Abstract
Stimulation of the amygdala produces pupil dilation in animal and human subjects. The present study examined whether the amygdala is sensitive to variations in the pupil size of others. Male subjects underwent event-related functional magnetic resonance imaging while passively viewing unfamiliar female faces whose pupils were either unaltered (natural variations in large and small pupils) or altered to be larger or smaller than their original size. Results revealed that the right amygdala and left amygdala/substantia innominata were sensitive to the pupil size of others, exhibiting increased activity for faces with relatively large pupils. Upon debrief, no subject reported being aware that the pupils had been manipulated. These results suggest a function for the amygdala in the detection of changes in pupil size, an index of arousal and/or interest on the part of a conspecific, even in the absence of explicit knowledge.
Keywords: amygdala, arousal, attractiveness, fMRI, pupil size
Introduction
Humans are well adapted to detect and interpret the subtle yet meaningful cues found within the human face. For example, eye widening is a signal of heightened vigilance and arousal on the part of the expresser, indicating that this individual has detected a salient event in the immediate environment. The human amygdala is sensitive to signals such as these, in part because of the outcomes these expressions have predicted for us in the past. For example, the widening eyes of a conspecific suggest a significant change in that individual's arousal state in response to a proximal environmental event, and communicate to the viewer that he or she may do well to adopt a similar state of arousal. Consistent with this notion, multiple neuroimaging studies have demonstrated that the amygdala is responsive to fearful facial expressions (Breiter et al. 1996; Morris et al. 1996; Whalen et al. 2001) and that subjective awareness of the expression is not necessary to produce this response (Whalen et al. 1998; Pessoa et al. 2006). Indeed, a particular sensitivity to the telltale, widened eyes of a fearful face may be the basis for this effect (Whalen et al. 2004).
Like eye widening, pupil dilation is considered another facial signal indicating heightened vigilance on the part of a conspecific. Indeed, data from both animal (Applegate et al. 1983; Ursin and Kaada 1960) and human (Gloor 1997) subjects show that electrical stimulation of the amygdala produces both eye-widening and pupil dilation. Based upon these findings, the present study was designed to investigate whether greater amygdala activity would be observed in response to presented faces with larger pupils compared with faces with smaller pupils, and, if so, whether this response would occur irrespective of participants' subjective awareness of the manipulation.
Interestingly, pupil dilation has long been thought to convey interest in conspecifics, as women of the Victorian Era and the Italian Renaissance purposefully dilated their pupils using a poisonous extract from the Belladonna plant to appear more attractive to male suitors. Because experimental research on this subject has offered conflicting reports as to whether pupil size affects the perceived attractiveness of presented faces (Hess and Polt 1960; Janisse 1973) and attractiveness ratings can be associated with amygdala activity (Winston et al. 2007), we thought it important to assess a subject group where attractiveness ratings could also be readily measured. To this end, we studied male subjects viewing images of female faces that depicted big or small pupils (Fig. 1). Such a design allows for an assessment of the relationship between amygdala activity and pupil size, where any impact of the perceived attractiveness of the faces can be determined.
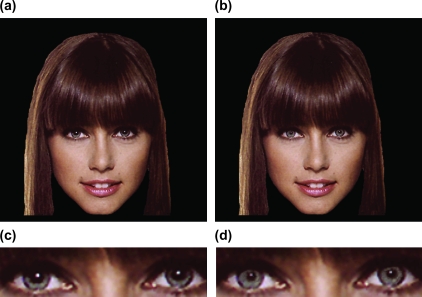
Figure 1.
Examples of big- and small-pupil faces. Face images depicted females with naturally big pupils and naturally small pupils. An altered big- or small-pupil counterpart of each face was created such that the same individual could be represented in either the big- or small-pupil condition. Shown here is a photograph depicting naturally big pupils (a, c) and its altered small-pupil counterpart (b, d).
Methods
Subjects
Twenty-seven right-handed males from the Dartmouth community between the ages of 18 and 33 (mean age = 22 years) participated in this experiment. No subjects reported abnormal neurological history and all had normal or corrected-to-normal visual acuity. Each subject provided informed consent in accordance with the guidelines set by the Committee for the Protection of Human Subjects at Dartmouth College, and received either course credit or monetary compensation for participating in this study.
Apparatus
Imaging was performed on a Philips Intera Achieva 3-Tesla scanner (Phillips Medical Systems, Bothell, WA) with a SENSE (SENSEitivity Encoding) head coil. During scanning, visual stimuli were generated with an Apple G3 Laptop computer running Psyscope software (Cohen et al. 1993). An Epson (model ELP-7000) LCD projector displayed stimuli on a screen positioned at the head end of the scanner bore which subjects were able to view through a mirror mounted on top of the head coil. Following scanning subjects were behaviorally tested via an Apple PowerBook G4 running Psyscope software.
Imaging
Anatomic images were acquired using a high-resolution 3D magnetization-prepared rapid gradient echo sequence (60 sagittal slices, time echo [TE] = 4.6 ms, time repetition [TR] = 9.9 ms, flip angle = 8°, voxel size = 1 × 1 x 1 mm). Functional images were collected in a single run using T2* fast field echo, echo planar functional images sensitive to blood oxygenation level–dependent (BOLD) contrast (TR = 2500 ms, TE= 35 ms, flip angle = 90°, 3 × 3 mm in-plane resolution). During the functional run, 190 sets of axial images (36 slices, 3.5-mm slice thickness, 0.5 mm skip between slices) were acquired parallel to the horizontal axis of the anterior and posterior commissures, allowing complete brain coverage.
Procedure
A total of 73 unfamiliar female faces were compiled from a standardized face database used in previous studies (Kelley et al. 1998; Wig et al. 2004). These forward-facing face images were originally sampled from the media and cropped below the chin line and around the outer hairline and were presented on a solid black background. Approximately half of the images (37) portrayed female faces with naturally large pupils; the remaining half (36) portrayed female faces with naturally small pupils as determined by 3 independent raters. Each image was digitally edited using Adobe Photoshop 7.0 (San Jose, CA) to create an altered big-pupil or small-pupil counterpart. In order to preserve the natural variations in pupil size within a biologically plausible range, naturally big pupils were reduced by 30% in area and naturally small pupils were enlarged by 30% in area (Fig. 1). This allowed each individual face to be presented in either its natural or altered state and ensured that faces comprising the big and small pupil conditions were made up of equal numbers of natural and altered faces. During scanning, subjects viewed only 1 version of the face. Face stimuli were further counterbalanced such that half of the subjects viewed the big-pupil version (e.g., naturally big) and the other half viewed the small-pupil version (e.g., altered small). The pupils of faces comprising the BIG condition were, on average, 52.4% of the entire iris size with sizes ranging from 2.54–4.60 mm, (mean = 3.44 mm, SD = 0.56 mm) whereas the pupils of faces comprising the SMALL condition were, on average, 36.5% of the entire iris, with sizes ranging from 1.52 to 4.24 mm (mean = 2.40 mm, SD = 0.49 mm). Pupil size did not differ between the left and right eyes of each face (BIG: mean right = 3.44 mm, mean left = 3.40 mm; t[72]=1.53, P = 0.131; SMALL: mean right = 2.40 mm, mean left = 2.36 mm; t[72] = 0.86, P = 0.393).
All faces were normalized on explicit measures of valence and arousal in a separate set of 15 male volunteers. Each subject viewed a single version of each face (natural or altered) and the stimuli were counterbalanced such that half of the subjects viewed the big-pupil version of a given face while the remaining half viewed the small-pupil version of that face. Explicit ratings of valence and arousal did not differ as a function of pupil size (VALENCE: t[14] = 0.96, P = 0.355; AROUSAL: t[14] = 1.44, P = 0.171).
During scanning, subjects passively viewed faces of each type (BIG and SMALL pupils) presented 1 at a time for 2000 ms each in an event-related functional run. Face trials were pseudorandomly intermixed with jittered periods of fixation, creating a variable interstimulus interval ranging from 0 to 7500 ms and allowing for computation of unique estimates of the hemodynamic response associated with viewing faces with BIG and SMALL pupils (Ollinger et al. 2001).
Although pupil dilation has been anecdotally linked to increases in perceived attractiveness, the extant experimental literature has been mixed (Hess and Polt 1960; Janisse 1973). Nonetheless, attractiveness ratings have been recently associated with changes in amygdala activity (Winston et al. 2007). To ensure that putative differences in amygdala activity in response to changes in pupil size were not confounded with changes in perceived attractiveness, each subject participated in a postscan behavioral session. Following scanning, subjects were asked to rate each face on a 9-point Likert scale of attractiveness (1 = extremely unattractive; 5 = average; 9 = extremely attractive). Faces were again presented in random order for 2000 ms followed by a 1500-ms fixation crosshair with the labeled scale appearing at the bottom of the screen below each face. Subjects were given 3500 ms to respond. Each participant 1st completed a practice session with a set of 15 novel faces in order to assimilate to the task, and then performed the task on the same faces they had viewed during scanning.
Assessment of Explicit Knowledge
To determine whether subjects were aware of the experimental manipulation, after viewing all faces, subjects were given a comprehensive list of 16 facial features (e.g., forehead, eyes, pupils, nose, nostrils) and were asked to recall if they noticed anything about the faces with respect to any of the listed features. After providing their responses, subjects were informed that 1 facial feature was in fact systematically varied. Subjects were then instructed to select 1 of the 16 facial features even if they felt that they were guessing.
Functional Magnetic Resonance Imaging Analysis
Functional magnetic resonance imaging (fMRI) data were analyzed using the general linear model for event-related designs in SPM2 (Wellcome Department of Cognitive Neurology, London, UK). Data were preprocessed to remove sources of noise and artifact, corrected for differences in acquisition time between slices for each whole-brain volume, realigned within the run to correct for head movement, and coregistered with each participant's anatomical data. Functional data were then transformed into a standard anatomical space (3-mm isotropic voxels) based on the ICBM 152 brain template (Montreal Neurological Institute) which approximates Talairach and Tournoux's (Talairach and Tournoux 1988) atlas space. Normalized data were then spatially smoothed (6-mm full-width-at-half-maximum) using a Gaussian kernel. Analyses took place at 2 levels: formation of statistical images and regional analysis of hemodynamic responses.
For each subject, a general linear model incorporating condition effects (modeled as events convolved with the canonical hemodynamic response function), and covariates of no interest (a session mean, a linear trend to account for low-frequency noise, and 6 movement parameters obtained from realignment) were used to compute parameter estimates (β) and t-contrast images (containing weighted parameter estimates) for each comparison at each voxel. These individual contrast images were then submitted to a 2nd-level, random-effects analysis to create mean t-images images (thresholded at P < 0.01, uncorrected, minimum cluster size = 5 voxels). Monte Carlo simulations indicate this threshold corresponds to a small-volume corrected alpha (P = 0.05) based upon the size of the amygdala bilaterally (Kim et al. 2003, 2004; Whalen et al. 2004; Johnstone et al. 2005).
In order to more closely explore the patterns of activity in the amygdala, region of interest (ROI) analyses were conducted. ROIs were defined functionally from a set of peak activations observed in the direct contrast of big- versus small-pupil faces (all contiguous voxels within 6 mm of the peak that exceeded P < 0.01). For each participant, signal intensities for face conditions relative to fixation baseline from ROIs were submitted to offline statistical analyses.
Results
fMRI Results
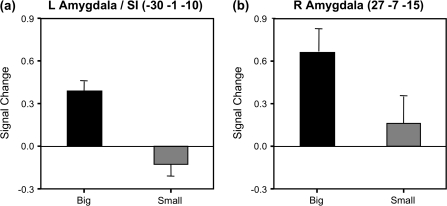
When compared directly, big-pupil faces yielded greater activity than small-pupil faces within the amygdaloid complex (Fig. 2). This effect was observed bilaterally, in the amygdala proper (right amygdala ROI: 27 −7 −15; t[26] = 3.32, P < 0.005) and extended into the substantia innominata within the ventral basal forebrain in the left hemisphere (Left Amygdala/SI ROI: −30 −1 −10; t[26] = 3.96, P < 0.005) (Fig. 3). ROI analyses further revealed that when each face condition was considered relative to the fixation baseline, activity significantly increased in response to big pupils (R Amygdala: t[26] = 4.67, P < 0.0001; L Amygdala/SI: t[26] = 5.34, P < 0.0001) and did not differ from baseline in response to small pupils (R Amygdala: t[26] = 0.85, P = 0.40; L Amygdala/SI: t[26] = −1.43, P = 0.17).
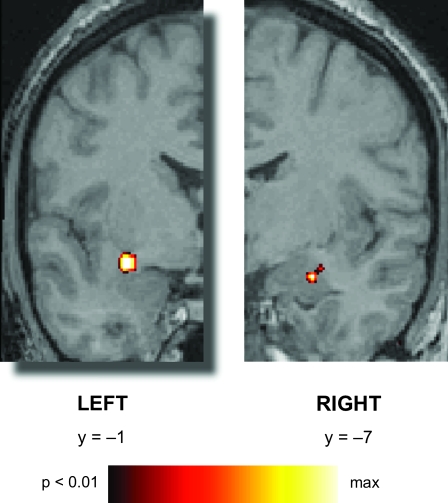
Figure 2.
Amygdala responses during passive viewing of big- and small-pupil faces. Coronal sections show greater activity for big- versus small-pupil faces. Images are coronal sections in Talairach and Tournoux (1988) atlas space. Colored pixels exceeded the statistical threshold (P < 0.01, uncorrected, minimum cluster size = 5 voxels) and are superimposed on corresponding anatomy images. The left side of the image corresponds to the left hemisphere at a y coordinate of −1 and the right side of the image corresponds to a y coordinate of −7. Greater activity to BIG versus SMALL pupils was observed in both the right amygdala (27 −7 −15) and the left amygdala extending into the substantia innominata within the ventral basal forebrain (−30 −1 −10).
Figure 3.
Signal change in left- (a) and right-amygdaloid (b) regions for big- and small-pupil faces. Signal intensities (arbitrary units) for each condition are plotted relative to a baseline control condition (fixating a crosshair). For both ROIs, activity was significantly greater than baseline when subjects viewed big-pupil faces but was no different from baseline when viewing small-pupil faces. Error bars indicate standard error of the mean.
Importantly, the greater amygdala activity for BIG versus SMALL pupils did not depend on whether pupils were viewed in their natural or altered state. When amygdala activity was considered as a function of both pupil size (BIG vs. SMALL) and image type (NATURAL vs. ALTERED), a 2 × 2 repeated measures ANOVA revealed a main effect of pupil size (R Amygdala: F1,26 = 10.35, P < 0.005; L Amygdala/SI: F1,26 = 30.06, P < 0.0001), no main effect of image type (both F's < 1), and no interaction (both F's < 1).
Although the amygdala was the sole a priori ROI herein, other brain regions identified exhibiting greater activity for big- versus small-pupil faces included the left superior frontal gyrus (Broadmann's area [BA] 8 and 11), the medial frontal gyrus (BA 10), the lingual gyrus (BA 19), a region of the left inferior parietal lobe (BA 40), and the left ventral lateral nucleus of the thalamus (Supplementary Table 1). The response in regions including the precuneus (BA 7), right inferior frontal gyrus (BA 45), and the inferior occipital gyrus (BA 18) was greater for viewing SMALL compared with BIG pupils (Supplementary Table 1).
Effect of Attractiveness
Behaviorally there were no differences in subsequent ratings of attractiveness between faces with big pupils and faces with small pupils (mean BIG = 4.65, SD BIG = 0.63, mean SMALL = 4.56, SD SMALL = 0.65; t[26] = 1.50, P = 0.15). Importantly, the difference in amygdala activity in response to BIG versus SMALL pupils did not reflect the perceived attractiveness of the faces. When attractiveness ratings were included as a separate covariate in the model, amygdala sensitivity to pupil size was preserved (R Amygdala ROI: 27 −7 −15; t[24] = 2.83, P < 0.01; L Amygdala/SI ROI: −30 −1 −10; t[24] = 4.84, P < 0.001).
Effect of Expression
A final potential factor we considered post hoc was whether facial expression influenced amygdala response to pupil size. Although facial expressions were not explicitly manipulated in the current study, the face stimuli employed here included both neutral (i.e., expressionless) and happy (i.e., smiling) faces. To explore this possibility, we conducted a 2nd 2 × 2 ANOVA examining the effects of pupil size and facial expression. Results of this analysis revealed a main effect of pupil size in both the left and right amygdala (R Amygdala: F1,26 = 9.33, P < 0.005; L Amygdala/SI: F1,26 = 25.67, P < 0.0001), no main effect of facial expression (R Amygdala: F1,26 = 2.24, P = 0.15; L Amygdala/SI: F < 1), and no interaction (R Amygdala: F < 1; L Amygdala/SI: F1,26 = 1.84, P = 0.19).
Debriefing Results
An important facet of the present study design was that big- and small-pupil images of the same facial identity were not presented within subject. This was done to mitigate subjects' awareness of the experimental manipulation of pupil size. When subjects were given a list of 16 face parts and asked to comment on any face feature they wished, none spontaneously reported that they had noticed anything about pupils. Further, when subjects were then informed that 1 facial feature had indeed been manipulated, none correctly guessed pupil size.
Discussion
In the present study, greater amygdala activity was observed in response to faces with big versus small pupils. This sensitivity to the larger pupil size of others was observed in bilateral regions across the amygdaloid complex and emerged even though subjects did not report explicit knowledge of the varying pupil sizes. Moreover, changes in pupil size were unrelated to changes in perceived attractiveness. Subjective ratings of attractiveness did not differ between big- and small-pupil faces, a finding that discounts attractiveness as a basis for the observed effect. Further, the amygdala's response to big- versus small-pupil faces did not differ as a function of the facial expressions considered here (i.e., neutral and happy faces).
Pupil dilation has been interpreted as a general indicator of increased vigilance, arousal, and/or interest (Hess 1965; Steinhauer et al. 2004), indexing behavioral responses as disparate as political interests, hunger, cognitive load, and attraction (Hess 1965; Steinhauer et al. 2004). The nonspecific nature of pupil dilation is relevant when considered in light of data showing that the amygdala is particularly responsive when cues predict multiple outcomes (Kapp et al. 1992; Whalen 1998; Holland and Gallagher 1999). In this way, amygdala activity potentiates neuronal responses in sensory cortical regions that may, in turn, facilitate subsequent information processing (Amaral et al. 1992; Kapp et al. 1992; Whalen 1998; Morris et al. 2001; Pessoa et al. 2006; Phelps 2006). Put simply, amygdala sensitivity to pupil dilation is usefully conceptualized as an alerting response related to the wide range of biologically relevant outcomes that this signal can predict.
Pupil dilation is produced through the sympathetic nervous system, whereas pupil constriction is produced by the parasympathetic nervous system. Specifically, dilation occurs via sympathetic nerve fibers arising from the 1st, 2nd, and 3rd thoracic nerves of the spinal cord, which innervate the radial muscles of the iris through connections in the superior cervical sympathetic trunk ganglia. By contrast, the parasympathetic nerve fibers responsible for pupil constriction arise from the Edinger–Westphal nucleus within the brain stem and innervate the circular muscles of the iris. Although it is anatomically plausible then that the amygdala facilitates pupil dilation by either stimulating sympathetic inputs or inhibiting parasympathetic inputs, research to date suggests that the amygdala's effect on dilation is likely an inhibition of parasympathetic input (Bitsios et al. 1996, 1998, 1999; Hourdaki et al. 2005). The existing data further suggest that the amygdala influences pupil dilation through an indirect route via the hypothalamus (Koss and Wang 1972; Saper et al. 1976; Holstege 1987) and/or locus coeruleus (Loewy et al. 1973; Breen et al. 1983; Koss et al. 1984). Still, direct modulation of pupillary kinetics is plausible as the amygdala is known to send extensive projections to these regions of the brain stem and spinal cord (Cedarbaum and Aghajanian 1978) that directly modulate pupil size.
Upon excitation of the sympathetic nervous system, the pupils obligatorily dilate, thus a conspecific's pupil dilation is often an indicator of his or her increased arousal. This notion holds similarly for eye-widening, another indicator of heightened arousal, and accordingly, these data support and extend the finding that the amygdala is also sensitive to the eye-region of the face (Adolphs et al. 2005) and more specifically, eye-widening (Morris et al. 2002; Whalen et al. 2004). Because stimulation of the amygdala produces an increase in nonspecific arousal that is accompanied by peripheral responses such as eye-widening and pupil dilation (Ursin and Kaada 1960; Applegate et al. 1983; Kapp et al. 1994), it will be important to investigate in a future study using a similar experimental design whether the pupil size of participants varies as a function of the size of observed pupils.
Indeed, a recent study demonstrated that amygdala activity and the size of an observer's pupil may be associated with the size of perceived pupils (Harrison et al. 2006). This study investigated whether amygdala activity in response to pupil size differences interacted with the emotional expression of the presented face (e.g., fearful, angry, sad and neutral). The results showed that the amygdala was more responsive to smaller pupils sizes, but only for sad faces, suggesting that this result (opposite to that observed here) may be related to an important interaction between facial expression, amygdala activity and pupil size. The challenge then for future research will be to disentangle the meaning of amygdala activity to the pupil size of a conspecific across differing experimental designs. For example, the findings of Harrison et al. (2006) showed that the amygdala was only sensitive to pupil size for faces with sad expressions; however, varying pupil sizes for sad faces also produced significant differences in explicit ratings of arousal and valence. Given the broad literature demonstrating that the amygdala is sensitive to changes in valence and arousal (Anderson et al. 2003; Kim et al. 2003, 2004; Small et al. 2003), an alternative explanation of the Harrison et al. (2006) findings is that the amygdala response observed in their work reflected a more general sensitivity to valence and/or arousal. Furthermore, they report that subjects' pupil size mirrored these effects, demonstrating contagion such that subjects' pupils constricted in response to the viewing small-pupil faces. Although we did not measure such changes in the present study, 1 critical caveat to consider is that direct stimulation of the amygdala is consistently associated with pupil dilation rather than constriction (Ursin and Kaada 1960; Koss and Wang 1972; Loewy et al. 1973; Saper et al. 1976; Cedarbaum and Aghajanian 1978; Applegate et al. 1983; Breen et al. 1983; Koss et al. 1984; Kapp et al. 1994; Holstege 1987; Bitsios et al. 1996; Bitsios et al. 1998, 1999; Gloor 1997; Hourdaki et al. 2005). Thus, it is somewhat unclear under what circumstances BOLD signal activation in the amygdala might index pupil constriction.
In the present experimental design, pupil size was manipulated within stimulus conditions that controlled for explicit valence, arousal, and attractiveness ratings. Indeed, when comparing the present results to other experimental designs, it will be critical to note that these findings were observed in male subjects while viewing female faces. Further, in the present study, subjects did not have any explicit knowledge of the differing pupil sizes between big- and small-pupil faces, perhaps owing to the biologically plausible range of pupil sizes presented and the slight overlap in size across conditions. Thus, it is possible that amygdala response to these changes was observed on an implicit basis in the present study. It is interesting to note that Harrison et al. (2006) utilized a much greater range of pupil sizes (i.e., 64–180% of natural size) which was likely more noticeable to subjects, offering another potential basis for the differential effects observed across these 2 initial studies. Clearly, then, both studies support the notion that the amygdala is sensitive to subtle changes in pupil size—findings that warrant future work aimed at determining the precise functional relationship between amygdala activity, pupil size, and implicit preference.
Conclusion
Though much is known about human amygdala responses to stimuli that produce a strong state of fear (LeDoux 1996; LaBar et al. 1998), more recent work has begun to elucidate a role for the amygdala in detecting biologically relevant stimuli that call for a more generalized (Hamann et al. 2002) or more subtle level of state change (Whalen et al. 1998; Davis and Whalen 2001). Here we offer additional data showing that pupil dilation is 1 such subtle signal of nonspecific arousal and vigilance to which the amygdala is sensitive. Importantly, the big- and small-pupil faces in our work did not differ in explicit ratings of arousal, valence, or attractiveness, yet the amygdala responded with increased activity to the big-pupil faces. Finally, this effect occurred without explicit awareness on the part of subjects. Future research could seek to define the effect of this implicit environmental monitoring in terms of behavioral outcomes for the organism of study.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Institute of Health (NIMH 069315 and NIMH 080716). Dartmouth Brain Imaging Center.
Supplementary Material
Acknowledgments
We thank Jasmin Cloutier, Jed Dobson, Howard Hughes, Jay Hull, Joe Moran, Tammy Moran, Leah Somerville, and Gagan Wig for their assistance. Conflict of Interest: None declared.
References
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory and mental dysfunction. New York: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav. 1983;31:353–360. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Langley RW, Szabadi E, Bradshaw CM. Comparison of the effects of clonidine on tyramine- and methoxamine-evoked mydriasis in man. Br J Clin Pharmacol. 1996;41:269–275. doi: 10.1046/j.1365-2125.1996.03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsios P, Langley RW, Tavernor S, Pyykko K, Scheinin M, Szabadi E, Bradshaw CM. Comparison of the effects of moclobemide and selegiline on tyramine-evoked mydriasis in man. Br J Clin Pharmacol. 1998;45:551–558. doi: 10.1046/j.1365-2125.1998.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsios P, Philpott A, Langley RW, Bradshaw CM, Szabadi E. Comparison of the effects of diazepam on the fear-potentiated startle reflex and the fear-inhibited light reflex in man. J Psychopharmacol. 1999;13:226–234. doi: 10.1177/026988119901300303. [DOI] [PubMed] [Google Scholar]
- Breen LA, Burde RM, Loewy AD. Brainstem connections to the Edinger-Westphal nucleus of the cat: a retrograde tracer study. Brain Res. 1983;261:303–306. doi: 10.1016/0006-8993(83)90633-9. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Aghajanian GK. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol. 1978;178:1–16. doi: 10.1002/cne.901780102. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. Psyscope: a new graphic interactive environment for designing psychology experiments. Behav Res Methods Instrum Comput. 1993;25:257–271. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Gloor P. The temporal lobe and limbic system. New York: Oxford University Press; 1997. [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol Sci. 2002;13:135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Singer T, Rotshtein P, Dolan RJ, Critchley HD. Pupillary contagion: central mechanisms engaged in sadness processing. Soc cogn Affect Neurosci. 2006;1:5–17. doi: 10.1093/scan/nsl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess EH. Attitude and pupil size. Sci Am. 1965;212:46. doi: 10.1038/scientificamerican0465-46. [DOI] [PubMed] [Google Scholar]
- Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science. 1960;132:349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Holstege G. Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: an HRP and autoradiographic tracing study in the cat. J Comp Neurol. 1987;260:98–126. doi: 10.1002/cne.902600109. [DOI] [PubMed] [Google Scholar]
- Hourdaki E, Giakoumaki SG, Grinakis V, Theou K, Karataraki M, Bitsios P. Parametric exploration of the fear-inhibited light reflex. Psychophysiology. 2005;42:447–455. doi: 10.1111/j.1469-8986.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- Janisse MP. Pupil size and affect—critical review of literature since 1960. Can Psychol. 1973;14:311–329. [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, Whalen PJ. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage. 2005;25:1112–1123. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Supple WF, Jr, Whalen PJ. Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behav Neurosci. 1994;108:81–93. doi: 10.1037//0735-7044.108.1.81. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Whalen PJ, Supple WF, Pascoe JP. Amygdaloid contributions to conditioned arousal and sensory information processing. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory and mental dysfunction. New York: Wiley-Liss; 1992. pp. 229–254. [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Koss MC, Gherezghiher T, Nomura A. CNS adrenergic inhibition of parasympathetic oculomotor tone. J Auton Nerv Syst. 1984;10:55–68. doi: 10.1016/0165-1838(84)90067-5. [DOI] [PubMed] [Google Scholar]
- Koss MC, Wang SC. Brainstem loci for sympathetic activation of the nictitating membrane and pupil in the cat. Am J Physiol. 1972;222:900–905. doi: 10.1152/ajplegacy.1972.222.4.900. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotional networks and motor control: a fearful view. Prog Brain Res. 1996;107:437–446. doi: 10.1016/s0079-6123(08)61880-4. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Araujo JC, Kerr FW. Pupillodilator pathways in the brain stem of the cat: anatomical and electrophysiological identification of a central autonomic pathway. Brain Res. 1973;60:65–91. doi: 10.1016/0006-8993(73)90851-2. [DOI] [PubMed] [Google Scholar]
- Morris JS, Buchel C, Dolan RJ. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage. 2001;13:1044–1052. doi: 10.1006/nimg.2000.0721. [DOI] [PubMed] [Google Scholar]
- Morris JS, deBonis M, Dolan RJ. Human amygdala responses to fearful eyes. Neuroimage. 2002;17:214–222. doi: 10.1006/nimg.2002.1220. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Japee S, Sturman D, Ungerleider LG. Target visibility and visual awareness modulate amygdala responses to fearful faces. Cereb Cortex. 2006;16:366–375. doi: 10.1093/cercor/bhi115. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Siegle GJ, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. Int J Psychophysiol. 2004;52:77–86. doi: 10.1016/j.ijpsycho.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Ursin H, Kaada BR. Functional localization within the amygdaloid complex in the cat. Electroencephalogr Clin Neurophysiol. 1960;12:1–20. doi: 10.1016/0013-4694(60)90058-4. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1998;7:177–188. [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Wig GS, Miller MB, Kingstone A, Kelley WM. Separable routes to human memory formation: dissociating task and material contributions in the prefrontal cortex. J Cogn Neurosci. 2004;16:139–148. doi: 10.1162/089892904322755629. [DOI] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.