior parietal cortex (BA 2 at 40%) | −34 | −46 | 64 | 3.61 | |||
| 2 | R anteromedial cerebellum/dentate nucleus | 0.000 | 10 704 | 14 | −52 | −32 | 5.25 |
| Cerebellar vermis | 6 | −64 | −40 | 4.18 | |||
| 3 | R postcentral gyrus (BA 2 at 40%) | 0.000 | 11 680 | 56 | −26 | 48 | 4.93 |
| R superior temporal gyrus | 66 | −24 | 10 | 4.74 | |||
| 4 | L middle cingulate cortex | 0.000 | 4608 | −2 | −10 | 44 | 4.60 |
| 5 | L superior temporal gyrus | 0.000 | 4160 | −58 | −22 | 8 | 4.59 |
| 6 | L thalamus | 0.008 | 1992 | −10 | −22 | 4 | 3.80 |
| IMAGERY > MOVEMENT (imagery-predominant) | |||||||
| 1 | L medial superior frontal gyrus (BA 6 at 70%) | 0.014 | 2096 | 0 | 10 | 58 | 4.33 |
| L superior frontal sulcus (BA 6 at 20%) | −16 | 6 | 58 | 3.85 | |||
| 2 | R precentral sulcus | 0.040 | 1488 | 42 | 0 | 40 | 4.26 |
| 3 | R fusiform gyrus | 0.014 | 2136 | 30 | −60 | −18 | 4.23 |
Note: BA, Brodmann area (with probability determined by SPM Anatomy Toolbox when available); medial frontal gyrus, medial aspect of the superior frontal gyrus.
Comparison between the IS- and Imagery-Related Activities
The 2 event-related activities without accompanying motor execution, IS-related activity versus imagery-related activity, were directly compared as they demonstrated a similar pattern of activity. No region showed significantly greater activity during the IS events than the imagery events, but a trend for difference (uncorrected P < 0.001) was found in the left superior occipital gyrus (x, y, z = –32, –86, 30; Z = 3.14).
Conversely, activity significantly greater during the imagery events than the IS events was found in the medial superior frontal gyrus around the VAC line, anterior cingulate cortex, right superior precentral sulcus, bilateral frontal and temporal opercular areas, left supramarginal gyrus, bilateral fusiform gyri, and right posterolateral cerebellum (Table 4, Figure 3C). A trend toward difference was also found in the left superior precentral sulcus corresponding to the pre-PMd (x, y, z = −30, −8, 54, Z = 3.65). Most of those areas overlapped with the common movement-and-imagery activity, except for the cingulate activity overlapping with the movement-predominant activity and right precentral sulcal activity overlapping with the imagery-predominant activity.
Table 4.
Comparison of the IS-related and imagery-related activities
| Regions (BA) | Cluster-level P corrected | Volume (mm3) | Coordinates |
Z value | |||
| x | y | z | |||||
| IMAGERY > IS | |||||||
| 1 | R frontal and temporal opercular areas | 0.001 | 3672 | 56 | 14 | −12 | 4.85 |
| 2 | L frontal and temporal opercular areas | 0.007 | 2704 | −46 | 14 | −16 | 4.53 |
| 3 | Medial superior frontal gyrus (BA 6 at 90%) | 0.000 | 10 880 | 0 | −2 | 60 | 4.50 |
| R anterior cingulate cortex | 6 | 18 | 32 | 3.73 | |||
| 4 | R precentral gyrus (BA 6 at 40%) | 0.000 | 2936 | 46 | 0 | 44 | 4.01 |
| R superior frontal gyrus (BA 6 at 20%) | 32 | −6 | 60 | 3.63 | |||
| 5 | L fusiform gyrus (BA 18/17 at 30/20%) | 0.000 | 6928 | −12 | −76 | −12 | 4.21 |
| L posterolateral cerebellum | −28 | −64 | −36 | 3.74 | |||
| R fusiform gyrus (BA 18/17 at 20/10%) | 16 | −78 | −12 | 3.50 | |||
| 6 | L supramarginal gyrus | 0.000 | 1512 | −64 | −30 | 26 | 3.53 |
| No activity for IS > IMAGERY | |||||||
Note: BA, Brodmann area (with probability determined by SPM Anatomy Toolbox when available); medial frontal gyrus, medial aspect of the superior frontal gyrus.
Delay Period Activity
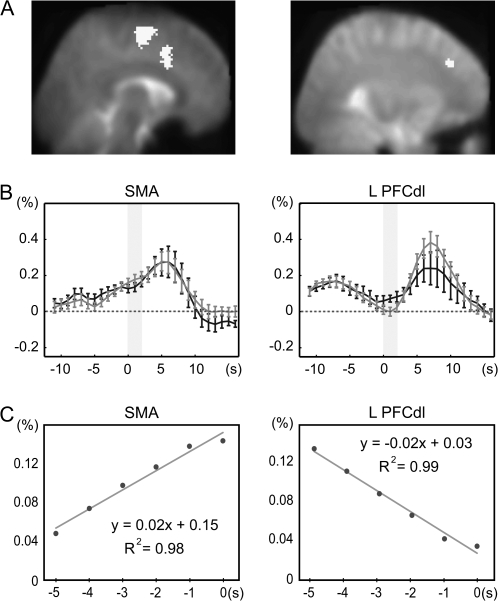
The medial superior frontal gyrus and cingulate zones showed statistically significant activity during the D1 period as compared with the D2 period (Table 5, Figure 4A). The medial superior frontal activity corresponded to the SMA, and its location was very close to the medial superior frontal zone where the common movement-and-imagery activity was shown in the conjunction analysis of cue stimulus–related activity. In addition, the left dorsolateral prefrontal cortex (x, y, z = –30, 34, 34; Z = 3.16) showed a nonsignificant trend toward D1 delay period activity (Figure 4A).
Table 5.
Delay period activity (D1 vs D2)
| Regions (BA) | Cluster-level P corrected | Volume (mm3) | Coordinates |
Z value | |||
| x | y | z | |||||
| 1 | R middle cingulate cortex | 0.002 | 1888 | 10 | −28 | 36 | 4.25 |
| 2 | L medial superior frontal gyrus (BA 6 at 100%) | 0.000 | 3416 | −4 | −8 | 58 | 4.21 |
| L anterior cingulate cortex | −8 | 12 | 38 | 3.72 | |||
Note: BA, Brodmann area (with probability determined by SPM Anatomy Toolbox when available); medial frontal gyrus, medial aspect of the superior frontal gyrus.
Figure 4.
D1 delay period activity. (A) Delay period activity (activity greater during the 1st delay periods than during the 2nd delay periods) in the supplementary motor areas (SMAs) from the 2nd-stage analysis, superimposed onto a sagittal slice of the PRESTO template image. The left dorsolateral prefrontal cortex (PFCdl), which showed mild but nonnegligible delay period activity, is also shown in the same format for comparison. (B) Time-course data aligned to the cue stimulus onset (time 0), sampled from the SMA that showed delay period activity (left panel). This area showed delay period activity that was building up toward the cue stimulus (gray shades), similar for the movement (black line) and imagery (gray line) events. Activity from PFCdl is shown for comparison (right panel). (C) The delay period activity just before the cue stimulus presentation was approximated by regression lines in both the SMA and PFCdl. The regression slope of the delay period activity was positive in the SMA (left panel), whereas it was negative in the PFCdl (right panel), which meant that the D1 activity was increasing toward the cue stimulus presentation in the SMA whereas it was decreasing toward it in the PFCdl.
At each individual's level, SMA activity during the D1 delay period was consistent across subjects (n = 12; uncorrected P < 0.001). A time-course analysis revealed that activity in the SMA was gradually building up during the D1 delay period, accompanying a transient increase in activity after the cue stimulus presentation (Figure 4B). On the other hand, the delay activity in the dorsolateral prefrontal cortex appeared to be decreasing toward the cue stimulus presentation and showed a transient cue stimulus–related activity from the baseline level. These findings were further investigated by analyzing the D1 period activity ranging from 5 s before the cue stimulus presentation to the cue stimulus presentation. This time window was selected to minimize the effect of the IS-related activity. As should be expected, in this delay period during which the subjects did not know which mode should be selected, activity did not differ between the MOVE and IMAGE trials (P > 0.5) in either the SMA or dorsolateral prefrontal cortex. Averaged across the subjects, the delay period activity just before the cue stimulus presentation was reasonably approximated by regression lines in both the SMA and dorsolateral prefrontal cortex (Figure 4C). The regression slopes significantly differed between the 2 regions (paired t-test, P < 0.001). Moreover, the regression slope of the delay period activity was significantly greater than zero in the SMA (1-sample t-test, P < 0.001), whereas it was negative in the dorsolateral prefrontal cortex (P < 0.002). These findings meant that the delay period activity was increasing toward the cue stimulus presentation in the SMA whereas it was decreasing in the dorsolateral prefrontal cortex, supporting the idea that the delay period in the SMA reflects a preparation process toward the cue stimulus events.
Time-Course Analysis of the Event-Related Activities
Time-course data were sampled from the frontoparietal cortical and cerebellar VOIs, which were predetermined according to our previous study (Hanakawa, Immisch, et al. 2003) as summarized in Table 6. An RM-ANOVA at the group level supported the classification of the activity type based on the cue stimulus–related activities: movement-predominant type I activity (time-by-task interaction significant at P < 0.001 for all 7 VOIs); movement-and-imagery type II activity (P > 0.05 for all 13 VOIs); and imagery-predominant type III activity (P < 0.005 for all 4 VOIs). Temporal profiles of task-relevant activity were assessed by realigning the time-course data to the IS onset.
Table 6.
Volumes of interests for time-course analysis
| Anatomical location | Functional areas | Confidence intervals |
IS activity (%) | Imagery activity (%) | Movement activity (%) | ||
| x | y | z | |||||
| Type Ia | |||||||
| L precentral gyrus-knob | M1 | −33/−38 | −26/−31 | 55/60 | 0.03 ± 0.11 | 0.03 ± 0.14 | 0.62 ± 0.19 |
| L postcentral gyrus | S1 | −41/−47 | −29/−34 | 55/60 | 0.01 ± 0.14 | 0.06 ± 0.21 | 0.78 ± 0.28 |
| L superior temporal gyrus | S2 | −51/−57 | −21/−27 | 7/13 | 0.05 ± 0.07 | 0.03 ± 0.10 | 0.60 ± 0.31 |
| R anteromedial cerebellum | 18/24 | −50/−58 | −32/−38 | 0.02 ± 0.10 | 0.03 ± 0.13 | 0.52 ± 0.30 | |
| Ib | |||||||
| L precentral gyrus-SPcS | PMd | −34/−42 | −11/−18 | 57/62 | 0.11 ± 0.14 | 0.28 ± 0.38 | 0.71 ± 0.51 |
| L anterior parietal cortex | −27/−35 | −44/−53 | 64/67 | 0.05 ± 0.18 | 0.11 ± 0.09 | 0.69 ± 0.26 | |
| L middle cingulate cortex | CCZ | −4/0 | −6/−15 | 45/50 | 0.07 ± 0.09 | 0.21 ± 0.19 | 0.60 ± 0.22 |
| Type II | |||||||
| L SPcS | PMd/pre-PMd | −25/−35 | −2/−8 | 55/64 | 0.18 ± 0.12 | 0.37 ± 0.19 | 0.34 ± 0.17 |
| L inferior precentral gyrus | PMv | −50/−58 | 4/9 | 29/37 | 0.31 ± 0.09 | 0.38 ± 0.23 | 0.33 ± 0.21 |
| Anterior cingulate cortex | RCZ | −1/3 | 16/22 | 32/38 | 0.19 ± 0.11 | 0.39 ± 0.12 | 0.46 ± 0.27 |
| Medial superior frontal gyrus | SMA | −4/−1 | 4/−5 | 51/60 | 0.13 ± 0.08 | 0.25 ± 0.17 | 0.26 ± 0.26 |
| L inferior parietal lobule | −35/−41 | −50/−58 | 51/58 | 0.27 ± 0.09 | 0.21 ± 0.19 | 0.40 ± 0.22 | |
| L supramarginal gyrus | −54/−62 | −34/−42 | 20/27 | 0.17 ± 0.15 | 0.30 ± 0.17 | 0.30 ± 0.15 | |
| R supramarginal gyrus | 60/66 | −35/−45 | 21/30 | 0.14 ± 0.13 | 0.36 ± 0.21 | 0.45 ± 0.21 | |
| R posterolateral cerebellum | 32/37 | −71/−78 | −36/−28 | 0.22 ± 0.16 | 0.39 ± 0.18 | 0.38 ± 0.23 | |
| L posterolateral cerebellum | −30/−37 | −71/−79 | −34/−29 | 0.28 ± 0.13 | 0.41 ± 0.19 | 0.41 ± 0.28 | |
| L middle frontal gyrus | PFCdl | −34/42 | 45/53 | 15/22 | 0.20 ± 0.09 | 0.40 ± 0.18 | 0.33 ± 0.13 |
| L superior parietal lobule | −16/−25 | −71/−82 | 40/49 | 0.23 ± 0.10 | 0.13 ± 0.09 | 0.20 ± 0.18 | |
| R superior parietal lobule | 20/30 | −70/−79 | 40/48 | 0.29 ± 0.14 | 0.28 ± 0.16 | 0.24 ± 0.17 | |
| L inferior frontal gyrus | Broca | −48/−56 | 8/20 | 23/30 | 0.35 ± 0.14 | 0.31 ± 0.17 | 0.28 ± 0.23 |
| R inferior frontal gyrus | 47/55 | 11/20 | 17/25 | 0.30 ± 0.07 | 0.20 ± 0.18 | 0.18 ± 0.17 | |
| Type III | |||||||
| L SPcS-caudal SFS | pre-PMd | −19/−27 | 1/10 | 54/62 | 0.14 ± 0.09 | 0.24 ± 0.13 | 0.05 ± 0.19 |
| Medial frontal gyrus | pre-SMA | −3/3 | 3/10 | 56/63 | 0.17 ± 0.09 | 0.32 ± 0.12 | 0.07 ± 0.16 |
| L SPcS-middle frontal gyrus | FEF | −45/−52 | −6/5 | 40/44 | 0.18 ± 0.09 | 0.20 ± 0.09 | 0.01 ± 0.10 |
| R SPcS-middle frontal gyrus | FEF | 48/56 | −2/5 | 39/46 | 0.13 ± 0.08 | 0.25 ± 0.10 | 0.02 ± 0.17 |
Note: Confidence intervals indicate 95% confidence intervals of stereotaxic coordinates. Activity indicates percent signal changes (mean ± SD) during 5–6 s after the IS or cue stimulus prompting movement or imagery performance. PFCdl, dorsolateral prefrontal cortex; SPcS, superior precentral sulcus; SFS, superior frontal sulcus.
Type I Activity
The type I activity was further subdivided into type Ia with minimal activity during the imagery events at the group level and type Ib with moderate imagery-related activity (Figure 5). The examples of type Ia areas were the left M1, left S1, 2nd somatosensory areas (S2), and right anteromedial cerebellum. In individual analyses, 1 subject out of 13 revealed statistically significant imagery-related activity at the hand M1. The type Ib areas included the PMd, middle cingulate cortex, and anterior part of the superior parietal lobule. Notably, the type Ib areas showed a greater degree of IS-related activity in comparison with the type Ia where IS-related activity was negligible. We carefully checked the location of the movement-predominant middle cingulate activity with regard to the cingulate and paracingulate sulci in each individual (Paus et al. 1996; Immisch et al. 2001). The paracingulate sulcus was observed in 7 out of 13 subjects (present or prominent). Type I activity was always situated within the cingulate sulcus or within a short sulcus branching from the cingulate sulcus and extending dorsally. This region probably corresponded to the CCZ (Picard and Strick 1996).
Figure 5.
Type I activity aligned to the IS onset. Activity related to the movement events (black line) and the imagery events (gray line) were aligned to the IS onset (time 0) and were averaged separately for the 1st delay period of 12, 15, and 18 s, corresponding to the 3 different gray and black lines. Gray shades indicate the period of time during which the number stimuli were presented. Type I areas showed marked movement-related activity. There was little imagery-related activity in the type Ia areas but were mild imagery-related as well as IS-related activities in the type Ib areas.
Type II Activity
The type II areas showed activity similar between the MOVE and IMAGE trials (Figure 6). The type II areas were exemplified by the multiple frontoparietal cortical areas, medial superior frontal cortex, anterior cingulate cortex (RCZ), basal ganglia, and posterolateral cerebellum. Generally, these areas were located rostral to the type I areas in the frontal cortex and caudal to them in the parietal cortex and cerebellum. The SMA showing delay period activity was classified as type II when cue stimulus–related activity was assessed. A general characteristic of the type II areas was salient coexistence of the IS-related activity, particularly prominent in the precuneus and inferior frontal cortex including Broca's area. However, RCZ, SMA, and supramarginal gyrus showed greater imagery-related activity than IS-related activity.
Figure 6.
Type II activity aligned to the IS onset. Note remarkable activity following the IS presentation (gray shades) for the type II areas. Type II areas included brain areas where there was relative exaggeration of the IS-related activity compared with the cue stimulus–related activity (e.g., right inferior frontal gyrus and right superior parietal lobule). See legends for Figure 5 for the display conventions. PMd/pre-PMd, intersection of the PMd and pre-PMd.
Type III activity
The pre-SMA, pre-PMd, and presumable FEF were classified into the type III activity (Figure 7). All type III areas revealed modest IS-related activity (ca. 0.2%) as well as imagery-related activity. The pre-SMA and FEF belonged to the areas where imagery-related activity was significantly greater than the IS-related activity in the statistical parametric mapping analysis.
Figure 7.
Type III activity aligned to the IS onset. Type III areas showed marked IS-related activity as did the type II areas. See legends for Figure 5 for the display conventions. pre-SMA, rostral part of the supplementary motor areas; pre-PMd, rostral and dorsal sector of the lateral premotor cortex; FEF, presumable frontal eye fields.
Relationship among the Instruction, Imagery, and Movement-Related Activities
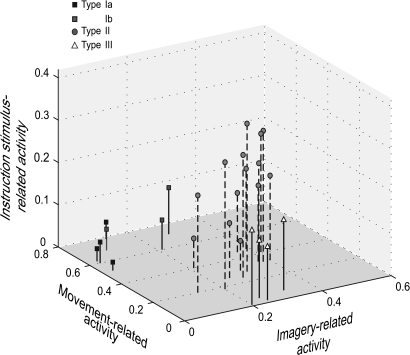
With all the sampled areas combined, there was significant correlation between the size of the IS-related activity and that of the imagery-related activity (r = 0.632, P = 0.001), whereas the movement-related activity was inversely correlated with the IS-related activity (r = –0.540, P = 0.005). There was no correlation between the size of movement- and imagery-related activities (r = –0.327, P = 0.111) (Figure 8).
Figure 8.
Three-dimensional plots of IS-, movement-, and imagery- related activity. The mean amplitude of each event-related activity was expressed in percent signal changes (see Table 6) and plotted against each other for each brain area sampled for the time-course analysis. The IS-related activity was correlated positively with the imagery-related activity (r = 0.632, P = 0.001) and inversely with the movement-related activity (r = −0.540, P = 0.005).
Discussion
The present dSMI task shares an important basic feature, objective evaluation of the imagery task performance, with the original SMI task (Hanakawa, Immisch, et al. 2003). In the dSMI task, accurate motor response to a MOVE stimulus after an IMAGE trial indicated accurate imagery performance in the IMAGE trial. First, more than 90% (96% after exclusion) of accurate responses guaranteed the subjects’ understanding of the dSMI task and reliable imagery performance. Second, this behavioral observation and the exclusion of less accurately performed runs should increase reliability of the inferences about imagery-related brain activity. Regrettably, online EMG monitoring was available only in half of the subjects. However, as the rest of the subjects were trained on the task beforehand under EMG monitoring, the ability of the subjects for motor imagery without activating muscles had been confirmed. Moreover, the presence of minor muscle activity during motor imagery mainly affects the interpretation of M1 and S1 activity. Because most of the subjects did not show significant M1 or S1 activity, the lack of online EMG monitoring in those subjects would not considerably affect the interpretation of the present results.
Motor imagery has both visual and kinesthetic aspects, the predominance of which may differentially modulate the participation of the neural substrates in the task (Stinear et al. 2006). We did not intend to design the experiment for discriminating those different aspects of motor imagery in this particular experiment as visual and kinesthetic aspects had been tightly coupled through visuomotor experience in sighted or late blind subjects (Imbiriba et al. 2006; Hagura et al. 2007).
Movement-Related Activity Versus Imagery-Related Activity
The movement-predominant activity in the present experiment perfectly overlapped with its counterpart in the previous experiment with the SMI task (Hanakawa, Immisch, et al. 2003). Imagery-related activity was not significant in the M1 or S1 at a population level, but 1 subject showed significant imagery-related activity there. There is indeed some inconsistency in the literature whether the M1 is active during motor imagery (Porro et al. 1996; Roth et al. 1996; Deiber et al. 1998; Gerardin et al. 2000; Dechent et al. 2004), and the role of the M1 for motor imagery remains a matter of debate. It is clear from several transcranial magnetic stimulation (TMS) studies that motor imagery, especially of the kinesthetic type, can enhance motor-evoked potentials (Fadiga et al. 1995; Facchini et al. 2002; Stinear et al. 2006). This modulation effect could occur at the level of premotor cortex, M1, or the spinal cord (Li et al. 2004). The M1 functionality seems essential for kinesthetic motor perception (“motor illusion”) induced by vibratory stimulation to muscle tendons (Naito et al. 2002). As such M1 activity could be present during any type of motor imagery even without accompanying muscle activity. However, this does not mean that M1 activity is necessary for generating motor imagery. Our series of studies with the SMI and dSMI tasks have indicated that a type of motor imagery can be achieved without significantly involving the M1. Consistently, M1 dysfunction did not critically impair motor imagery, at least of the visual type, as shown by recent “virtual lesion” studies with TMS (Sauner et al. 2006; Bode et al. 2007). To integrate those seemingly contradictory findings, a more comprehensive theory about the role of the M1 for cognitive and executive motor behavior should be developed. However, it seems likely that the type Ia areas (M1, S1, S2, and anteromedial cerebellum) are mainly important for motor execution and analysis of the afferent sensory information. As compared with the type Ia activity, some movement-predominant areas (type Ib) such as PMd, CCZ, and anterior parietal cortex showed mild but clear imagery-related activity. The difference between the type Ia and Ib supports the concept that motor imagery is represented in the distributed motor network with a functional gradient (Hanakawa, Immisch, et al. 2003).
Imagery-predominant activity was found more prominently in the present study with the dSMI task than in the previous one with the SMI task. This outcome is consistent with our hypothesis that the disparity between movement and motor imagery would become clearer when sensory–cognitive components in the tasks are reduced. The imagery-predominant activity was observed in the pre-PMd, pre-SMA, and FEF, which supported our previous finding of a trend toward imagery predominance in these areas (Hanakawa,Immisch, et al. 2003). These areas are important for visuospatial information processing for subsequent actions (Picard and Strick 2001; Rizzolatti and Luppino 2001). The present finding signifies their roles in cognitive motor control, which seems to have some relevance to motor imagery more than immediate motor execution.
Even after sensory–cognitive components were reduced, many brain areas still demonstrated similar activity during movement and motor imagery as shown by the conjunction analysis of movement and imagery. These shared areas included the parts of nonprimary motor areas, Broca's area, cingulate areas, frontal and temporal opercular areas, inferior and superior parietal cortices, visual areas, basal ganglia, and posterior cerebellum. It is unlikely that these areas directly control movement execution because they do not have direct access to the M1 or the spinal cord. Activity of the posterior cerebellum is also localized in its nonmotor part (Allen et al. 1997; Middleton and Strick 2000). An emerging question is then which function of those areas is relevant to both actual and potential movements.
Delay Period Activity
The delay period activity probably represents preparatory neural activity for both motor execution and motor imagery. This delay period activity shares its characteristics with the contingent negative variation (CNV), which is a slow negative brain potential occurring between 2 successive stimuli only when the 2 stimuli are associated with or contingent with each other (Walter et al. 1964). Recording with subdural electrodes, a slow negative potential preceding the 2nd sensory stimulus, most likely late CNV, was found on the medial aspect of the superior frontal cortex consistent with the SMA (Ikeda et al. 1996). A similar preparatory activity in the SMA was shown in a recent neuroimaging study (Cavina-Pratesi et al. 2006).
The dorsolateral prefrontal cortex, which has strong connections with the pre-SMA that linked with SMA (Luppino et al. 1993), also demonstrated modest delay period activity. The delay-period activity in the prefrontal cortex was decreasing toward the cue stimulus presentation, whereas that in the SMA was increasing to it. Such temporal relationship of the prefrontal and medial frontal delay period activity implies that the dorsolateral prefrontal cortex may be located upstream to the pre-SMA and SMA in the information flow during the preparation period.
IS- and Imagery-Related Activities
To our knowledge, this is the 1st study to report detailed dynamic temporal characteristics of brain activity during motor imagery and execution together with their planning and preparation processes over time. The fact that even the most imagery-predominant areas (type III activity) showed considerable movement-related activity supported the well-accepted concept that motor imagery is founded on the motor-related brain network. Moreover, the assessment of the IS-related activity in reference to the movement- and imagery-related activities has provided new knowledge about the neural mechanisms of motor imagery. The time-course analysis clearly showed that the many nodes of the motor network represented all the 3 event-related activities. However, these areas were not homogenous. The type Ia areas almost exclusively showed movement-related activity, most of the type II areas showed all the 3 activities to a similar degree, and the type III areas showed substantial IS- and imagery-related activities but only mild movement-related activity. By reflecting this distributional gradient of activity, the correlation analysis across the 3 event-related activities indicated a close relationship of the imagery-related activity with the IS-related activity but not with the movement-related activity (Figure 8).
Our idea was that a distributed network with a functional gradient for motor behaviors (some more executive and some more imaginative) might be the correlate of motor imagery (Hanakawa, Immisch, et al. 2003). Within the motor network, the IS-related activity would reflect the collection, integration, and encoding of the information necessary for motor performance. A core factor of this process may be called motor planning (Hoshi and Tanji 2007), but the IS-related process would also include sensory–motor mapping, sequence encoding, and update of motor plans. Here, we collectively call these factors preexecutive processes of motor behavior, as opposed to executive processes, for convenience. We interpreted the present finding as indicating that motor imagery is more closely related to preexecutive processes of a movement than its actual execution. These 2 critical functions of motor-related areas, pre-executive processing and motor execution, are not completely segregated but rather distributed with a functional gradient from more planning-oriented areas such as the pre-SMA to more output-oriented areas as the M1 (Geyer et al. 2000). This functional gradient concept in motor-related areas has been supported by neuroimaging studies (Tyszka et al. 1994; Hanakawa, Immisch, et al. 2003). This notion also agrees with a psychological concept that “Covert and overt stages thus represent a continuum …” (Jeannerod 2001).
There is also neurophysiological evidence to support the functional gradient in motor-related areas. The movement mode of the present dSMI task is very similar to instructed delay motor tasks studied extensively in nonhuman primates (Wise 1985; di Pellegrino and Wise 1993; Johnson et al. 1996; Crammond and Kalaska 2000). Such studies have found a population of neurons that respond to the instruction sensory stimuli (“signal-related” neurons) in premotor and parietal areas. Supportive evidence has been accumulated from human neuroimaging studies using instructed delay tasks (Toni et al. 1999; Cavina-Pratesi et al. 2006). In monkeys, there is a transition from signal-related to movement-related activity in the rostral-to-caudal direction in the premotor cortex and in the opposite direction in the superior parietal cortex (Johnson et al. 1996). This agrees with the rostral-to-caudal transition of the activity in the precentral regions: prominent IS-related and imagery-predominant activities in the pre-PMd and the movement-predominant activity in the M1. It seems likely that “signal-related” activity is similar to the IS-related activity observed in the present experiment. Recently, accumulating evidence has shown the functional difference between the PMv and PMd, which constitute the ventral–dorsal axis of functional gradient within the motor-related areas. In Figure 6, PMv responded similarly to all the IS, imagery, and movement events whereas PMd/pre-PMd was more active with the imagery and movement events than the IS events. This finding suggests that, as compared with PMd, PMv is involved more with the IS-related process. Such PMv activity can be interpreted as reflecting a variant of direct sensorimotor mapping (Hoshi and Tanji 2007). For example, the IS “3” can be interpreted as a number sequence of 1–2–3, which actually instructed a subject to map the number sequence of 1–2–3 onto the tapping sequence of the 1st, 2nd, then 3rd fingers. This could be considered as atypical direct mapping in a sense that the stimulus unambiguously determines the movement, although the stimulus is not the direct target of movement as in the typical direct mapping. Alternatively, the number stimulus may be linguistically recoded before mapping onto the fingers. A region of PMv representing both finger and mouth movements and their imagery (Hanakawa et al. 2005) could mediate the mapping of a linguistically recoded stimulus onto a motor plan of fingers. The PMd activity enhanced during imagery and movement performance might reflect the function of PMd for motor and nonmotor sequence generation (Ohbayashi et al. 2003; Abe et al. 2007).
The IS-related activity should concern information processing for both motor execution and motor imagery. That is, subjects would semiautomatically link the presented IS to the required tapping pattern in a form applicable to both execution and imagery (mapping or planning). This stimulus–response linkage process may be regarded as “sensory-triggered” activation of motor representations or implicit-type motor imagery. Sensory-triggered or implicit motor imagery may underlie cognitive tasks (Parsons et al. 1995; Frak et al. 2001) and a process to understand actions being performed by other agents (Grafton et al. 1996; Buccino et al. 2001; Rizzolatti et al. 2002). It is possible that not only concrete visual stimuli inherently specifying movements (i.e., other agent's movement) but also arbitrary stimuli (i.e., numbers or colors) may induce activation of the corresponding motor representations after acquisition of arbitrary stimulus–response linkage (Wise and Murray 2000; Cisek and Kalaska 2004).
The sensory-triggered imagery concept might partly explain the overall similarity between the IS-related activity and the imagery-related activity. Nonetheless, the areas showing significantly greater imagery-related activity than IS-related activity indicates a larger role of these areas in mental rehearsal of actions, which is regarded as a voluntary process, than sensory-triggered passive-type motor imagery. Nonprimary motor areas (pre-PMd/PMd and pre-SMA/SMA) and parietal cortex (supramarginal gyrus) have been implicated to play an important role in motor imagery (Grezes and Decety 2001). The fusiform cortex activity, which also showed a trend toward imagery-predominant (imagery > movement) cue-related activity, suggests involvement of visual form imagery, probably of the fingers, in the present motor imagery task. From the standpoint of the emulation theory of representation (Grush 2004), what is present in motor imagery, but not in the IS-related processes, is probably willed generation of efference copy and analysis of “mock” sensory (mainly proprioceptive and partly visual) signals. It is possible that nonprimary motor areas such as SMA are related to the willed generation of efference copy without activating the motor apparatus, and supramarginal gyrus, fusiform gyrus, and posterolateral cerebellum are in charge of analyzing “mock” sensory signals.
Physical movements do not necessarily follow the IS in the present task. In this regard, the present task resembles a Go/NoGo choice reaction time paradigm (Kalaska and Crammond 1995; Cavina-Pratesi et al. 2006). Actually, it is possible that imagery-related activity partially included activity to inhibit movement execution, if subjects primarily prepared for physical movement as a default. Nonetheless, because subjects are required to imagine (not just to abort) the instructed finger tapping, imagery-related activity should also reflect the underlying imagery processes. This assumption is supported by the different distribution of the present imagery-related activity from that of the inhibition-related activity reported previously (Garavan et al. 1999). The issue of motor imagery versus motor inhibition will require further clarification.
Concluding Remarks
Motor imagery likely corresponds to activation of the neural representations of a “potential” movement, which may be triggered by sensory stimuli or retrieved volitionally from motoric memory (simulation, emulation, or rehearsal). Although the motor-related areas are suggested to subserve motor imagery, it has remained unclear which aspects of the motor area functions are important for generating motor imagery. By incorporating the instructed delay period, the present study showed that activity of the motor network during motor imagery was associated more closely with that during preexecutive stage of movement than that during movement execution stage and analysis of sensory afferents. A gradient of imagery- and movement-related activity in the frontoparietal cortex may reflect the extent to which each area contributes more to preexecutive processing or executive processing. As the functions of the motor-related areas at the preexecution stage may be applicable to behaviors not directly associated with movement (Hanakawa et al. 2002; Abe et al. 2007), further studies to clarify the roles of preexecutive aspects of motor network will be warranted.
Funding
Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH); NINDS, NIH (Intramural Competitive Fellowship to T.H.); Ministry of Education, Culture, Sports, Science, and Technology, Japan (Grant-in-Aid on Fundamental Research (C) (17500210) to T.H.); Priority Areas (Mobiligence Project 17022023); Pfizer grant to the Foundation for the NIH (to M.A.D.).
Acknowledgments
We thank Devera G. Schoenberg, MSc, for skillful editing. Conflict of Interest: None declared.
References
- Abe M, Hanakawa T, Takayama Y, Kuroki C, Ogawa S, Fukuyama H. Functional coupling of human prefrontal and premotor areas during cognitive manipulation. J Neurosci. 2007;27:3429–3438. doi: 10.1523/JNEUROSCI.4273-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- Amiez C, Kostopoulos P, Champod AS, Petrides M. Local morphology predicts functional organization of the dorsal premotor region in the human brain. J Neurosci. 2006;26:2724–2731. doi: 10.1523/JNEUROSCI.4739-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode S, Koeneke S, Jancke L. Different strategies do not moderate primary motor cortex involvement in mental rotation: a TMS study. Behav Brain Funct. 2007;3:38. doi: 10.1186/1744-9081-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig (Germany): Barth; 1909. [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Bushara KO, Hanakawa T, Immisch I, Toma K, Kansaku K, Hallett M. Neural correlates of cross-modal binding. Nat Neurosci. 2003;6:190–195. doi: 10.1038/nn993. [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Valyear KF, Culham JC, Kohler S, Obhi SS, Marzi CA, Goodale MA. Dissociating arbitrary stimulus-response mapping from movement planning during preparatory period: evidence from event-related functional magnetic resonance imaging. J Neurosci. 2006;26:2704–2713. doi: 10.1523/JNEUROSCI.3176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of mental rehearsal in dorsal premotor cortex. Nature. 2004;431:993–996. doi: 10.1038/nature03005. [DOI] [PubMed] [Google Scholar]
- Crammond DJ. Motor imagery: never in your wildest dream. Trends Neurosci. 1997;20:54–57. doi: 10.1016/s0166-2236(96)30019-2. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol. 2000;84:986–1005. doi: 10.1152/jn.2000.84.2.986. [DOI] [PubMed] [Google Scholar]
- Decety J. The neurophysiological basis of motor imagery. Behav Brain Res. 1996;77:45–52. doi: 10.1016/0166-4328(95)00225-1. [DOI] [PubMed] [Google Scholar]
- Dechent P, Merboldt KD, Frahm J. Is the human primary motor cortex involved in motor imagery? Brain Res Cogn Brain Res. 2004;19:138–144. doi: 10.1016/j.cogbrainres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Honda M, Sadato N, Raman R, Hallett M. Cerebral processes related to visuomotor imagery and generation of simple finger movements studied with positron emission tomography. NeuroImage. 1998;7:73–85. doi: 10.1006/nimg.1997.0314. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Pelisson D, Grethe JS, Alexander GE, Urquizar C, Prablanc C, Grafton ST. Functional adaptation of reactive saccades in humans: a PET study. Exp Brain Res. 2000;132:243–259. doi: 10.1007/s002210000342. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Wise SP. Visuospatial versus visuomotor activity in the premotor and prefrontal cortex of a primate. J Neurosci. 1993;13:1227–1243. doi: 10.1523/JNEUROSCI.13-03-01227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Geyer S, Naito E. Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J Neurophysiol. 2003;90:3304–3316. doi: 10.1152/jn.01113.2002. [DOI] [PubMed] [Google Scholar]
- Facchini S, Muellbacher W, Battaglia F, Boroojerdi B, Hallett M. Focal enhancement of motor cortex excitability during motor imagery: a transcranial magnetic stimulation study. Acta Neurol Scand. 2002;105:146–151. doi: 10.1034/j.1600-0404.2002.1o004.x. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Frak V, Paulignan Y, Jeannerod M. Orientation of the opposition axis in mentally simulated grasping. Exp Brain Res. 2001;136:120–127. doi: 10.1007/s002210000583. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline J. To smooth or not to smooth? Bias and efficiency in fMRI time-series analysis. NeuroImage. 2000;12:196–208. doi: 10.1006/nimg.2000.0609. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. NeuroImage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardin E, Sirigu A, Lehericy S, Poline JB, Gaymard B, Marsault C, Agid Y, Le Bihan D. Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex. 2000;10:1093–1104. doi: 10.1093/cercor/10.11.1093. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K. Functional neuroanatomy of the primate isocortical motor system. Anat Embryol (Berl) 2000;202:443–474. doi: 10.1007/s004290000127. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res. 1996;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Grezes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum Brain Mapp. 2001;12:1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grush R. The emulation theory of representation: motor control, imagery, and perception. Behav Brain Sci. 2004;27:377–396. doi: 10.1017/s0140525x04000093. discussion 396–442. [DOI] [PubMed] [Google Scholar]
- Hagura N, Takei T, Hirose S, Aramaki Y, Matsumura M, Sadato N, Naito E. Activity in the posterior parietal cortex mediates visual dominance over kinesthesia. J Neurosci. 2007;27:7047–7053. doi: 10.1523/JNEUROSCI.0970-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Okada T, Fukuyama H, Shibasaki H. Neural correlates underlying mental calculation in abacus experts: a functional magnetic resonance imaging study. Neuroimage. 2003;19:296–307. doi: 10.1016/s1053-8119(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Sawamoto N, Okada T, Yonekura Y, Fukuyama H, Shibasaki H. The role of rostral Brodmann area 6 in mental-operation tasks: an integrative neuroimaging approach. Cereb Cortex. 2002;12:1157–1170. doi: 10.1093/cercor/12.11.1157. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Zito G, Dimyan MA, Hallett M. Brain activity during visuomotor behavior triggered by arbitrary and spatially constrained cues: an fMRI study in humans. Exp Brain Res. 2006;172:275–282. doi: 10.1007/s00221-005-0336-z. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Immisch I, Toma K, Dimyan MA, van Gelderen P, Hallett M. Functional properties of brain areas associated with motor execution and imagery. J Neurophysiol. 2003;89:989–1002. doi: 10.1152/jn.00132.2002. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Parikh S, Bruno MK, Hallett M. Finger and face representations in the ipsilateral precentral motor areas in humans. J Neurophysiol. 2005;93:2950–2958. doi: 10.1152/jn.00784.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. NeuroImage. 1998;7:754. [Google Scholar]
- Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr Opin Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Luders HO, Collura TF, Burgess RC, Morris HH, Hamano T, Shibasaki H. Subdural potentials at orbitofrontal and mesial prefrontal areas accompanying anticipation and decision making in humans: a comparison with Bereitschaftspotential. Electroencephalogr Clin Neurophysiol. 1996;98:206–212. doi: 10.1016/0013-4694(95)00239-1. [DOI] [PubMed] [Google Scholar]
- Imbiriba LA, Rodrigues EC, Magalhaes J, Vargas CD. Motor imagery in blind subjects: the influence of the previous visual experience. Neurosci Lett. 2006;400:181–185. doi: 10.1016/j.neulet.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Immisch I, Waldvogel D, van Gelderen P, Hallett M. The role of the medial wall and its anatomical variations for bimanual antiphase and in-phase movements. NeuroImage. 2001;14:674–684. doi: 10.1006/nimg.2001.0856. [DOI] [PubMed] [Google Scholar]
- Ives JR, Warach S, Schmitt F, Edelman RR, Schomer DL. Monitoring the patient's EEG during echo planar MRI. Electroencephalogr Clin Neurophysiol. 1993;87:417–420. doi: 10.1016/0013-4694(93)90156-p. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The representing brain. Neural correlates of motor intention and imagery. Behav Brain Sci. 1994;17:187–245. [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. NeuroImage. 2001;14:S103–S109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Deciding not to GO: neuronal correlates of response selection in a GO/NOGO task in primate premotor and parietal cortex. Cereb Cortex. 1995;5:410–428. doi: 10.1093/cercor/5.5.410. [DOI] [PubMed] [Google Scholar]
- Li S, Kamper DG, Stevens JA, Rymer WZ. The effect of motor imagery on spinal segmental excitability. J Neurosci. 2004;24:9674–9680. doi: 10.1523/JNEUROSCI.2781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre- SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Naito E, Roland PE, Ehrsson HH. I feel my hand moving: a new role of the primary motor cortex in somatic perception of limb movement. Neuron. 2002;36:979–988. doi: 10.1016/s0896-6273(02)00980-7. [DOI] [PubMed] [Google Scholar]
- Ohbayashi M, Ohki K, Miyashita Y. Conversion of working memory to motor sequence in the monkey premotor cortex. Science. 2003;301:233–236. doi: 10.1126/science.1084884. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CC, Jerabek PA, Lancaster JL. Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature. 1995;375:54–58. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;34:475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Paus T, Tomaiuolo F, Otaky N, MacDonald D, Petrides M, Atlas J, Morris R, Evans AC. Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map. Cereb Cortex. 1996;6:207–214. doi: 10.1093/cercor/6.2.207. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation [review] Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, Bazzocchi M, di Prampero PE. Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci. 1996;16:7688–7698. doi: 10.1523/JNEUROSCI.16-23-07688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. NeuroImage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol. 2002;12:149–154. doi: 10.1016/s0959-4388(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Roth M, Decety J, Raybaudi M, Massarelli R, Delon-Martin C, Segebarth C, Morand S, Gemignani A, Decorps M, Jeannerod M. Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. Neuroreport. 1996;7:1280–1284. doi: 10.1097/00001756-199605170-00012. [DOI] [PubMed] [Google Scholar]
- Sauner D, Bestmann S, Siebner HR, Rothwell JC. No evidence for a substantial involvement of primary motor hand area in handedness judgements: a transcranial magnetic stimulation study. Eur J Neurosci. 2006;23:2215–2224. doi: 10.1111/j.1460-9568.2006.04731.x. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol. 1995;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD, Steyvers M, Levin O, Swinnen SP. Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp Brain Res. 2006;168:157–164. doi: 10.1007/s00221-005-0078-y. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Toni I, Schluter ND, Josephs O, Friston K, Passingham RE. Signal-, set- and movement-related activity in the human brain: an event-related fMRI study [published erratum appears in Cereb Cortex 1999;9(2):196] Cereb Cortex. 1999;9:35–49. doi: 10.1093/cercor/9.1.35. [DOI] [PubMed] [Google Scholar]
- Tyszka JM, Grafton ST, Chew W, Woods RP, Colletti PM. Parceling of mesial frontal motor areas during ideation and movement using functional magnetic resonance imaging at 1.5 Tesla [see comments] Ann Neurol. 1994;35:746–749. doi: 10.1002/ana.410350617. [DOI] [PubMed] [Google Scholar]
- van Gelderen P, Ramsey NF, Liu G, Duyn JH, Frank JA, Weinberger DR, Moonen CT. Three-dimensional functional magnetic resonance imaging of human brain on a clinical 1.5-T scanner. Proc Natl Acad Sci USA. 1995;92:6906–6910. doi: 10.1073/pnas.92.15.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: an electrical sign of sensorimotor association and expectency in the human. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Wise SP. The primate premotor cortex: past, present, and preparatory. Annu Rev Neurosci. 1985;8:1–19. doi: 10.1146/annurev.ne.08.030185.000245. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA. Arbitrary associations between antecedents and actions. Trends Neurosci. 2000;23:271–276. doi: 10.1016/s0166-2236(00)01570-8. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]